Abstract
Alzheimer's disease (AD), one of the neurodegenerative disorders that may develop in the elderly, is characterized by the deposition of β-amyloid protein (Aβ) and extensive neuronal cell death in the brain. Neuregulin-1 (Nrg1)-mediated intercellular and intracellular communication via binding to ErbB receptors regulates a diverse set of biological processes involved in the development of the nervous system. In the present study, a linear correlation was identified between Nrg1 and phosphorylated ErbB (pNeu and pErbB4) receptors in a human cortical tissue microarray. In addition, increased expression levels of Nrg1, but reduced pErbB receptor levels, were detected in the frontal lobe of a patient with AD. Western blotting and immunofluorescence staining were subsequently performed to uncover the potential preventive role of Nrg1 in cortical neurons affected by the neurodegenerative processes of AD. It was observed that the expression of Nrg1 increased as the culture time of the cortical neurons progressed. In addition, H2O2 and Aβ1–42, two inducers of oxidative stress and neuronal damage, led to a dose-dependent decrease in Nrg1 expression. Recombinant Nrg1β, however, was revealed to exert a pivotal role in preventing oxidative stress and neuronal damage from occurring in the mouse cortical neurons. Taken together, these results suggest that changes in Nrg1 signaling may influence the pathological development of AD, and exogenous Nrg1 may serve as a potential candidate for the prevention and treatment of AD.
Keywords: Alzheimer's disease, neuregulin-1, frontal lobe, ErbB receptors, cortical neurons
Introduction
Alzheimer's disease (AD) is the most common age-associated disorder, accounting for ~60–80% of all cases of dementia (1). Previous studies have shown that atrophy of the hippocampus and amygdala may occur in AD, even at the preclinical stages (2–4). AD is typically characterized by a progressive loss of memory, impairment of higher cognitive functions and major degeneration in the brain cortex. This degeneration includes the production and deposition of β-amyloid (Aβ) peptide, intracellular neurofibrillary tangles (5,6) and extensive neuronal cell death (7) in specific cortical and subcortical zones. AD shares a number of common pathological features with other neurodegenerative diseases, including activated apoptotic biochemical cascades, up-regulated oxidative stress levels, abnormal protein processing, and so forth (8). Age-associated oxidative insults have been associated with neurodegenerative diseases, including AD and Parkinson's disease (9,10). Aβ peptide fragments are capable of inducing neuronal cell death directly or indirectly (11–13). In addition, transgenic mice with mutant amyloid precursor protein are considered a valuable animal model to test preventative and therapeutic interventions for AD due to the occurrence of biochemical, behavioral and histopathological changes that are similar to those observed in patients with AD (14). Although current therapeutic candidates for the treatment of AD that are similar to cholinesterase inhibitors such as meserine (15) and memantine (16) may modestly improve memory and cognitive function in this transgenic mouse model, these drugs do not show disease-modifying effects in patients. To date, the available therapies for AD only serve the purpose of ameliorating disease symptoms, and there are no effective therapeutic approaches that address the underlying pathological processes of AD (17).
Neuregulin-1 (Nrg1), a protein encoded by the NRG1 gene, has been identified as an active epithelial growth factor (EGF) family member (18). At least 31 isoforms and six types of Nrg1, including Nrg1α and Nrg1β, types I to VI, have been identified, due to alternative splicing (19). These types, or isoforms, perform a broad spectrum of functions. Nrg1 has been implicated in glioma malignancy (20), gastrointestinal systems (21) and prolactin secretion (22–24). Specific direct binding of Nrg1 to ErbB receptors, including ErbB3 and ErbB4 (25,26), activates a diverse set of biological processes, including myelination, neurite outgrowth, cell proliferation, differentiation and protection against apoptosis (27,28). Nrg1, as well as its receptor ErbB tyrosine kinase, is expressed in the developing nervous system and the adult brain, where they exert a key role in regulating the development and regeneration of the nervous system (29–31). Nrg1 has been reported to prevent brain injury following stroke (32), and to exert a protective role for dopaminergic neurons in a mouse model of Parkinson's disease (33). A burgeoning body of evidence suggests that Nrg1 is associated with traumatic brain injury (34) and AD (35).
Given the alteration of Nrg1 signaling in patients with AD and the protective role of Nrg1 in the lesioned nervous system (29,30), it was hypothesized that Nrg1 may exert a preventive role in the maintenance of cell survival-associated signaling under the pathological conditions present in AD. In the present study, it has been shown that the levels of Nrg1 are altered in response to hydrogen peroxide (H2O2)- or Aβ1–42-induced oxidative stress and neuronal damage in an attempt to protect the cortical neurons from abnormal changes in cell signaling. Notably, exogenous Nrg1 was revealed to have a pivotal role in preventing neurons from oxidative damage and in triggering changes in Nrg1-ErbB signaling in response to the harmful situation. These results demonstrated that Nrg1 signaling is perturbed under the pathological conditions of AD, and this alteration may be partially reversed by the exogenous application of Nrg1. Taken together, these data indicate a neuroprotective role of Nrg1 against pathological damage during the development of AD.
Materials and methods
Tissue microarray
Human brain tissue microarray containing 4-μm-thick cortical tissues was purchased from Shaanxi Chaoying Biotechnology Co., Ltd. (BN 126; Xi'an, Shanxi, China). In addition, human brain frontal lobe sections from a normal individual (cat. no. ab4304; Abcam, Cambridge, MA, USA) and from a patient with AD (cat. no. ab4582; Abcam) at a thickness of 5 μm were used.
Animals
Female and male C57BL/6 mice (n=20; age, 3 months) were purchased from Guangdong Medical Laboratory Animal Center (Foshan, Guangdong, China) and maintained in the animal center of Shantou University Medical College (SUMC). All the animals were housed in the SUMC animal center at 25°C in a reversed 12/12 h dark-light cycle, and food and water were provided ad libitum. All experiments conducted on animals were reviewed and approved by the Animal Ethics Committee of SUMC and the authorities of the Guangdong Province. All efforts were made to minimize the suffering of animals and to reduce the number of animals used in these experiments.
Preparation of recombinant Nrg1β (rNrg1β) and oligomeric Aβ1–42
The Escherichia coli-derived rNrg1β (CYT-407; ProSpec-Tany TechnoGene Ltd., Ness Ziona, Israel) was dissolved in phosphate-buffered saline (PBS, pH 7.4) and used for in vitro cell culture experiments.
Synthetic Aβ1–42 powder (cat. no.1932-2-15; ChinaPeptides Co., Ltd., Shanghai, China) was dissolved in 0.1% dimethyl sulfoxide and diluted 1:100 in Dulbecco's modified Eagle's medium (DMEM)/F-12 culture medium (HyClone™; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The diluted solution was subsequently incubated at 4°C for 24 h prior to centrifugation at 14,000 g for 10 min. The supernatant was used as oligomeric Aβ1–42 for in vitro cell culture experiments.
Primary culture of the mouse cortical neurons
Mouse frontal cortical tissues were obtained from postnatal C57BL/6 mice on day 0 (P0) and crudely homogenized by chopping following the removal of the vessels and meninges. The tissues were kept on ice in DMEM/F-12 culture medium (HyClone™; Thermo Fisher Scientific, Inc.) without serum, and subsequently digested with 0.125% trypsin (Solarbio Biotech Corp., Beijing, China) at 37°C in a humidified 5% CO2 atmosphere for 30 min. The finely separated cortical neurons were seeded in a volume of 200 μl at a density of 2×105 cells per well in 48-well cell culture plates pre-coated with 100 μg/ml poly-D-lysine (C0312; Beyotime Institute of Biotechnology, Shanghai, China). Cells were cultured in DMEM/F-12 culture medium supplemented with 10% fetal bovine serum (FBS; Sijiqing Biotech Corp., Hangzhou, China) and 1% penicillin/streptomycin (Solarbio Biotech Corp.) for 6 h to enable cell adhesion to the plates. The medium was subsequently aspirated and replaced with Neurobasal-A (cat. no. 21103-049; Gibco; Thermo Fisher Scientific, Inc.) culture medium supplemented with 2% B-27 (cat. no. 17504-001, Gibco, Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin.
To investigate changes in Nrg1 signaling in vitro at the protein level, the primary cortical neurons were treated using two different paradigms: i) The cortical neurons were maintained at 37°C in a humidified 5% CO2 atmosphere for 24 h, and subsequently the culture medium was replaced with Neurobasal-A medium containing H2O2 at various concentrations: 0, 1, 2.5, 5, 10 and 20 μM for 24 h; ii) the cells were cultured for 6 h, 1, 3, 6 and 10 days. Cells cultured for 6 h were used as a control (0 days). At the indicated time points, whole-cell lysates were collected.
To study the neuroprotective role of rNrg1β in regulating Nrg1 signaling at the protein level, cortical neurons were utilized with two different cell models: i) Cortical neurons were treated with 0, 5 or 10 nM rNrg1β for 2 h, followed by an exposure to 2.5 μM H2O2 for 24 h; ii) after a 24-h incubation period, cells were treated with 0, 5 or 10 nM rNrg1β for 2 h prior to incubation with a sublethal dose of 10 μM oligomeric Aβ1–42 for 24 h. Finally, whole-cell lysates were collected for western blotting.
Immunofluorescence staining
Paraffin-embedded human brain cortical tissue microarray and human frontal cortical sections from a normal individual and a patient with AD were deparaffinized, rehydrated via a graded array of ethanol to PBS, and antigen retrieval was then performed using 10 mM citrate buffer (pH 6.0) for 40 min. Non-specific protein binding sites were blocked by incubation with 10% normal donkey serum diluted in PBS at room temperature (RT, 25°C) for 1 h. The sections were incubated at 4°C overnight with mouse monoclonal anti-Nrg1 antibody (1:200, cat. no. MS-272-P1, Thermo Fisher Scientific, Inc.) without or with either rabbit polyclonal anti-phosphorylated (p)ErbB4 antibody (1:200, cat. no. sc-33040, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or rabbit polyclonal anti-pNeu antibody (1:200, cat. no. sc-12352-R, Santa Cruz Biotechnology, Inc.). Mouse monoclonal anti-β-III tubulin (1:1,000, cat. no. sc-80016, Santa Cruz Biotechnology, Inc.) was applied to indicate the mouse cortical neurons. Following washing with PBS three times (5 min each wash), the samples were incubated with a donkey anti-mouse secondary antibody conjugated to Dylight™ 488 (1:1,000) and a donkey anti-rabbit secondary antibody conjugated to Dylight™ 594 (1:1,000) at RT in the dark for 90 min. The samples were finally mounted using ProLong® Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; P36935, Gibco; Thermo Fisher Scientific, Inc.). Double-immunofluorescence images were acquired using an Olympus laser confocal system (FV-1000; Olympus, Tokyo, Japan). DAPI was excited at 405 nm. Dylight™ 488 and Dylight™ 594 were excited at 488 and 594 nm, respectively, in a multi-track configuration.
The protein levels of Nrg1, pNeu and pErbB4 in the human brain cortical tissue microarray were evaluated using integrated fluorescence intensity (IFI). The IFI at each tissue point was obtained using the MultiImage Light Cabinet CY3 filter for Nrg1, and the CY5 filter of the FluorChem HD2 gel-imaging system for pErbB4 or pNeu (Alpha Innotech, San Leandro, CA, USA). The IFI was analyzed using Image Tool II software 3.0 (University of Texas Health Science Center, San Antonio, TX, USA).
Immunohistochemical staining
Deparaffinized human frontal cortical sections from a normal individual and a patient with AD were rehydrated via a graded array of ethanol to PBS. Subsequently, hit-induced antigen retrieval was performed in citrate buffer (10 mM, pH 6.0) at 95°C for 40 min, followed by cooling down to RT for at least 60 min. Sections were then incubated in a 3% H2O2 solution for endogenous peroxidase clearance at RT for 10 min. Sections were subsequently washed in PBS for 5 min three times. Following blocking in 10% PBS-buffered normal goat serum for 30 min, sections were incubated with primary antibodies, including mouse monoclonal anti-Nrg1 antibody (1:200, cat. no. MS-272-P1, Thermo Fisher Scientific, Inc.), rabbit polyclonal anti-pErbB4 antibody (1:200, cat. no. sc-33040, Santa Cruz Biotechnology, Inc.), rabbit polyclonal anti-pNeu antibody (1:200, cat. no. sc-12352-R, Santa Cruz Biotechnology, Inc.) and rabbit polyclonal anti-Aβ1–42 antibody (1:1,000, cat. no. ab39377, Abcam) overnight at 4°C, followed by incubation with an Enhanced Polymer DAB Detection kit (cat. no. PV-900; ZSGB-Bio, Beijing, China) and an AEC kit (cat. no. ZLI-9036; ZSGB-Bio). Stained sections were mounted on slides, dehydrated and sealed with coverslips using a commercial water-soluble mounting kit (cat. no. AR1018; Boster Biological Technology, Wuhan, China). Counterstaining was performed with Mayer's hematoxylin in certain of the tissue sections.
Congo red reagent (cat. no. DG0025, Beijing Leagene Biotech. Co., Ltd., Beijing, China) was used to confirm the formation of amyloid plaques in frontal lobe sections from a patient with AD, according to the manufacturer's protocol.
Western blotting analysis
Tissue lysates of the frontal lobe from either normal adults (cat. no. ab29969, Abcam) or the patient with AD (cat. no. ab29971, Abcam) and the whole-cell lysates from cultured mouse cortical neurons were mixed with 20% sample loading buffer [0.125 M Tris/HCl (pH 6.8), 20% glycerol, 10% sodium dodecylsulfate, 0.1% bromophenol blue and 5% β-mercaptoethanol]. All samples were heated for 15 min at 95°C. Protein samples were resolved using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequently electroblotted onto polyvinylidene difluoride membranes (Millipore Corp., Billerica, USA). Incubation in 5% non-fat milk or bovine serum albumin diluted in Tris/HCl saline buffer supplemented with 0.1% Tween-20 (TBST, pH 7.4) for 1 h was used to block non-specific protein-binding sites. Membranes were incubated with antibodies specific for mouse monoclonal anti-Nrg1 antibody (1:1,000, cat. no. MS-272-P1, Thermo Fisher Scientific, Inc.), rabbit polyclonal anti-pErbB4 antibody (1:500, cat. no. sc-33040, Santa Cruz Biotechnology, Inc.), rabbit polyclonal anti-ErbB4 antibody (1:1,000, cat. no. sc-283, Santa Cruz Biotechnology, Inc.), rabbit polyclonal anti-pNeu antibody (1:500, cat. no. sc-12352-R, Santa Cruz Biotechnology, Inc.), mouse monoclonal anti-Neu antibody (1:1,000, cat. no. sc-33684, Santa Cruz Biotechnology, Inc.), mouse monoclonal anti-phosphorylated extracellular signal-regulated kinase 1/2 (anti-pErk1/2) antibody (1:1,000, cat. no. sc-7383, Santa Cruz Biotechnology, Inc.), mouse monoclonal anti-Erk1/2 antibody (1:1,000, cat. no. sc-135900, Santa Cruz Biotechnology, Inc.), mouse monoclonal anti-pAkt1 antibody (1:1,000, cat. no. sc-81433, Santa Cruz Biotechnology, Inc.), mouse monoclonal anti-Akt1 antibody (1:1,000, cat. no. sc-55523, Santa Cruz Biotechnology, Inc.) and mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GADPH) antibody (1:1,000, cat. no. sc-365062, Santa Cruz Biotechnology, Inc.) overnight at 4°C. After washing the membrane with TBST three times at RT (5 min each wash), membranes were further incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:1,000, cat. no. BA1051, Boster Biological Technology, Wuhan, China) or anti-rabbit secondary antibody (1:1,000, cat. no. BA1055, Boster Biological Technology) for 1 h. Subsequently, membranes were washed in TBST three times (5 min each wash) at RT. The immunoreactive bands were visualized using an enhanced chemiluminescence kit (Bio-Rad Laboratories, Richmond, CA, USA) and an imaging system (Alpha Innotech, San Leandro, CA, USA). The signal intensity was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analyses were performed with SPSS 17.0 software (Chicago, IL, USA). Data were expressed as the mean ± standard error of the mean and analyzed with one-way analysis of variance (ANOVA) with Tukey's post-hoc test for independent samples. P<0.05 was considered to indicate a statistically significant difference.
Results
Co-immunostaining correlation analysis of Nrg1 with the phosphorylation levels of ErbB4 and Neu receptors in a human cortical tissue microarray
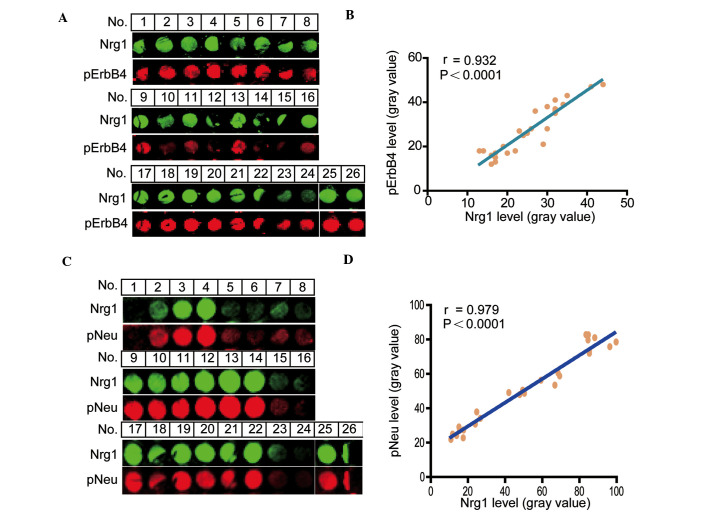
To determine a functional correlative relationship between the level of Nrg1 and the phosphorylation levels of either the ErbB4 or the Neu receptors, the co-localization of Nrg1 with either pErbB4 or pNeu receptors was examined. The signal intensity for Nrg1 with pErbB4 receptors was revealed by double immunofluorescence (Fig. 1A), and a positive correlation between Nrg1 and pErbB4 (r=0.932, P<0.0001) (Fig. 1B) was identified. Similarly, the signal intensity for Nrg1 with Neu receptors was also revealed by double immunofluorescence (Fig. 1C) and an apparent positive correlation between Nrg1 and pNeu (r=0.979, P<0.0001) (Fig. 1D) was revealed in the human cortical region.
Figure 1.
Linear correlation analyses for Nrg1 with either pErbB4 (A and B) or pNeu (C and D) based on integrated fluorescence intensity of the human frontal cortex tissue array. Double immunofluorescence staining of Nrg1 with either pErbB4 (A) or pNeu (C) at each tissue point is shown. Linear correlation graph depicting the association between Nrg1 and pErbB4 (B) or pNeu (D). Each corresponding Pearson correlation coefficient (r) is shown. Nrg1, neuregulin 1; pErbB4, phosphorylated ErbB4; pNeu, phosphorylated Neu.
Detection of Nrg1 signaling in the frontal lobe of a human AD brain
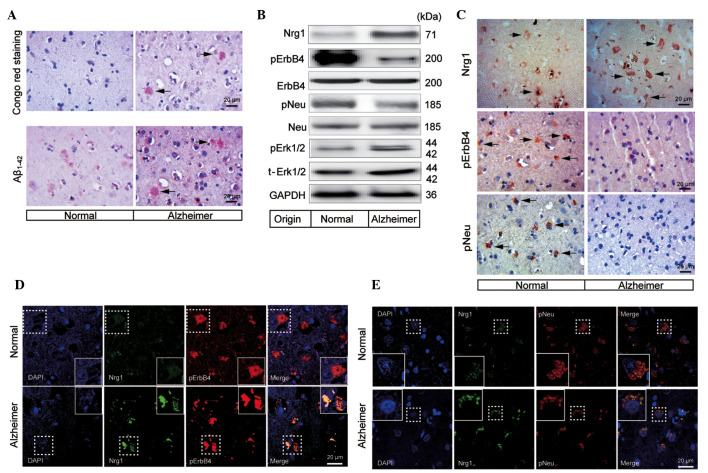
Congo red staining was used to confirm the formation of the amyloid plaques in the frontal lobe of the brain of a human patient with AD. The results demonstrated that there were numerous amyloid plaques distributed in the frontal lobe of an AD brain, whereas no amyloid plaque was detected in the normal control (Fig. 2A). In addition, immunohistochemical staining also revealed the formation of Aβ1–42 positive plaques in the frontal cortical zone, whereas few Aβ1–42 positive plaques were found in the normal individual (Fig. 2A).
Figure 2.
Changes in the Nrg1 signaling pathway molecules in the frontal lobe of a human AD brain. (A) Congo red staining and abnormal aggregation of Aβ1–42, indicating the formation of the amyloid plaques (indicated by the arrowheads) in the frontal cortical gray matter of a human AD brain. The scale bar represents 20 μm. (B) Western blotting analysis of Nrg1, phosphorylation levels of ErbB4, Neu and Erk1/2 in the frontal lobe of a human AD patient brain. (C) Immunohistochemical detection of Nrg1, pErbB4 and pNeu (indicated by the arrowheads) in the frontal cortical gray matter from either the normal indivdual or the human AD patient. Double immunofluorescence staining images are shown for co-localization of Nrg1 with either (D) pErbB4 or (E) pNeu in the frontal cortical gray matter from either the normal individual or the human AD patient. The scale bar represents 20 μm. AD, Alzheimer's disease; Aβ, β-amyloid; Nrg1, neuregulin 1; pErbB4, phosphorylated ErbB4; pNeu, phosphorylated Neu; Erk, extracellular-signal regulated kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To study the changes in Nrg1 signaling in the frontal cortex of an AD brain, western blotting was used to determine the protein levels of Nrg1 and the phosphorylation levels of ErbB4 and Neu. The protein level of Nrg1 was increased in the frontal cortical gray matter from an AD brain (Fig. 2B). In contrast, the levels of pErbB4 and pNeu showed a tendency towards a decrease when compared with a normal control. Notably, the phosphorylation level of Erk1/2, which is involved in downstream Nrg1-ErbB signaling, was increased when compared with that in the normal control (Fig. 2B).
To further investigate changes in Nrg1 signaling under the conditions of AD, Nrg1, pErbB4 and pNeu in the frontal cortical gray matter from a patient with AD and a normal control were immunohistochemically stained. A tendency for there to be an increased level of Nrg1 was observed in the gray matter of an AD brain compared with a normal control (Fig. 2C). By contrast, the staining intensities for pErbB4 and pNeu were clearly reduced (Fig. 2C).
To evaluate the expression and potential co-localization of Nrg1 with pErbB4 or pNeu, co-immunostaining of Nrg1 with these receptors was performed using the frontal cortical tissue from an AD brain. Co-localization of Nrg1 (green) with pErbB4 (red) or with pNeu (red) was observed. In the human AD brain, a tendency towards an up-regulation of the levels of Nrg1 was observed, whereas the levels of pErbB4 and pNeu were not up-regulated compared with those of normal brain tissue (Fig. 2D and E). In addition, pErbB4 and pNeu were detected in a smaller number of the neuronal cells in the AD-affected cerebral cortex, which may explain, in part, the reduced levels of the two molecules observed in the western blots.
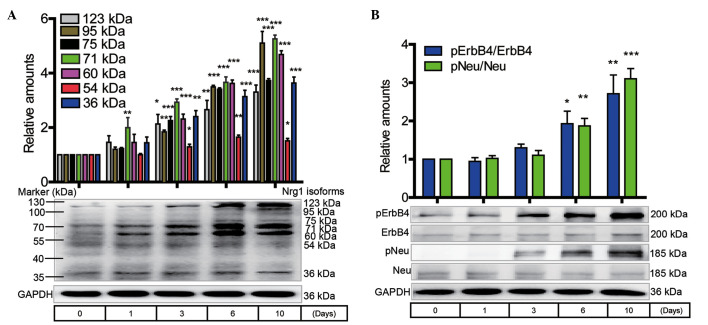
Western blotting analysis of Nrg1 isoform expression and the ErbB receptor phosphorylation level in primary cortical neurons during the progression of cell senescence
The primary cortical neurons were routinely cultured for 1, 3, 6 and 10 days without any treatment. The expression of Nrg1 isoforms, including the 123-, 95-, 75-, 71-, 60-, 54- and 36-kDa variants, showed a time-dependent increase that reached statistical significance at 3, 6 and 10 days when compared with the 0-day control (Fig. 3A). A marked increase in the protein levels of the pNeu and pErbB4 receptors was observed at 1 to 10 days when compared with the 0-day control (Fig. 3B).
Figure 3.
Western blot analysis of Nrg1, pNeu and pErbB4 in primary mouse cortical neurons in response to cell senescence. (A) Protein levels of Nrg1 isoforms and (B) the phosphorylation levels of Neu and ErbB4 in cortical neurons cultured for 0 to 10 days (n=5, one-way analysis of variance with Tukey's post-hoc test; data are expressed as the mean ± standard error of the mean). *P<0.05, **P<0.01 and ***P<0.001 compared with the 0-day control. Nrg1, neuregulin 1; pErbB4, phosphorylated ErbB4; pNeu, phosphorylated Neu; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Investigation of the protective role of Nrg1β in primary cortical neuronal cultures oxidatively stressed by H2O2
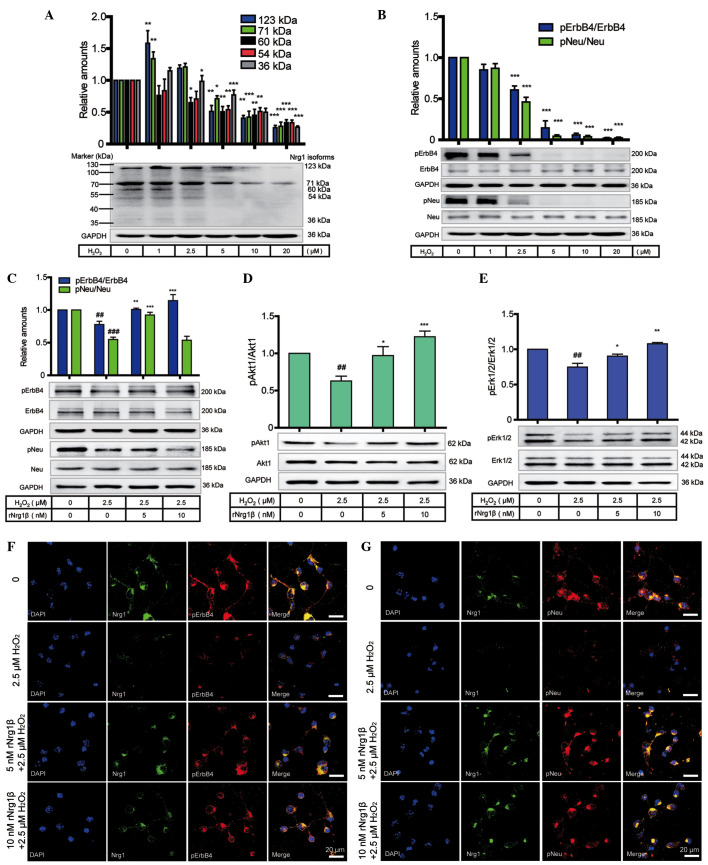
To determine the role of Nrg1 in preventing oxidative insults at the protein level, western blotting was employed to analyze the protein level of Nrg1 and the phosphorylation levels of Neu and ErbB4 receptors in primary cortical neurons in response to a 24 h treatment with H2O2 at concentrations ranging from 0 to 20 μM. Multiple isoforms of Nrg1, including the 123-, 71-, 60-, 54- and 36-kDa variants, were expressed in the primary cortical neurons. Compared with the vehicle control, all Nrg1 isoforms showed a dose-dependent decrease in response to the gradually increased concentrations of H2O2 (Fig. 4A). In comparison with the vehicle control, the expression of Nrg1 was down-regulated, accompanied by a marked reduction in the receptor levels of pNeu and pErbB4 (Fig. 4B).
Figure 4.
Protective role of rNrg1β in primary mouse cortical neurons in response to oxidative stress and neuraxon damage. (A) Protein levels of Nrg1 isoforms and (B) phosphorylation levels of Neu and ErbB4 receptors in primary cortical neurons after a 24 h treatment with 0-20 μM H2O2 (n=6, one-way ANOVA with Tukey's post-hoc test; data are expressed as the mean ± SEM). *P<0.05, **P<0.01 and ***P<0.001 compared with the control group. (C) The phosphorylation levels of Neu and ErbB4, and (D) the pAkt1 levels and (E) the pErk1/2 levels in primary cortical neurons pretreated with rNrg1β at a concentration of 5 or 10 nM for 2 h prior to a 24-h treatment with 2.5 μM oligomeric H2O2 (n=5, one-way ANOVA with Tukey's post-hoc test; data are expressed as the mean ± SEM). ##P<0.01 and ###P<0.001 vs. the vehicle control and *P<0.05, **P<0.01 and ***P<0.001 vs. the H2O2-treated group. Double immunofluorescence staining of Nrg1 with either (F) pErbB4 or (G) pNeu in the cortical neurons pretreated with rNrg1β at a concentration of 5 or 10 nM for 2 h prior to a 24 h treatment with 2.5 μM oligomeric H2O2. Scale bars=20 μm. ANOVA, analysis of variance; SEM, standard error of the mean; H2O2, hydrogen peroxide; rNRG1β, recombinant neuregulin 1β, pErbB4, phosphorylated ErbB4; pNeu, phosphorylated Neu; pErk1/2, phosphorylated extracellular-regulated signal kinase 1/2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Furthermore, in order to explore the role of rNrg1β in alleviating oxidative stress and axonal damage, western blotting was conducted to evaluate whether the Nrg1-ErbB signaling pathway was involved in the preventive mechanism on exposure to H2O2. Based on the data from a previous study (Chen et al, unpublished) a concentration of 2.5 μM was adopted as the optimal concentration of H2O2 for treatment of the cortical neurons following pretreatment with 0, 5 or 10 nM rNrg1β for 2 h. It was revealed that the levels of pErbB4 and pNeu were markedly decreased following treatment with H2O2, and this effect was reversed on addition of rNrg1β, to a maximal extent at 10 nM for pErbB4 and at 5 nM for pNeu (Fig. 4C). The levels of Akt1 and Erk1/2 activation exhibited a similar trend to that of pErbB4 (Fig. 4D and E).
Double immunofluorescence staining was subsequently used to further confirm these observations. It was revealed that, compared with non-stressed neurons, H2O2-treated neurons exhibited diminished levels of neurite outgrowth, with the detection of reduced levels of Nrg1 and of pNeu or pErbB4. In contrast, pretreatment with 5 and 10 nM rNrg1β prior to H2O2 exposure was able to partially reverse these effects by increasing the levels of both pNeu and pErbB4, with more clearly recognizable effects observed at a concentration of 10 nM (Fig. 4F and G).
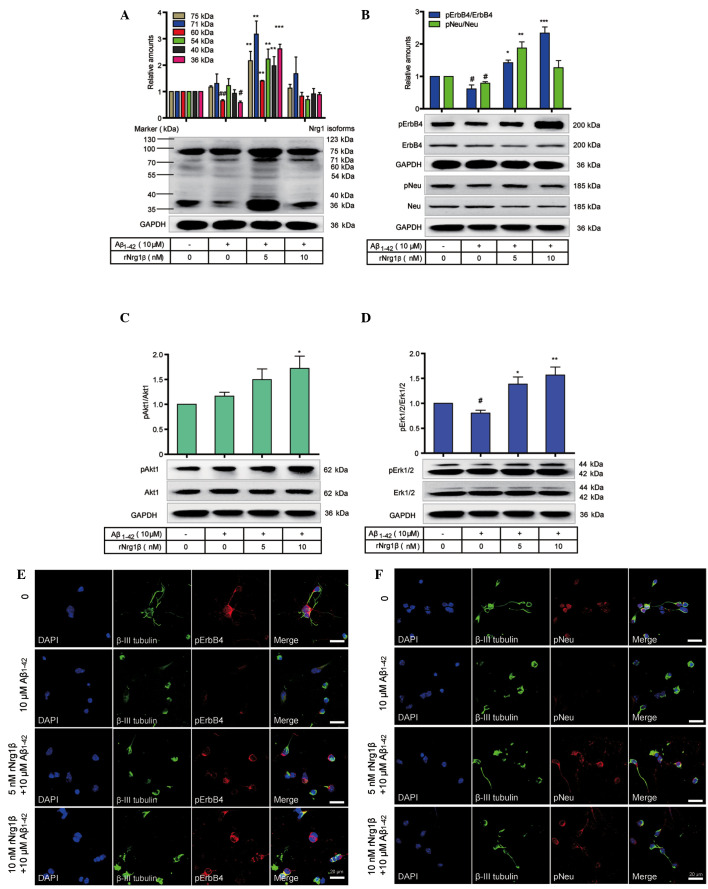
The preventive role of rNrg1β in counteracting the effects of Aβ1–42 on mouse cortical neurons
The protective role of rNrg1β in mouse cortical neurons treated with Aβ1–42 was subsequently investigated. Western blotting was utilized to evaluate the influence of rNrg1β pretreatment on the phosphorylation levels of Neu and ErbB4 and on the downstream signaling pathways in primary cortical neurons following a 24 h incubation with 10 μM oligomeric Aβ1–42. Administration of 10 μM oligomeric Aβ1–42 significantly downregulated the protein levels of several Nrg1 isoforms, including the 60 kDa and 36 kDa Nrg1, whereas pretreatment with 5 nM rNrg1β counteracted the effects of Aβ1–42 by increasing the Nrg1 isoforms. By contrast, pretreatment with 10 nM rNrg1β showed no apparent effects on the function of Aβ1–42 (Fig. 5A). It was also demonstrated that the relative levels of pErbB4 and pNeu were markedly decreased following treatment with Aβ1–42, and this effect on pErbB4 was compensated for by pretreatment with 10 nM rNrg1β and on pNeu by pretreatment with 5 nM rNrg1β (Fig. 5B). In addition, pAkt1 levels were increased, and pErk levels were decreased when treated with Aβ1–42 alone (Fig. 5C and D). However, rNrg1β pretreatment upregulated the levels of pAkt1 and pErk in Aβ1–42-challenged cortical neurons (Fig. 5C and D).
Figure 5.
Effects of rNrg1β pretreatment on the Nrg1 signaling in primary mouse cortical neurons exposed to Aβ1–42. The (A) relative levels of the Nrg1 isoforms, (B) phosphorylation levels of Neu and ErbB4, (C) pAkt1 levels and (D) pErk1/2 levels in primary cortical neurons pretreated with rNrg1β at a concentration of 5 or 10 nM for 2 h prior to a 24-h treatment with 10 μM oligomeric Aβ1–42 are shown (n=5, one-way analysis of variance with Tukey's post-hoc test; data are expressed as the mean ± standard error of the mean). Double immunofluorescence staining of β-III tubulin with either (E) pErbB4 or (F) pNeu in the cortical neurons pretreated with rNrg1β at a concentration of 5 or 10 nM for 2 h prior to a 24 h treatment with 10 μM oligomeric Aβ1–42. Scale bars=20 μm. rNRG1β, recombinant neuregulin 1β, pErbB4, phosphorylated ErbB4; pNeu, phosphorylated Neu; pErk1/2, phosphorylated extracellular-regulated signal kinase 1/2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Aβ, amyloid-beta; DAPI, 4′,6-diamidino-2-phenylindole. #P<0.05 and ##P<0.01 vs. vehicle control and *P<0.05, **P<0.01 and ***P<0.001 compared with the control group.
We subsequently applied double immunofluorescence staining to further confirm these observations. It was observed that, compared with non-stressed neurons, Aβ1–42-treated neurons demonstrated a diminished neurite outgrowth, with reduced levels of pNeu or pErbB4 detected. In contrast, pretreatment with 5 or 10 nM rNrg1β prior to Aβ1–42 exposure was able to partially reverse these effects by increasing the levels of both pNeu and pErbB4, with the most marked effects being observed with 10 nM rNrg1β for pErbB4 and 5 nM rNrg1β for pNeu (Fig. 5E and F).
Discussion
It is widely acknowledged that the Nrg1-ErbB signaling pathway exerts a crucial role in multiple biological processes, including cell differentiation, organ development and tumorigenesis. Receptors for Nrg1 signaling undergo phosphorylation of their cytoplasmic tyrosine residues, which elicits downstream effects and biological responses (36). The binding of the ligand results in the dimerization and activation of ErbB receptors. Phosphorylation of the intracellular domains creates docking sites for adaptor proteins, including growth factor receptor-bound protein 2 (Grb2) and Shc for the activation of the Erk pathway, and p85 for the activation of the phosphoinositide 3-kinase pathway (37). A previous study revealed an association between Nrg1 and ErbB4 immunoreactivity and the formation of neuritic plaques in patients with AD in a transgenic animal model of AD (35). In the present study, a linear correlation was observed between Nrg1 and the phosphorylation of Neu and ErbB4 receptors in a normal human cortical tissue microarray. To elucidate the exact mechanism by which Nrg1 contributes to AD development, two cell models were applied. Based on our results using cortical neurons under the pathological conditions of AD, multiple isoforms of Nrg1 were altered, including the 123-, 95-, 75-, 71-, 60-, 54-, 40- and 36-kDa proteins. These bands represent alternatively spliced products of the NRG1 gene, post-translationally modified forms of the proteins, and/or a shedding of the ectodomains from the initial precursors. All isoforms of Nrg1 contain an epidermal growth factor (EGF)-like signaling domain that is required for activation of the receptors (38). In addition, the interaction of Nrg1 with its receptors was shown to be associated with the activation of intracellular signaling pathways that are associated with the development and regeneration of the nervous system (29,30). In the present study, it has been demonstrated that the changes in Nrg1 isoform expression and receptor phosphorylation are highly influenced by the pathological conditions observed in AD. Thus, expression changes in Nrg1 isoforms appear to be associated with the pathological development of AD, suggesting that Nrg1 may be a critical molecule in the development of AD.
Oxidative stress plays an essential role in the onset and development of AD (39,40), and cellular oxidative stress levels are increased in vulnerable regions of the AD brain (41,42). The brain is particularly sensitive to oxidative stress due to special cellular features, including a large dependence on oxidative phosphorylation for energy production, low antioxidant concentrations, low levels of membrane lipids and high levels of iron, which are associated with free radical injury (43–45). Previous studies reported that Nrg1 was up-regulated following nerve injury, and it served as an essential agent to protect the neurons from ischemic damage (34,46,47). In addition, Nrg proteins attenuated the release of free radicals and protected neuronal cells from H2O2-induced apoptosis (48,49). In the present study, the protein levels of multiple Nrg1 isoforms and the phosphorylation of their receptors were observed to increase in a time-dependent manner. Changes in Nrg1 signaling in cortical neurons exposed to oxidative stress were further investigated. It was observed that protein levels of Nrg1, and the phosphorylation of its receptors, were down-regulated in response to high concentrations of H2O2. Although the Nrg1 protein level was up-regulated at low concentrations of H2O2 compared with the control, no up-regulation of receptor phosphorylation was identified. This suggested that the interactions between Nrg1 and the ErbB receptors were perturbed under conditions of oxidative stress. By contrast, exogenous rNrg1β was able to protect the cortical neurons from oxidative stress and neuraxonal damage via the up-regulation of the Nrg1-ErbB cell signaling pathway. Cui et al (50) proposed that endogenous Nrg1 was increased in response to the production of Aβ to protect the neurons against damage. However, the injured neurons were not capable of expressing sufficient Nrg1β1 to adapt to prolonged damage, which ultimately led to apoptosis. Increased oxidative stress occurs in response to increased Aβ levels (51); therefore, in the present study, it was originally hypothesized that the up-regulation of endogenous Nrg1 in primary cortical neurons exposed to H2O2 may be an initial, local protective response against abnormal cell signaling. However, the cortical neurons were unable to express sufficient Nrg1 over time, eventually resulting in the dysfunction of Nrg1 signaling. These results suggest the existence of an intrinsic self-protective mechanism in which injured cortical neurons may adapt to, and automatically counteract, neuronal injury.
Aβ is associated with the generation of reactive oxygen species, which cause cell damage, apoptosis, mitochondrial dysfunction and the peroxidation of membrane lipids (52,53). In addition, the accumulation of Aβ peptides has been identified as a key step in the multiple pathogenic changes associated with neurodegeneration and dementia (54,55). Previous studies demonstrated that the neurotoxicity induced by Aβ1–42 may lead to apoptotic cell death (56), and that Aβ is able to disrupt signaling pathways, including those involving Erk1/2 and Akt in the primary rat cortical neurons (57,58). In the present study, it was observed that exposure of primary cortical neurons to Aβ1–42 caused an up-regulation in the level of Nrg1 protein and in Akt1 phosphorylation, and a down-regulation of Neu/ErbB4 phosphorylation and pErk1/2 levels. In vitro studies have demonstrated that Nrg1β treatment may protect neuronal cells (59–62). In the present study, Aβ1–42 treatment increased the levels of Nrg1 and Akt1 phosphorylation, and decreased the phosphorylated levels of ErbB4, Neu and Erk1/2. Moreover, the Aβ1–42-induced reduction in the levels of pErbB4, pNeu, pAkt1 and pErk1/2 was antagonized by rNrg1β pretreatment. Recombinant human Nrg1 contains an EGF-like domain that is essential for the phosphorylation-dependent activation of Neu/ErbB4 receptors (63). Nrg1 is able to signal to target cells via interactions with transmembrane tyrosine kinase receptors of the ErbB family. The interaction of Nrg1 with ErbB receptors may result in the dimerization of receptors, tyrosine phosphorylation, and activation of intracellular signaling pathways (59,64). Activation of ErbB4 by Nrg1 may induce a marked increase in ErbB4 phosphorylation (65) and lead to a sustained activation of Akt and Erk (66). In addition, the Akt and Erk1/2 signaling cascades have an essential role in regulating gene expression and in preventing apoptosis (67). A wide spectrum of in vivo and in vitro studies have demonstrated that phosphorylation of Erk facilitates cell survival (68), and that the dephosphorylation of Akt is involved in the development of AD (58,69,70). These results indicated that Nrg1 signaling in mouse cortical neurons is altered in response to the accumulation of Aβ, suggesting that Nrg1 may function as a crucial candidate for the prevention and treatment of AD.
In view of these observations, it was our hypothesis that, although Nrg1 is up-regulated in cortical neurons during the early stages of AD to protect against abnormal changes in cell signaling, the phosphorylation levels of its receptors are relatively less responsive due to some unknown interrupting factors. As a consequence, Nrg1 signaling is not able to function properly when ErbB4 is not adequately activated. In addition, sufficient levels of Nrg1 are not expressed when the damage is prolonged, thus failing to prevent the development and progression of AD. Notably, the present study revealed that pretreatment of neural cells with rNrg1β partially reversed the neurotoxicity of Aβ1–42. These findings have provided a foundational basis for Nrg1 signaling as a potential therapeutic target for the prevention, and possibly the treatment, of AD.
Acknowledgments
We would like to thank the National Natural Science Foundation of China (grant nos. 81171138 and 81471279 to W-J. Z.) for support. This work was also supported by a Talent Support Grant from Shantou University Medical College (grant no. 250122 0118 to W-J. Z.).
References
- 1.Alzheimer's Association 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Golebiowski M, Barcikowska M, Pfeffer A. Magnetic resonance imaging-based hippocampal volumetry in patients with dementia of the Alzheimer type. Dement Geriatr Cogn Disord. 1999;10:284–288. doi: 10.1159/000017133. [DOI] [PubMed] [Google Scholar]
- 3.Heun R, Mazanek M, Atzor KR, Tintera J, Gawehn J, Burkart M, Gänsicke M, Falkai P, Stoeter P. Amygdala-hippocampal atrophy and memory performance in dementia of Alzheimer type. Dement Geriatr Cogn Disord. 1997;8:329–336. doi: 10.1159/000106651. [DOI] [PubMed] [Google Scholar]
- 4.Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 5.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 6.D'Aniello A, Fisher G, Migliaccio N, Cammisa G, D'Aniello E, Spinelli P. Amino acids and transaminases activity in ventricular CSF and in brain of normal and Alzheimer patients. Neurosci Lett. 2005;388:49–53. doi: 10.1016/j.neulet.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 8.Culmsee C, Landshamer S. Molecular insights into mechanisms of the cell death program: Role in the progression of neurodegenerative disorders. Curr Alzheimer Res. 2006;3:269–283. doi: 10.2174/156720506778249461. [DOI] [PubMed] [Google Scholar]
- 9.Honda K, Casadesus G, Petersen RB, Perry G, Smith MA. Oxidative stress and redox-active iron in Alzheimer's disease. Ann N Y Acad Sci. 2004;1012:179–182. doi: 10.1196/annals.1306.015. [DOI] [PubMed] [Google Scholar]
- 10.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S26–S36. doi: 10.1002/ana.10483. discussion S36–S38. [DOI] [PubMed] [Google Scholar]
- 11.Su JH, Anderson AJ, Cummings BJ, Cotman CW. Immunohistochemical evidence for apoptosis in Alzheimer's disease. Neuroreport. 1994;5:2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Yagami T, Ueda K, Asakura K, Sakaeda T, Nakazato H, Kuroda T, Hata S, Sakaguchi G, Itoh N, Nakano T, et al. Gas6 rescues cortical neurons from amyloid beta protein-induced apoptosis. Neuropharmacology. 2002;43:1289–1296. doi: 10.1016/S0028-3908(02)00333-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang DM, Li SQ, Zhu XY, Wang Y, Wu WL, Zhang XJ. Protective effects of hesperidin against amyloid-β (Aβ) induced neurotoxicity through the voltage dependent anion channel 1 (VDAC1)-mediated mitochondrial apoptotic pathway in PC12 cells. Neurochem Res. 2013;38:1034–1044. doi: 10.1007/s11064-013-1013-4. [DOI] [PubMed] [Google Scholar]
- 14.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 15.Shao BY, Xia Z, Xie Q, Ge XX, Zhang WW, Sun J, Jiang P, Wang H, Le WD, Qiu ZB, et al. Meserine, a novel carbamate AChE inhibitor, ameliorates scopolamine-induced dementia and alleviates amyloidogenesis of APP/PS1 transgenic mice. CNS Neurosci Ther. 2014;20:165–171. doi: 10.1111/cns.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu MY, Wang S, Yao WF, Zhang ZJ, Zhong X, Sha L, He M, Zheng ZH, Wei MJ. Memantine improves spatial learning and memory impairments by regulating NGF signaling in APP/PS1 transgenic mice. Neuroscience. 2014;273:141–151. doi: 10.1016/j.neuroscience.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Sadowski M, Wisniewski T. Disease modifying approaches for Alzheimer's pathology. Curr Pharm Des. 2007;13:1943–1954. doi: 10.2174/138161207781039788. [DOI] [PubMed] [Google Scholar]
- 18.Ritch PS, Carroll SL, Sontheimer H. Neuregulin-1 enhances survival of human astrocytic glioma cells. Glia. 2005;51:217–228. doi: 10.1002/glia.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao WJ, Schachner M. Neuregulin 1 enhances cell adhesion molecule l1 expression in human glioma cells and promotes their migration as a function of malignancy. J Neuropathol Exp Neurol. 2013;72:244–255. doi: 10.1097/NEN.0b013e3182863dc5. [DOI] [PubMed] [Google Scholar]
- 21.Zhao WJ. The expression and localization of neuregulin-1 (Nrg1) in the gastrointestinal system of the rhesus monkey. Folia Histochem Cytobiol. 2013;51:38–44. doi: 10.5603/FHC.2013.006. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W, Ren SG. Neuregulin-1 (Nrg1) is mainly expressed in rat pituitary gonadotroph cells and possibly regulates prolactin (PRL) secretion in a juxtacrine manner. J Neuroendocrinol. 2011;23:1252–1262. doi: 10.1111/j.1365-2826.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao W, Shen Y, Ren S. Endogenous expression of Neuregulin-1 (Nrg1) as a potential modulator of prolactin (PRL) secretion in GH3 cells. Cell Tissue Res. 2011;344:313–320. doi: 10.1007/s00441-011-1157-y. [DOI] [PubMed] [Google Scholar]
- 24.Zhao WJ, Jiang Q, Mei JP. Neurohypophyseal Neuregulin 1 is derived from the hypothalamus as a potential prolactin modulator. Neuroendocrinology. 2015;102:288–299. doi: 10.1159/000431377. [DOI] [PubMed] [Google Scholar]
- 25.Wilson TR, Lee DY, Berry L, Shames DS, Settleman J. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell. 2011;20:158–172. doi: 10.1016/j.ccr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Zhao WJ, Ren SG. Endogenous neuregulin-1 expression in the anterior pituitary of female Wistar-Furth rats during the estrous cycle. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:921–927. In Chinese. [PubMed] [Google Scholar]
- 27.Liu J, Kern JA. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol. 2002;27:306–313. doi: 10.1165/rcmb.4850. [DOI] [PubMed] [Google Scholar]
- 28.Puricelli L, Proietti CJ, Labriola L, Salatino M, Balañá ME, Aguirre Ghiso J, Lupu R, Pignataro OP, Charreau EH, Bal de Kier Joffé E, Elizalde PV. Heregulin inhibits proliferation via ERKs and phosphatidyl-inositol 3-kinase activation but regulates urokinase plasminogen activator independently of these pathways in metastatic mammary tumor cells. Int J Cancer. 2002;100:642–653. doi: 10.1002/ijc.10533. [DOI] [PubMed] [Google Scholar]
- 29.Falls DL. Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/S0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 30.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- 32.Xu Z, Croslan DR, Harris AE, Ford GD, Ford BD. Extended therapeutic window and functional recovery after intraarterial administration of neuregulin-1 after focal ischemic stroke. J Cereb Blood Flow Metab. 2006;26:527–535. doi: 10.1038/sj.jcbfm.9600212. [DOI] [PubMed] [Google Scholar]
- 33.Carlsson T, Schindler FR, Höllerhage M, Depboylu C, Arias-Carrión O, Schnurrbusch S, Rösler TW, Wozny W, Schwall GP, Groebe K, et al. Systemic administration of neuregulin-1β1 protects dopaminergic neurons in a mouse model of Parkinson's disease. J Neurochem. 2011;117:1066–1074. doi: 10.1111/j.1471-4159.2011.07284.x. [DOI] [PubMed] [Google Scholar]
- 34.Tokita Y, Keino H, Matsui F, Aono S, Ishiguro H, Higashiyama S, Oohira A. Regulation of neuregulin expression in the injured rat brain and cultured astrocytes. J Neurosci. 2001;21:1257–1264. doi: 10.1523/JNEUROSCI.21-04-01257.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhury AR, Gerecke KM, Wyss JM, Morgan DG, Gordon MN, Carroll SL. Neuregulin-1 and erbB4 immunoreactivity is associated with neuritic plaques in Alzheimer disease brain and in a transgenic model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:42–54. doi: 10.1093/jnen/62.1.42. [DOI] [PubMed] [Google Scholar]
- 36.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 37.Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83:27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanzaki H, Mizobuchi S, Obata N, Itano Y, Kaku R, Tomotsuka N, Nakajima H, Ouchida M, Nakatsuka H, Maeshima K, Morita K. Expression changes of the neuregulin 1 isoforms in neuropathic pain model rats. Neurosci Lett. 2012;508:78–83. doi: 10.1016/j.neulet.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: Can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Mattson MP. Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer's disease. J Neurovirol. 2002;8:539–550. doi: 10.1080/13550280290100978. [DOI] [PubMed] [Google Scholar]
- 43.Facecchia K, Fochesato LA, Ray SD, Stohs SJ, Pandey S. Oxidative toxicity in neurodegenerative diseases: Role of mitochondrial dysfunction and therapeutic strategies. J Toxicol. 2011;2011:683728. doi: 10.1155/2011/683728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viegas CM, Tonin AM, Zanatta A, Seminotti B, Busanello EN, Fernandes CG, Moura AP, Leipnitz G, Wajner M. Impairment of brain redox homeostasis caused by the major metabolites accumulating in hyperornithinemia-hyperammonemia-homocitrullinuria syndrome in vivo. Metab Brain Dis. 2012;27:521–530. doi: 10.1007/s11011-012-9327-5. [DOI] [PubMed] [Google Scholar]
- 45.Herbert V, Shaw S, Jayatilleke E, Stopler-Kasdan T. Most free-radical injury is iron-related: It is promoted by iron, hemin, holoferritin and vitamin C, and inhibited by desferoxamine and apoferritin. Stem Cells. 1994;12:289–303. doi: 10.1002/stem.5530120305. [DOI] [PubMed] [Google Scholar]
- 46.Guo WP, Fu XG, Jiang SM, Wu JZ. Neuregulin-1 regulates the expression of Akt, Bcl-2, and Bad signaling after focal cerebral ischemia in rats. Biochem Cell Biol. 2010;88:649–654. doi: 10.1139/O09-189. [DOI] [PubMed] [Google Scholar]
- 47.Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, Bennett DL. Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci. 2010;30:5437–5450. doi: 10.1523/JNEUROSCI.5169-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erlich S, Goldshmit Y, Lupowitz Z, Pinkas-Kramarski R. ErbB-4 activation inhibits apoptosis in PC12 cells. Neuroscience. 2001;107:353–362. doi: 10.1016/S0306-4522(01)00350-5. [DOI] [PubMed] [Google Scholar]
- 49.Dimayuga FO, Ding Q, Keller JN, Marchionni MA, Seroogy KB, Bruce-Keller AJ. The neuregulin GGF2 attenuates free radical release from activated microglial cells. J Neuroimmunol. 2003;136:67–74. doi: 10.1016/S0165-5728(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 50.Cui W, Tao J, Wang Z, Ren M, Zhang Y, Sun Y, Peng Y, Li R. Neuregulin1beta1 antagonizes apoptosis via ErbB4-dependent activation of PI3-kinase/Akt in APP/PS1 transgenic mice. Neurochem Res. 2013;38:2237–2246. doi: 10.1007/s11064-013-1131-z. [DOI] [PubMed] [Google Scholar]
- 51.Yu W, Bonnet M, Farso M, Ma K, Chabot JG, Martin E, Torriglia A, Guan Z, McLaurin J, Quirion R, Krantic S. The expression of apoptosis inducing factor (AIF) is associated with aging-related cell death in the cortex but not in the hippocampus in the TgCRND8 mouse model of Alzheimer's disease. BMC Neurosci. 2014;15:73. doi: 10.1186/1471-2202-15-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cecchi C, Fiorillo C, Baglioni S, Pensalfini A, Bagnoli S, Nacmias B, Sorbi S, Nosi D, Relini A, Liguri G. Increased susceptibility to amyloid toxicity in familial Alzheimer's fibroblasts. Neurobiol Aging. 2007;28:863–876. doi: 10.1016/j.neurobiolaging.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 53.Miranda S, Opazo C, Larrondo LF, Muñoz FJ, Ruiz F, Leighton F, Inestrosa NC. The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer's disease. Prog Neurobiol. 2000;62:633–648. doi: 10.1016/S0301-0082(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 54.Hardy JA, Higgins GA. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 55.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: An appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 56.Thangnipon W, Puangmalai N, Chinchalongporn V, Jantrachotechatchawan C, Kitiyanant N, Soi-Ampornkul R, Tuchinda P, Nobsathian S. N-benzylcinnamide protects rat cultured cortical neurons from β-amyloid peptide-induced neurotoxicity. Neurosci Lett. 2013;556:20–25. doi: 10.1016/j.neulet.2013.09.071. [DOI] [PubMed] [Google Scholar]
- 57.Chen TJ, Wang DC, Chen SS. Amyloid-beta interrupts the PI3K-Akt-mTOR signaling pathway that could be involved in brain-derived neurotrophic factor-induced Arc expression in rat cortical neurons. J Neurosci Res. 2009;87:2297–2307. doi: 10.1002/jnr.22057. [DOI] [PubMed] [Google Scholar]
- 58.Tong L, Balazs R, Thornton PL, Cotman CW. Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J Neurosci. 2004;24:6799–6809. doi: 10.1523/JNEUROSCI.5463-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parkinson DB, Langner K, Namini SS, Jessen KR, Mirsky R. beta-Neuregulin and autocrine mediated survival of Schwann cells requires activity of Ets family transcription factors. Mol Cell Neurosci. 2002;20:154–167. doi: 10.1006/mcne.2002.1109. [DOI] [PubMed] [Google Scholar]
- 60.Xu Z, Jiang J, Ford G, Ford BD. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophys Res Commun. 2004;322:440–446. doi: 10.1016/j.bbrc.2004.07.149. [DOI] [PubMed] [Google Scholar]
- 61.Xu Z, Ford GD, Croslan DR, Jiang J, Gates A, Allen R, Ford BD. Neuroprotection by neuregulin-1 following focal stroke is associated with the attenuation of ischemia-induced pro-inflammatory and stress gene expression. Neurobiol Dis. 2005;19:461–470. doi: 10.1016/j.nbd.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Yu Y, Schachner M, Zhao W. Neuregulin 1-β regulates cell adhesion molecule L1 expression in the cortex and hippocampus of mice. Biochem Biophys Res Commun. 2013;441:7–12. doi: 10.1016/j.bbrc.2013.09.102. [DOI] [PubMed] [Google Scholar]
- 63.An T, Zhang Y, Huang Y, Zhang R, Yin S, Guo X, Wang Y, Zou C, Wei B, Lv R, et al. Neuregulin-1 protects against doxorubicin-induced apoptosis in cardiomyocytes through an Akt-dependent pathway. Physiol Res. 2013;62:379–385. doi: 10.33549/physiolres.932516. [DOI] [PubMed] [Google Scholar]
- 64.Burden S, Yarden Y. Neuregulins and their receptors: A versatile signaling module in organogenesis and oncogenesis. Neuron. 1997;18:847–855. doi: 10.1016/S0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 65.Vaskovsky A, Lupowitz Z, Erlich S, Pinkas-Kramarski R. ErbB-4 activation promotes neurite outgrowth in PC12 cells. J Neurochem. 2000;74:979–987. doi: 10.1046/j.1471-4159.2000.0740979.x. [DOI] [PubMed] [Google Scholar]
- 66.Di Segni A, Farin K, Pinkas-Kramarski R. ErbB4 activation inhibits MPP+-induced cell death in PC12-ErbB4 cells: Involvement of PI3K and Erk signaling. J Mol Neurosci. 2006;29:257–267. doi: 10.1385/JMN:29:3:257. [DOI] [PubMed] [Google Scholar]
- 67.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D'Assoro AB, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 68.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 69.Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Aβ(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 70.Ma T, Du X, Pick JE, Sui G, Brownlee M, Klann E. Glucagon-like peptide-1 cleavage product GLP-1(9-36) amide rescues synaptic plasticity and memory deficits in Alzheimer's disease model mice. J Neurosci. 2012;32:13701–13708. doi: 10.1523/JNEUROSCI.2107-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]