Abstract
STUDY QUESTION
Does supplementation with co-enzyme Q10 (CoQ10) improve the oocyte mitochondrial abnormalities associated with obesity in mice?
SUMMARY ANSWER
In an obese mouse model, CoQ10 improves the mitochondrial function of oocytes.
WHAT IS KNOWN ALREADY
Obesity impairs oocyte quality. Oocytes from mice fed a high-fat/high-sugar (HF/HS) diet have abnormalities in mitochondrial distribution and function and in meiotic progression.
STUDY DESIGN, SIZE, DURATION
Mice were randomly assigned to a normal, chow diet or an isocaloric HF/HS diet for 12 weeks. After 6 weeks on the diet, half of the mice receiving a normal diet and half of the mice receiving a HF/HS diet were randomly assigned to receive CoQ10 supplementation injections for the remaining 6 weeks.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Dietary intervention was initiated on C57Bl6 female mice at 4 weeks of age, CoQ10 versus vehicle injections were assigned at 10 weeks, and assays were conducted at 16 weeks of age. Mice were super-ovulated, and oocytes were collected and stained to assess mitochondrial distribution, quantify reactive oxygen species (ROS), assess meiotic spindle formation, and measure metabolites. In vitro fertilization was performed, and blastocyst embryos were transferred into control mice. Oocyte number, fertilization rate, blastulation rate and implantation rate were compared between the four cohorts. Bivariate statistics were performed appropriately.
MAIN RESULTS AND THE ROLE OF CHANCE
HF/HS mice weighed significantly more than normal diet mice (29 versus 22 g, P< 0.001). CoQ10 supplementation did not influence weight. Levels of ATP, citrate, and phosphocreatine were lower and ROS levels were higher in HF/HS mice than in controls (P< 0.001). CoQ10 supplementation significantly increased the levels of metabolites and decreased ROS levels in oocytes from normal diet mice but not in oocytes from HF/HS mice. However, CoQ10 completely prevented the mitochondrial distribution abnormalities observed in the HF/HS mice. Overall, CoQ10 supplementation significantly increased the percentage of normal spindle and chromosome alignment (92.3 versus 80.2%, P= 0.039). In the sub-analysis by diet, the difference did not reach statistical significance. When undergoing IVF, there were no statistically significant differences in the number of mature oocytes, the fertilization rate, blastocyst formation rates, implantation rates, resorption rates or litter size between HF/HS mice receiving CoQ10 or vehicle injections.
LIMITATIONS, REASONS FOR CAUTION
Experiments were limited to one species and strain of mice. The majority of experiments were performed after ovulation induction, which may not represent natural cycle fertility.
WIDER IMPLICATIONS OF THE FINDINGS
Improvement in oocyte mitochondrial distribution and function of normal, chow-fed mice and HF/HS-fed mice demonstrates the importance of CoQ10 and the efficiency of the mitochondrial respiratory chain in oocyte competence. Clinical studies are now needed to evaluate the therapeutic potential of CoQ10 in women's reproductive health.
STUDY FUNDING/COMPETING INTEREST(S)
C.E.B. received support from the National Research Training Program in Reproductive Medicine sponsored by the National Institute of Health (T32 HD040135-13) and the Scientific Advisory Board of Vivere Health. K.H.M received support from the American Diabetes Association and the National Institute of Health (R01 HD083895). There are no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
This study is not a clinical trial.
Keywords: co-enzyme Q10, antioxidant, mitochondria, obesity, oocyte quality, electron transport complex, spindle and chromosome alignment
Introduction
Obesity has devastating effects on nearly all aspects of women's reproductive health; it is associated with anovulation, polycystic ovarian syndrome and pregnancy complications such as miscarriage, pre-eclampsia, and gestational diabetes (Jungheim and Moley, 2010). Furthermore, obesity is associated with infertility and poor assisted reproductive technology outcomes (Bellver et al., 2006). Although the pathophysiology behind these effects is not fully known, strong evidence suggests that obesity impairs oocyte quality. For example, analysis of a large dataset from the Society of Assisted Reproductive Technology Clinic Online Reporting System revealed that obese women using autologous oocytes were less likely to achieve a clinical pregnancy than their normal-weight peers, while obese women who used donor oocytes had pregnancy rates similar to their normal-weight peers (Luke et al., 2011). Research investigating the effects of obesity on human oocytes has shown that mature oocytes from obese women were smaller and had more abnormal spindles and chromosome misalignment than oocytes from women with a normal body mass index (Marquard et al., 2010; Machtinger et al., 2012). Another study showed an association between elevated levels of free fatty acids in the follicular fluid and abnormal cumulus–oocyte complex morphology (Jungheim et al., 2011). However, studies using human oocytes are limited given their sparse availability and thus, the utilization of immature oocytes or oocytes that failed fertilization.
Obese mouse models have served as surrogates for human oocyte studies as similar findings have been demonstrated in the mouse oocytes. High-fat diet (HFD)-induced obesity has been associated with fetal growth restriction and abnormal neurodevelopment (Luzzo et al., 2012). When embryos from HFD mice were transferred into lean controls, abnormal fetal development persisted, suggesting that an obese uterine environment is not required for developmental defects. This concept is further supported by recent work demonstrating impaired fetal and placental growth after pre-gestational exposure to a maternal HFD and a normal gestational environment (Sasson et al., 2015).
Several defects have been observed in oocytes from HFD-exposed mice. First, these oocytes have spindle and chromosome alignment defects, which correlate with the observed increased incidence of aneuploidy. Second, oocytes from HFD-exposed mice have abnormalities in mitochondrial morphology and function (Wang et al., 2009; Luzzo et al., 2012). The high oxidative stress in an obese, hyperglycemic environment likely disturbs oocyte metabolism and thus hinders mitochondrial function (Gu et al., 2015; Seidler and Moley, 2015). As a result, the oocyte mitochondria produce less ATP and more reactive oxygen species (ROS). This increase in ROS production is associated with metabolic enzyme deficiencies, increased fatty acid oxidation and apoptosis (Balaban et al., 2005; Bentov and Casper, 2013). In the present study, a diet high in both fat and sugar (HF/HS) was administered as it induces obesity and insulin resistance, which is representative of the effects seen in humans from the consumption of the typical Western society diet. This HF/HS diet has previously been shown to illicit the above effects on oocyte mitochondria in a diet-induced obese mouse model (Wang et al., 2009; Luzzo et al., 2012).
Oocytes from aging mice show several of the same impairments as oocytes from HFD-exposed mice (Bentov et al., 2010). To assess whether or not these impairments could be ameliorated, Ben-Meir et al. supplemented mice with co-enzyme Q10 (CoQ10), an antioxidant component of the electron transport chain. They chose CoQ10 because a decline in CoQ10 concentration in serum has been associated with genetic mutations, medication use and aging (Pignatti et al., 1980; Bentov et al., 2010). These authors found that aged mice receiving CoQ10 supplements had significantly improved mitochondrial function, spindle formation and chromosome alignment (Ben-Meir et al., 2015a). The objective of this study was to determine whether supplementation with CoQ10 could alleviate the mitochondrial dysfunction in oocytes from obese mice and improve their reproductive outcomes.
Materials and Methods
Ethical approval
The Animal Studies Committee at Washington University School of Medicine approved all animal experiments. Animals were maintained in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals provided by the Institute for Laboratory Animal Research.
Animals and diet
Female C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) at 4 weeks of life. The experimental timeline is shown in Fig. 1A. Briefly, groups of 100 mice were randomly assigned to either a normal diet (PicoLab Rodent diet 20 (TestDiet, St. Louis, MO, USA); 13% fat, 3.2% sucrose, 25% protein by weight) or an isocaloric high-fat/high-sugar (HF/HS) diet (Test Diet 58R3 (TestDiet); 59% fat, 17% sucrose, 15% protein by weight). At 10 weeks of age, 50 of the mice receiving a normal diet and 50 of the mice receiving a HF/HS diet were randomly assigned to receive CoQ10 3 times per week (22 mg/kg dissolved in sesame oil, subcutaneously; Sigma-Aldrich, St. Louis, MO, USA) and the remaining mice received vehicle injections of sesame oil (Sigma-Aldrich).
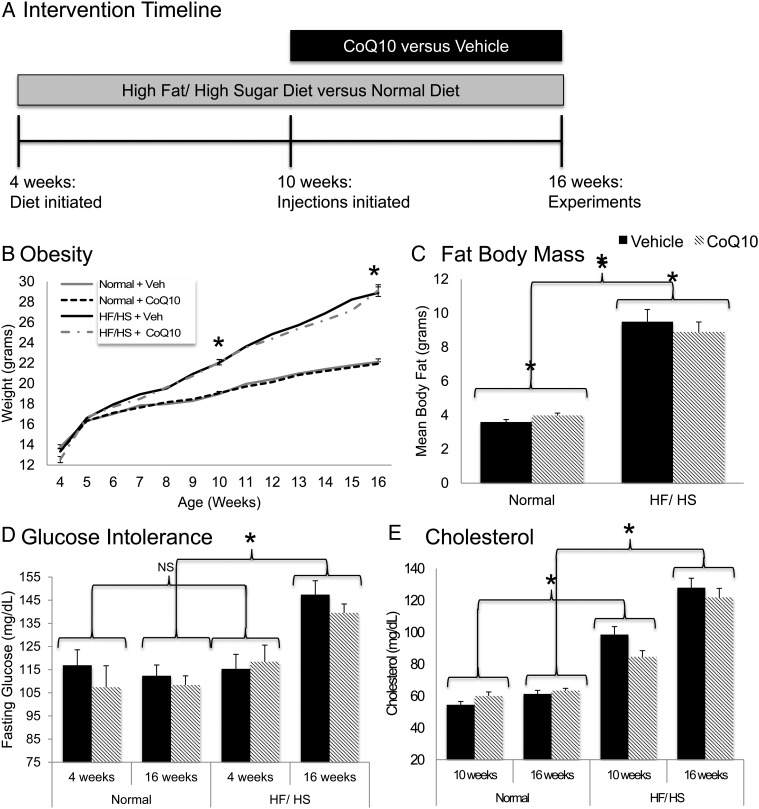
Figure 1.
Metabolic phenotype: (A) mice (n = 200, 50 per group) were on the high-fat/high-sugar (HF/HS) diet or a normal diet for 12 weeks and received subcutaneous, injections of co-enzyme Q10 (CoQ10, 22 mg/kg) or vehicle (Veh) for the latter 6 weeks. (B) Weekly weights (n = 50 per group) were obtained starting at 4 weeks until 16 weeks. (C) Prior to sacrifice at 16 weeks, body composition (n = 73) was assessed by EchoMRI. (D) Glucose levels (n = 50) were measured using venous tail blood after 4–5 h of fasting at 4 weeks, prior to diet exposure and again at 16 weeks. (E) Serum cholesterol (n = 40) concentration was obtained at 10 and 16 weeks. Values are represented as mean with error bars indicating+SEM. Statistical analyses were performed using Student's t-test or one-way ANOVA. *P < 0.05.
Body weights were recorded weekly. Fasting glucose levels were measured at 4-, 10- and 16-week time points (n = 50). All oocyte experiments were performed at 16 weeks of age, after 12 weeks on the assigned diet and 6 weeks of injections. Before sacrifice at 16 weeks, EchoMRI analysis of body compositions was performed to quantify adiposity (n = 73), and fasting serum was obtained to measure cholesterol and triglyceride concentrations (n = 40). Separate sets of mice were used for metabolic assays (n = 20); imaging of ROS, mitochondrial distribution (n = 20), and spindle/chromosome alignment (n = 20); and in vitro fertilization studies (n = 60).
Oocyte collection
To collect germinal vesicle (GV)-stage oocytes, mice were stimulated by intraperitoneal injection of 5 IU of pregnant mare serum gonadotrophin (PMSG; Harbor-UCLA Research and Education, LA, CA, USA). Forty-eight hours later, mice were sacrificed, and ovaries were dissected and placed in M2 media (Sigma-Aldrich) with milrinone (5 µm; EMD Millipore, Billerica, MA, USA) to prevent meiotic progression. Follicles were punctured and oocytes were mechanically denuded of cumulus cells.
To collect Metaphase II oocytes, mice were primed with PMSG (5 IU) and human chorionic gonadotrophin (hCG; Harbor-UCLA Research and Education, LA) (10 IU). Fourteen hours after hCG injection, oviducts were collected and the ampulla punctured to release ovulated oocytes. Cumulus cells were removed by brief incubation with hyaluronidase (1 mg/ml) (Sigma-Aldrich) and gentle pipetting.
Metabolite microanalytic assays
GV oocytes (n = 60) were frozen on a glass slide by dipping in isopentane equilibrated with liquid nitrogen. After freeze-drying overnight under vacuum at 35°C, the oocytes were extracted in a nanoliter volume under oil as described previously (Chi et al., 1988, 2002 ). Adenosine triphosphate (ATP), citrate and phosphocreatine (PCr) were measured by using an enzyme-linked assay as previously described (Chi et al., 1988, 2002).
Determination of mitochondrial distribution
GV oocytes (n = 340) were cultured in milrinone-supplemented M2 media containing 500 nM MitoTracker Red CMXRos (Molecular Probes, ThermoFisher, Waltham, MA, USA) in a dark, humidified atmosphere for 30 min at 37°C. Oocytes were then fixed with 4%, v/v, paraformaldehyde for 30 min, permeabilized (0.5%, v/v, Triton X-100; Sigma-Aldrich) for 20 min, blocked (1%, w/v, bovine serum albumin (BSA) (Sigma-Aldrich) by weight supplemented phosphate buffered saline (PBS)) for 1 h at room temperature, and incubated at 4°C overnight with anti-HSP60 antibody (Santa Cruz Biotechnology, Dallas, TX, USA). After washing, oocytes were incubated with secondary antibody and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Samples were mounted in Vectashield (Vector Labs, Burlingame, CA, USA) and analyzed by fluorescence microscopy (Leica LASX SPE Scanning Confocal Microscope, Buffalo Grove, IL, USA) with a 63× oil immersion objective. Mitochondrial distribution in each oocyte was blindly categorized as perinuclear, homogenous or aggregating.
Measurement of reactive oxygen species
GV oocytes (n = 438) were cultured in milrinone-supplemented M2 media containing 5 µm MitoSOX Red (Molecular Probes, ThermoFisher, Waltham, MA, USA) in a dark, humidified atmosphere for 10 min at 37°C. After washing in milrinone-supplemented EmbryoMax KSOM Embryo Culture (EMD Millipore) under low light, live oocytes were imaged via fluorescence microscopy (Leica LASX SPE Scanning Confocal Microscope). Mean signal intensity of each oocyte was measured by a blinded examiner and adjusted for oocyte size (ImageJ, Version 2.0.0-rc-31/1.49v, National Institutes of Health, Bethesda, MD, USA).
Assessment of spindle and chromosome alignment
M2 oocytes (n = 146) were fixed with 4% paraformaldehyde for 30 min, permeabilized (0.5%, v/v, Triton X-100) for 20 min, and blocked (1%, w/v, BSA-supplemented PBS) for 1 h at room temperature. Samples were then incubated with anti-α tubulin fluorescein isothiocyanate antibody (Sigma-Aldrich) and counterstained with DAPI for 1 h. After washing, M2 oocytes were mounted in Vectashield and analyzed by the fluorescence microscopy as above. Alignment of spindles and chromosomes in each oocyte was blindly categorized as normal or abnormal.
In vitro fertilization
M2 oocytes (n = 977) were recovered from super-ovulated female mice. Sperm were collected from cauda epididymides of 10-week-old male mice and capacitated in vitro at 37°C for 1 h as described previously (Kim and Moley, 2008). Capacitated sperm (100 000 sperm/ml) were co-incubated with M2 oocytes for 6 h, and unbound sperm were subsequently washed away. After 24 h, the development of 2-cell stage embryos was considered successful fertilization. These embryos (n = 770) were then cultured for three additional days to the blastocyst stage. On embryonic day 4.5, 15 blastocysts were transferred into each pseudo-pregnant ICR (Jackson Laboratory) control recipient (n = 17). On embryonic Day 14.5, mice were sacrificed and uteri dissected. Implantation rate, resorption rate and pregnancy rate were recorded. Each experiment was performed in triplicate for a total of 15 mice per group.
Statistical analyses
Statistical analyses were performed in SPSS Statistics (Version 23.0; IBM Corp., Armonk, NY, USA). For parametric analysis of continuous variables, the Student t-test or one-way ANOVA was used. Homogeneity of variance of each sample was confirmed and the post hoc Tukey–Kramer test was performed. Mann–Whitney U test, Kruskal–Wallis and nonparametric analog ANOVA were used for non-parametric variables. The χ2 test was used for categorical analysis. Results are expressed as mean and standard error of the mean unless otherwise indicated. P < 0.05 was considered significant.
Results
CoQ10 supplementation does not prevent HF/HS diet-induced obesity
As expected, HF/HS-exposed mice weighed significantly more than mice on the control diet at both the 10- and 16-week time points (Fig. 1B). At 16 weeks, HF/HS-exposed mice had a higher mean body fat (Fig. 1C), were more glucose intolerant (Fig. 1D), and had higher serum levels of cholesterol (Fig. 1E) than mice on control diet. None of these parameters were altered by CoQ10 supplementation (Fig. 1B–E).
Effects of CoQ10 supplementation on oocyte mitochondrial function and distribution
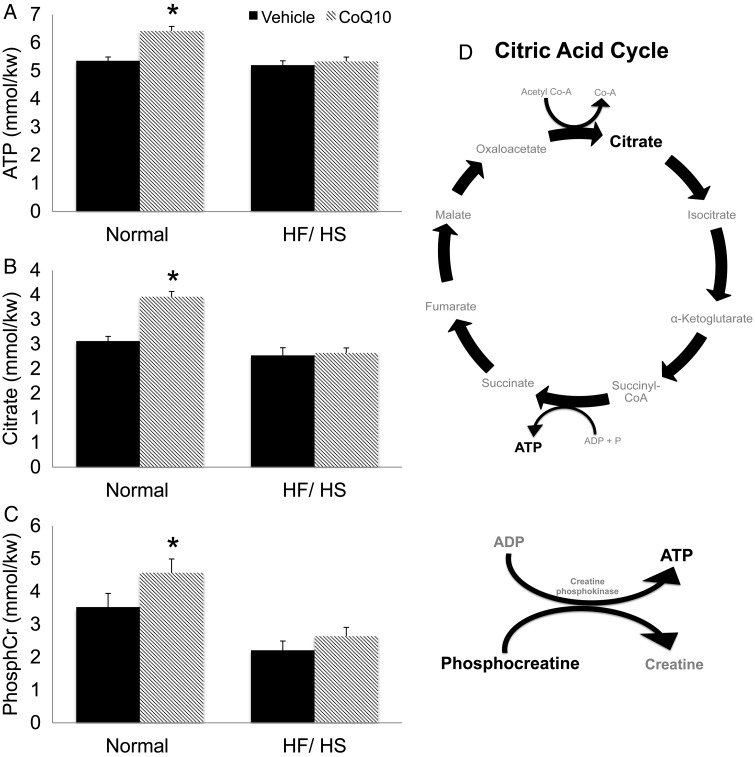
To assess the effects of CoQ10 on mitochondrial function, we performed microanalytical assays of energy substrates and metabolites in single GV oocytes. Oocytes from mice on a HF/HS diet had lower levels of ATP, citrate and creatine phosphate (PCr) than those of mice on a normal diet (Fig. 2). Supplementation with CoQ10 significantly increased oocyte levels of ATP, citrate and PCr in oocytes of mice on the normal diet but not in those on the HF/HS diet (Fig. 2).
Figure 2.
Metabolites (A–C) were measured from single GV oocytes using microanalytical assays as described in the Materials and Methods section. Sixty oocytes from 5 mice per group were used to quantify levels of ATP, citrate and Creatine phosphate (PhosphCr). Mean metabolite levels are expressed as millimoles/kilograms wet weight (mmol/kg wet wt.). (D) Depiction of citric acid cycle and its metabolites. Statistical analyses were performed using Student's t-test or one-way ANOVA. *P< 0.05. Error bars indicate + SEM. HF/HS, high-fat/high-sugar; CoQ10, co-enzyme q10.
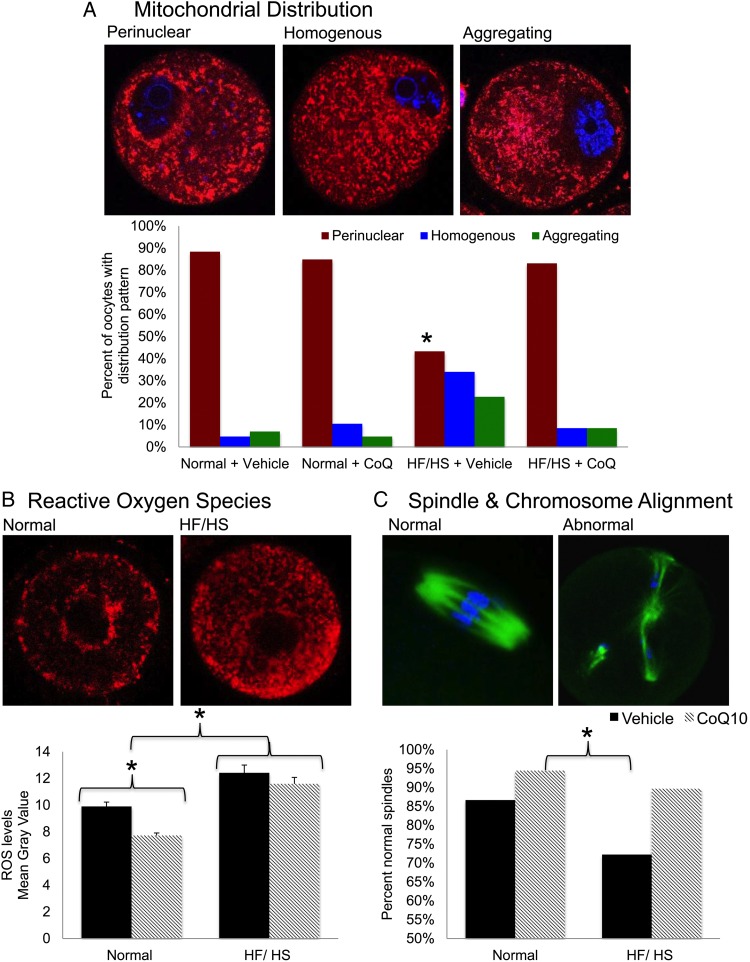
The previous work demonstrated that mitochondrial distribution in the developing oocyte is important for ATP production and oocyte competence and can be impaired by diet-induced obesity (Wang et al., 2009; Igosheva et al., 2010; Yu et al., 2010). High-quality oocytes typically have a perinuclear distribution of mitochondria (Wang et al., 2009; Ge et al., 2012). Conversely, the appearance of aggregating mitochondria in oocytes is associated with diminished embryo competence (Bellone et al., 2009; Igosheva et al., 2010). To assess the effects of CoQ10 supplementation on mitochondrial distribution, we stained oocytes with MitoTracker Red (Fig. 3A, top) and blindly categorized the mitochondria as perinuclear, homogenous or aggregating. We found that oocytes from HF/HS-exposed mice had a significantly lower percentage of oocytes with perinuclear mitochondria than normal diet-exposed mice. However, supplementation with CoQ10 completely prevented this distribution abnormality (Fig. 3A, bottom).
Figure 3.
Mitochondria: (A) MitoTracker Red CMXRos (red) probes were used to localize mitochondria in germinal vesicle (GV) oocytes and 4′,6′-diamidino-2-phenylindole (DAPI, blue) to stain DNA. Oocytes were imaged with fluorescence microscopy. Distribution was blindly categorized as perinuclear (normal), homogenous (abnormal) or aggregating (abnormal) in 340 oocytes from 20 mice (five per group). Statistical analyses were performed using χ2 test. *Significantly different from normal and HF/HS + CoQ10, P < 0.05. (B) Reactive oxygen species levels were quantified by MitoSOX Red staining and live imaging of 438 GV oocytes from 20 mice (5 per group) using a confocal microscope. Mean signal intensity was adjusted for oocyte size and expressed as mean gray value. Student's t-test and one-way ANOVA were utilized for statistical analyses. *P < 0.05. Error bars indicate + SEM. (C) Mature, ovulated oocytes were immunolabeled with anti-α tubulin antibody (green) and counterstained with DAPI (blue). A blind, qualitative analysis of confocal images was performed to assess the proportion of normally aligned spindles and chromosomes (n = 146 oocytes from 20 mice [5per group]). Statistical analyses were performed using χ2 test. *P < 0.05.
As another measure of mitochondrial function, we quantified the levels of ROS in individual GV oocytes (Fig. 3B). Oocytes from HF/HS diet-exposed mice had higher levels of ROS than oocytes as detected by mean signal intensity (arbitrary units) from mice fed a normal diet (12 ± 0.38 versus 8.63 ± 0.19, P < 0.001). Supplementation with CoQ10 significantly reduced levels of ROS in oocytes of mice on the normal diet (7.72 ± 0.19 versus 9.9 ± 0.33, P < 0.001) but not in those on the HF/HS diet (11.6 ± 0.48 versus 12.42 ± 0.58, P= NS).
CoQ10 supplementation improves spindle and chromosome alignment
In addition to mitochondrial defects, exposure to HF/HS diet impairs meiotic spindle assembly and chromosome alignment during oocyte maturation. To assess the ability of CoQ10 supplementation to prevent these defects, we immunolabeled M2 oocytes with an anti-α tubulin antibody to visualize the spindle, which in a healthy oocyte is barrel-shaped, and counterstained with DAPI to visualize chromosomes, which normally are well aligned at the metaphase plate. Overall, oocytes from mice receiving CoQ10 had a higher percentage of normal spindles and chromosome alignment than those from mice receiving vehicle injections (92.3 versus 80.2%, P = 0.039). As shown in Fig. 3C, mice fed a normal diet and supplemented with CoQ10 had the highest proportion of normal spindles and chromosomes (94.4%). This was significantly higher than mice on a HF/HS diet receiving vehicle injections (72.2%, P =0.01).
In vitro fertilization
Given that supplementation with CoQ10 was able to improve oocyte mitochondrial distribution and spindle and chromosome alignment, we wondered whether it could improve oocyte function. To test this, we performed in vitro fertilization. As shown in Table I, we evaluated the number of mature oocytes per female, fertilization rates and blastulation rates. However, none of the differences noted between HF/HS-exposed mice receiving CoQ10 and HF/HS-exposed mice receiving the vehicle injections were statistically significant. We then transferred 15 blastocysts into control, pseudo-pregnant recipients. One of the recipients of HF/HS + Veh blastocysts and one of the recipients of Normal + CoQ10 blastocysts had complete failed implantation. Additionally, both of these cohorts, had high-resorption rates (88.9 and 81.3%, respectively).
Table I.
In vitro fertilization outcomes.
| M2 oocytes per female | Fertilization rate (%) | Blastulation rate (%) | No of transfers | Failed implantation | Implantation rate (%) | Resorption rate (%) | Mean litter size | |
|---|---|---|---|---|---|---|---|---|
| Normal + Veh | 17.6 | 73.0 | 79.0 | 4 | 0 | 55.0 | 52.9 | 4.0 |
| Normal + CoQ10 | 15.1 | 76.0 | 79.3 | 3 | 1 | 22.2 | 81.3 | 1.5 |
| HF/HS + Veh | 15.9 | 77.3 | 82.7 | 4 | 1 | 28.3 | 88.9 | 0.3 |
| HF/HS + CoQ10 | 19.9 | 84.3 | 87.0 | 6 | 0 | 40.0 | 63.3 | 2.0 |
Discussion
Consumption of a HF/HS diet induces obesity in mice and has profound effects on oocyte quality, including impaired metabolism, abnormal mitochondrial distribution, increased levels of ROS, and impaired spindle formation and chromosomal alignment. We show here that supplementation with CoQ10 prevented some, but not all, of these effects. Most notably, CoQ10 supplementation improved mitochondrial distribution, spindle formation and chromosome alignment in oocytes from HF/HS-exposed mice.
CoQ10 supplementation also had significant effects on oocytes in mice on the normal diet; oocytes from supplemented mice had higher levels of ATP, PCr and citrate and lower levels of ROS than non-supplemented mice. One possibility is that the housing, handling or weekly injections induced above-baseline stress to these experimental mice, and CoQ10 compensated for these stressors. However, CoQ10 supplementation did not have statistically significant effect on the levels of these molecules in oocytes of mice exposed to the HF/HS diet. These results suggest that a CoQ10 deficiency is not the sole source of these effects or that these abnormalities occur as a result of oxidative and lipotoxic damage that cannot be prevented by CoQ10 supplementation. The idea that oocyte defects are caused by oxidative and lipotoxic damage is supported by recent work from our laboratory showing that weight loss reversed the obese phenotype in mice but did not improve oocyte quality (Reynolds et al., 2015). Diet and obesity most likely impair oocyte quality through multiple pathways, and additional work is needed to identify effective interventions.
Whether obesity is associated with decreased synthesis or increased consumption of CoQ10, which is required for the function of the electron transport chain, is not known. However, decreased concentrations of CoQ10 have been associated with several pathological conditions such as aging, several neurological disorders and mitochondrial disease. Recently, Mourier et al. (2015) showed that mice lacking mitofusin 2, a protein essential for the mitochondrial morphology and optimal respiratory chain function, had decreased levels of CoQ10, and CoQ10 supplementation could partially rescue the pathological defects in these mice. Similarly, Ben-Meir et al. (2015a,b) showed that supplementation with CoQ10 dramatically reversed the mitochondrial defects seen in oocytes of aged mice and improved metabolism in human granulosa cells from older women.
One strength of this study is that we compared oocytes from mice in the intervention group with those from mice in the control group. All experiments were performed on an in-bred strain of C57Bl6 female mice at identical ages after being housed in the same environment, thus reducing the possible number of variables. We used the same CoQ10 weight-based dosing regimen that has previously been shown to improve mitochondrial function and reproductive performance in aged mice (Ben-Meir et al., 2015a).
Limitations of the study include the use of an animal model and a single mouse strain, thus limiting generalizability of the findings. Although the obese mouse model is representative of human physiology (Collins et al., 2004), clinical studies are needed to define the effects of diet, obesity and antioxidant supplementation on oocyte quality and subsequent reproductive outcomes in humans. Another possible limitation is that we administered CoQ10 subcutaneously rather than orally, which would be more translatable to humans. Subcutaneous injections allowed for accurate, weight-based dosing in our mice and absorption of dietary CoQ10 is limited given its hydrophobicity and large molecular weight (Bhagavan and Chopra, 2006). Oral dose recommendations in humans vary based on pathology and range from 100 to 1200 mg/day. The absorption of CoQ10 is only 2–3% of the given dose. The dose administered to mice in our study would theoretically convert into ∼200 mg/day in a 60-kg woman. However, sparse pharmacokinetic data are available to guide the appropriate dose conversion from subcutaneous to oral administration, especially when translating from mouse to human.
The majority of experiments were performed after ovulation induction, which may not represent natural cycle fertility. An additional limitation is the small sample size used in the IVF experiments. Although they were performed in triplicate, yielding a large number of oocytes and embryos, more mice would be required to obtain a sample size adequate enough for statistical comparison of IVF outcomes such as litter, fetal and placental size.
In conclusion, consumption of a HF/HS diet and the resulting obesity clearly impair oocyte quality in mice. CoQ10 supplementation improves several aspects of oocyte quality but does not completely prevent the effects of obesity. Translational studies are now needed to investigate whether CoQ10 biosynthesis is impaired in oocytes of obese women or whether increased consumption of CoQ10 improves oocyte metabolism. While large societal and epidemiological efforts and clinical strategies to prevent obesity and encourage healthy diet are needed, our results also suggest that clinical trials to evaluate the effects of CoQ10 in obese patients are warranted.
Authors' roles
C.E.B. was involved in conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the article, and final approval of the submitted manuscript. A.B. was involved in protocol development, data acquisition and critical discussion. W.Z. performed microanalytical metabolic assays and approved the final manuscript. A.D. performed the IVF and approved the final manuscript. K.H.M contributed to conception and design of the study, interpretation of data, critical discussion, and revision and approval of the manuscript.
Funding
C.E.B. received support from the National Research Training Program in Reproductive Medicine sponsored by the National Institute of Health (T32 HD040135-13) and the Scientific Advisory Board of Vivere Health. K.H.M. received support from the American Diabetes Association and the National Institute of Health (R01 HD083895).
Conflict of interest
None declared.
Acknowledgements
We thank the Diabetes Models Phenotyping Core of the Diabetes Research Center at Washington University in St. Louis (NIH P60 DK 20579) for determining mouse body composition. We also thank Dr Deborah Frank for critical reading and editing of the manuscript.
References
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005;120:483–495. [DOI] [PubMed] [Google Scholar]
- Bellone M, Zuccotti M, Redi CA, Garagna S. The position of the germinal vesicle and the chromatin organization together provide a marker of the developmental competence of mouse antral oocytes. Reproduction 2009;138:639–643. [DOI] [PubMed] [Google Scholar]
- Bellver J, Busso C, Pellicer A, Remohi J, Simon C. Obesity and assisted reproductive technology outcomes. Reprod BioMed Online 2006;12:562–568. [DOI] [PubMed] [Google Scholar]
- Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015. a;14:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Meir A, Yahalomi S, Moshe B, Shufaro Y, Reubinoff B, Saada A. Coenzyme Q-dependent mitochondrial respiratory chain activity in granulosa cells is reduced with aging. Fertil Steril 2015. b;104:724–727. [DOI] [PubMed] [Google Scholar]
- Bentov Y, Casper RF. The aging oocyte--can mitochondrial function be improved. Fertil Steril 2013;99:18–22. [DOI] [PubMed] [Google Scholar]
- Bentov Y, Esfandiari N, Burstein E, Casper RF. The use of mitochondrial nutrients to improve the outcome of infertility treatment in older patients. Fertil Steril 2010;93:272–275. [DOI] [PubMed] [Google Scholar]
- Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmokinetics. Free Radic Res 2006;40:445–453. [DOI] [PubMed] [Google Scholar]
- Chi MM, Manchester KJ, Yang VC, Curato AD, Strickler RC, Lowry OH. Contrast in levels of metabolic enzymes in human and mouse ova. Biol Reprod 1998;39:295–307. [DOI] [PubMed] [Google Scholar]
- Chi MM, Hoehn A, Moley KH. Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. Am J Physiol Endocrinol Metab 2002;283:E226–E232. [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav 2004;81:243–248. [DOI] [PubMed] [Google Scholar]
- Ge H, Tollner TL, Hu Z, Dai M, Li X, Guan H, Shan D, Zhang X, Lv J, Huang C et al. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev 2012;79:392–401. [DOI] [PubMed] [Google Scholar]
- Gu L, Liu H, Gu X, Boots C, Moley KH, Wang Q. Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci 2015;72:251–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters activity and redox status in mouse oocytes and zygotes. Plos One 2010;5:e10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Moley KH. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol 2010;203:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Macones GA, Odem RR, Patterson BW, Lanzendorf SE, Ratts VS, Moley KH. Associations between free fatty acids, cumulus oocytes complex morphology and ovarian function during in vitro fertilization. Fertil Steril 2011;95:1970–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Moley KH. Paternal effect on embryo quality in diabetic mice is related to poor sperm quality and associated with decreased glucose transporter expression. Reproduction 2008;136:313–322. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R, SART Writing Group. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod 2011;26:245–252. [DOI] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One 2012;7:e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R, Combelles CMH, Missmer SA, Correia KF, Fox JH, Racowsky C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reprod 2012;27:3198–3207. [DOI] [PubMed] [Google Scholar]
- Marquard KL, Stephens SM, Jungheim ES, Ratts VS, Odem RR, Lanzendorf S, Moley KH. Polycycstic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril 2010;95:2146–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourier A, Motori E, Brandt T, Lagouge M, Atanassov I, Galinier A, Rappl G, Brodesser S, Hultenby K, Dieterich C et al. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J Cell Biol 2015;208:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatti C, Cocchi M, Weiss H. Coenzyme Q10 levels in rat heart of different age. Biochem Exp Biol 1980;16:39–42. [PubMed] [Google Scholar]
- Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev 2015;27:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson IE, Vitins AP, Mainigi MA, Moley KH, Simmons RA. Pre-gestation vs gestational exposure to maternal obesity differentially programs the offspring in mice. Diabetologia 2015;58:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler EA, Moley KH. Metabolic determinants of mitochondrial function in oocytes. Semin Reprod Med 2015;33:896–400. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol 2009;23:1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol 2010;224:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]