Abstract
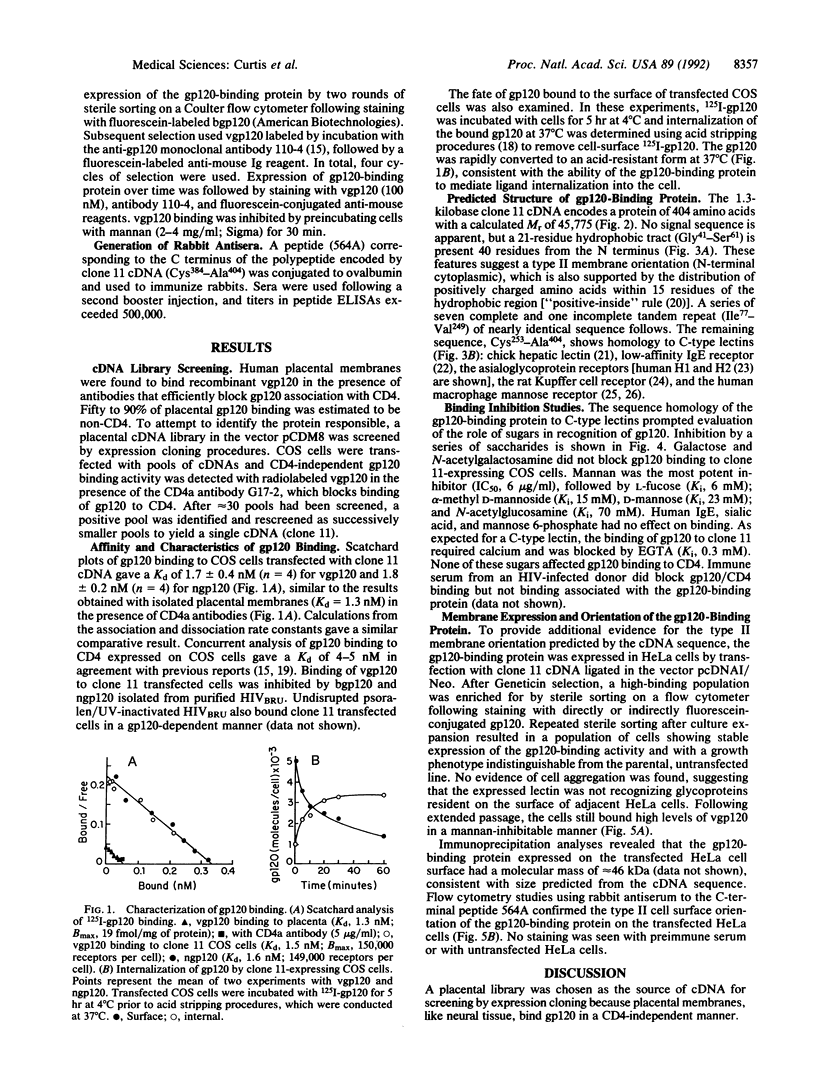
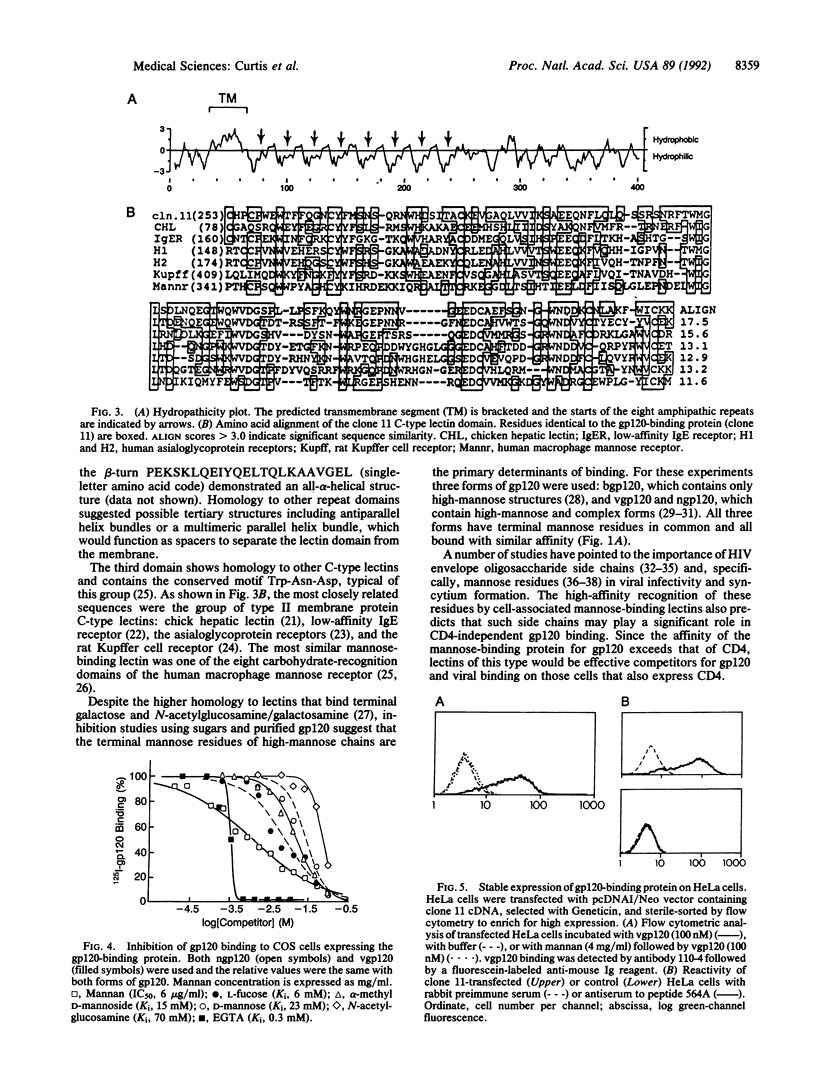
The binding of the human immunodeficiency virus (HIV) envelope glycoprotein gp120 to the cell surface receptor CD4 has been considered a primary determinant of viral tropism. A number of cell types, however, can be infected by the virus, or bind gp120, in the absence of CD4 expression. Human placenta was identified as a tissue that binds gp120 in a CD4-independent manner. A placental cDNA library was screened by expression cloning and a cDNA (clone 11) encoding a gp120-binding protein unrelated to CD4 was isolated. The 1.3-kilobase cDNA predicts a protein of 404 amino acids with a calculated M(r) of 45,775 and organized into three domains: an N-terminal cytoplasmic and hydrophobic region, a set of seven complete and one incomplete tandem repeat, and a C-terminal domain with homology to C-type (calcium-dependent) lectins. A type II membrane orientation (N-terminal cytoplasmic) is predicted both by the cDNA sequence and by the reactivity of C-terminal peptide-specific antiserum with the surface of clone 11 transfected cells. Native and recombinant gp120 and whole virus bind transfected cells. gp120 binding is high affinity (kd, 1.3-1.6 nM) and inhibited by mannan, D-mannose, and L-fucose; once bound, gp120 is internalized rapidly. Collectively, these data demonstrate that the gp120-binding protein is a membrane-associated mannose-binding lectin. Proteins of this type may play an important role in the CD4-independent association of HIV with cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amerongen H. M., Weltzin R., Farnet C. M., Michetti P., Haseltine W. A., Neutra M. R. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4(8):760–765. [PubMed] [Google Scholar]
- Bourinbaiar A. S., Phillips D. M. Transmission of human immunodeficiency virus from monocytes to epithelia. J Acquir Immune Defic Syndr. 1991;4(1):56–63. [PubMed] [Google Scholar]
- Brenneman D. E., Westbrook G. L., Fitzgerald S. P., Ennist D. L., Elkins K. L., Ruff M. R., Pert C. B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988 Oct 13;335(6191):639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Cao Y. Z., Friedman-Kien A. E., Huang Y. X., Li X. L., Mirabile M., Moudgil T., Zucker-Franklin D., Ho D. D. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol. 1990 Jun;64(6):2553–2559. doi: 10.1128/jvi.64.6.2553-2559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Widmer M. B., deRoos P., Qwarnstrom E. E. IL-1 and its receptor are translocated to the nucleus. J Immunol. 1990 Feb 15;144(4):1295–1303. [PubMed] [Google Scholar]
- Dreyer E. B., Kaiser P. K., Offermann J. T., Lipton S. A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990 Apr 20;248(4953):364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Complete amino acid sequence of a membrane receptor for glycoproteins. Sequence of the chicken hepatic lectin. J Biol Chem. 1981 Jun 10;256(11):5827–5839. [PubMed] [Google Scholar]
- Drickamer K. Multiple subfamilies of carbohydrate recognition domains in animal lectins. Ciba Found Symp. 1989;145:45-58, discussion 58-61. doi: 10.1002/9780470513828.ch4. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Kuhlman M., Groopman J. E., Byrn R. A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J Exp Med. 1989 Jan 1;169(1):185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sastry K., Bailly P., Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990 Dec 1;172(6):1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouillet E., Gluckman J. C., Bahraoui E. Role of N-linked glycans of envelope glycoproteins in infectivity of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2841–2848. doi: 10.1128/jvi.64.6.2841-2848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattegno L., Ramdani A., Jouault T., Saffar L., Gluckman J. C. Lectin-carbohydrate interactions and infectivity of human immunodeficiency virus type 1 (HIV-1). AIDS Res Hum Retroviruses. 1992 Jan;8(1):27–37. doi: 10.1089/aid.1992.8.27. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer H., Holschbach C., Hunsmann G., Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988 Aug 25;263(24):11760–11767. [PubMed] [Google Scholar]
- Hansen J. E., Nielsen C. M., Nielsen C., Heegaard P., Mathiesen L. R., Nielsen J. O. Correlation between carbohydrate structures on the envelope glycoprotein gp120 of HIV-1 and HIV-2 and syncytium inhibition with lectins. AIDS. 1989 Oct;3(10):635–641. doi: 10.1097/00002030-198910000-00003. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Kunsch C., Hartle H. T., Laughlin M. A., Hoxie J. A., Wigdahl B., Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G. W., Hill R. L. Molecular cloning and sequencing of a cDNA for a carbohydrate binding receptor unique to rat Kupffer cells. J Biol Chem. 1988 Jun 5;263(16):7487–7492. [PubMed] [Google Scholar]
- Hsieh P., Robbins P. W. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984 Feb 25;259(4):2375–2382. [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Kozlowski M. R., Sandler P., Lin P. F., Watson A. Brain-derived cells contain a specific binding site for Gp120 which is not the CD4 antigen. Brain Res. 1991 Jul 12;553(2):300–304. doi: 10.1016/0006-8993(91)90838-m. [DOI] [PubMed] [Google Scholar]
- Kunsch C., Hartle H. T., Wigdahl B. Infection of human fetal dorsal root ganglion glial cells with human immunodeficiency virus type 1 involves an entry mechanism independent of the CD4 T4A epitope. J Virol. 1989 Dec;63(12):5054–5061. doi: 10.1128/jvi.63.12.5054-5061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M., Childs R. A., Matthews T. J., Thiel S., Mizuochi T., Lawson A. M., Savill J. S., Haslett C., Diaz R., Feizi T. Oligosaccharide-mediated interactions of the envelope glycoprotein gp120 of HIV-1 that are independent of CD4 recognition. AIDS. 1989 Dec;3(12):793–798. doi: 10.1097/00002030-198912000-00003. [DOI] [PubMed] [Google Scholar]
- Larsen A., Davis T., Curtis B. M., Gimpel S., Sims J. E., Cosman D., Park L., Sorensen E., March C. J., Smith C. A. Expression cloning of a human granulocyte colony-stimulating factor receptor: a structural mosaic of hematopoietin receptor, immunoglobulin, and fibronectin domains. J Exp Med. 1990 Dec 1;172(6):1559–1570. doi: 10.1084/jem.172.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson J., Coutré S., Huang E., Engleman E. Role of envelope glycoprotein carbohydrate in human immunodeficiency virus (HIV) infectivity and virus-induced cell fusion. J Exp Med. 1986 Dec 1;164(6):2101–2106. doi: 10.1084/jem.164.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A., Kinney-Thomas E., Hu S. L. Effects of anti-gp120 monoclonal antibodies on CD4 receptor binding by the env protein of human immunodeficiency virus type 1. J Virol. 1988 Oct;62(10):3695–3702. doi: 10.1128/jvi.62.10.3695-3702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews T. J., Weinhold K. J., Lyerly H. K., Langlois A. J., Wigzell H., Bolognesi D. P. Interaction between the human T-cell lymphotropic virus type IIIB envelope glycoprotein gp120 and the surface antigen CD4: role of carbohydrate in binding and cell fusion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5424–5428. doi: 10.1073/pnas.84.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi T., Matthews T. J., Kato M., Hamako J., Titani K., Solomon J., Feizi T. Diversity of oligosaccharide structures on the envelope glycoprotein gp 120 of human immunodeficiency virus 1 from the lymphoblastoid cell line H9. Presence of complex-type oligosaccharides with bisecting N-acetylglucosamine residues. J Biol Chem. 1990 May 25;265(15):8519–8524. [PubMed] [Google Scholar]
- Mizuochi T., Spellman M. W., Larkin M., Solomon J., Basa L. J., Feizi T. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese-hamster ovary cells. Biochem J. 1988 Sep 1;254(2):599–603. doi: 10.1042/bj2540599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Schröder H. C., Reuter P., Maidhof A., Uhlenbruck G., Winkler I. Polyclonal antibodies to mannan from yeast also recognize the carbohydrate structure of gp120 of the AIDS virus: an approach to raise neutralizing antibodies to HIV-1 infection in vitro. AIDS. 1990 Feb;4(2):159–162. doi: 10.1097/00002030-199002000-00010. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Brenner D. A., de Grandpre L. Y., Roebuck K. A., Richman D. D., Kagnoff M. F. HIV-1 infection and expression in human colonic cells: infection and expression in CD4+ and CD4- cell lines. AIDS. 1991 Mar;5(3):275–281. doi: 10.1097/00002030-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Peterson A., Seed B. Genetic analysis of monoclonal antibody and HIV binding sites on the human lymphocyte antigen CD4. Cell. 1988 Jul 1;54(1):65–72. doi: 10.1016/0092-8674(88)90180-8. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Montefiori D. C., Mitchell W. M. Evidence that mannosyl residues are involved in human immunodeficiency virus type 1 (HIV-1) pathogenesis. AIDS Res Hum Retroviruses. 1987 Fall;3(3):265–282. doi: 10.1089/aid.1987.3.265. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Weiss R. A. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1988 Mar 11;52(5):631–633. doi: 10.1016/0092-8674(88)90397-2. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Roth J., Burrows A., Folks T. M., Kehrl J. H., Koenig S., Berman P., Fauci A. S. Characterization of GP120 binding to CD4 and an assay that measures ability of sera to inhibit this binding. J Immunol. 1988 Dec 15;141(12):4181–4186. [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. Sequence of a second human asialoglycoprotein receptor: conservation of two receptor genes during evolution. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6465–6469. doi: 10.1073/pnas.82.19.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. E., Conary J. T., Lennartz M. R., Stahl P. D., Drickamer K. Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J Biol Chem. 1990 Jul 25;265(21):12156–12162. [PubMed] [Google Scholar]
- Weber J., Clapham P., McKeating J., Stratton M., Robey E., Weiss R. Infection of brain cells by diverse human immunodeficiency virus isolates: role of CD4 as receptor. J Gen Virol. 1989 Oct;70(Pt 10):2653–2660. doi: 10.1099/0022-1317-70-10-2653. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]



