Abstract
STUDY QUESTION
Is the female 2th- to 4th-finger ratio (2D:4D) associated with fecundity as measured by time-to-pregnancy (TTP)?
SUMMARY ANSWER
Our study does not support an association between female 2D:4D and TTP.
WHAT IS KNOWN ALREADY
The 2th- to 4th-finger ratio (2D:4D) has been proposed as a potential indicator of greater androgen exposure during fetal development. Women exposed in utero to unbalanced steroid hormones may have impaired fecundity in the adulthood. Fecundity is often measured by TTP, an epidemiological tool commonly used to assess the impact of environmental factors in human conception.
STUDY DESIGN, SIZE, DURATION
The Maternal-Infant Research on Environmental Chemicals (MIREC) Study is a pregnancy and birth cohort of 2001 women recruited before 14 weeks of gestation in 10 cities across Canada between 2008 and 2011. The present analysis is part of MIREC-CD Plus, a follow-up study in a subsample of some 800 MIREC mothers and their children from 2012 to 2015.
PARTICIPANTS/MATERIALS, SETTING, METHODS
TTP and maternal characteristics were collected from questionnaires administered during the first trimester of pregnancy as part of the MIREC study. Digital pictures of the ventral surface of both hands were obtained in the MIREC mothers at the MIREC-CD Plus follow-up study. The 2D:4D was calculated as the ratio of the second and fourth fingers of each hand. The exposure of interest was the 2D:4D of the women categorized by tertiles, or dichotomized as ≥1 (index finger longer than the ring finger) or <1 (ring finger longer than the index finger, implying greater androgen exposure during fetal development). The final sample included 696 mothers. Statistical analyses included discrete-time Cox proportional hazard models, allowing adjustment for potential confounding factors.
MAIN RESULTS AND THE ROLE OF CHANCE
There was no evidence of diminished/increased fecundability according to the 2D:4D, neither on the right nor on the left hand. In our analysis by tertiles, the smallest 2D:4D (i.e. higher androgen exposure during fetal life) resulted in FORs higher than 1 (i.e. shorter TTP) in both hands, although this was not statistically significant (FOR 1.19 [95% CI 0.93, 1.51] in the right hand and 1.16 [95% CI 0.91, 1.47] in the left hand). In the dichotomous analysis, 2D:4D <1 resulted in FORs higher than 1 (i.e. shorter TTP), but this was also not statistically significant (FOR 1.08 [95% CI 0.88, 1.33] in the right hand and 1.14 [95% CI 0.92, 1.42] in the left hand). Our large sample size resulted in a high statistical power to exclude an association between female 2D:4D and TTP.
LIMITATIONS, REASONS FOR CAUTION
The MIREC Study is a cohort of pregnant women, and therefore, women with infertility were excluded by design from our study.
WIDER IMPLICATIONS OF THE FINDINGS
Our data do not provide evidence for an association between female 2D:4D and fecundity as measured by TTP. Whether the female 2D:4D is a marker of in utero androgen exposure and whether it is associated with fecundity have yet to be determined.
STUDY FUNDING/COMPETING INTEREST
The MIREC Study was funded by Health Canada's Chemicals Management Plan, the Canadian Institute of Health Research (CIHR grant # MOP - 81285), and the Ontario Ministry of the Environment. MIREC-CD Plus was funded by Health Canada's Chemicals Management Plan Research Fund. The 2D:4D component was funded by a research grant from the CIHR-Quebec Training Network in Perinatal Research (QTNPR). M.P. Vélez was supported by a CIHR Fellowship Award, and a QTNPR scholarship. P. Monnier is supported by the Research Institute of the McGill University Health Centre. W.D Fraser is supported by a CIHR Canada Research Chair. There are no conflicts of interest to declare.
Keywords: developmental origins of health and disease, digit ratios, time to pregnancy, fecundity, prenatal androgen exposure
Introduction
There is a paucity of endocrine-sensitive end-points that allow the epidemiological assessment of diverse life-course stressors on developmental and reproductive functions (Arbuckle et al., 2008). A novel end-point in humans that has been proposed as an indicator of sexual development is the 2nd (index) to 4th (ring) finger digit ratio (2D:4D). The 2D:4D ratio reflects sexual differentiation early in life and may be an end-point for the organizational effects of prenatal androgens in the human body. The 2D:4D is a sexually dimorphic trait, with males having relatively shorter 2nd than 4th digit lengths (Manning et al., 1998). As such, lower 2D:4Ds could be potential indicators of greater androgen exposure during fetal development (Manning et al., 1998). Associations between digit ratios and several health outcomes including male fertility, sexual orientation, physical performance, hand preference and autism have been reported (Breedlove, 2010). The direction and magnitude of these associations as illustrated below, however, have not been consistent.
Since the 2D:4D is sexually dimorphic at birth, and under the hypothesis that this difference is related to in utero androgens levels, several studies have evaluated the association between 2D:4D as a marker of prenatal androgen exposure and different reproductive outcomes across the life course. Several cross-sectional studies have reported low sperm counts, decreased testicular volume and impaired hormonal status in adult men having higher digit ratios (i.e. implying low androgen exposure during fetal life) (Klimek et al., 2014; Oh et al., 2014). However, other studies have failed to replicate the associations between 2D:4D and hormone levels (Honekopp et al., 2007) or a number of male reproductive end-points including semen quality (Firman et al., 2003; Bang et al., 2005).
In females, two retrospective studies in adult women have reported associations between lower digit ratios (i.e. implying greater androgen exposure during fetal development) and delayed age at menarche (Matchock, 2008; Manning and Fink, 2011), while a prospective cohort in pre-menarcheal girls aged 5–12 years found lower digit ratios as predictors of early age at menarche (Oberg and Villamor, 2012). The evidence concerning female fertility is sparse and has usually been assessed by indirect markers. For example, lower female digit ratios have been associated with low offspring counts (Manning and Fink, 2008), congenital adrenal hyperplasia (Oswiecimska et al., 2012) and polycystic ovary syndrome (PCOS) (Cattrall et al., 2005), although this latter finding was challenged by Lujan et al. (2010a,b).
Time-to-pregnancy (TTP) is an epidemiological metric widely used for the assessment of human fecundity (Buck Louis, 2011). One study has reported decreased fecundity (i.e. longer TTP) at a higher 2D:4D in males (Auger and Eustache, 2011). However, the predictive role of 2D:4D on TTP has never been assessed in females.
The purpose of this study is to evaluate the association between female 2D:4D and fecundity as measured by TTP in women from the MIREC Study, a Canadian pregnancy and birth cohort.
Materials and Methods
Population and study design
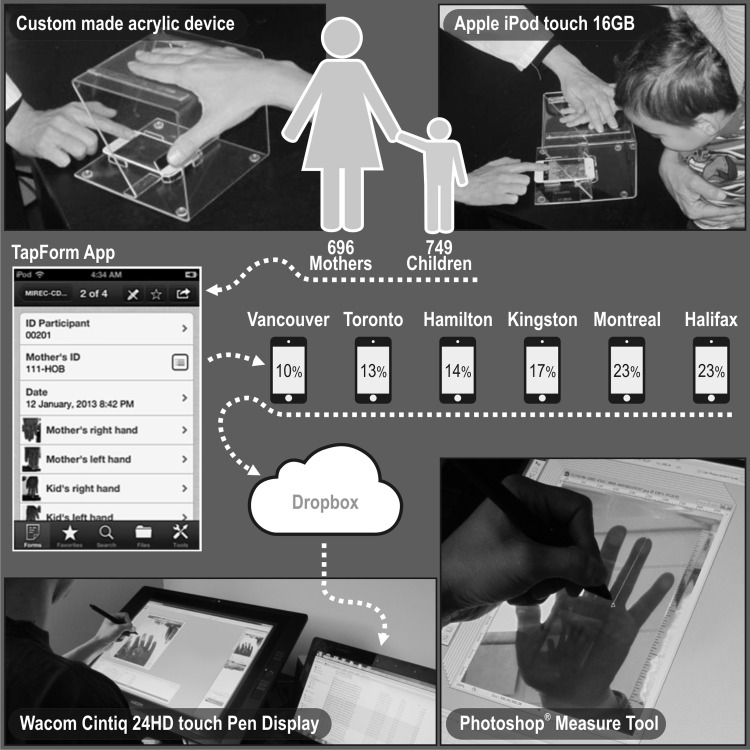
The Maternal-Infant Research on Environmental Chemicals (MIREC) study is a pregnancy cohort conducted in 10 cities across Canada between 2008 and 2011 (Arbuckle et al., 2013). Approximately 2000 women attending prenatal clinics were recruited during the first trimester of pregnancy and followed up to 10 weeks post-partum. Baseline demographic and lifestyle characteristics of the women were obtained from data collected at the first trimester visit. The present analysis is part of MIREC-CD Plus, a follow-up study conducted to measure growth and development in a subsample of some 800 MIREC children. One component of MIREC-CD Plus was the measurement of the index (second, 2D) and ring (fourth, 4D) digit lengths in mothers to calculate the 2D:4D ratio. Digital pictures of the ventral surface of both hands were obtained using a 16-GB iPOD TOUCH (Apple, Inc., CA, USA), placed in a custom made acrylic device specially designed to standardize this procedure (Fig. 1). Measurements of the second and fourth digit lengths were conducted by a single experienced research assistant with the aid of Adobe Photoshop 8 software (Adobe Systems Incorporated, San Jose, CA, USA). The lengths of the second and fourth fingers of each hand were measured using mouse-controlled calipers. Calipers were positioned midline along the finger's basal crease and expanded to the edge of the finger tip. 2D:4D was calculated as the ratio of the second and fourth fingers of each hand. In order to assess intra-observer reliability, each measurement was performed twice after a period of two weeks, and the intraclass correlation coefficients (ICCs) were calculated in a convenience subsample of 80 mother and children pairs. The reliability of the two repeated digit lengths measures performed by the same examiner was very high, resulting in ICCs higher than 0.97 for the finger lengths with narrow 95% confidence intervals (Table I).
Figure 1.
Finger digital length image capture and measurement procedure.
Table I.
Intraclass correlation coefficient subsample (n = 80).
| Mothers |
Children |
|||
|---|---|---|---|---|
| ICCa | 95% CIb | ICCa | 95% CIb | |
| Right hand | ||||
| 2: index finger | 0.98 | 0.97, 0.99 | 0.98 | 0.97, 0.99 |
| 4: ring finger | 0.99 | 0.98, 0.99 | 0.99 | 0.98, 0.99 |
| Left hand | ||||
| 2: index finger | 0.97 | 0.95, 0.98 | 0.98 | 0.97, 0.99 |
| 4: ring finger | 0.99 | 0.99, 0.99 | 0.99 | 0.98, 0.99 |
aIntraclass correlation coefficient.
bConfidence interval.
A total of 808 follow-up visits were conducted in MIREC-CD Plus. There were 24 mothers did not have their anthropometric measurements taken, including the hand pictures and 6 mothers were not available at the time of the visit. The hand pictures of one mother were excluded because of visible malformations (i.e. syndactyly). Another mother refused to have the pictures taken. There were 15 mothers were excluded due to technical problems related to their pictures (i.e. poor quality, failed uploading or transfer from the study site to the study coordinating center). Furthermore, 56 mothers were further excluded because the index pregnancy was the consequence of a birth control failure, which made them ineligible for TTP analyses, and 9 women did not have information on their TTP, resulting in a total sample of 696 women for the present analysis. The MIREC Study was approved by the Research Ethics Board of Health Canada, the research ethics committee of the coordinating center of Ste-Justine's Hospital in Montreal, and the academic and hospital ethics committees of the study sites across Canada. All the participants signed informed consent forms.
Operational definition of variables
The exposure of interest was the 2D:4D of the women categorized by tertiles, or dichotomized as ≥1 (index finger longer than the ring finger) or <1 (ring finger longer than the index finger, implying greater androgen exposure during fetal development). The end-point was TTP (i.e. number of months of trying to conceive). TTP was assessed in the MIREC cohort by asking women during the first trimester of pregnancy the following question: ‘How long did it take you to get pregnant with this pregnancy?’ (number of months). In MIREC, women were asked about the type of birth control they used before this pregnancy. If a method was indicated, women were then asked if they had stopped it before the pregnancy started or if there was a birth control failure. Women who answered that there was a birth control failure were excluded from the present analysis. Potential confounders were self-reported maternal age and smoking, ethnicity, household income, maternal education and BMI before pregnancy. We conducted a sensitivity analysis in women who had undergone infertility treatment (including nine patients whose male partners required some infertility treatment) to assess whether the results were different in this population.
Statistical analysis
The reliability of the digit length measurement was evaluated by calculating the ICC using ANOVA analysis. The descriptive phase of the analysis included assessment of the distributions of the baseline characteristics of the mothers and their children. Fecundability odds ratios (FORs) were estimated using the Cox model, modified for discrete time data. FORs estimate the odds of becoming pregnant each cycle, given the exposure of interest, conditional on not being pregnant in the previous cycle. FORs <1 denote reduction in fecundity or longer TTP, and FORs >1 denote a shorter TTP. TTP was censored at the 13th month. Linearity and proportional hazard assumptions were verified (Allison, 2010). Statistical analysis was performed using STATA 10.0 (Stata Corporation, College Station, TX, USA) and SAS 9.4 (SAS Institute, Inc., Cary, NC, USA), specifically for the discrete-time Cox proportional models. Our large sample size resulted in a statistical power higher than 95% to detect FOR = 0.85 (i.e. decreased fecundability).
Results
The mean maternal age at recruitment was 33.45 years (SD 4.71), with a median gestational age of 12 weeks (Table II). The mean maternal 2D:4D was similar in both hands (right 0.985 ± 0.036 and left 0.978 ± 0.036). Most of the women were White, two-thirds had a university degree, more than half had a prior pregnancy with a live birth, and 14.5% were obese prior to the index pregnancy. There were 82 women (11.8%) who reported being smokers or having quit after learning they were pregnant. Ethnicity was associated with female 2D:4D, with Black women having smaller ratios in both hands. Education was significantly associated with maternal 2D:4D in the right hand, with women who had high school or less education having a smaller ratio. With respect to TTP, increasing age was statistically associated with a longer time to conceive. Differences in TTP were also present by ethnicity with Latin-American women reporting longer time to conceive; however, the small number of participants from countries other than Canada precluded conclusions about this estimate.
Table II.
Digit ratio and estimated time to pregnancy according to baseline characteristics in 696 women: the MIREC study.
| Characteristic | n (%) | Digit ratio |
TTP |
||||
|---|---|---|---|---|---|---|---|
| Right hand |
Left hand |
Median (25–75%)a | Pe | ||||
| Mean | Pd | Mean | Pd | ||||
| Age, years | 0.90 | 0.24 | <0.001 | ||||
| <30 | 160 (23.0) | 0.986 | 0.976 | 2 (1–4) | |||
| 30–34 | 274 (39.4) | 0.984 | 0.978 | 2 (1–5) | |||
| 35–39 | 210 (30.2) | 0.984 | 0.980 | 2 (1–6) | |||
| ≥40 | 52 (7.4) | 0.986 | 0.987 | 5.5 (1–18) | |||
| Education | 0.03 | 0.33 | 0.88 | ||||
| High school or less | 39 (5.6) | 0.975 | 0.972 | 1 (1–7) | |||
| Some college classes | 28 (4.0) | 0.989 | 0.988 | 2.5 (1–5.5) | |||
| College trade diploma | 143 (20.5) | 0.991 | 0.978 | 2 (1–6) | |||
| University degree | 486 (69.8) | 0.983 | 0.979 | 2 (1–5) | |||
| Ethnicity | <0.001 | 0.01 | 0.05 | ||||
| White | 590 (84.8) | 0.986 | 0.979 | 2 (1–5) | |||
| Black | 20 (2.8) | 0.953 | 0.952 | 2 (1–3) | |||
| Latin | 18 (2.6) | 0.978 | 0.971 | 8.5 (2–12) | |||
| Chinese | 11 (1.6) | 0.978 | 0.986 | 1 (1–6) | |||
| Other | 57 (8.2) | 0.982 | 0.982 | 4 (1–8) | |||
| Household income | 0.16 | 0.13 | 0.32 | ||||
| <$60 000 | 129 (18.5) | 0.978 | 0.973 | 2 (1–5) | |||
| $60 001–100 000 | 257 (37.0) | 0.985 | 0.978 | 2 (1–5) | |||
| >$100 000 | 287 (41.2) | 0.987 | 0.981 | 2 (1–6) | |||
| No response | 23 (3.3) | 0.989 | 0.983 | 2 (1–3) | |||
| Parity conditional on gravidity | 0.80 | 0.38 | 0.06 | ||||
| No prior pregnancy | 206 (29.5) | 0.983 | 0.976 | 3 (1–6) | |||
| Prior pregnancy without live birth(s) | 101 (14.5) | 0.985 | 0.977 | 2 (1–7) | |||
| Prior pregnancy with live birth(s) | 389 (56.0) | 0.985 | 0.980 | 2 (1–5) | |||
| Maternal smoking | 0.63 | 0.56 | 0.21 | ||||
| Never | 451 (64.8) | 0.984 | 0.978 | 2 (1–6) | |||
| Former | 163 (23.4) | 0.984 | 0.981 | 1 (1–4) | |||
| Currentb | 82 (11.8) | 0.988 | 0.976 | 2 (1–6) | |||
| Pre-pregnancy BMIc | 0.15 | 0.35 | 0.09 | ||||
| <24.9 | 423 (60.8) | 0.986 | 0.979 | 2 (1–5) | |||
| 25–29.9 | 130 (18.7) | 0.979 | 0.974 | 2 (1–6) | |||
| >30 | 101 (14.5) | 0.987 | 0.979 | 3 (1–7) | |||
aMedian TTP in months (25–75 percentiles).
bIncludes women who quit during the first trimester of pregnancy.
cn = 654 because of missing values.
dANOVA F test.
eLog-rank test.
The probabilities of pregnancy at 1, 6 and 12 months were 0.40 (95% CI 0.36–0.44), 0.80 (95% CI 0.76–0.83) and 0.91 (95% CI 0.89–0.93), respectively. As presented in Table III, in our analysis by tertiles, the highest FOR was associated with the smallest ratio in both hands, although the 95% CI included the null effect (FOR 1.19 [95% CI 0.93, 1.51] in the right hand and 1.16 [95% CI 0.91, 1.47] in the left hand). In the dichotomous analysis, 2D:4D < 1 (i.e. implying greater androgen exposure during fetal development) resulted in FOR higher than 1 (i.e. shorter TTP), although this was not statistically significant (FOR 1.08 [95% CI 0.88, 1.33] in the right hand and 1.14 [95% CI 0.92, 1.42] in the left hand). In the adjusted models, age, ethnicity (Latin-American women), and high BMI were associated with decreased fecundability (i.e. longer TTP).
Table III.
Fecundability odds ratios (FORs) according to digit ratio in 696 women from the MIREC study.
| Right hand |
Left hand |
|||
|---|---|---|---|---|
| Crude FOR [95% CI] | Adjusteda (n = 654)b FOR [95% CI] | Crude FOR [95% CI] | Adjusteda (n = 654)b FOR [95% CI] | |
| Digit ratio tertile (right/left medians) | ||||
| 1 (0.952/0.954) | 1.17 [0.94, 1.47] | 1.19 [0.93–1.51] | 1.15 [0.92, 1.44] | 1.16 [0.91–1.47] |
| 2 (0.982/0.972) | 1.05 [0.84, 1.31] | 0.97 [0.77–1.23] | 1.07 [0.86, 1.34] | 1.01 [0.80–1.28] |
| 3 (1.021/1.000) | Reference | Reference | Reference | Reference |
| Age | 0.95 [0.93–0.97] | 0.95 [0.93–0.97] | ||
| Ethnicity | ||||
| White | Reference | Reference | ||
| Black | 1.03 [0.55–1.92] | 1.02 [0.55–1.92] | ||
| Latin | 0.43 [0.23–0.80] | 0.44 [0.24–0.82] | ||
| Chinese | 1.21 [0.54–2.68] | 1.21 [0.55–2.67] | ||
| Other | 0.76 [0.54–1.07] | 0.75 [0.53–1.06] | ||
| Pre-pregnancy body mass index | ||||
| <24.9 | Reference | Reference | ||
| 25–29.9 | 0.88 [0.68–1.13] | 0.87 [0.68–1.12] | ||
| >30 | 0.74 [0.57–0.97] | 0.75 [0.58–0.99] | ||
| Digit ratio dichotomized | ||||
| <1 | 1.12 [0.92, 1.36] | 1.08 [0.88–1.33] | 1.17 [0.95, 1.44] | 1.14 [0.92–1.42] |
| ≥1 | Reference | Reference | Reference | Reference |
| Age | 0.95 [0.93–0.97] | 0.95 [0.93–0.97] | ||
| Ethnicity | ||||
| White | Reference | Reference | ||
| Black | 1.06 [0.57–1.98] | 1.04 [0.55–1.95] | ||
| Latin | 0.46 [0.25–0.85] | 0.46 [0.25–0.85] | ||
| Chinese | 1.23 [0.56–2.74] | 1.18 [0.53–2.61] | ||
| Other | 0.74 [0.53–1.04] | 0.74 [0.52–1.03] | ||
| Pre-pregnancy body mass index | ||||
| <24.9 | Reference | Reference | ||
| 25–29.9 | 0.87 [0.68–1.12] | 0.88 [0.69–1.13] | ||
| >30 | 0.75 [0.58–0.99] | 0.75 [0.57–0.98] | ||
aAdjusted by age, ethnicity and pre-pregnancy body mass index.
bDue to missing values of pre-pregnancy body mass index.
Discussion
Our objective was to evaluate the association between the female 2D:4D as a marker of in utero androgen exposure, and fecundity as measured by TTP. However, we were unable to find an association between female 2D:4D and TTP.
In the univariate analysis, ethnicity and education were associated with female 2D:4D. Mean 2D:4D varied across ethnic groups with higher ratios for White women and lower ratios for Black women. Chinese and Latin-American women had similar ratios, just slightly lower than those of White women but higher than those of Black women. In a large Internet survey using self-reported direct finger measurements, White women had higher ratios than Black and Chinese women, which is consistent with our study. Latin-American women were not included in that survey (Manning et al., 2007). Education was associated with 2D:4D on the right hand, the hand that shows greater sex difference (Honekopp and Watson, 2010), with lower ratios in women having only high school or less education. In a recent study on gender inequalities across nations, female secondary education was not associated with 2D:4D (Manning et al., 2014). Further studies should address this subject.
Several critical and sensitive windows essential for human reproduction and development are present as early as in the intrauterine period (Buck Louis, 2011). If the 2D:4D is defined in utero by the homeostasis of reproductive hormones, we should have been able to demonstrate some association between 2D:4D and TTP. Moreover, one would have expected lower FORs (delayed TTP) in women with the lowest 2D:4D, but we observed an inverse trend in the analysis by tertiles (i.e. the highest FORs were associated with the lowest 2D:4Ds), as well as in the dichotomous analysis (i.e. FORs >1 for 2D:4Ds <1), although these differences were not statistically significant. These findings also disagree with the hypothesis that increased 2D:4D in females may be associated with increased fertility/fecundity, as Sutcliffe et al. (2010) proposed after finding higher 2D:4D in girls born following intracytoplasmic sperm injection (ICSI) compared with naturally conceived controls.
There are some potential explanations for our failure to support this hypothesized association. First, 2D:4D may not be a precise measure of intrauterine exposure to testosterone in women. Although a prenatal reproductive hormonal imbalance might affect female fecundity, these hormonal changes might be too subtle to produce a detectable association between female 2D:4D and TTP. Second, some methodological aspects of our study need to be considered. The MIREC Study is a cohort of pregnant women, and therefore, women who are infertile and have no access to or success in infertility treatments were excluded by design from our study. If the 2D:4D is different in women able to conceive compared with those who are infertile, our study would not have been able to capture this association. We did, however, conduct sensitivity analysis in women who had undergone infertility treatment for the index pregnancy, and the results remained negative for any association between 2D:4D and TTP. Another aspect that needs to be considered is the retrospective nature of the assessment of TTP in our study. However, studies have reported reasonable validity of recall data if collected in the short term as was done in our study (Cooney et al., 2009; Radin et al., 2015). Moreover, known determinants of fecundity, such as age, ethnicity and obesity (Buck Louis, 2011) were indicators of a longer TTP in the crude analysis, which give us confidence about the reliability of our data. Smoking however was not associated with TTP, which could be explained by the low prevalence of smoking in the cohort (11.8%). In addition, concerns about the stability of the 2D:4D may arise, especially since the maternal 2D:4Ds were measured 2–5 years after pregnancy; however, 2D:4D is reported stable during development (McIntyre et al., 2005; Trivers et al., 2006) and through the menstrual cycle (Barrett et al., 2015), and unrelated to levels of circulation sex steroid hormones (Honekopp et al., 2007).
The method used to measure digit length could also account for the differences among studies. Most of the studies have measured finger lengths by a physical method, while we used digital imaging of the hands. There is evidence suggesting that computer-assisted measurements of the 2D:4D are more accurate than physical measurements, photocopies, or printed scanned images measured with Vernier calipers (Allaway et al., 2009). Our mean maternal 2D:4Ds (right 0.985 ± 0.036 and left 0.978 ± 0.036) are similar to those reported in Lujan et al. (2010b) who also used digital hand scans to assess 2D:4D in 96 women with PCOS (right 2D:4D 0.981 ± 0.028 and left 2D:4D 0.982 ± 0.030) compared with 48 female controls (right 2D:4D 0.972 ± 0.028 and left 2D:4D 0.974 ± 0.037).
A major strength of our study is the large sample size, giving us a high statistical power to exclude an association between female 2D:4D and TTP. A limitation of the present analysis is the absence of information on the hormonal profile of the study participants, and the presence of subjacent pathologies associated with TTP and androgen levels like PCOS. The MIREC biobank offers the future possibility of measuring these hormones in women and their children.
In summary, our data do not provide evidence for an association between female 2D:4D and fecundity as measured by TTP. Thus, whether the female 2D:4D is a marker of in utero androgen exposure, and associated with fecundity, is yet to be determined.
Authors' roles
M.P.V., T.E.A., P.M. and WD.F. were all involved in the conception and design of the study. M.P.V. carried out analysis and interpretation of data, in addition to drafting the manuscript. T.E.A. and WD.F. are the co-principal investigators of the MIREC Study, and along with PM, contributed to data interpretation and review of the manuscript.
Funding
The MIREC Study was funded by Health Canada's Chemicals Management Plan, the Canadian Institute of Health Research (CIHR grant # MOP - 81285), and the Ontario Ministry of the Environment. MIREC-CD Plus is funded by Health Canada's Chemicals Management Plan Research Fund. The 2D:4D component was funded by a research grant from the CIHR-Quebec Training Network in Perinatal Research (QTNPR). M.P.V. was supported by a CIHR Fellowship Award, and a QTNPR scholarship. P.M. is supported by the Research Institute of the McGill University Health Centre. W.D.F. is supported by a CIHR Canada Research Chair.
Conflict of interest
None declared.
Acknowledgements
This work would not have been possible without the generous collaboration of the MIREC participants (i.e. women, partners, and children). Special thanks to Stéphanie Bastien and Nicole Lupien for their assistance during the 2D:4D component, and to Gabriel Abad who designed and produced the hand scan devices and measured the digit lengths. The authors acknowledge the contributions of the MIREC Study Group, especially the site investigators and their staff.
References
- Allaway HC, Bloski TG, Pierson RA, Lujan ME. Digit ratios (2D:4D) determined by computer-assisted analysis are more reliable than those using physical measurements, photocopies, and printed scans. Am J Hum Biol 2009;21:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD. Survival Analysis Using SAS: A Practical Guide, 2nd edn Cary, NC: SAS Institute, Inc., 2010. [Google Scholar]
- Arbuckle TE, Hauser R, Swan SH, Mao CS, Longnecker MP, Main KM, Whyatt RM, Mendola P, Legrand M, Rovet J et al. . Meeting report: measuring endocrine-sensitive endpoints within the first years of life. Environ Health Perspect 2008;116:948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TE, Fraser WD, Fisher M, Davis K, Liang CL, Lupien N, Bastien S, Velez MP, von Dadelszen P, Hemmings DG et al. . Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr Perinat Epidemiol 2013;27:415–425. [DOI] [PubMed] [Google Scholar]
- Auger J, Eustache F. Second to fourth digit ratios, male genital development and reproductive health: a clinical study among fertile men and testis cancer patients. Int J Androl 2011;34 (4 Pt 2):e49–e58. [DOI] [PubMed] [Google Scholar]
- Bang AK, Carlsen E, Holm M, Petersen JH, Skakkebaek NE, Jorgensen N. A study of finger lengths, semen quality and sex hormones in 360 young men from the general Danish population. Hum Reprod 2005;20:3109–3113. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Swan SH. Stability of proposed biomarkers of prenatal androgen exposure over the menstrual cycle. J Dev Orig Health Dis 2015;6:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM. Minireview: Organizational hypothesis: instances of the fingerpost. Endocrinology 2010;151:4116–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM. Fecundity and Fertility. In: Buck GM, Platt RW (eds). Reproductive and Perinatal Epidemiology. New York: Oxford University Press, 2011,16–61. [Google Scholar]
- Cattrall FR, Vollenhoven BJ, Weston GC. Anatomical evidence for in utero androgen exposure in women with polycystic ovary syndrome. Fertil Steril 2005;84:1689–1692. [DOI] [PubMed] [Google Scholar]
- Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology 2009;20:56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firman RC, Simmons LW, Cummins JM, Matson PL. Are body fluctuating asymmetry and the ratio of 2nd to 4th digit length reliable predictors of semen quality. Hum Reprod 2003;18:808–812. [DOI] [PubMed] [Google Scholar]
- Honekopp J, Watson S. Meta-analysis of digit ratio 2D:4D shows greater sex difference in the right hand. Am J Hum Biol 2010;22:619–630. [DOI] [PubMed] [Google Scholar]
- Honekopp J, Bartholdt L, Beier L, Liebert A. Second to fourth digit length ratio (2D:4D) and adult sex hormone levels: new data and a meta-analytic review. Psychoneuroendocrinology 2007;32:313–321. [DOI] [PubMed] [Google Scholar]
- Klimek M, Galbarczyk A, Nenko I, Alvarado LC, Jasienska G. Digit ratio (2D:4D) as an indicator of body size, testosterone concentration and number of children in human males. Ann Hum Biol 2014;41:518–523. [DOI] [PubMed] [Google Scholar]
- Lujan ME, Bloski TG, Chizen DR, Lehotay DC, Pierson RA. Digit ratios do not serve as anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Hum Reprod 2010. a;25:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan ME, Podolski AJ, Chizen DR, Lehotay DC, Pierson RA. Digit ratios by computer-assisted analysis confirm lack of anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Reprod Biol Endocrinol 2010. b;8:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JT, Fink B. Digit ratio (2D:4D), dominance, reproductive success, asymmetry, and sociosexuality in the BBC Internet Study. Am J Hum Biol 2008;20:451–461. [DOI] [PubMed] [Google Scholar]
- Manning JT, Fink B. Is low digit ratio linked with late menarche? Evidence from the BBC internet study. Am J Hum Biol 2011;23:527–533. [DOI] [PubMed] [Google Scholar]
- Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod 1998;13:3000–3004. [DOI] [PubMed] [Google Scholar]
- Manning JT, Churchill AJ, Peters M. The effects of sex, ethnicity, and sexual orientation on self-measured digit ratio (2D:4D). Arch Sex Behav 2007;36:223–233. [DOI] [PubMed] [Google Scholar]
- Manning JT, Fink B, Trivers R. Digit ratio (2D:4D) and gender inequalities across nations. Evol Psychol 2014;12:757–768. [PubMed] [Google Scholar]
- Matchock RL. Low digit ratio (2D:4D) is associated with delayed menarche. Am J Hum Biol 2008;20:487–489. [DOI] [PubMed] [Google Scholar]
- McIntyre MH, Ellison PT, Lieberman DE, Demerath E, Towne B. The development of sex differences in digital formula from infancy in the Fels Longitudinal Study. Proc Biol Sci 2005;272:1473–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg AS, Villamor E. Low digit ratio predicts early age at menarche in Colombian schoolgirls. Paediatr Perinat Epidemiol 2012;26:448–455. [DOI] [PubMed] [Google Scholar]
- Oh JK, Kim KT, Yoon SJ, Kim SW, Kim TB. Second to fourth digit ratio: a predictor of adult testicular volume. Andrology 2014;2:862–867. [DOI] [PubMed] [Google Scholar]
- Oswiecimska JM, Ksiazek A, Sygulla K, Pys-Spychala M, Roczniak GR, Roczniak W, Stojewska M, Ziora K. Androgens concentrations and second-to fourth-digit ratio (2D:4D) in girls with congenital adrenal hyperplasia (21-hydroxylase deficiency). Neuro Endocrinol Lett 2012;33:787–791. [PubMed] [Google Scholar]
- Radin RG, Rothman KJ, Hatch EE, Mikkelsen EM, Sorensen HT, Riis AH, Fox MP, Wise LA. Maternal recall error in retrospectively reported time-to-pregnancy: an assessment and bias analysis. Paediatr Perinat Epidemiol 2015;29:576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe AG, Manning JT, Katalanic A, Ludwig A, Mehta M, Lim J, Basatemur E, Ludwig M. Perturbations in finger length and digit ratio (2D:4D) in ICSI children. Reprod Biomed Online 2010;20:138–143. [DOI] [PubMed] [Google Scholar]
- Trivers R, Manning J, Jacobson A. A longitudinal study of digit ratio (2D:4D) and other finger ratios in Jamaican children. Horm Behav 2006;49:150–156. [DOI] [PubMed] [Google Scholar]