Abstract
STUDY QUESTION
Does advanced maternal age (AMA) in mice affect cardiometabolic health during post-natal life in offspring derived from an assisted reproduction technology (ART) procedure?
SUMMARY ANSWER
Offspring derived from blastocysts collected from aged female mice displayed impaired body weight gain, blood pressure, glucose metabolism and organ allometry during post-natal life compared with offspring derived from blastocysts from young females; since all blastocysts were transferred to normalized young mothers, this effect is independent of maternal pregnancy conditions.
WHAT IS KNOWN ALREADY
Although studies in mice have shown that AMA can affect body weight and behaviour of offspring derived from natural reproduction, data on the effects of AMA on offspring cardiometabolic health during post-natal development are not available. Given the increasing use of ART to alleviate infertility in women of AMA, it is pivotal to develop ART–AMA models addressing the effects of maternal aging on offspring health.
STUDY DESIGN, SIZE, DURATION
Blastocysts from old (34–39 weeks) or young (8–9 weeks) C57BL/6 females mated with young CBA males (13–15 weeks) were either subjected to differential cell staining (inner cell mass and trophectoderm) or underwent embryo transfer (ET) into young MF1 surrogates (8–9 weeks) to produce young (Young-ET, 9 litters) and old (Old-ET, 10 litters) embryo-derived offspring. Offspring health monitoring was carried out for 30 weeks.
PARTICIPANTS/MATERIALS, SETTING, METHODS
All animals were fed with standard chow. Blood pressure was measured at post-natal Weeks 9, 15 and 21, and at post-natal Week 30 a glucose tolerance test (GTT) was performed. Two days after the GTT mice were killed for organ allometry. Blastocyst cell allocation variables were evaluated by T-test and developmental data were analysed with a multilevel random effects regression model.
MAIN RESULTS AND THE ROLE OF CHANCE
The total number of cells in blastocysts from aged mice was decreased (P < 0.05) relative to young mice due to a lower number of cells in the trophectoderm (mean ± SEM: 34.5 ± 2.1 versus 29.6 ± 1.0). Weekly body weight did not differ in male offspring, but an increase in body weight from Week 13 onwards was observed in Old-ET females (final body weight at post-natal Week 30: 38.5 ± 0.8 versus 33.4 ± 0.8 g, P < 0.05). Blood pressure was increased in Old-ET offspring at Weeks 9–15 in males (Week 9: 108.5 ± 3.13 versus 100.8 ± 1.5 mmHg, Week 15: 112.9 ± 3.2 versus 103.4 ± 2.1 mmHg) and Week 15 in females (115.9 ± 3.7 versus 102.8 ± 0.7 mmHg; all P < 0.05 versus Young-ET). The GTT results and organ allometry were not affected in male offspring. In contrast, Old-ET females displayed a greater (P < 0.05) peak glucose concentration at 30 min during the GTT (21.1 ± 0.4 versus 17.8 ± 1.16 mmol/l) and their spleen weight (88.2 ± 2.6 ± 105.1 ± 4.6 mg) and several organ:body weight ratios (g/g × 103) were decreased (P < 0.05 versus Young-ET), including the heart (3.7 ± 0.06 versus 4.4 ± 0.08), lungs (4.4 ± 0.1 versus 5.0 ± 0.1), spleen (2.4 ± 0.06 versus 3.2 ± 0.1) and liver (36.4 ± 0.6 versus 39.1 ± 0.9).
LIMITATIONS, REASONS FOR CAUTION
Results from experimental animal models cannot be extrapolated to humans. Nevertheless, they are valuable to develop conceptual models that can produce hypotheses for eventual testing in the target species (i.e. humans).
WIDER IMPLICATIONS OF THE FINDINGS
Our data show that offspring from mouse embryos from aged mothers can develop altered phenotypes during post-natal development compared with embryos from young mothers. Because all embryos were transferred into young mothers for the duration of pregnancy to normalize the maternal in vivo environment, our findings indicate that adverse programming via AMA is already established at the blastocyst stage. Whilst human embryos display increased aneuploidy compared with mouse, we believe our data have implications for women of AMA undergoing assisted reproduction, including surrogacy programmes.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported through the European Union FP7-CP-FP Epihealth programme (278418) to T.P.F. and the BBSRC (BB/F007450/1) to T.P.F. The authors have no conflicts of interest to declare.
Keywords: maternal aging, blastocysts, developmental programming, offspring health, gender effects
Introduction
Advanced maternal age (AMA), generally referred to as childbearing in women aged 35 years or older, is an increasing worldwide phenomenon (Laopaiboon et al., 2014; Fall et al., 2015; Sauer, 2015). It is generally accepted that as maternal age increases there is a decline in the population of primordial follicles in the ovaries (Nelson et al., 2013) and an increase in the presence of chromosomal abnormalities in oocytes (Jones and Lane, 2013) with consequences for fertility. Indeed, epidemiological studies in humans have revealed that AMA is associated with reduced capacity to conceive (Dunson et al., 2004) and increased risk of miscarriage (Khalil et al., 2013). Adverse pregnancy outcomes have also been reported, and although the evidence is not entirely consistent, a wealth of data from human studies supports the notion that AMA is a risk factor for congenital abnormalities (Csermely et al., 2015), stillbirth (Laopaiboon et al., 2014), preterm birth (Kenny et al., 2013), perinatal death (Laopaiboon et al., 2014), macrosomia (Kenny et al., 2013), large for gestational age (Kenny et al., 2013), small for gestational age (Khalil et al., 2013), low birthweight (Fall et al., 2015) and Caesarean delivery (Khalil et al., 2013).
Although inconsistencies among studies exist, epidemiological studies have also shown that AMA can affect human offspring during post-natal life. For instance, there is evidence supporting an increased risk of behavioural alterations in children of women of AMA, including autism spectrum disorders (Lee and McGrath, 2015). The risk of developing diabetes has been examined in a meta-analysis of 30 studies which revealed that children born to women of AMA have an increased risk of type 1 diabetes (Cardwell et al., 2010). In young adults with mothers of AMA, this latter association was not observed, but AMA was linked with the risk of early onset type 2 diabetes (Lammi et al., 2007). A recent study reported that young adults with mothers of AMA displayed increased fasting plasma glucose concentrations (Fall et al., 2015). Cardiovascular function can also be affected, as shown by the positive association observed between maternal age at conception and blood pressure in children aged 5–7 years (Whincup et al., 1989; Lawlor et al., 2004), and the high blood pressure found in newborns of mothers of AMA (Gillman et al., 2004). There are also indications that AMA may decrease the lifespan (Wilding et al., 2014) and reproductive capacity of human offspring (Tarin et al., 2001; Smits et al., 2002).
In contrast, positive effects attributed to AMA have been reported in human studies. For example, several studies indicate that AMA seems to exert a positive effect on some behavioural and cognitive outcomes in children (Tearne, 2015). A UK-based study found that children of mothers of AMA had a decreased risk of unintentional injuries and admissions to hospital, better language development, increased likelihood of having a complete immunization schedule by age 9 months, and improved ability to deal with social and emotional difficulties (Sutcliffe et al., 2012). Children of women of AMA were also taller, slimmer and displayed decreased abdominal adiposity and plasma concentrations of insulin-like growth factor-2 (Savage et al., 2013). Moreover, a study conducted in 73 overweight middle-age males reported that as the age of their mothers at childbearing increased (range 18–45 years), the better the metabolic and cardiovascular phenotype they showed, including improved insulin sensitivity, decreased fasting concentrations of insulin and glucose, and lower blood pressure (Albert et al., 2015). Similarly, another recent study found an inverse relationship between maternal age at childbearing and fasting glucose levels in young adult offspring (Verroken et al., 2015). However, it has been suggested that these positive effects of AMA are probably caused by environmental factors and may not be directly related to biological mechanisms of reproductive aging. In this context, in some populations, older mothers will have better socioeconomic status and/or greater knowledge about healthy lifestyles (e.g. breastfeeding, balanced nutrition, physical activity, etc.), which, in turn, will give an advantage for the implementation of a healthier and safer environment for child-rearing with long-term positive consequences in adulthood (Wilding et al., 2014; Albert et al., 2015; Tearne, 2015). Some authors believe that environmental factors may also be the cause for some of the adverse effects attributed to AMA in human studies, as the negative effect of AMA on offspring longevity and body weight (i.e. obesity) lost significance after controlling for factors such as maternal education, socioeconomic status and early parental loss (Myrskylä and Fenelon, 2012; Myrskylä et al., 2014).
Besides the difficulty in controlling confounding factors, human retrospective studies cannot delineate the long-term effects of AMA from those attributed to delayed fatherhood (Myrskylä and Fenelon, 2012; Albert et al., 2015). This highlights the importance of experimental animal models. Experimental work in mice indicated that AMA can affect post-natal development of offspring derived from natural reproduction. Accordingly, AMA was associated with higher pup mortality in the first 3 days after birth (Tarin et al., 2003), lower body weight during the preweaning period (Tarin et al., 2003), behavioural alterations at post-natal Weeks 8–10 (Lerch et al., 2015) or 24–37 (Tarin et al., 2003), lower body weight during post-natal Weeks 40–90, and decreased lifespan (Tarin et al., 2005). However, there is a lack of information on the long-term effects of AMA on metabolic and cardiovascular function of offspring in experimental animal models. Furthermore, given the increasing trend in the use of assisted reproduction technology (ART) to alleviate infertility in women of AMA (Cabry et al., 2014; Jackson et al., 2015), it is imperative to develop experimental ART models of AMA. These studies will discriminate between AMA effects on the preimplantation embryo itself from those that may derive from the maternal tract and systemic environment of the pregnancy following embryo transfer (ET).
In the present study, using a murine ET model, we examine the effects of AMA on the post-natal development of offspring under an ART-derived procedure. To avoid potential adverse programming from ovulation induction, IVF and extended embryo culture, and paternal conditions (Watkins et al., 2007; El Hajj and Haaf, 2013) and to focus attention on outcomes mainly related to maternal age of embryos, we used aged or young females naturally mated to young males, with subsequent collection and immediate transfer of blastocysts to young recipients. We provide evidence that offspring of aged mice display alterations in body weight gain, blood pressure, glucose metabolism and organ allometry during post-natal development that predominantly affect females. Furthermore, our data demonstrate that these adverse phenotypes are already programmed by the blastocyst stage.
Materials and Methods
Animals
All animal experimentation was conducted in accordance with the UK Home Office Animal (Scientific Procedures) Act 1986 and local ethics committee at the University of Southampton. All animals were bred in-house (University of Southampton, Biomedical Research Facility) and kept on a 0700–1900 h light cycle at a temperature of 20–22°C. Water and standard laboratory chow (Special Diet Services, Ltd, Witham, Essex, UK) was provided ad libitum to all animals used in the study.
In vivo production of blastocysts
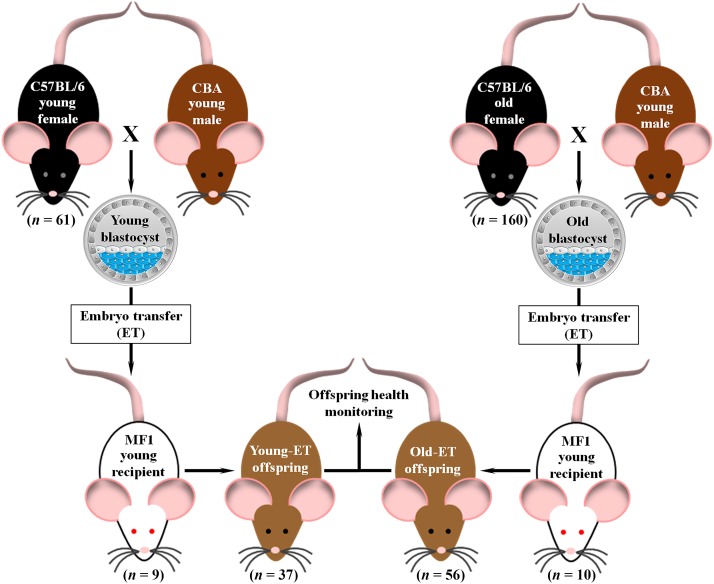
Non-superovulated virgin young (8–9 weeks) and old (34–39 weeks) female mice (C57BL/6) were mated with young CBA males (10–15 weeks; Fig. 1). Male–female pairs were housed overnight and the presence of a vaginal plug the following morning was regarded as a sign of successful mating. Females were considered to be on embryonic Day 0.5 (E0.5) at midday on the day the vaginal plug was detected. On E3.5 mice were killed by cervical dislocation and uterine horns were immediately dissected and placed in warm (37°C) saline solution (BR0053G, OXOID, UK). Each uterine horn was then gently flushed on an empty petri dish, under a stereomicroscope, with 1 ml of H6 medium supplemented with 4 mg/ml bovine serum albumin (BSA, A3311, Sigma, UK; Nasr-Esfahani et al., 1990). Arrested and/or fragmented embryos at the 2- to 8-cell stage were classified as degenerated embryos. Morula and blastocyst with clear signs of fragmentation were also considered degenerated embryos. All non-fragmented 1-cell structures (i.e. absence of first cleavage division) were classified as unfertilized oocytes (i.e. ova; Wu et al., 2010). All embryos in the stage of morula or blastocyst with no signs of fragmentation were classified as viable embryos. Viability (total viable embryos/total embryos × 100), fertilization (total embryos/total embryos + ova × 100) and degeneration (degenerated embryos/total embryos × 100) rates were calculated. Blastocysts were washed three times in H6-BSA medium to remove debris. Collected blastocyst were either subjected to differential cell staining or transferred to 30 µl drops (10–15 blastocysts per drop) of synthetic oviductal medium enriched with potassium medium (Summers et al., 2000) and incubated under 5% CO2 in air at 37°C for 2–3 h until the time of ET.
Figure 1.
Murine model of AMA used in the study. Young (8–9 weeks) or old (34–39 weeks) C57BL/6 females were mated with young CBA males (10–15 weeks) to obtain young or old blastocysts (Day 3.5 of pregnancy). Blastocysts were then transferred to young MF1 recipients (8–9 weeks) to obtain young (Young-ET) or old (Old-ET) embryo-derived offspring. ET, embryo transfer.
Differential cell staining in blastocysts
Blastocysts with a blastocoel cavity greater than half of the volume of the embryo (i.e. expanded blastocysts) were subjected to differential nuclear labelling according to the protocol developed by Hardy et al. (1989) with some modifications. Unless otherwise indicated, embryos were processed in 50 µl drops. After removing the zona pellucida in 1 ml of warm (37°C) acid Tyrode's solution (T1788, Sigma), blastocysts were washed for 15–20 min in 1 ml H6-BSA followed by 10 min incubation in 10% trinitrobenzenesulfonic acid solution (TNBS, P-2297, Sigma) at room temperature. H6 medium supplemented with 1% polyvinylpyrrolidone (PVP, P0930, Sigma) was used to prepare the 10% TNBS solution. Blastocysts were then washed three times in H6-PVP and incubated for 10 min in 0.4 mg/ml goat anti-dinitrophenyl antibody (D9781, Sigma) in H6-PVP at room temperature. After washing three times in H6-PVP, blastocysts were incubated in 50 µl of reconstituted (1:10 dilution with H6-BSA) Low-Tox® guinea pig complement (CL4051, Cedarlane, Canada) supplemented with 4 µl propidium iodide (1 mg/ml, P4170, Sigma) for 15 min at 37°C. Blastocysts were washed again three times with H6-BSA and fixed in 1 ml ice-cold ethanol supplemented with 1% Bisbenzimide H 33258 (2.5 mg/ml, B2883, Sigma) at 4°C for 1 h. For cell quantification, blastocysts were washed in 1 ml ice-cold fresh ethanol and mounted onto a glass microscope slide in a ∼4 µl drop of glycerol (G5516, Sigma) and coverslipped. Digital photographs of blastocysts were obtained with an inverted epifluorescence microscope (Axiovert 200M, Carl Zeiss Ltd) in a darkened room. Cell nuclei were manually counted with the MetaMorph software (Version 6.2r6, Molecular Devices).
Embryo transfer
Blastocysts were washed three times in M2 medium before ET (Watkins et al., 2007). The uterine horn was exposed by flank laparotomy and six expanding blastocysts were transferred with a minimal amount of medium into the uterine cavity of MF1 pseudo-pregnant (E2.5) young recipients (8–9 weeks; Fig. 1). The uterine horn was then placed back into the abdominal cavity and the incision site closed. The procedure was repeated in the opposite flank where another six expanding blastocysts were transferred. Following ET, recipients were placed individually in clean cages to recover from anaesthesia in a warm room (28–30°C). Females were then moved to a quiet room where they were kept for the rest of the pregnancy and lactation.
Offspring analysis
ET-derived offspring were weaned 3 weeks after birth, separated according to sex, and their body weight recorded weekly for 30 weeks. Systolic blood pressure was measured with a standard protocol at post-natal Weeks 9, 15 and 21 by tail-cuff plethysmography with the Non-Invasive Blood Pressure Monitor (NIBP-8, Columbus Instruments, Columbus, OH, USA) in a warm room (28–30°C; Watkins et al., 2007, 2008). Five readings with good waveforms and good overall quality were obtained per mouse (Williams et al., 2011). At post-natal Week 30 a standard protocol for glucose tolerance test (GTT) was performed in unrestrained conscious mice following a 15 h overnight fast. A blood glucose metre (Accu-Chek, Aviva, Roche Diagnostics GmbH, Germany) was used to measure glucose in small drops of blood collected by tail tipping. Twenty minutes before starting the GTT, anaesthetic cream (Lidocaine 5%, Teva, UK) was applied to the tail. After recording of the baseline glucose level (0 min), a glucose (G8270, Sigma) solution (20%, in sterile, distilled water) was administered by i.p. injection at a dose of 2 g/kg. Glucose levels were measured 15, 30, 60 and 120 min after glucose administration (Constantinou et al., 2014; Weidemann et al., 2016). Water was provided ad libitum during fasting and GTT. Immediately after the GTT, mice were placed in clean cages with food and water ad libitum. Two days after the GTT, mice were killed by cervical dislocation and organs (i.e. spleen, liver, left and right kidneys, heart and lungs) and carcass weighed.
Statistics
Statistical analysis was performed with the IBM SPSS Statistics Software, Version 21 (IBM Corporation UK Ltd, Hampshire, England). All data were tested for normal distribution with the Shapiro–Wilk test. Fertility and blastocyst cell allocation variables were analysed by T-test or χ2 as appropriate. Percentage data analysed with T-test were arcsine transformed before analysis. Litter size and sex allocation of pups following ET was analysed with T-test and binomial test, respectively. The rest of the post-natal data were analysed with a multilevel random effects regression model taking into consideration the random effects of mother and litter size. Post-natal data subjected to regression analysis were not normally distributed and Fisher–Yates transformations were carried out on the outcome variables before analysis (Crozier et al., 2012). Of the five blood pressure readings taken at each time point per mouse, the lowest and highest values were discarded and the mean of the three middle values was used for statistical analysis (Williams et al., 2011). Area under the curve values were calculated for GTT data by the trapezoidal rule (Matthews et al., 1990). Data are presented as mean ± SEM unless otherwise indicated.
Results
Maternal aging can alter cell allocation of blastocysts in mice
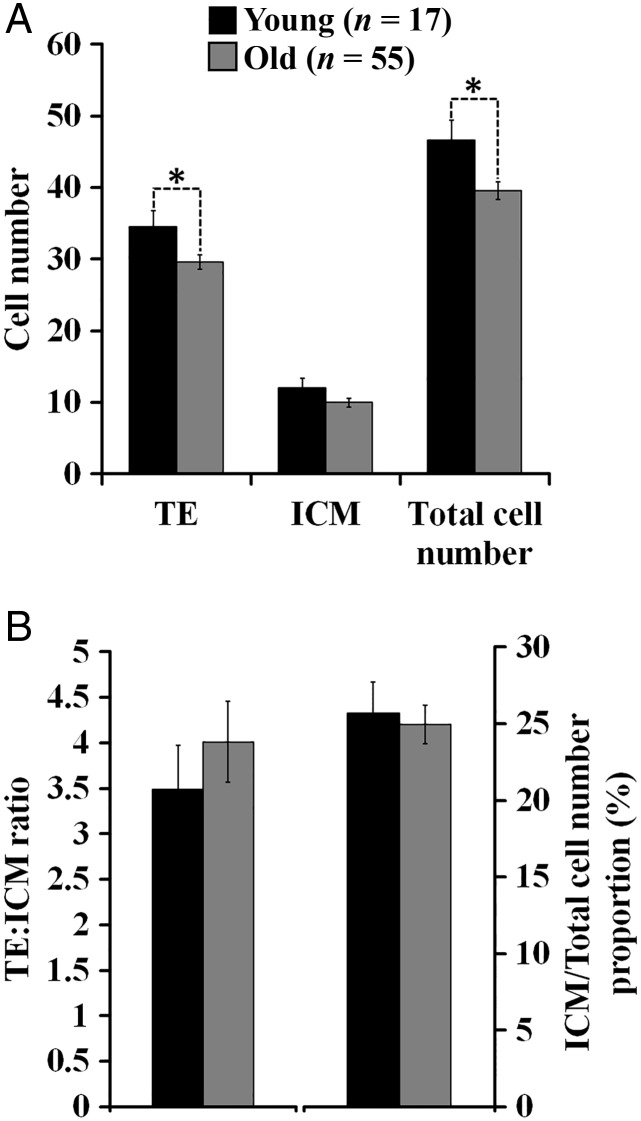
The percentage of plug-positive females that failed to yield embryos (Fig. 2A) and the number of embryos produced per donor was not different between the age groups (Fig. 2C). However, the fertilization rate was decreased in aged females (Fig. 2B). Some of the embryos were subjected to differential cell staining and the analysis revealed a decrease in the total number of cells in blastocysts of aged mice due to a lower number of cells in the trophectoderm (Fig. 3A). Other variables of cell allocation were not affected (Fig. 3B).
Figure 2.
Effects of AMA on fertility outcome in mice. (A) Percentage of non-superovulated mice with a vaginal plug that failed to yield embryos (n = females mated; χ2). Larger numbers of replicates were generated for the old treatment group reflecting additional samples required for future studies and researcher training. (B and C) Embryo production variables (n = females mated that yielded embryos; T-test). Arrested and/or fragmented embryos at the 2- to 8-cell stage along with morula and blastocyst with clear signs of fragmentation were classified as degenerated embryos. All non-fragmented 1-cell structures were classified as unfertilized oocytes (i.e. ova). All embryos in the stage of morula or blastocyst with no signs of fragmentation were classified as viable embryos. *Indicates a significant difference (P < 0.05) between the embryo donors (Young versus Old).
Figure 3.
Effects of AMA on cell number of blastocysts in mice. Blastocyst stained had a blastocoel cavity greater than half of the volume of the embryo (i.e. expanded blastocysts). (A) Cell number in trophectoderm (TE) and inner cell mass (ICM; n = embryos analysed from 6 Young and 18 Old mothers; T-test). (B) Cell allocation variables. *Indicates a significant difference (P < 0.05) between the embryo donors (Young versus Old).
Offspring derived from blastocysts collected from aged mice show an increased weight gain during post-natal life in a sex-specific manner
To examine the effects of AMA on the post-natal development of offspring under an ART-derived procedure, we transferred blastocysts from old mothers into young surrogates and the resultant offspring (Old-ET) was compared with offspring derived from blastocysts collected from young mothers that were transferred into equivalent young surrogates (Young-ET; Fig. 1). Following ET, the number of pups per litter was not different between the groups (Fig. 4A), nor the efficiency of ET to yield live offspring (Old 66%; Young 65%). Similarly, the male to female proportion was not affected by origin of the embryo (Fig. 4B). Monitoring of body weight every week revealed no differences in weight gain in male offspring across treatments at any time point during the experimental trial (Fig. 5A). In contrast, Old-ET females were heavier than Young-ET females during most of post-natal development. The difference in body weight started to become apparent at post-natal Week 13 and remained significant for virtually the rest of the study (Fig. 5C).
Figure 4.
Effects of AMA on ET outcome in mice. (A) Number of pups per litter following ET (n = litters analysed; T-test). (B) Sex allocation of pups following ET (n = total offspring number; Binomial test). Offspring is the result of the transfer of blastocysts collected from young (Young-ET) or old (Old-ET) embryo donors into young embryo recipients.
Figure 5.
AMA can affect growth and cardiovascular function in mice. Post-natal body weight and blood pressure in male (A and B) and female (C and D) offspring derived from the transfer of blastocysts collected from young (Young-ET, 9 litters) or old (Old-ET, 10 litters) mothers into young embryo recipients. n = number of animals. Multilevel random effects regression analysis. *Indicates a significant difference (P < 0.05) between the groups (Young-ET versus Old-ET).
Offspring derived from blastocysts collected from aged mice develop hypertension during post-natal life in a non-sex-specific manner
Non-invasive systolic blood pressure measurements were carried out in the offspring at post-natal Week 9, 15 and 21. The analysis showed a significant increase in blood pressure in Old-ET male offspring at Weeks 9 and 15 compared with Young-ET counterparts (Fig. 5B). However, by Week 21 systolic blood pressure values were not significantly different between the groups (Fig. 5B). A similar scenario was observed in female offspring, but the difference was only significant at post-natal Week 15 (Fig. 5D). The average of the three measurements was higher in Old-ET offspring for both sexes (Males: 106.6 ± 1.4 versus 102.6 ± 0.9 mmHg; Females: 105.0 ± 1.4 versus 101.5 ± 0.7 mmHg), but it did not reach statistical significance (P < 0.10).
Offspring derived from blastocysts collected from aged mice display altered glucose homeostasis in a sex-specific manner
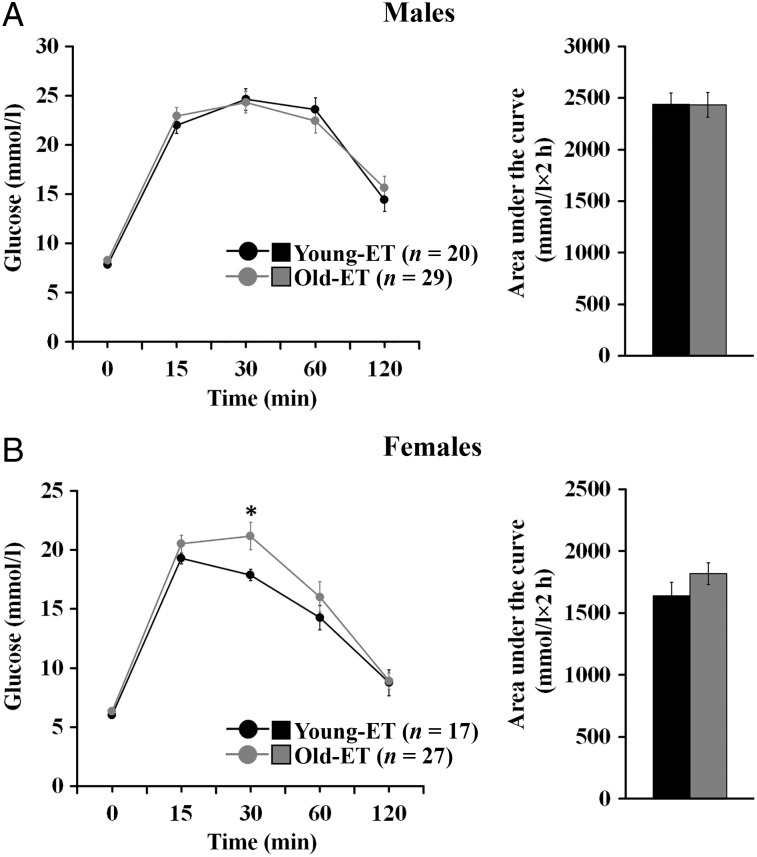
Offspring were subjected to an i.p. GTT at the end of the study on post-natal Week 30. Basal glucose concentrations following overnight fasting (i.e. 0 min) were not different between the groups in both sexes (Fig. 6A and B). In male offspring, glucose levels at different time points after glucose administration and the area under the curve were not affected by the origin of the offspring (Fig. 6A). In contrast, Old-ET females showed a greater peak glucose concentration 30 min after glucose injection compared with Young-ET counterparts (Fig. 6B). However, 60 min after glucose administration the difference started to lose significance (P = 0.050), and by 120 min post glucose injection glucose levels have returned to basal values and were very similar between the groups. The area under the curve in Old-ET females (Fig. 6B) was higher compared with Young-ET counterparts, but the difference was not significant (P < 0.10).
Figure 6.
AMA can affect glucose metabolism in mice. Intraperitoneal GTT outcome in male (A) and female (B) offspring derived from the transfer of blastocysts collected from young (Young-ET, 9 litters) or old (Old-ET, 10 litters) mothers into young embryo recipients. n = number of animals. Multilevel random effects regression analysis. *Indicates a significant difference (P < 0.05) between the groups (Young-ET versus Old-ET).
Offspring derived from blastocysts collected from aged mice exhibit altered organ allometry in a sex-specific manner
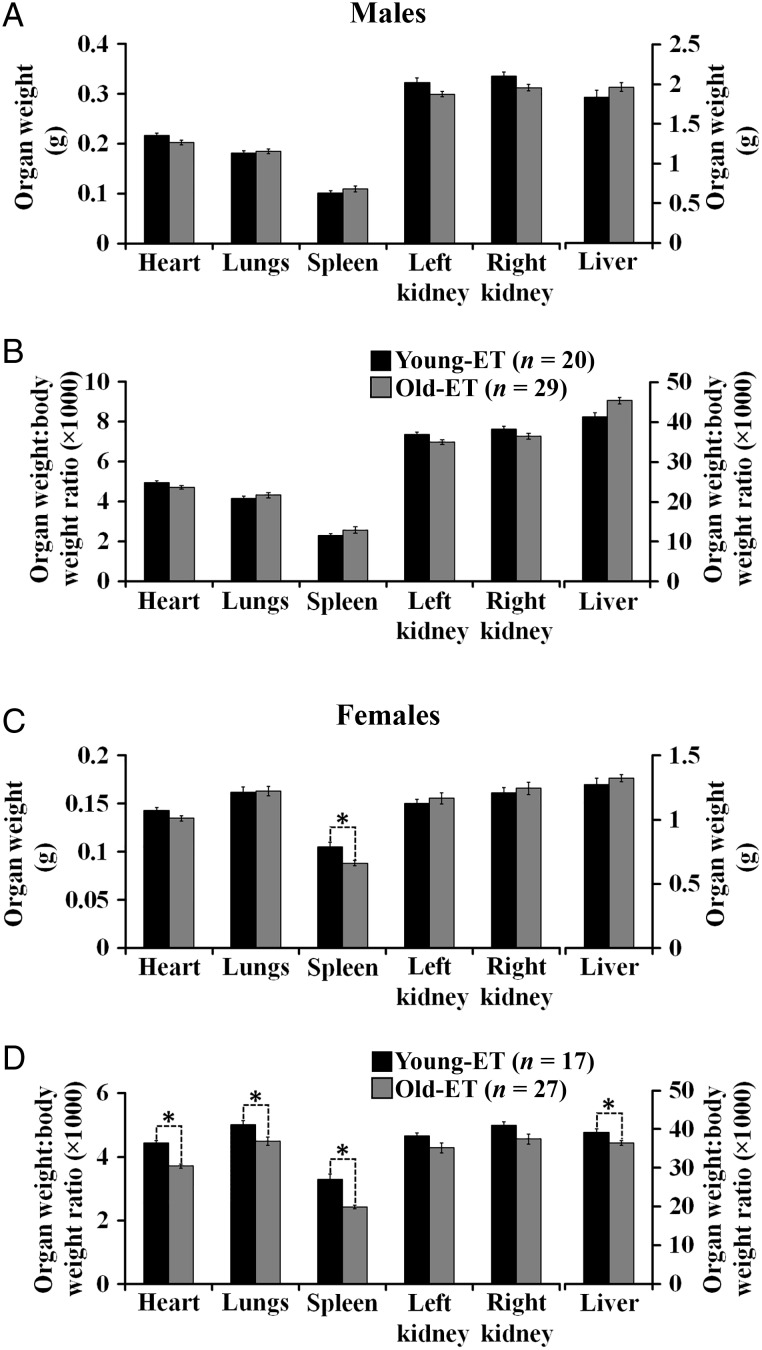
Analysis of organ allometry was carried out at the end of the study on post-natal Week 30. No significant differences in carcass weight were observed in males (38.5 ± 0.5 and 40.0 ± 1.0 g for Old-ET and Young-ET offspring, respectively). In contrast, the carcass weight of females was higher in the Old-ET group (32.9 ± 0.6 versus 29.3 ± 0.7 g, P < 0.05). In male offspring, organ weights and organ weight:body weight ratios were not different between the groups (Fig. 7A and B). However, in Old-ET females the spleen was lighter compared with Young-ET females (Fig. 7C). Furthermore, the organ weight:body weight ratio for heart, lungs, spleen and liver were decreased in Old-ET females (Fig. 7D). Most of the internal organs of female offspring were of similar weight between the groups. Hence, with the exception of the spleen, the undersized organs in Old-ET females are more related to their increased body weight.
Figure 7.
AMA can affect organ size in mice. Organ allometry variables in male (A and B) and female (C and D) offspring derived from the transfer of blastocysts collected from young (Young-ET, 9 litters) or old (Old-ET, 10 litters) mothers into young embryo recipients. n = number of animals. Multilevel random effects regression analysis. *Indicates a significant difference (P < 0.05) between the groups (Young-ET versus Old-ET).
Discussion
In this study, we have investigated the long-term effects of AMA on offspring development during post-natal life. Using an ET model our data show that offspring derived from embryos collected from aged female mice (mated with young males) and transferred into young embryo recipients display alterations in body weight gain, blood pressure, glucose metabolism and organ allometry during post-natal development. Most of the altered phenotypes were observed in a sex-specific manner, where females were more affected. Our study is the first to examine metabolic and cardiovascular function in offspring of aged female mice under an ART-related procedure. The fact that phenotypic alterations were observed under a normalized maternal in vivo environment following ET indicates that some of the adverse programming induced by AMA is already established by the time the embryo reaches the blastocyst stage and independent of subsequent conditions of pregnancy. In support of our current findings, experimental evidence in rodents and ruminants has shown that altered phenotypes during post-natal life can be programmed during the periconceptional period when critical reproductive events such as ovarian folliculogenesis and/or preimplantation embryo development are exposed to undernutrition (Sinclair et al., 2007; Watkins et al., 2008), overnutrition (Rattanatray et al., 2010; Kleemann et al., 2015), ART-related procedures (Watkins et al., 2007; Rexhaj et al., 2013; Donjacour et al., 2014; López-Cardona et al., 2015) or inflammation-like events (Williams et al., 2011).
We used 34–39-week-old mice in our AMA model in an effort to reflect the onset of reproductive aging in middle-aged females rather than substantial loss of reproductive function during advanced senescence. Indeed, in our study the number of embryos recovered from non-superovulated old mice was not affected, which is comparable with the lack of effect of AMA on litter size following natural mating reported in AMA studies with mice of similar ages (Lopes et al., 2009; Yue et al., 2012). Although cases of pregnancies in women in their 60s have been reported (Cutas and Smajdor, 2015), we believe our model is more akin with the current trend in delayed childbearing, in which middle-aged women rather than elderly individuals are more likely to delay motherhood. Human embryos from aging mothers are known to show an increase in aneuploidy, contributing to reduced fertility with age (Munné, 2006; Jones and Lane, 2013). In comparison, depending on the mouse strain (Yun et al., 2014), murine embryos are less likely to display aneuploidy (Carbone and Chavez, 2015), especially in the age range used in the present study (Jones and Lane, 2013). This distinction, although reflecting a difference to the conditions normally encountered during human assisted conception practice, is to our advantage in the current mouse model since it permits increased survival to screen post-natal health in relation to maternal age. Indeed, it is believed that aneuploidy is the main cause of human IVF failure, resulting in implantation failure, miscarriage or birth of offspring with congenital disorders (Campbell et al., 2013; Franasiak and Scott, 2014). Moreover, from a clinical practice perspective, our model relates to conditions of human gestational surrogacy where AMA embryos are transferred to a younger recipient and the potential programming of such embryos mediated through donor maternal age.
Our results indicate that maternal aging can induce a decrease in the number of cells at the blastocyst stage, especially in the trophectoderm. A recent study in rats reported that growth-restricted females with AMA at conception produced blastocysts with decreased cell number attributed to a lower cell number in trophectoderm (Master et al., 2015). Low cell number in blastocysts has been associated with decreased placental and fetal weight in a mouse model of mitochondrial dysfunction (Wakefield et al., 2011). We did not analyse fetal development in our study, but a reduction in embryo size and somite number without alterations in litter size has been reported in a murine model of AMA (Lopes et al., 2009). In our study, birthweight was not examined, but our data suggest that deviation in cell proliferation at early stages of development is an early sign of impaired post-natal health. Indeed, rodent offspring developing high blood pressure in adulthood showed alterations in cell number at the blastocyst stage after exposure to undernutrition or to an in vitro environment during the preimplantation period (Kwong et al., 2000; Watkins et al., 2007).
The subtle impairment of glucose homeostasis found in female offspring of old mice in our study gives partial support to a recent multi-country study that suggested that AMA can induce increased plasma glucose concentrations in young adults (Fall et al., 2015). However, in that epidemiological human study the impaired glucose metabolism was not sex-specific (Fall et al., 2015), which contrast with our results. It will be interesting to test under experimental conditions if this lack of gender specificity on post-natal glucose metabolism is due to an added effect of paternal aging. Previous epidemiological human studies also failed to detect an effect of AMA on offspring body weight (Myrskylä and Fenelon, 2012) or blood pressure (Fall et al., 2015; Verroken et al., 2015) during adulthood. Here, it is important to highlight that in epidemiological studies it is extremely difficult to control relevant confounding factors and to collect accurate long-term information on lifestyle habits and health status. Under well-controlled experimental conditions in mice, we found that maternal aging can programme the development of high blood pressure in adulthood in a non-sex-specific manner. However, the high blood pressure observed in Old-ET offspring was accompanied by increased body weight only in females. Similarly, in previous experiments examining the effects of undernutrition (Watkins et al., 2008) and in vitro culture (Watkins et al., 2007) during the periconceptional period in mice, we found increases in blood pressure in male offspring without noticeable changes in body weight during post-natal development. The lack of association between cardiac activity and body conformation has also been observed in another model of developmental programming in which male offspring of obese mice showed alterations during post-natal development in several variables associated with cardiovascular function independently of body weight (Blackmore et al., 2014).
It is believed that sex chromosome dosage differences between males and females are behind the gender-specific offspring phenotypes found on programming trajectories (Laguna-Barraza et al., 2012). In this scenario, gene dosage differences on sex chromosomes would affect differently the transcriptional activity of autosomal genes, resulting in sex-biased modulatory interactions in gene networks that would affect a large fraction of the genome, the ‘sexome’, which in turn will produce sex-specific phenotypes (Arnold and Lusis, 2012). Sex-specific differences in gene expression, mitochondrial function and epigenetic function are present at the preimplantation stage that could be expected to last beyond embryogenesis (Laguna-Barraza et al., 2012).
Interestingly, our AMA model showed several similarities with our previous study examining the long-term effects of extended in vitro embryo culture in mice (Watkins et al., 2007). In that study, a low cell number in both the trophectoderm and the inner cell mass was associated with increased body weight exclusively in females and high blood pressure in offspring regardless of sex (Watkins et al., 2007). However, even brief embryo culture (2 h) before blastocyst transfer was sufficient to minimally increase offspring blood pressure (Watkins et al., 2007). Hence, a combined effect of maternal aging of embryos and their in vitro culture and transfer most probably are behind the altered post-natal phenotype observed in the present study. Nevertheless, our aim was to develop a model that reflects to a certain extent the current use of ART in women of AMA (Cabry et al., 2014; Jackson et al., 2015). The use of ET in our study most probably accounts for the increase in body weight, which contrasts with previous reports of lower post-natal body weight in offspring of normally mated aged mice, coupled with the aged maternal tract environment (Tarin et al., 2005). However, notably, in both models the deviation in body weight after weaning was observed, not early, but later during post-natal development. Moreover, the decreased spleen weight associated with AMA we observed in our study was also found in mice offspring derived from natural pregnancies of old mothers (Albert et al., 1965). It is unknown at present if this decreased spleen weight will impact the functioning of the immune system.
Mitochondrial dysfunction associated with increased oxidative stress in oocytes has been suggested as a possible mechanism behind the long-term effects of aging (Tarin et al., 2003). Indeed, several studies in mice and humans have found altered mitochondrial function in oocytes from aged females (Kujjo and Perez, 2012). Epigenetic modification has been also suggested as a potential mechanism underlying the periconceptional programming of disease occurrence in adulthood (Lucas, 2013) and several epigenetic modifications in oocytes from aged mice and humans have been documented (Ge et al., 2015). However, a direct association between these oocyte alterations and long-term programming of disease has not been reported. In addition, although experimental evidence has indicated that uterine aging is accompanied by molecular and morphological changes that can be detrimental for fertility (Nelson et al., 2013), the contribution of the microenvironment in the oviduct and uterus of aged mothers to long-term programming of phenotypes during post-natal life is largely unknown. Interestingly, a recent study with reciprocal ovarian transplants between young and old mothers indicated that the uterine environment, but not the oocyte, plays a crucial role in the development of congenital heart defects in offspring of old mice (Schulkey et al., 2015).
Given that the impaired oocyte quality and the development of congenital heart disease attributed to AMA can be ameliorated with nutritional or exercise interventions (Nehra et al., 2012; Schulkey et al., 2015), it is critical to determine if the risk of developing altered post-natal phenotypes associated with AMA can be decreased with similar interventions. We recognize that data from animal models cannot be extrapolated to humans, but they are valuable to develop conceptual models that can produce verifiable hypotheses for eventual testing in the target species (i.e. humans).
In conclusion, in a murine ART–AMA model, we have shown that maternal aging can programme the development of impaired metabolic and cardiovascular function during post-natal life. Furthermore, our data also indicate that the preimplantation period is a developmental window in which adverse programming can be exerted by AMA and be independent of the maternal tract and systemic environment where pregnancy subsequently occurs. Hence, whilst human embryos display increased aneuploidy compared with mouse, we believe our data have implications for women of AMA undergoing assisted reproduction, including surrogacy programmes.
Authors' roles
M.A.V. performed experiments, analysed data and wrote the manuscript. C.G.C.S. planned and performed experiments. N.R.S. performed experiments, and edited the paper. C.O. provided statistical expertise and edited the paper. T.P.F. conceived and designed the study, and edited the paper.
Funding
This work was supported through the European Union FP7-CP-FP Epihealth programme (278418) to T.P.F. and the BBSRC (BB/F007450/1) to TPF. Funding to pay the Open Access publication charges for this article was provided by University of Southampton.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
We are grateful for the technical support of the Biomedical Research Facility at the University of Southampton.
References
- Albert S, Wolf PL, O'Mara C, Barany W, Pryjma I. Influence of maternal age and parity on development of lymphoreticular organs of offspring in mice. J Gerontol 1965;20:530–535. [PubMed] [Google Scholar]
- Albert BB, De Bock M, Derraik JG, Brennan CM, Biggs JB, Hofman PL, Cutfield WS. Increasing parental age at childbirth is associated with greater insulin sensitivity and more favorable metabolic profile in overweight adult male offspring. Am J Hum Biol 2015;27:380–386. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Lusis AJ. Understanding the sexome: measuring and reporting sex differences in gene systems. Endocrinology 2012;153:2551–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore HL, Niu Y, Fernandez-Twinn DS, Tarry-Adkins JL, Giussani DA, Ozanne SE. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology 2014;155:3970–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabry R, Merviel P, Hazout A, Belloc S, Dalleac A, Copin H, Benkhalifa M. Management of infertility in women over 40. Maturitas 2014;78:17–21. [DOI] [PubMed] [Google Scholar]
- Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod Biomed Online 2013;27:140–146. [DOI] [PubMed] [Google Scholar]
- Carbone L, Chavez SL. Mammalian pre-implantation chromosomal instability: species comparison, evolutionary considerations, and pathological correlations. Syst Biol Reprod Med 2015;61:321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell CR, Stene LC, Joner G, Bulsara MK, Cinek O, Rosenbauer J, Ludvigsson J, Jane M, Svensson J, Goldacre MJ et al. . Maternal age at birth and childhood type 1 diabetes: a pooled analysis of 30 observational studies. Diabetes 2010;59:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C, Mpatsoulis D, Natsos A, Petropoulou PI, Zvintzou E, Traish AM, Voshol PJ, Karagiannides I, Kypreos KE. The low density lipoprotein receptor modulates the effects of hypogonadism on diet-induced obesity and related metabolic perturbations. J Lipid Res 2014;55:1434–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM; SWS Study Group. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women's Survey. Am J Clin Nutr 2012;96:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely G, Czeizel AE, Veszpremi B. Distribution of maternal age and birth order groups in cases with unclassified multiple congenital abnormalities according to the number of component abnormalities: a national population-based case–control study. Birth Defects Res A Clin Mol Teratol 2015;103:67–75. [DOI] [PubMed] [Google Scholar]
- Cutas D, Smajdor A. Postmenopausal motherhood reloaded: advanced age and in vitro derived gametes. Hypatia 2015;30:386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod 2014;90:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004;103:51–56. [DOI] [PubMed] [Google Scholar]
- El Hajj N, Haaf T. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertil Steril 2013;99:632–641. [DOI] [PubMed] [Google Scholar]
- Fall CH, Sachdev HS, Osmond C, Restrepo-Mendez MC, Victora C, Martorell R, Stein AD, Sinha S, Tandon N, Adair L et al. . Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low-income and middle-income countries (COHORTS collaboration). Lancet Glob Health 2015;3:e366–e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franasiak JM, Scott RT Jr. Embryonic aneuploidy: overcoming molecular genetics challenges improves outcomes and changes practice patterns. Trends Mol Med 2014;20:499–508. [DOI] [PubMed] [Google Scholar]
- Ge ZJ, Schatten H, Zhang CL, Sun QY. Oocyte ageing and epigenetics. Reproduction 2015;149:R103–R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr 2004;144:240–245. [DOI] [PubMed] [Google Scholar]
- Hardy K, Handyside AH, Winston RM. The human blastocyst: cell number, death and allocation during late preimplantation development in vitro. Development 1989;107:597–604. [DOI] [PubMed] [Google Scholar]
- Jackson S, Hong C, Wang ET, Alexander C, Gregory KD, Pisarska MD. Pregnancy outcomes in very advanced maternal age pregnancies: the impact of assisted reproductive technology. Fertil Steril 2015;103:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Lane SI. Molecular causes of aneuploidy in mammalian eggs. Development 2013;140:3719–3730. [DOI] [PubMed] [Google Scholar]
- Kenny LC, Lavender T, McNamee R, O'Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One 2013;8:e56583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol 2013;42:634–643. [DOI] [PubMed] [Google Scholar]
- Kleemann DO, Kelly JM, Rudiger SR, McMillen IC, Morrison JL, Zhang S, MacLaughlin SM, Smith DH, Grimson RJ, Jaensch KS et al. . Effect of periconceptional nutrition on the growth, behaviour and survival of the neonatal lamb. Anim Reprod Sci 2015;160:12–22. [DOI] [PubMed] [Google Scholar]
- Kujjo LL, Perez GI. Ceramide and mitochondrial function in aging oocytes: joggling a new hypothesis and old players. Reproduction 2012;143:1–10. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000;127:4195–4202. [DOI] [PubMed] [Google Scholar]
- Laguna-Barraza R, Bermejo-Alvarez P, Ramos-Ibeas P, de Frutos C, Lopez-Cardona AP, Calle A, Fernandez-Gonzalez R, Pericuesta E, Ramirez MA, Gutierrez-Adan A. Sex-specific embryonic origin of postnatal phenotypic variability. Reprod Fertil Dev 2012;25:38–47. [DOI] [PubMed] [Google Scholar]
- Lammi N, Moltchanova E, Blomstedt P, Eriksson JG, Taskinen O, Sarti C, Tuomilehto J, Karvonen M. The effect of birth order and parental age on the risk of type 1 and 2 diabetes among young adults. Diabetologia 2007;50:2433–2438. [DOI] [PubMed] [Google Scholar]
- Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel JP, Souza JP, Gulmezoglu AM. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG 2014;121 (Suppl 1):49–56. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Najman JM, Sterne J, Williams GM, Ebrahim S, Davey Smith G. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater-University study of pregnancy and its outcomes. Circulation 2004;110:2417–2423. [DOI] [PubMed] [Google Scholar]
- Lee BK, McGrath JJ. Advancing parental age and autism: multifactorial pathways. Trends Mol Med 2015;21:118–125. [DOI] [PubMed] [Google Scholar]
- Lerch S, Brandwein C, Dormann C, Gass P, Chourbaji S. Mice age: does the age of the mother predict offspring behaviour. Physiol Behav 2015;147:157–162. [DOI] [PubMed] [Google Scholar]
- Lopes FL, Fortier AL, Darricarrere N, Chan D, Arnold DR, Trasler JM. Reproductive and epigenetic outcomes associated with aging mouse oocytes. Hum Mol Genet 2009;18:2032–2044. [DOI] [PubMed] [Google Scholar]
- López-Cardona AP, Fernández-González R, Pérez-Crespo M, Alén F, de Fonseca FR, Orio L, Gutierrez-Adan A. Effects of synchronous and asynchronous embryo transfer on postnatal development, adult Health, and behavior in Mice. Biol Reprod 2015;93:85. [DOI] [PubMed] [Google Scholar]
- Lucas E. Epigenetic effects on the embryo as a result of periconceptional environment and assisted reproduction technology. Reprod Biomed Online 2013;27:477–485. [DOI] [PubMed] [Google Scholar]
- Master JS, Thouas GA, Harvey AJ, Sheedy JR, Hannan NJ, Gardner DK, Wlodek ME. Low female birth weight and advanced maternal age programme alterations in next-generation blastocyst development. Reproduction 2015;149:497–510. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J 1990;300:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné S. Chromosome abnormalities and their relationship to morphology and development of human embryos. Reprod Biomed Online 2006;12:234–253. [DOI] [PubMed] [Google Scholar]
- Myrskylä M, Fenelon A. Maternal age and offspring adult health: evidence from the health and retirement study. Demography 2012;49:1231–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrskylä M, Elo IT, Kohler IV, Martikainen P. The association between advanced maternal and paternal ages and increased adult mortality is explained by early parental loss. Soc Sci Med 2014;119:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr-Esfahani M, Johnson MH, Aitken RJ. The effect of iron and iron chelators on the in-vitro block to development of the mouse preimplantation embryo: BAT6 a new medium for improved culture of mouse embryos in vitro. Hum Reprod 1990;5:997–1003. [DOI] [PubMed] [Google Scholar]
- Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, Pan AH, Guo L, Rodig SJ, Tilly JL et al. . Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 2012;11:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update 2013;19:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanatray L, MacLaughlin SM, Kleemann DO, Walker SK, Muhlhausler BS, McMillen IC. Impact of maternal periconceptional overnutrition on fat mass and expression of adipogenic and lipogenic genes in visceral and subcutaneous fat depots in the postnatal lamb. Endocrinology 2010;151:5195–5205. [DOI] [PubMed] [Google Scholar]
- Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, Fuster DG, Anderegg M, Somm E, Bouillet E, Allemann Y, Sartori C, Scherrer U. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest 2013;123:5052–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril 2015;103:1136–1143. [DOI] [PubMed] [Google Scholar]
- Savage T, Derraik JG, Miles HL, Mouat F, Hofman PL, Cutfield WS. Increasing maternal age is associated with taller stature and reduced abdominal fat in their children. PLoS One 2013;8:e58869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkey CE, Regmi SD, Magnan RA, Danzo MT, Luther H, Hutchinson AK, Panzer AA, Grady MM, Wilson DB, Jay PY. The maternal-age-associated risk of congenital heart disease is modifiable. Nature 2015;520:230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA et al. . DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA 2007;104:19351–19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits LJ, Zielhuis GA, Jongbloet PH, Van Poppel FW. Mother's age and daughter's fecundity. An epidemiological analysis of late 19th to early 20th century family reconstitutions. Int J Epidemiol 2002;31:349–358. [PubMed] [Google Scholar]
- Summers MC, McGinnis LK, Lawitts JA, Raffin M, Biggers JD. IVF of mouse ova in a simplex optimized medium supplemented with amino acids. Hum Reprod 2000;15:1791–1801. [DOI] [PubMed] [Google Scholar]
- Sutcliffe AG, Barnes J, Belsky J, Gardiner J, Melhuish E. The health and development of children born to older mothers in the United Kingdom: observational study using longitudinal cohort data. Br Med J 2012;345:e5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin JJ, Vidal E, Perez-Hoyos S, Cano A, Balasch J. Delayed motherhood increases the probability of sons to be infertile. J Assist Reprod Genet 2001;18:650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin JJ, Gomez-Piquer V, Manzanedo C, Minarro J, Hermenegildo C, Cano A. Long-term effects of delayed motherhood in mice on postnatal development and behavioural traits of offspring. Hum Reprod 2003;18:1580–1587. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Gomez-Piquer V, Rausell F, Navarro S, Hermenegildo C, Cano A. Delayed motherhood decreases life expectancy of mouse offspring. Biol Reprod 2005;72:1336–1343. [DOI] [PubMed] [Google Scholar]
- Tearne JE. Older maternal age and child behavioral and cognitive outcomes: a review of the literature. Fertil Steril 2015;103:1381–1391. [DOI] [PubMed] [Google Scholar]
- Verroken C, Kaufman J-M, Goemaere S, Toye K, Lapauw B. Maternal age at childbirth is associated with glucose metabolism in adult men. Endocr Rev 2015;36:FRI-616-FRI-616. [Google Scholar]
- Wakefield SL, Lane M, Mitchell M. Impaired mitochondrial function in the preimplantation embryo perturbs fetal and placental development in the mouse. Biol Reprod 2011;84:572–580. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Platt D, Papenbrock T, Wilkins A, Eckert JJ, Kwong WY, Osmond C, Hanson M, Fleming TP. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci USA 2007;104:5449–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, Wilkins A, Perry VH, Sheth B, Kwong WY et al. . Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod 2008;78:299–306. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Lovas A, Rauch A, Andreas N, von Maltzahn J, Riemann M, Weih F. Classical and alternative NF-κB signaling cooperate in regulating adipocyte differentiation and function. Int J Obes (Lond) 2016;40:452–459. [DOI] [PubMed] [Google Scholar]
- Whincup PH, Cook DG, Shaper AG. Early influences on blood pressure: a study of children aged 5–7 years. Br Med J 1989;299:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding M, Coppola G, De Icco F, Arenare L, Di Matteo L, Dale B. Maternal non-Mendelian inheritance of a reduced lifespan? A hypothesis. J Assist Reprod Genet 2014;31:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Teeling JL, Perry VH, Fleming TP. Mouse maternal systemic inflammation at the zygote stage causes blunted cytokine responsiveness in lipopolysaccharide-challenged adult offspring. BMC Biol 2011;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 2010;151:5438–5445. [DOI] [PubMed] [Google Scholar]
- Yue MX, Fu XW, Zhou GB, Hou YP, Du M, Wang L, Zhu SE. Abnormal DNA methylation in oocytes could be associated with a decrease in reproductive potential in old mice. J Assist Reprod Genet 2012;29:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Y, Holt JE, Lane SI, McLaughlin EA, Merriman JA, Jones KT. Reduced ability to recover from spindle disruption and loss of kinetochore spindle assembly checkpoint proteins in oocytes from aged mice. Cell Cycle 2014;13:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]