Abstract
The aim of the present study was to examine and understand changes in platelet functions prior to and after the treatment of primary immune thrombocytopenia (ITP) in children. An automatic hematology analyzer and whole blood flow cytometry were used to detect immature platelet fraction (IPF), IPC and membrane glycoproteins (CD62p, PAC-1 and CD42b) in ITP children (ITP group), children with complete response after ITP treatment (ITP-CR group) and children with elective surgery (normal control group). The results showed that, levels of platelet count (PLT) and plateletcrit in the ITP group were lower alhtough the levels of mean platelet volume, platelet distribution width and platelet-large cell ratio (P-LCR) were higher than those in the normal control and ITP-CR groups. PLT in the ITP-CR group was lower than that in the normal controls. Additionally, IPF% was higher in the normal control and ITP-CR groups, IPC was lower in the ITP group compared to the normal control and ITP-CR groups. Furthermore, prior to ADP activation, the expression levels of CD62p, PAC-1 and CD42b in the ITP group were lower in ITP group than those in the normal control and ITP-CR groups. The expression level of PAC-1 was lower in the ITP-CR and normal control groups. No differences were identified in CD62p and CD42b expression levels. Following ATP activation, CD62p, PAC-1 and CD42b expression in the ITP group was lower than that in the normal control and ITP-CR groups. PAC-1 expression was lower while CD62p expression was higher in the ITP-CR group compared to the normal control group. In conclusion, the activation of platelets in ITP children was low. Decreased platelet function, platelet parameters and platelet glycoproteins may be used as markers for monitoring the treatment efficacy in ITP children.
Keywords: primary immune thrombocytopenia, platelet parameters, platelet function
Introduction
Primary immune thrombocytopenia (ITP) is a common acquired autoimmune hemorrhagic disorder, accounting for 30% of the total hemorrhagic disorders (1). The annual incidence of ITP in children was approximately 1.9–6.4/100,000 (2). The clinical manifestations of ITP were predominantly spontaneous skin and mucosal bleeding, while the complication of intracranial hemorrhage occurred in severe patients, leading to death. The majority of children presented acute course, while approximately 20% children were transformed into chronic ITP (3).
The main mechanism of pathogenesis of this disease was increased platelet destruction and insufficient thrombo cytopoiesis due to abnormal cellular and humoral immunity (4,5). The complicated pathogenesis mechanism of ITP was recently identified (6). Investigators found apoptosis of platelets in ITP children, and speculated that platelet apoptosis was also a pathogenesis mechanism of ITP (7,8). Currently, there is no uniform 'gold standard' mechanism of pathogenesis. Clinical diagnosis is mainly an exclusive procedure and is dependent on bone marrow aspiration, which is traumatic and not routinely recommended. Previous findings (9,10) showed that changes of the platelet-associated parameters [mean platelet volume (MPV), platelet distribution width (PDW), platelet-large cell ratio (P-LCR), immature platelet fraction (IPF)%] were used to diagnose thrombocytopenia due to various causes, and used as indicators of relapse and efficacy. Long-term clinical diagnosis and treatment also indicated that the hemorrhagic symptoms of some children were not proportional to the reduction of the platelet count (PLT), which indicated that hemorrhage in ITP children was asssociated with the reduction of platelet count, as well as abnormal platelet function. Therefore, only platelet count is not sufficient to evaluate disease severity in children, and monitoring of individual platelet function should also be considered. Previously employed methods for the detection of platelet function were limiting and were not optimal to assess the many characteristics of platelet. Therefore, the development of a rapid, sensitive and convenient detection method is important to assess this disease, and understand platelet function.
The development of flow cytometry (FCM) and various monoclonal antibodies that could be labeled with fluorescein, led to several tools for the detection of platelet function becoming available.
Whole blood FCM was used to measure the percentage or fluorescence intensity of fluorescence-labelled platelet-specific membrane glycoproteins and activation markers, in order to assess the activation of circulating platelets and the in vitro response to activators by platelets, and evaluate platelet function. This technique simplified the treatment procedure of samples, avoided the artificial activation of platelet in vitro and prevented the loss of platelet subset. In addition, the blood sample volume was small, thus this technique was especially useful in the evaluation of the platelet function in the children with thrombocytopenia. Regarding relevant antibodies, Chen et al (11) compared the monoclonal antibody-specific immobilization of platelet antigens (MAIPA) and FCM in the detection of the sensitivity and specificity of GPIIb/IIIa and GPIIb/IIIa antibodies, and identified no significant differences. However, the sensitivity of FCM was higher than that of MAIPA. This observation indicated that FCM could be used as a new clinical diagnostic method (12–14).
Alterations in platelet function in ITP patients is controversial. Wang et al (15) demonstrated decreased activation and lower function of platelet in vitro and in vivo in ITP patients. Psaila et al (16) compared the expression of membrane glycoproteins in the platelets in ITP patients and MDS patients, and found high activation and in vitro response of the platelet in ITP patients. Therefore, further investigation of the platelet function change was required. The abovementioned study detected activation markers of GPIIb/IIIa on the platelet surface, such as PAC-1, granule membrane glycoprotein (CD62p), CD42b and IPF, evaluated the function of peripheral platelet and the change of new platelet in ITP patients prior to and after treatment, and assessed the change of platelet function in ITP patients after initial diagnosis and treatment.
The aim of the present study was to detect PAC-1, CD62p, CD42b and IPF by trace whole blood FCM, evaluate the function of peripheral platelet and the change of new platelet in ITP patients before and after treatment, assess the change of platelet function in ITP patients after initial diagnosis and treatment, analyze the change of platelet-associated parameters, such as platelet count (PLT), MPV, plateletcrit (PCT), PDW and platelet-large cell ratio, and provide the basis of the diagnosis, and the interpretation of disease course and efficacy.
Materials and methods
Materials
Fluorescein-labeled anti-platelet monoclonal antibodies used were: fluorescein isothiocyanate (FITC)-labeled anti-platelet GPIIb/IIIa monoclonal mouse antibody (PAC-1-FITC; cat. no. 340507); phycoerythrin (PE)-labeled anti-platelet GMP-140 mouse monoclonal antibody (CD62p-PE; cat. no. 348107); peridinin chlorophyll protein-labeled GPIIIa monoclonal mouse antibody (CD61-PercP; cat. no. 340506); PE-labeled mouse IgG (MIgG-PE; cat. no. 349043); phycocyanin-labeled anti-platelet GPIb-IX monoclonal mouse antibody (CD42b-APC; cat. no. 551061); and phycocyanin-labeled mouse IgG (MIgG-APC; cat. no. 555751). Thiazole orange (TO) (1.0 µg/ml; Sigma, St. Louis, MO, USA); RGDS (Arg-Gly-Asp-Ser) peptide as a blocker of PAC-1, and synthetic arginyl-glycyl-Asp-serine peptide, (Sigma) were also used. Other monoclonal antibodies were purchased from Becton-Dickinson (Franklin Lakes, NJ, USA). The following were also used: 0.2 mol/l ADP (Sigma); 1% paraformaldehyde solution; phosphate-buffered saline (PBS); FCM, a BD FACSCanto™ II high-speed sorter manufactured by Becton-Dickinson; and an automatic hematology analyzer, BC-6800, Mindray (Shenzhen, China).
Subjects
All the subjects were inpatients at the Affiliated Hospital of Luzhou Medical College (Sichuan, China) between March 2014 and February 2015. All the patients agreed to participate in the current study and signed written informed consent. The patients were divided into three groups: i) Normal control: 17 children prior to elective surgery at the Department of Pediatric Surgery, including 12 male and 5 female patients, with a mean age of 4.05±1.89 years. The children had inguinal hernia, with a mean platelet count of 309.2±54.06 ×109/l. None of the children had a previous history of circulatory diseases, immunological diseases, malignancy or transfusion. None of the children had any infection signs 1 week prior to hospitalization, and had no drugs 1 week prior to blood sampling. The children had normal complete blood count and blood samples were collected prior to surgery; ii) primary ITP: all the children met the diagnostic criteria of ITP (17), including 12 male and 6 female patients. The mean age of 3.8±1.4 years, the mean onset time (from the onset of disease to presentation in hospital) was 1–6 days and the mean platelet count was 28.61±10.42 ×109/l. No children had drugs that could affect platelet function 3 months prior to admission, such as corticosteroids, immunosuppressants, heparin, or any relevant treatment. This disease was not accompanied by significant infectious symptoms, and required no other anti-infection drugs. ITP was confirmed by the morphological analysis of bone marrow cells, which were collected prior to drug administration; and iii) complete response to primary ITP (ITP-CR): the children met the efficacy criteria (18) with a mean platelet count of 236.9±40.9 ×109/l. The differences in the age and gender between the treatment and control groups were not statistically significant (p>0.05).
Approval of the study was obtained from the Ethics Committee of the Affiliated Hospital of Luzhou Medical College.
Detection methods
Platelet membrane glycoproteins, such as PAC-1, CD62p and CD42b, were measured. Venous blood (3 ml) was collected and the middle section of 1.8 ml whole blood was added into an anticoagulation tube containing sodium citrate, mixed gently and processed for analysis. Platelet activation was carried out by adding 50 µl ADP into a Falcon tube (Becton-Dickinson Company, Franklin Lakes, NJ, USA) followed by 450 µl blood with anticoagulant, mixed gently by vortexing and incubated at 37°C for 10 min. Expression of PAC-1, CD62p and CD42b was measured by three-color fluorescence analysis with FCM. In separate tubes, 20 µl of PE-isotype control antibody, CD61-PercP, PAC-1-FITC and MIgG-APC, and 10 µl RGDS or 20 µl CD62p-PE, CD61-PercP, PAC-1-FITC and CD42b-APC were added. Then, 5 µl non-activated and activated whole blood was added into the tubes of the treatment groups, and 5 µl non-activated whole blood was added into the control tube, mixed gently, and incubated at room temperature (25°C) in the dark for 20 min. Subsequently, 1% paraformaldehyde was added (2–8°C, 1 ml) into each tube, mixed completely, and placed at 2–8°C in the dark for 30 min. Using FCM, signal for 10,000 platelets was acquired, and the positive expression rate of CD62p, PAC-1 and CD42b was calculated (19).
Platelet-associated parameters (PLT, MPV, PDW, PCT and P-LCR) were measured. Venous blood (4-ml) was collected into an anticoagulant tube containing EDTA with a 5-ml syringe, 2-ml whole blood was reserved, and the remaining blood was used in the measurement of platelet-associated parameters by an automatic hematology analyzer. Whole blood (5-µl) was placed into the treatment tubes, followed by 5 µl CD42b-APC and 50 µl TO. Whole blood (5-µl) was placed into the control tube, followed by 5 µl CD42b-APC and 50 µl PBS. The tubes were mixed gently, incubated at room temperature in the dark for 15 min; and then, 1% paraformaldehyde was added (2–8°C, 1 µl) into each tube, and mixed completely. The samples were analyzed within 45 min. Signals for 10,000 platelets were obtained, IPC was measured and IPF% was calculated.
Statistical analysis
Experimental data were presented as mean ± SD and analyzed using SPSS 11.5 software (SPSS Inc., Chicago, IL, USA). The Student's t-test or rank-sum test was used for comparisons between the two treatment groups. The one-sample t-test or rank-sum test was used for the comparison between the treatment and control groups. P≤0.05 was considered to indicate a statistically significant difference.
Results
Measurement of platelet parameters
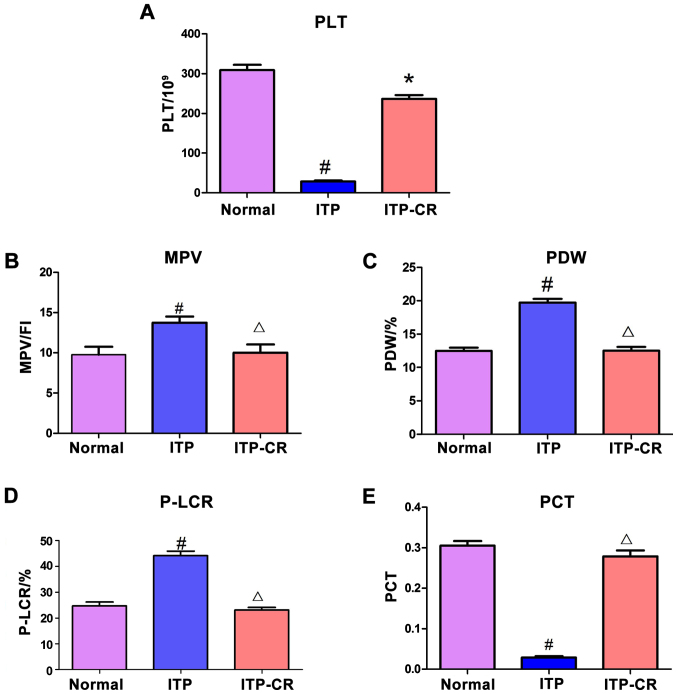
As shown in Fig. 1, PLT and PCT in the ITP group had a lower expression level compared to those in the normal control and ITP-CR groups (p<0.05), while the MPV, PDW and P-LCR in ITP group were higher than those in the normal control and ITP-CR groups (p<0.05). Differences in the expression of MPV, PDW, PCT and P-LCR between the ITP-CR and normal control groups were not statistically significant (p>0.05), whereas PLT was lower in the ITP-CR group (p<0.05).
Figure 1.
Comparison of platelet parameters. (A) The expression of PLT in 3 groups, (B) the expression of MPV in 3 groups, (C) the expression of PDW in 3 groups, (D) the expression of P-LCR in 3 groups, and (E) the expression of PCT in 3 groups. #P<0.05, ITP-CR vs. normal control group; *P<0.05, in comparison to the normal control group; ΔP>0.05, in comparison to the normal control group. PLT, platelet count; MPV, mean platelet volume; PDW, platelet distribution width; P-LCR, platelet-large cell ratio; PCT, plateletcrit.
Measurement of IPF% and IPC
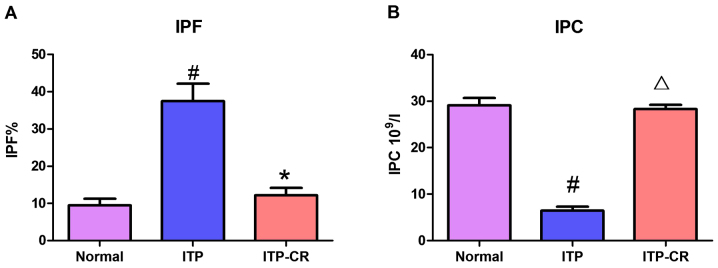
As shown in Figs. 2 and 7, IPF% in the ITP group was higher than that in the ITP-CR and normal control groups (p<0.05). IPC in the ITP group was lower than that in the ITP-CR and normal control groups (p>0.05). IPF% was higher (p<0.05) and IPC was lower in ITP-CR group in comparison to the normal control group. The differences were not statistically significant.
Figure 2.
Expression of IPF% and IPC. (A) Expression of IPF% in 3 groups, and (B) expression of IPC in 3 groups. #P<0.05, ITP-CR vs. normal control group; *P<0.05, in comparison to the normal control group; Δp>0.05, in comparison to the normal control group. IPF, immature platelet fraction.
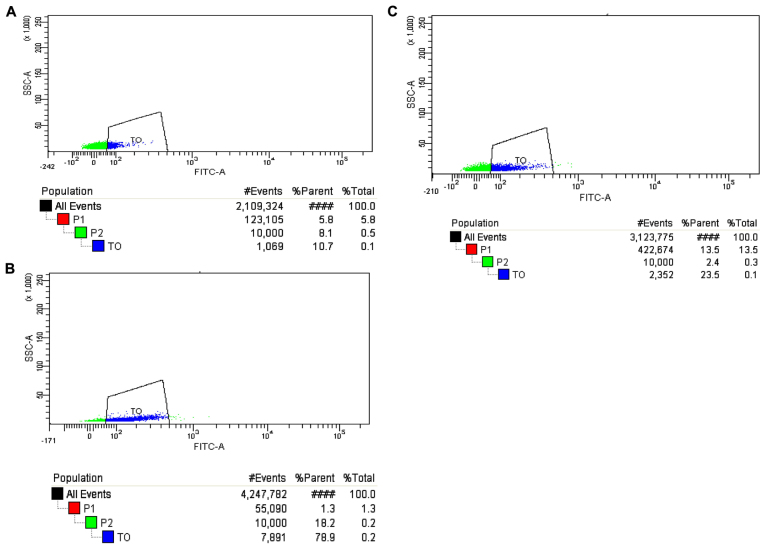
Figure 7.
Expression of IPF% in different stages of the ITP and normal control groups. TO represented reticulated platelets. (A) The polygon is the IPF% in the normal control group; (B) the polygon is the IPF% in the ITP group; (C) the polygon represented IPF% in the ITP-CR group. IPF, immature platelet fraction; ITP, immune thrombocytopenia; TO, thiazole orange.
Measurement of platelet membrane glycoproteins prior to and after platelet activation by ADP
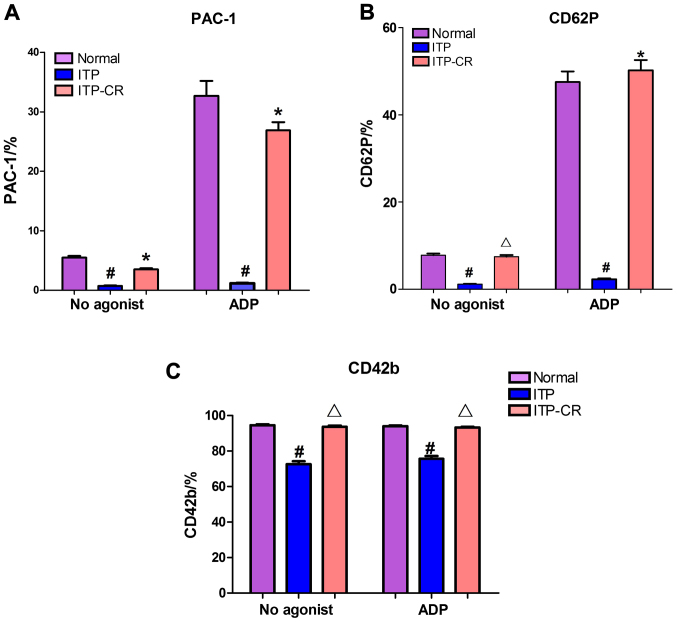
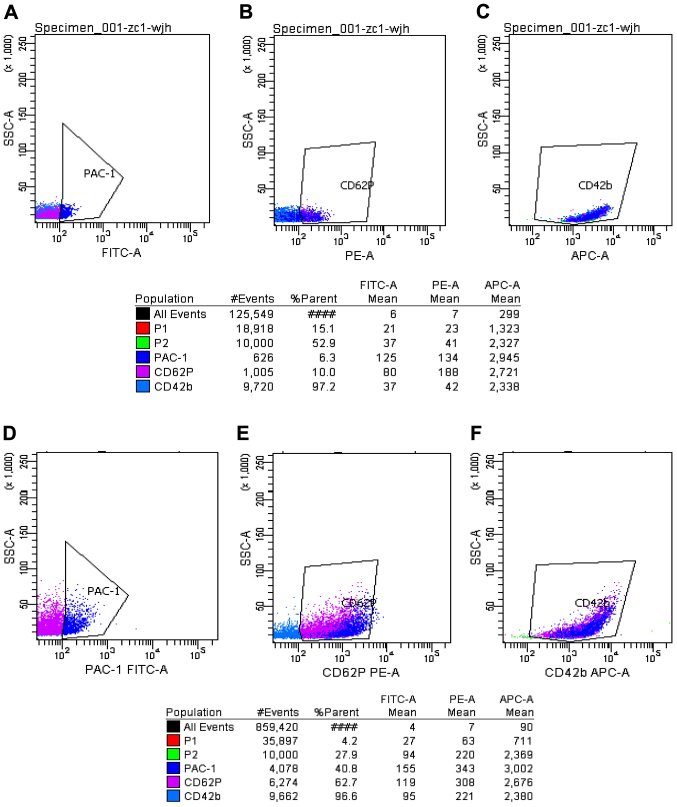
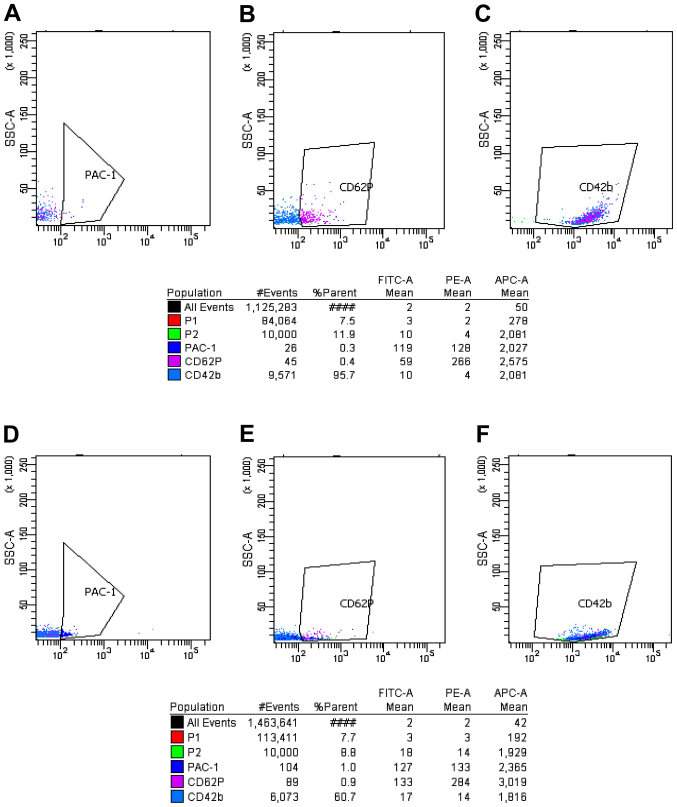
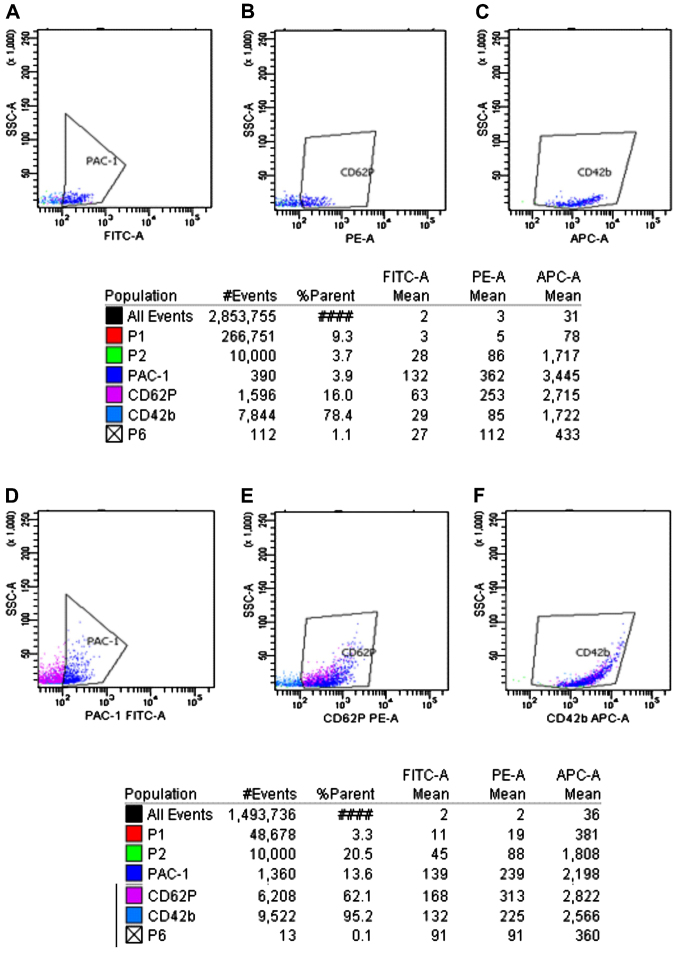
As shown in Figs. 3–6, prior to platelet activation by ADP, the expression levels of CD62p, PAC-1 and CD42b in the ITP group were lower than those in ITP-CR and normal control groups (p<0.05). PAC-1 was lower in the ITP-CR compared to that in the normal control group (p<0.05). Differences in CD62p and CD42b were not statistically significant (p>0.05). After platelet activation by ADP, the expression levels of CD62p, PAC-1 and CD42b in the ITP group were lower than those in the ITP-CR and normal control groups (p<0.05). PAC-1 was lower and CD62p was higher in the ITP-CR group than that in the normal control group (p<0.05). Differences in CD42b were not statistically significant (p>0.05).
Figure 3.
Expression of (A) PAC-1, (B) CD62p and (C) CD42b. #P<0.05, ITP-CR group vs. normal control group; *p<0.05, in comparison to normal control group; and Δp>0.05, in comparison to normal control group.
Figure 4.
Expression of PAC-1, CD62p and CD42b in the normal control group before and after platelet activation by ADP. P2 indicated 10,000 platelets obtained. The polygon in panels (A–C) is the expression of PAC-1, CD62p and CD42b before platelet activation by ADP in the normal control group. The polygon in panels (D–F) shows the expression of PAC-1, CD62p and CD42b after platelet activation by ADP in the normal control group.
Figure 5.
Expression of PAC-1, CD62p and CD42b in the ITP group before and after platelet activation by ADP (FCM). P2 indicated 10,000 platelet obtained. The polygon in panels (A–C) shows the expression of PAC-1, CD62p and CD42b before platelet activation by ADP in the normal control group. The polygon in panels (D–F) shows the expression of PAC-1, CD62p and CD42b after platelet activation by ADP in the normal control group. FCM, flow cytometry; ITP immune thrombocytopenia.
Figure 6.
The expression of PAC-1, CD62p and CD42b in the ITP group before and after platelet activation by ADP (FCM). P2 indicated 10,000 platelet obtained. The polygon in panels (A–C) represented the expression of PAC-1, CD62p and CD42b before platelet activation by ADP in normal control group. The polygon in panels (D–F) represented the expression of PAC-1, CD62p and CD42b after platelet activation by ADP in normal control group. ITP, immune thrombocytopenia; FCM, flow cytommetry.
Discussion
Platelet is the smallest cell in whole blood cells, originating from mature megakaryocytes cytoplasm. It has the functions of adhesion, accumulation and release, and plays an important role in hemostasis (16). Platelet membrane glycoprotein (GP) is a main component of platelet membrane proteins, and plays major roles in the activation of platelet. Platelet GPs can be classified into plasma membrane and granule membrane glycoproteins based on the distribution location (19). Plasma membrane glycoproteins are predominantly located on the cell membrane of resting platelets, major GPs including the GPIb (CD42b)-IX-V complex and GPIIb/IIIa complex. CD42b (GPIb) is an important platelet adhesion receptor that promotes platelet to secret platelet-derived growth factors and 5-HT, accelerating the deformation and aggregation of platelets. GpIIb/IIIa complex is the most abundant membrane glycoprotein and can mediate the binding of platelet and fibrinogen (Fg), which is the final pathway of platelet aggregation (20). PAC-1 is the monoclonal antibody in the exposed Fg-binding site after the activation of GpIIb/IIIa, and is the specific marker of the activation of GPIIb/IIIa complex and the early marker of platelet activation (21). Granule membrane glycoproteins comprise CD62p and CD63p, with CD62p also known as P-selectin. Since the increase of CD62p is not altered with time prolongation, it is considered the 'gold standard' of platelet activation markers (22). Therefore, we investigated the change of platelet function by detecting the expression of PAC-1, CD62p and CD42b (23).
Hemorrhage due to decreased platelets caused by disrupted platelet autoimmunity is currently considered as the pathogenesis mechanism of ITP (7). However, platelet function was less investigated. The present study measured the expression percentage and fluorescence intensity of three membrane glycoproteins, i.e., PAC-1, CD62p and CD42b with FCM to analyze the in vitro and in vivo activation of platelets in ITP patients. The percentage of membrane glycoproteins indicated the total amount of activated platelets, and the mean fluorescence intensity (MFI) indicated the expression of membrane glycoproteins in single-activated platelets. These factors provide an understanding of the activation capability of individual platelet. In the current study, the expression levels of PAC-1, CD62p and CD42b in ITP children were significantly lower those in the normal control group prior to and after platelet activation by ADP. This result was consistent with that by Liu and Qian (6), which demonstrated low platelet activation in vitro and in vivo in ITP children. Other studies reported that some autoantibodies may affect platelet function (24–26). As autoantibodies may bind to the antigenic epitopes on GPIIb/IIIa and GPIb (CD42b), prevent the binding of added fluorescent mAb, the expression levels of PAC-1 and GPIb (CD42b) were decreased. Furthermore, inhibition of the initial activation steps prevented the exposure of intracellular granule membrane glycoproteins on the surface of plasma membrane, thus decreasing the expression level of CD62p. The present study also demonstrated normal MFI of CD62p in vivo and lower MFI of CD62p after platelet activation in vitro. This result demonstrated low in vivo activation of platelets in ITP children. Previous findings have shown that the inhibition or dysfunction of megakaryocytes in ITP children may cause abnormal membrane glycoproteins in new platelets in a qualitative and quantitative manner (27).
Platelet activation from the internal to the external compartment is a series of complicated physiological process, in which any section affects normal platelet activation. Further studies are required to determine which section may cause low platelet activation in ITP children. Our study also demonstrated that the expression levels of three glycoproteins gradually increased with the recovery of patients, although the expression level of PAC-1 in the recovery phase was lower than that in the normal group. This result may be related to the fact that platelet activation was enhanced and some antibodies were present due to the release of new platelets in the recovery phase in ITP children. The expression level of CD42b in the recovery phase was similar to that in the normal control group, which may be due to less membrane glycoprotein GPIb (CD42b) in ITP patients. The percentage and MFI of platelet membrane glycoprotein CD62p were higher than those in normal children after activation by ADP. This result was consistent with Bhoria et al (28), who demonstrated enhanced platelet function in the ITP children who were in the recovery phase.
Previous studies have demonstrated enhanced platelet function in ITP children. Wang et al (29) found the positive expression rate of PAC-1 in ITP patients was significantly higher than that in healthy and non-ITP patients. Psaila et al (30) observed high intrinsic platelet activity and intrinsic platelet reactivity due to high IPF, increased GPIb on the membrane surface of circulating platelets and increased expression of activated GPIIb/IIIa and GPIb. The finding was inconsistent with our results due to: i) ITP was a group of heterogeneous diseases with complicated etiology and pathogenesis mechanisms. Activation of the individual platelet with different pathogenesis mechanism may be different. ii) The stage of disease in the selected subject may be different, as some patients may have experienced in vitro activation (31); and iii) artificial agitation in the blood sample collection, the set-up of the instruments and sample treatment, and the time of sample treatment may have affected the expression of membrane glycoproteins and the final result. A limitation of the study is that, the samples collected were limited. Thus, a larger sample size may be required in future studies.
Platelet parameter was a simple non-invasive examination, that was capable of reflecting the proliferation kinetics of platelets in vivo (32), and was significant for the early diagnosis, risk assessment and efficacy interpretation of disease. Platelet parameters included PLT, P-LCR, MPV, PCT and PDW. PLT was a common indicator for the assessment of treatment protocol and disease severity. PLT in the ITP children was significantly lower than that in the normal control group at first visit due to the disruption of platelets by autoimmunity. MPV reflected the size and metabolism of platelets and the proliferation capability of macrophage in bone marrow (33,34). It was therefore useful in the assessment of platelet function and the reason for thrombocytopenia. Balduini and Noris (35) identified that the specificity of MPV in the differentiation of ITP and congenital thrombocytopenia was up to 91%, and was dependent on the precision of the instrument. Korkmaz et al (9) demonstrated MPV in a relapsed patient was significantly higher than that in the patient with a primary disease, and suggested that MPV could be used to predict the relapse of ITP, while PDW was a parameter that reflected the difference of platelet volume and was positively associated with MPV when marrow proliferation was normal. Ntaios et al (10) compared the thrombocytopenia caused by ITP with that by myelosuppression, and found MPV and PDW were higher when the disruption of peripheral platelets was increased. PDW and MPV were beneficial to the diagnosis of ITP. P-LCR refers to the percentage of blood large platelets (volume of >20 fl). An increase in P-LCR indicates an elevation in neonatal platelet count, and although the total number of platelets remained unchanged, it indirectly led to an increase in platelet destruction (34). PCT was the product of PLT and MPV, and the change of PCT was consistent with the change of PLT. The present findings demonstrated that PLT and PCR in the ITP patients were significant lower, whereas PDW, MPV and P-LCR in the ITP patients were significantly higher. Following treatment, PLT and PCR gradually increased, whereas PDW, MPV and P-LCR gradually decreased. PLT was negatively related to MPV, while MPV was positively related to PDW and P-LCR. These results were consistent with those of Fan and Wei (36), which suggested that the change of platelet parameters was beneficial to the diagnosis and efficacy interpretation of ITP patients. However, some factors may affect the detection of platelet parameters, including the time of sample storage, the method for blood collection, anticoagulant EDTA and enlarged platelet volume due to the binding of platelet to granulocytes or monocytes.
Reticulated platelets (RPs) are immature platelets that enter the blood from bone marrow, and are in the naïve phase of the process from megakaryocyte to platelet. RPs are an important indicator in assessing the ability of bone marrow to produce platelets. Various indicators can be used to identify thrombocytopenia (37–40), and be used to predict the recovery of platelets after hematopoietic stem cell transplantation (41). Liu et al (42) suggested that IPF% was superior to PA IgG in the diagnosis of ITP. It was reported that IPF was associated with the response to ITP treatment (43). The current results have demonstrated that the IPF% in ITP children prior to treatment was significantly increased in comparison to the normal control group. IPF% decreased with the relief of disease following treatment. The results were consistent with those by Adly et al (44), which suggested that the disruption of peripheral platelets in ITP children was increased, and although the bone marrow hyperplasia was normal, the compensatory hyperplasia of megakaryocytes caused the release of a large number of new platelets, leading to increased IPF% and subsequent increased PLT. As a result, the stimulation to megakaryocytes was weak and IPF% gradually decreased. IPC was the product of PLT and IPF%, and represented the absolute value of reticulated platelets in unit volume of peripheral blood. The present findings demonstrated that IPC prior to treatment in the ITP children was significantly decreased in comparison to the normal control group, and the response period after ITP treatment was significantly longer than that prior to treatment. IPC was in inverse proportion to IPF%. This may be associated with the short life-span of new platelets or the disruption of new platelets following release into the blood. Barsam et al (45) suggested that IPC reflected the balance between the compensatory production of platelets by megakaryocytes in ITP children and the disruption rate of peripheral platelets, and could be used as the efficacy indicator following treatment of thrombopoietin in chronic ITP patients. Greene et al (46) suggested that IPC could be used to assess the hemorrhage risk in ITP patients better than platelet parameters, such as PLT and MPV.
Currently, physicians tend to focus on the relationship between the change of platelet count and diseases, while ignoring the significance of other platelet parameters in the diagnosis and treatment in diseases. The findings of the current study have demonstrated that the test of platelet parameters is convenient and reliable, and valuable to the diagnosis and follow up in ITP children as it avoids the pain from bone marrow biopsy, repeated tests, and good patient compliance and dynamic assessment of the recovery in ITP children is possible. Findings of the present study indicated that a good platelet count is important for proper assessment of platelet parameters (47). However, IPF is not affected by platelet count, thus it is complementary to platelet parameters in the etiology of thrombocytopenia and the evaluation of efficacy.
Acknowledgments
The present study was funded by the Sichuan Department of Education Scientific and Development Funds (2011ZA150).
References
- 1.Min Z, Lin X. The advancement in the treatment of the children with refractory idiopathic thrombocytopenic purpura. J Clin Pediatr. 2008;26:727–730. [Google Scholar]
- 2.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85:174–180. doi: 10.1002/ajh.21616. [DOI] [PubMed] [Google Scholar]
- 3.Hu Q. Diagnosis of idiopathic thrombocytopenic purpura in children. Journal of Applied Clinical Pediatrics. 2010;25:159–161. In Chinese. [Google Scholar]
- 4.Li P, Shi T, Gao J, Zhao D, Xiao A. The immunization in the children with acute immune thrombocytopenia. Chinese Journal of Practical Pediatrics. 2014;29:548–550. In Chinese. [Google Scholar]
- 5.Provan D, Newland AC. Newl and current management of primary immune thrombocytopenia. Adv Ther. 2015;32:875–887. doi: 10.1007/s12325-015-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Qian X. Interpretation of the recommendations for the diagnosis and treatment of pediatric primary immune thrombocytopenia. Chin J Obstet Gynecol Pediatr. 2015;21:245–248. [Google Scholar]
- 7.Winkler J, Kroiss S, Rand ML, Azzouzi I, Annie Bang KW, Speer O, Schmugge M. Platelet apoptosis in paediatric immune thrombocytopenia is ameliorated by intravenous immunoglobulin. Br J Haematol. 2012;156:508–515. doi: 10.1111/j.1365-2141.2011.08973.x. [DOI] [PubMed] [Google Scholar]
- 8.Yin J, Wang J, Chen XQ, Yu WJ, Liao JY, Wang DX. Investigation on platelet apoptosis in patients with chronic idiopathic thrombocytopenic purpura. J Diagn Concepts & Pract. 2010;9:236–241. In Chinese. [Google Scholar]
- 9.Korkmaz S, Uslu AU, Aydin B, Dogan O, Sencan M. Pre-treatment and post-treatment changes in platelet indices in patients with immune thrombocytopenia. Saudi Med J. 2013;34:591–596. [PubMed] [Google Scholar]
- 10.Ntaios G, Papadopoulos A, Chatzinikolaou A, Saouli Z, Karalazou P, Kaiafa G, Girtovitis F, Kontoninas Z, Savopoulos C, Hatzitolios A, et al. Increased values of mean platelet volume and platelet size deviation width may provide a safe positive diagnosis of idiopathic thrombocytopenic purpura. Acta Haematol. 2008;119:173–177. doi: 10.1159/000135658. [DOI] [PubMed] [Google Scholar]
- 11.Chen JF, Yang LH, Chang LX, Feng JJ, Liu JQ. The clinical significance of circulating B cells secreting anti-glycoprotein IIb/IIIa antibody and platelet glycoprotein IIb/IIIa in patients with primary immune thrombocytopenia. Hematology. 2012;17:283–290. doi: 10.1179/1607845412Y.0000000014. [DOI] [PubMed] [Google Scholar]
- 12.Thomas MR, Wijeyeratne YD, May JA, Johnson A, Heptinstall S, Fox SC. A platelet P-selectin test predicts adverse cardiovascular events in patients with acute coronary syndromes treated with aspirin and clopidogrel. Platelets. 2014;25:612–618. doi: 10.3109/09537104.2013.863858. [DOI] [PubMed] [Google Scholar]
- 13.He Y, Zhao YX, Zhu MQ, Wu Q, Ruan CG. Detection of auto-antibodies against platelet glycoproteins in patients with immune thrombocytopenic purpura by flow cytometric immunobead array. Clin Chim Acta. 2013;415:176–180. doi: 10.1016/j.cca.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Xun L, Zhu WB, Wu JS, Cai XY, Liu X, Han YS, Yang HZ, Zheng CC, Li Q. Diagnosing ITP by Measuring the CD 61 and PAIg with FCM. Chin J Thromb Hemost. 2011;17:8–12. In Chinese. [Google Scholar]
- 15.Wang T, Zhang C, Lu J. Platelet parameter and platelet membrane glycoprotein in childhood acute lymphoblastic leukemia. J Chin Pediatr Blood Cancer. 2006;11:12–16. In Chinese. [Google Scholar]
- 16.Psaila B, Bussel JB, Frelinger AL, Babula B, Linden MD, Li Y, Barnard MR, Tate C, Feldman EJ, Michelson AD. Differences in platelet function in patients with acute myeloid leukemia and myelodysplasia compared to equally thrombocytopenic patients with immune thrombocytopenia. J Thromb Haemost. 2011;9:2302–2310. doi: 10.1111/j.1538-7836.2011.04506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subspecialty Group of Hematology, The Society of Pediatrics Chinese Medical Association. Editorial Board, Chinese Journal of Pediatrics Recommendations for diagnosis and treatment of primary immune thrombocytopenia in children. Zhonghua Er Ke Za Zhi. 2013;51:382–384. In Chinese. [PubMed] [Google Scholar]
- 18.Xu Q, Liu WJ. Advances in the diagnosis and treatment of primary immune thrombocytopenia in children. Chin J Pract Pediatr. 2013;28:646–652. In Chinese. [Google Scholar]
- 19.Huang Z, Liu WJ, Guo QL, Liu CY. Platelet parameters and expression of platelet membrane glycoprotein in childhood acute lymphoblastic leukemia. Genet Mol Res. 2015;14:16074–16089. doi: 10.4238/2015.December.7.20. [DOI] [PubMed] [Google Scholar]
- 20.Sarratt KL, Chen H, Zutter MM, Santoro SA, Hammer DA, Kahn ML. GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood. 2005;106:1268–1277. doi: 10.1182/blood-2004-11-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Z, Liu W. Progress in research on platelet glycoproteins of thrombocytopenia in children. J Appl Clin Pediatr. 2014;29:227–230. In Chinese. [Google Scholar]
- 22.Mutlu A, Gyulkhandanyan AV, Freedman J, Leytin V. Concurrent and separate inside-out transition of platelet apoptosis and activation markers to the platelet surface. Br J Haematol. 2013;163:377–384. doi: 10.1111/bjh.12529. [DOI] [PubMed] [Google Scholar]
- 23.Serebruany V, Malinin A, Aradi D, Kuliczkowski W, Norgard NB, Boden WE. The in vitro effects of niacin on platelet biomarkers in human volunteers. Thromb Haemost. 2010;104:311–317. doi: 10.1160/TH10-01-0015. [DOI] [PubMed] [Google Scholar]
- 24.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 25.Dong NZ, Cui RJ, Ruan CG. Generation of human Fab antibody against platelet membrane glycoprotein iib/iia and its effect on platelet aggregation. Chin J Cell Mol Immunol. 2009;25:65–67. In Chinese. [PubMed] [Google Scholar]
- 26.Yang G. research and application of platelet glycoprotein iib/iiia acceptor antagonist. Clin Med Eng. 2010;17:149–151. [Google Scholar]
- 27.Kashiwagi H, Tomiyama Y. Pathophysiology and management of primary immune thrombocytopenia. Int J Hematol. 2013;98:24–33. doi: 10.1007/s12185-013-1370-4. [DOI] [PubMed] [Google Scholar]
- 28.Bhoria P, Sharma S, Varma N, Malhotra P, Varma S, Luthra-Guptasarma M. Effect of steroids on the activation status of platelets in patients with Immune thrombocytopenia (ITP) Platelets. 2015;26:119–126. doi: 10.3109/09537104.2014.888546. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Niu A, Lu Z, Yang X, Wang L, Zou X. The clinical significances of PAIgG, PAC-1 and CD62P in patients with idiopathic thrombocytopenic purpura. Zhejiang J Lab Med. 2005;3:7–10. In Chinese. [Google Scholar]
- 30.Psaila B, Bussel JB, Linden MD, Babula B, Li Y, Barnard MR, Tate C, Mathur K, Frelinger AL, Michelson AD. In vivo effects of eltrombopag on platelet function in immune thrombocytopenia: no evidence of platelet activation. Blood. 2012;119:4066–4072. doi: 10.1182/blood-2011-11-393900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinholt PJ, Hvas AM, Nybo M. An overview of platelet indices and methods for evaluating platelet function in thrombocytopenic patients. Eur J Haematol. 2014;92:367–376. doi: 10.1111/ejh.12262. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Gang W. Study on clinical significance of platelet relevant parameters in diagnosis of thrombocytopenia. J Clin Exp Med. 2013;12:1533–1535. [Google Scholar]
- 33.Bai J, Liu W. Changes of platelet parameters and platelet function in primary immune thrombocytopenia. Chin J Pract Pediatr. 2015;30:150–153. In Chinese. [Google Scholar]
- 34.Adly AA, Ragab IA, Ismail EA, Farahat MM. Evaluation of the immature platelet fraction in the diagnosis and prognosis of childhood immune thrombocytopenia. Platelets. 2015;26:645–650. doi: 10.3109/09537104.2014.969220. [DOI] [PubMed] [Google Scholar]
- 35.Balduini CL, Noris P. Mean platelet volume for distinguishing between inherited thrombocytopenias and immune thrombocytopenia - response to Beyan. Br J Haematol. 2013;163:413–414. doi: 10.1111/bjh.12504. [DOI] [PubMed] [Google Scholar]
- 36.Fan L, Wei W. Platelet parameters in children with idiopathic thrombocytopenic purpura clinical application analysis. J Clin Exp Med. 2014;13:124–126. [Google Scholar]
- 37.Strauss G, Vollert C, von Stackelberg A, Weimann A, Gaedicke G, Schulze H. Immature platelet count: A simple parameter for distinguishing thrombocytopenia in pediatric acute lymphocytic leukemia from immune thrombocytopenia. Pediatr Blood Cancer. 2011;57:641–647. doi: 10.1002/pbc.22907. [DOI] [PubMed] [Google Scholar]
- 38.Pons I, Monteagudo M, Lucchetti G, Muñoz L, Perea G, Colomina I, Guiu J, Obiols J. Correlation between immature platelet fraction and reticulated platelets. Usefulness in the etiology diagnosis of thrombocytopenia. Eur J Haematol. 2010;85:158–163. doi: 10.1111/j.1600-0609.2010.01468.x. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W, Jiang H, Wu Y, Zhang Z, Chen X. The variation of immature platelet fraction in patients with thrombocytopenic diseases. Lab Med. 2013;28:47–50. [Google Scholar]
- 40.Yang J, Zhao Y, Wu W, Hua B, Zhu Z, Yang R, Wang S. Value of Reticulated Platelet Countsin Identifying Thrombocytopenia Aetiology. J Exp Hematol. 2010;18:482–485. In Chinese. [PubMed] [Google Scholar]
- 41.Michur H, Maślanka K, Szczepiński A, Mariańska B. Reticulated platelets as a marker of platelet recovery after allogeneic stem cell transplantation. Int J Lab Hematol. 2008;30:519–525. doi: 10.1111/j.1751-553X.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Wang Z, Yuan B, Wang X, Wu Q, Yuan H. Role of reticulated platelets and platelet-associated antibody in differential diagnosis of idiopathic thrombocytopenic purpura. J Exp Hematol. 2011;19:979–982. In Chinese. [PubMed] [Google Scholar]
- 43.Xiao M, Liu J, Wu P, Yuan Q. Significance of platelet parameters measurement in thrombocytopenia disease. Int J Lab Med. 2013;34:418–420. In Chinese. [Google Scholar]
- 44.Adly AA, Ragab IA, Ismail EA, Farahat MM. Evaluation of the immature platelet fraction in the diagnosis and prognosis of childhood immune thrombocytopenia. Platelets. 2015;26:645–650. doi: 10.3109/09537104.2014.969220. [DOI] [PubMed] [Google Scholar]
- 45.Barsam SJ, Psaila B, Forestier M, Page LK, Sloane PA, Geyer JT, Villarica GO, Ruisi MM, Gernsheimer TB, Beer JH, et al. Platelet production and platelet destruction: assessing mechanisms of treatment effect in immune thrombocytopenia. Blood. 2011;117:5723–5732. doi: 10.1182/blood-2010-11-321398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greene LA, Chen S, Seery C, Imahiyerobo AM, Bussel JB. Beyond the platelet count: Immature platelet fraction and thromboelastometry correlate with bleeding in patients with immune thrombocytopenia. Br J Haematol. 2014;166:592–600. doi: 10.1111/bjh.12929. [DOI] [PubMed] [Google Scholar]
- 47.Banfi G, Germagnoli L. Preanalytical phase in haematology. J Med Biochem. 2008;27:348–353. doi: 10.2478/v10011-008-0014-3. [DOI] [Google Scholar]