Abstract
The aim of the present study was to investigate regulatory relationships among hypoxia-inducible factor-1α (HIF-1α), microRNA and erythroid transcription factors. K562 cells were transfected with HIF-1α knockout or with overexpression lentivirus of plasmid (MOI 10). The cells were divided into 3 groups: the negative control, overexpressing and interference groups. The cells were cultured under normoxia and hypoxia. Expression of miR-17*, miR-363 and miR-574-5p in the three groups was determined by quantitative PCR. Expression levels of erythroid transcription factor mRNAs such as GATA-1/GATA-2 and nuclear factor-erythroid 2 (NF-E2) were measured using RT-qPCR while the protein expression was studied using western blot analysis. Under normoxia or hypoxia, the levels of miR-17*, miR-363 and miR-574-5p in the overexpression group were higher than those in the other groups. Differences were statistically significant (P<0.05). Under hypoxia, the level of miR-363 in the interference group was less than that in the negative control group and difference was statistically significant (P<0.05). The level of GATA-1 mRNA in the overexpression group was higher than that in the negative control group, however, in the interference group the level was lower than that in the overexpression group under both normoxic and hypoxic conditions. The level of GATA-2 mRNA in the interference group was higher than that in other two groups under normoxic or hypoxic conditions. The NF-E2 mRNA was reversely related to GATA-2. The levels of HIF-1α, GATA-1 and NF-E2 mRNAs in the negative control under hypoxia were higher than those of normoxia. The level of HIF-1α mRNA in the overexpression group in hypoxia was lower than that in normoxia, while the GATA-1 and GATA-2 mRNA showed a reverse association. The levels of HIF-1α and GATA-2 mRNA in the interference group under hypoxia were higher compared to those of normoxia. Differences were statistically significant (P<0.05). Western blot results suggested that GATA-1, GATA-2 and NF-E2 protein expression correlated with changes in their respective mRNA transcription levels. The results therefore suggested that GATA-l and miR-363 were involved in the regulation of hematopoiesis via the HIF-1α pathway in K562 cells under hypoxic condition. The hsa-miR-17* and hsa-miR-574-5p were not entirely dependent on HIF-1α, suggesting possible complex regulatory mechanisms involved in hypoxia.
Keywords: microRNA, erythroid transcription factors, hypoxic
Introduction
The process of hematopoietic stem cells (HSCs) to red blood cells is regulated by external factors such as interleukin-3 (IL-3), stem sell factor (SCF), granulocyte macrophage colony-stimulating factor (GM-CSF) and erythropoietin (EPO) (1). Other factors such as intracellular factors Myc, Myb, GATA-1, GATA-2, hypoxia-inducible factor-1α (HIF-1α) and microRNAs (miRNAs) may play a role in the process (2). miRNAs comprise a class of small non-coding RNAs that regulate gene expression by degradation of mRNAs or translational repression. The biosynthesis of miRNAs is a multistep process (3). They are typically transcribed by RNA polymerase II (Pol II) and commonly arise from the introns of coding genes or from intergenic long non-coding RNAs known as primary miRNAs (pri-miRNAs). Pri-miRNAs contain one or more miRNAs within hairpins. These hairpins are usually cleaved from the pri-miRNA transcript in the nucleus by the microprocessor complex, which consists of the RNA-binding protein (RBP) DGCR8 and the RNA endonuclease Drosha. The resulting pre-miRNA hairpins are transported to the cytoplasm where they are further processed into approximately 21-nucleotide long double-stranded RNAs (dsRNAs) by the endonuclease Dicer. Mature single-stranded miRNAs are transferred back into nucleus to form nucleoprotein complex formation-induced silencing complex (miRNP/RNA-induced silencing complex, miRNP/RISC). These structures can bind to the 3′UTR region of target mRNAs and inhibit the translation or initiate the degradation process of target mRNA (4). The degree of pairing between miRNA and target mRNA determines the action mode of the miRNA/RISC-inhibiting target mRNA (5,6).
A wide variety of transcription factors are involved in the establishment of hematopoietic cell lineages. GATA transcription factors are characterized by a conserved dual zinc finger domain. Transcription factor GATA-2 is essential for the early stages of hematopoiesis. Primitive and definitive hematopoiesis is abrogated when the GATA-2 gene is deleted, and it appears to play a role in the proliferation of the early precursors rather than in their differentiation (7,8). GATA-2 is abundantly expressed during embryogenesis and plays an important role in the specification of the hematopoietic lineage during embryogenesis (9). Haematopoietic transcription factor GATA-1 is the founding member of the GATA family transcription factors, which is expressed in primitive and definitive erythroid cells, megakaryocytes, eosinophils, mast cells and the Sertoli cells of the testis (10). GATA-1 is essential for normal erythropoiesis (11,12) and is directly involved in cell survival. It activates transcription of the erythropoietin receptor (EPOR) (13). EPO signaling is essential for the survival of erythroid progenitors (14). Transcription nuclear factor-erythroid 2 (NF-E2) is a target for RUNX1 which is essential for the regulation of erythroid, megakaryocytic maturation and differentiation as well as globin expression (15). RUNX1 and NF-E2 upregulation is not specific for MPNs, but is also seen in polycythemic disorders with enhanced HIF signaling (16). The detected miRNAs are not less consistent because different laboratories selected distinct cell sources. However, miR-223, miR-144, miR-451, miR-17, miR-210 and miR-23R are closely related to the regulation of erythropoiesis and play important roles in erythroid-directed differentiation, proliferation and maturation of red blood cells. Those miRNAs act on the target genes downstream of erythropoiesis-related and regulated development and biological function in red blood cells (2). There are many studies on miRNA regulation during erythroid differentiation. The erythroid-specific transcription factors, such as GATA-1, LMO2, EKLF, and c-kit as miRNA downstream target genes or target proteins coordinate to regulate erythroid differentiation. The GATA-1 and NF-E2 transcription factors also act as upstream factors regulating miRNA199b-5p, miRNA199b-5p-27a and miRNA-24 (17,18).
Previous studies focused on classic gene expression and erythropoiesis regulation. However, to the best of our knowledge, studies on the association between miRNA and erythropoiesis are relatively rare. Even fewer studies are available concerning hypoxic condition. In the present study, we treated K562 cells with HIF-1α lentiviral overexpression vector to identify the mechanism of miRNAs and erythroid transcription factors in K562 cells under hypoxia to expand the theory of erythropoiesis regulation.
Materials and methods
Cell lines and cell culture
The K562 cells lines (frozen by the Qinghai Provincial People's Hospital of Hematology Research) were cultured in RMPI-1640 complete medium (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS) (Sijiqing, Hangzhou, China) and penicillin/streptomycin (Ybiotech, Shanghai, China). The cells were maintained at 37°C with 5% CO2 and the medium was changed every 2–3 days. The cells were divided into 2–3 flasks and cultured sequentially. The cells in the logarithmic growth phase were used for subsequent experiments.
Cell transfection
For transfection with plasmids, logarithmic growth phase K562 (105) cells were introduced into 12-well culture plates (final volume of 1 ml) at 60% confluency and were incubated for 8 h in an incubator (Thermo Fisher Scientific, Waltham, MA, USA) with 5% CO2 prior to transfection with HIF-1α knockout or HIF-1α lentiviral overexpression vector (MOI 10). The transfection reagent lentivirus (Cyanogen, Inc., Seattle, WA, USA) was used according to the manufacturer's instructions. The K562 cells were divided into 3 groups: i) control group with lentiviral negative control; ii) interference group with HIF-1α knockout lentivirus; and iii) overexpression group with HIF-1α lentiviral overexpression vector. Polybrene (Cyanogen, Inc.) was added to the groups (final concentration of 5 µg/ml). Fresh medium was added (final volume 2 ml) after 8 h incubation with 5% CO2. After 72 h, the cells were observed using an inverted fluorescence microscope (Olympus, Tokyo, Japan) and then transferred to 6-well plates. Puromycin (final concentration 1 µg/ml) (Solarbio, Beijing, China) was added after 72 h and 6-well plates were screened for positive cells (19). For hypoxic exposure, the positively transfected cells were placed in an incubator with 5% CO2. The incubator chamber was tightly sealed and thoroughly flushed with 1% O2/5% CO2/balance nitrogen and set at 37 °C. The cells were harvested after 72 h. All the experiments were repeated three times.
RNA extraction, reverse transcription and quantitative PCR
Total RNA was extracted using TRIzol reagent (Ambion, Carlsbad, CA, USA) and was quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The first strand of cDNA was produced using an M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. mRNA was quantified by qPCR using TransScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) with the ABI 7500 Real-Time PCR detection system (Applied Biosystems Life Technologies, Foster City, CA, USA). PCR reactions were performed in triplex tubes, and GAPDH was used as an endogenous control to standardize the amount of the sample mRNA. Using 20 µl of the reaction system, the reaction condition was: Two-Step PCR amplification, pre-denaturing conditions were 95°C for 30 sec; 95°C reaction for 5 sec, 60°C annealing for 31 sec with a total of 40 cycles. The quantification data were analyzed with ABI 7500 software (Applied Biosystems Life Technologies) (20). The primers were synthesized according to the designed sequence by Shenggong (Shanghai, China) and were used for quantitative PCR: HIF-1α forward, 5′-ATACATGGTACCCACGAAGTGTTCCTTTG-3′ and reverse, 5′-ATACATCTCGAGAAAGAGACAAGTCCA-3′; GATA-1 forward, 5′-ATCACAAGATGAATGGGCAGAA-3′ and reverse, 5′-CACAGTGTCGTGGTGGTCGT-3′; GATA-2 forward, 5′ CATCAAGCCCAAGCGAAGA-3′ and reverse, 5′-CACAGGCGTTGCAGACAGG-3′; NF-E2 forward, 5′-TGGGACCATCTTCCTTGTG-3′ and reverse 5′-TTGCCATTGTCATCCTCTTCT-3′; GAPDH forward, 5′-ATCAAGAAGGTGGTGAAGCA-3′ and reverse, 5′-CAAAGGTGGAGGAGTGGGT-3′.
miRNA reverse transcription and PCR
Total RNA was extracted using the TRIzol reagent. For miRNA reverse a TaqMan® MicroRNA Reverse Transcription kit (Applied Biosystems Life Technologies) was used. The RT primer was produced by Applied Biosystems Life Technologies and the corresponding miRNA.
Reaction system was produced using 7 µl master mix I: 100 mM dNTPs with dTTP 0.15 µl, MultiScribe™ Reverse Transciptase 50 U/µl 1.00 µl, 10X reverse transciptase buffer 1.5 µl, RNase inhibitor, 20 U/µl 0.19 µl, nuclease-free water 4.16 µl, 3 µl of 5X RT primer, and 5 µl RNA sample.
Reverse conditions were: 16°C for 30 min, 42°C for 30 min, 85°C for 5 min. The quantitative PCR reaction system (20 µl) was produced using 1.00 µl TaqMan® Small Assay (20X), product from 1.33 µl RT reaction, 10 µl TaqMan Universal PCR master mix II (2X), and 7.67 µl nuclease-free water. The reaction conditions were: Option AmpErase UNG activity 50°C for 2 min, enzyme activation 95°C for 10 min, 40 cycles, denaturation at 95°C for 15 sec, annealing/extension at 60°C for 60 sec.
Protein extraction and western blot assay
The cells were collected and washed twice with cold PBS. Cells (1×106) were added to 1 ml of RIPA lysis buffer (including 10 µl of 10 mg/ml PMSF) (Solarbio). The cell samples were transferred to an Eppendorf tube (Axygen, LA, USA) following incubation on ice for 30 min. Cell lysate supernatant was collected, divided and stored at −20°C. Subsequently, the cells were centrifuged at 2,000 × g for 15 min. Total cell extracts were quantified using the BCA Protein Assay kit (Vigorous, Beijing, China) within Synergy 4 (BioTek, Winooski, VT, USA). Electrophoresis sample buffer (4X) (volume = 1/3 of lysates volume) was added to the same quality of the protein lysates (volume × protein concentration) and placed in a hot water bath for 5 min. Cell extracts were fractionated by electrophoresis on 10% SDS polyacrylamide gels and proteins were transferred to polyvinylidene difluoride membranes (Millipore Corp., Billerica, MA, USA). The membranes were blocked with 5% non-fat dry milk solution for 2 h and incubated with one of the following monoclonal antibodies: anti-HIF-1α (ab75186), anti-GATA-1 (ab76121), anti-GATA-2 (ab109241) and anti-NF-E2 (ab140598) overnight. The antibodies were rabbit mAb to human with a dilution of 1:1000, 1:3000, 1:1000 and 1:1000, respectively. Peroxidase-conjugated AffiniPure goat anti-rabbit IgG (Solarbio) was subsequently added. After washing with TBS-T buffer three times, the membrane was treated with Immobilon™ Western Chemiluminescent HRP Substrate (Beyotime Institute of Biotechnology, Shanghai, China) and exposed to a gel imaging system camera with Image Lab™ software version 2.0 (Bio-Rad, Berkeley, CA, USA).
Statistical analyses
Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Data are presented as the means ± standard deviation (SD) and were analyzed using one-way ANOVA in three groups and Student's t-test (two-tailed). P<0.05 was considered to indicate statistically significant results.
Results
Transfection efficiency of recombinant lentivirus to K562 cells
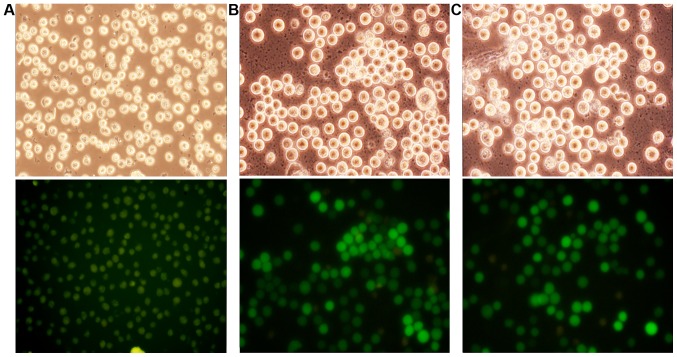
As shown in Fig. 1, the optimal MOI was 10 and >90% of the cells were transfected.
Figure 1.
Transfection efficiency. (A) Negative control group (×100), (B) overexpression group (×100), and (C) interference group (×100). The transfection efficiency of recombinant lentivirus in K562 cells, optimal MOI=10, >90% of the cells were transfected.
Erythroid miRNA levels in the negative, overexpression and interference groups
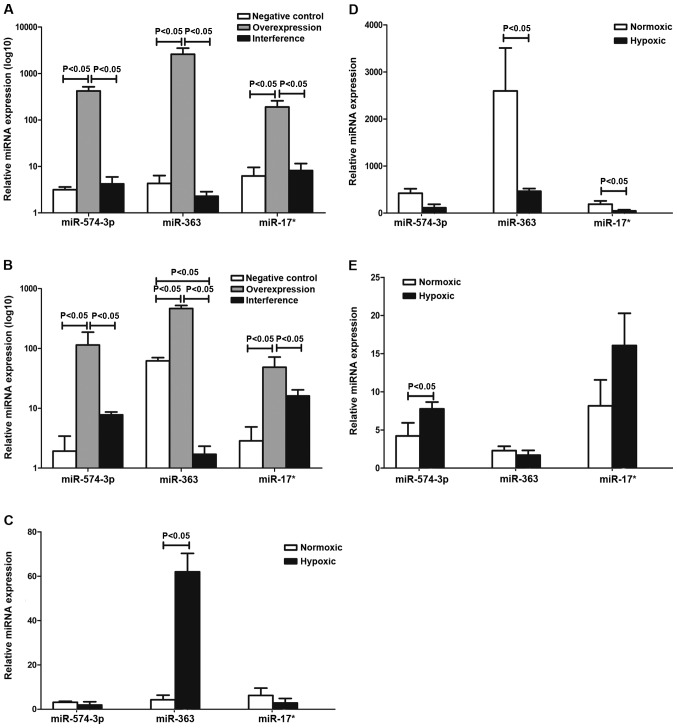
The results in Table I and Fig. 2 show that the expression of miR-17*, miR-363 and miR-574-5p of the negative, overexpression and interference groups were determined by qPCR. The results suggested that miR-363 was involved in the regulation of hematopoiesis via the HIF-1α pathway in K562 cells under hypoxia. miR-17* and miR-574-5p were not entirely dependent on HIF-1α.
Table I.
Erythroid miRNA levels in the negative control, overexpression and interference groups (means ± SD, n=9).
| Group | Normoxic
|
Hypoxic
|
||||
|---|---|---|---|---|---|---|
| miR-574-3p | miR-363 | miR-17a | miR-574-3p | miR-363 | miR-17a | |
| Negative | 3.16±0.46 | 4.31±2.06 | 6.25±3.30 | 1.92±1.50 | 62.00±8.29c | 2.85±2.03 |
| Overexpression | 422.27±95.42a | 2597.27±912.72a | 190.32±68.96a | 114.18±72.64a,c | 465.43±57.29a,c | 48.45±23.20a,c |
| Interference | 4.21±1.73b | 2.29±0.57b | 8.16±3.40b | 7.76±0.90b,c | 1.70±0.62b,b | 16.07±4.22b |
| F | 84.480 | 32.308 | 28.071 | 9.077 | 227.683 | 11.792 |
| P-value | 0.000001 | 0.000078 | 0.000135 | 0.006964 | 0.000001 | 0.003059 |
Compared to the negative control group P<0.05;
compared to the overexpression group P<0.05;
compared to normoxia P<0.05. miRNA, microRNA.
Figure 2.
Erythroid miRNA levels in the negative control, overexpression and interference groups. (A) Quantitative PCR results under normoxia in the three groups. Expression of miR-17*, miR-363 and miR-574-5p in the overexpression group was higher than that in the negative control and interference groups. Differences were statistically significant (P<0.05). (B) Quantitative PCR results under hypoxia in the three groups. The miR-17*, miR-363 and miR-574-5p expression level in the overexpression group was the highest. miR-363 expression in the interference group was less than that in the negative control group. miR-574-5p expression in the interference group was slightly higher than that in the negative group but significantly lower than that in the overexpression group. Differences were statistically significant (P<0.05). (C) miRNA expression in the negative control group under normoxia and hypoxia. miR-363 expression under hypoxia was higher compared to normoxia and the difference was statistically significant (P<0.05). (D) miRNA expression in the overexpression group under normoxia and hypoxia. miR-574-3p, miR-363 and miR-17* expression levels under hypoxia were lower than normoxia. Differences were statistically significant (P<0.05). (E) miRNA expression in the interference group under normoxia and hypoxia. miR-574-3p in hypoxia was higher than that in normoxia. Differences were statistically significant (P<0.05).
Erythroid transcription factors mRNA and protein expression levels
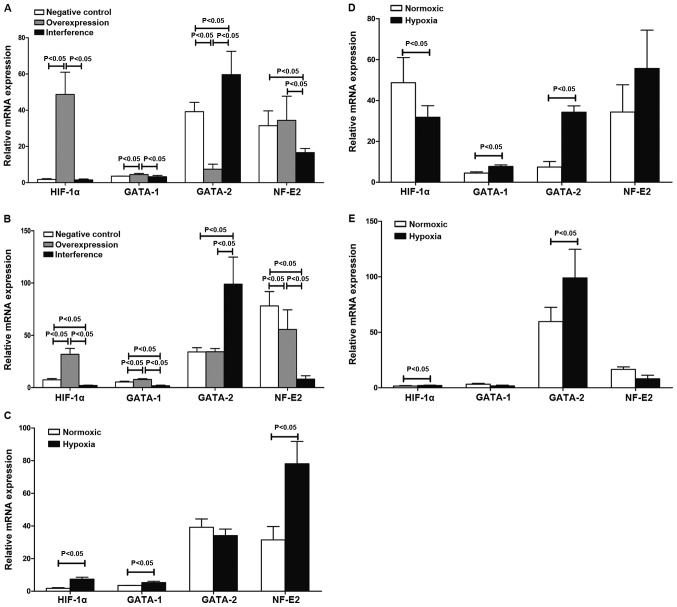
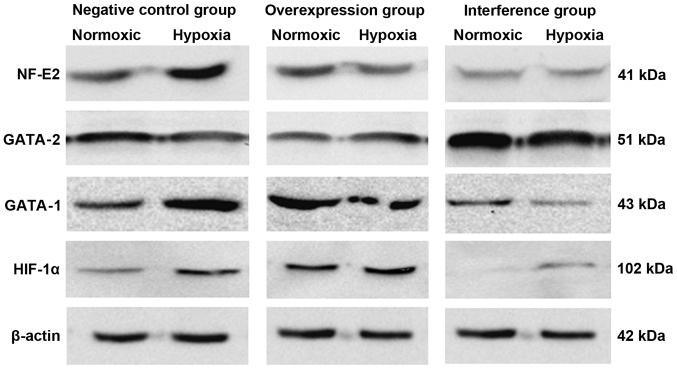
The levels of HIF-1α, GATA-1, GATA-2 and NF-E2 mRNA in the negative, overexpression and interference groups were determined using quantitative PCR. The results in Table II and Fig. 3 show that HIF-1α mediated GATA-1/GATA-2 induction involved in the regulation of hematopoiesis in K562 cells under hypoxia. HIF-1α promoted the expression of NF-E2 mRNA involvement in hematopoietic regulation. The western blot results suggested that the expression of GATA-1, GATA-2 and NF-E2 proteins was substantially consistent with changes in their respective mRNAs (Fig. 4).
Table II.
Erythroid transcription factor miRNA levels in the negative control, overexpression and interference groups (means ± SD, n=9).
| Group | Normoxic
|
Hypoxic
|
||||||
|---|---|---|---|---|---|---|---|---|
| HIF-1α | GATA-1 | GATA-2 | NF-E2 | HIF-1α | GATA-1 | GATA-2 | NF-E2 | |
| Negative | 1.77±0.43 | 3.57±0.05 | 39.20±5.10 | 31.44±8.20 | 7.40±1.21c | 5.33±0.76c | 34.11±4.01 | 78.13±13.68c |
| Overexpression | 48.69±12.28a | 4.48±0.61a | 7.45±2.72a | 34.37±13.33 | 31.79±5.65ac | 7.73±0.76a,c | 34.28±3.05c | 55.62±18.74a |
| Interference | 1.52±0.48b | 3.29±0.73b | 59.60±12.90a,b | 16.56±2.29a,b | 2.06±0.28 a–c | 1.72±0.61a,b | 98.91±25.84a–c | 8.09±3.25a,b |
| F | 58.569 | 5.023 | 41.498 | 4.369 | 90.048 | 71.638 | 24.160 | 27.960 |
| P-value | 0.000007 | 0.037090 | 0.000029 | 0.047209 | 0.000001 | 0.000003 | 0.000241 | 0.000138 |
Compared to the negative control group P<0.05;
compared to the overexpression group P<0.05;
compared to normoxia P<0.05. miRNA, microRNA; HIF-1α, hypoxia-inducible factor-1α; NF-E2, nuclear factor-erythroid 2.
Figure 3.
Erythroid transcription factor mRNA levels in the three groups. (A) Quantitative PCR under normoxia. HIF-1α mRNA levels in the negative control and interference groups were lower than that in the overexpression group while HIF-1α mRNA levels in the interference group was lower than those in the negative control group. GATA-1 mRNA expression in the overexpression group was higher than that in the negative group. GATA-1 mRNA expression in the interference group was lower than that in the overexpression group. GATA-2 mRNA expression in the interference group was higher than that in the negative and overexpression groups. GATA-2 mRNA expression in the overexpression group was lower than that in the interference group. NF-E2-mRNA expression in the interference group was lower than the other groups. Differences were statistically significant (P<0.05). (B) Quantitative PCR under hypoxic condition. HIF-1α mRNA level in the negative control and interference groups was lower than that in the overexpression group. HIF-1α mRNA level in the interference group was lower than that in the negative control group. GATA-1 mRNA in the overexpression group was the highest. GATA-2 mRNA in the interference group was the highest. NF-E2 mRNA level in the interference group was the lowest while NF-E2 mRNA level in the overexpression group was lower than that in the negative control group. Differences were statistically significant (P<0.05). (C) The transcription level of the four transcription factors in the negative control group under normoxia and hypoxia. HIF-1α, GATA-1 and NF-E2 mRNAs in the negative control group under hypoxia were higher than that in normoxia. Differences were statistically significant (P<0.05). (D) The transcription level of the four transcription factors in the overexpression group under normoxia and hypoxia. HIF-1α mRNA level in the overexpression group under hypoxia was lower compared to that under normoxis. GATA-1 and GATA-2 transcription under hypoxia was higher than under normoxia. Differences were statistically significant (P<0.05). (E) Transcription factor mRNA levels in the interference group under normoxia and hypoxia. HIF-1α and GATA-2 mRNA levels under hypoxia were higher than under normoxia. The differences were statistically significant (P<0.05). HIF-1α, hypoxia-inducible factor-1α; NF-E2, nuclear factor-erythroid 2.
Figure 4.
Erythroid transcription factor protein expression levels in the negative control, overexpression and interference groups. Western blot results suggested that the expression of GATA-1, GATA-2 and NF-E2 proteins was substantially consistent with the level of their respective mRNA. NF-E2, nuclear factor-erythroid 2.
Discussion
Hematopoietic differentiation is a process through which HSCs differentiate into progenitor cells of each chain and then divide into a variety of different forms of mature blood cells. Hematopoietic differentiation is an extremely complex regulatory process with regard to the regulation of epigenetic and transcriptional and post-transcriptional, translation and post-translational levels (21). Erythroid differentiation is an important part of hematopoietic differentiation and an important way through which the body can produce mature red blood cells. It is regulated by specific transcription factors that have strong spatial and temporal specificity. For example, the transcription factor GATA-2 has a high expression level in erythroid precursor cells to maintain the characteristics of stem cells in erythroid differentiation. GATA-1 expression gradual enhancement promotes the erythroid progenitor cells into mature red blood cells (22–26). By the end of differentiation, the involvement of EKLF and NF-E2 transcription factors was detected (12). Previous findings also showed that miRNA was a universal mechanism of post-transcriptional gene regulation that controlled precise gene expression (27,28). miRNA was involved in the whole process of erythropoiesis regulation, including the differentiation of erythroid lineage, appreciation of erythroid progenitor cells, terminal differentiation and denucleation of red blood cells (29–35). Some researchers obtained a more comprehensive interaction atlas between human CD34+ HSC miRNA and mRNA by analyzing miRNA expression and binding of the prediction miRNA target gene and corresponding mRNA expression profiles of CD34 HSC in human peripheral blood and bone marrow, respectively (36). Other studies suggest the integration of the miRNA-mRNA analysis (37).
In the present study, K562 cells were transfected with lentiviral-overexpressed and interference HIF-1α gene. Expression of miR-17*, miR-363 and miR-574-5p was determined by quantitative PCR. The results suggested that miR-17*, miR-363 and miR-574-5p expression levels in the overexpression group were higher than those in the negative control and the interference groups under normoxia and hypoxia. miR-363 expression in the interference group was lower than that in the negative control group in hypoxic conditions. The results of the present study showed that miR-363 was involved in the regulation of hematopoiesis via the HIF-1α pathway in K562 cells under hypoxia. We also showed that hsa-miR-17* and hsa-miR-574-5p were not entirely dependent on HIF-1α. Other factors may be involved in the regulation of the abovementioned three miRNAs under hypoxia.
Quantitative PCR and western blot results showed that GATA-1, GATA-2 and NF-E2 transcription level variations correlated well with the expression levels of their respective proteins. Our results showed that GATA-1 and NF-E2 were involved in hematopoiesis regulation via HIF-1α.
On erythroid differentiation regulation in hypoxia, erythroid transcription factor and miRNA have also made great progress. EPO-EPOR signaling and GATA-1 are necessary to generate the normal erythroid cells and regulate the progress of erythroid cell appreciation, differentiation and maturation (38–40). Under hypoxic conditions, the overexpression of GATA-1 promoted the expression of erythroid surface markers CD71 and CD235a by increasing HIF-1α in umbilical cord blood CD34+ and K562 cells (41). Results from prior studies revealed that hematopoietic GATA-1 as an EPOR promoter was involved in the transcriptional regulation of various genes in erythrocytes (13,17). Previous experiments showed that GATA-1 regulated erythroid-related globulin, heme biosynthetic enzymes, membrane protein and erythroid transcription factor genes (42,43). The experimental data suggested that GATA-1 and hsa-miR-363 were regulated by HIF-1α under hypoxia. Nevertheless, the association between GATA-1 and hsa-miR-363 is unclear and in need of further clarification.
Based on the HIF-1α/GATA-l/miR-363/GATA-2 regulatory pathways, important gene expression associated with erythroid differentiation is regulated by the GATA-1 transcription factor and the miRNA of HIF-1α regulation. The regulatory networks of hematopoietic differentiation are more dynamic and complex due to miRNA involvement. There are many studies on the differentiation of GATA-l/GATA-2 regulation through the GATA-1/2 switch (22,23,44). Regulation of erythroid transcription factor and miRNA under hypoxia condition have been the subject of at least one study (45). It was shown that miRNA-17 enhanced expansion and promoted erythroid differentiation via HIF-1α in cord blood CD34+ cells (45). Under hypoxic conditions, miRNA-210 overexpression increased the expression of globin genes and promoted the maturation of erythroids in K562 cells and erythroid progenitor cells in thalassemia. The opposite occurred after the expression of miRNA-210 was suppressed (46,47). Our results suggest that GATAl and miR-363 were involved in the regulation of hematopoiesis via the HIF-1α pathway in K562 cells under hypoxic condition. We found that hsa-miR-17* and hsa-miR-574-5p were not entirely dependent on HIF-1α and there may be more complex regulatory mechanisms involved under hypoxia.
Acknowledgments
The present study was funded by Regional Projects of the National Science Foundation (project code no. 81360084), the Project of 2014 Qinghai Talent 'Little Heights' and the National Key Disciplines (Hematology) in Qinghai Provincial People's Hospital.
References
- 1.Hattangadi SM, Wong P, Zhang L, et al. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118:6258–6268. doi: 10.1182/blood-2011-07-356006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Undi RB, Kandi R, Gutti RK. MicroRNAs as haematopoiesis regulators. Adv Hematol. 2013;2013:695–754. doi: 10.1155/2013/695754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 4.Zhao S, Liu M. The progress of microRNA mechanism and research. China Science C Series. 2009;39:109–113. [Google Scholar]
- 5.Hashimoto K, Otero M, Imagawa K, de Andrés MC, Coico JM, Roach HI, Oreffo RO, Marcu KB, Goldring MB. Regulated transcription of human matrix metalloproteinase 13 (MMP13) and interleukin-1β (IL1B) genes in chondrocytes depends on methylation of specific proximal promoter CpG sites. J Biol Chem. 2013;288:10061–10072. doi: 10.1074/jbc.M112.421156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai L. Epigenetic frontier (M) Beijing. Tsinghua University Press; 2012. pp. 133–142. 2012. [Google Scholar]
- 7.Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 8.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636–3643. [PubMed] [Google Scholar]
- 9.Vicente C, Conchillo A, García-Sánchez MA, Odero MD. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol Hematol. 2012;82:1–17. doi: 10.1016/j.critrevonc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira R, Ohneda K, Yamamoto M, Philipsen S. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol. 2005;25:1215–1227. doi: 10.1128/MCB.25.4.1215-1227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D'Agati V, Orkin SH, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 12.Pevny L, Lin CS, D'Agati V, Simon MC, Orkin SH, Costantini F. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 1995;121:163–172. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 13.Zon LI, Youssoufian H, Mather C, Lodish HF, Orkin SH. Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc Natl Acad Sci USA. 1991;88:10638–10641. doi: 10.1073/pnas.88.23.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacombe C, Mayeux P. The molecular biology of erythropoietin. Nephrol Dial Transplant. 1999;14(Suppl 2):22–28. doi: 10.1093/ndt/14.suppl_2.22. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, Li X, Deng C, Ney PA, Huang S, Bungert J. USF and NF-E2 cooperate to regulate the recruitment and activity of RNA polymerase II in the beta-globin gene locus. J Biol Chem. 2010;285:15894–15905. doi: 10.1074/jbc.M109.098376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapralova K, Lanikova L, Lorenzo F, Song J, Horvathova M, Divoky V, Prchal JT. RUNX1 and NF-E2 upregulation is not specific for MPNs, but is seen in polycythemic disorders with augmented HIF signaling. Blood. 2014;123:391–394. doi: 10.1182/blood-2013-10-534222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Bai H, Zhang Z, Li W, Dong L, Wei X, Ma Y, Zhang J, Yu J, Sun G, et al. The up-regulation of miR-199b-5p in erythroid differentiation is associated with GATA-1 and NF-E2. Mol Cells. 2014;37:213–219. doi: 10.14348/molcells.2014.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Zhu Y, Guo L, Dong L, Liu H, Yin H, Zhang Z, Li Y, Liu C, Ma Y, et al. A regulatory circuit comprising GATA1/2 switch and microRNA-27a/24 promotes erythropoiesis. Nucleic Acids Res. 2014;42:442–457. doi: 10.1093/nar/gkt848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Iyer N, Huettner JE, Sakiyama-Elbert SE. A puromycin selectable cell line for the enrichment of mouse embryonic stem cell-derived V3 interneurons. Stem Cell Res Ther. 2015;6:220. doi: 10.1186/s13287-015-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118:6258–6268. doi: 10.1182/blood-2011-07-356006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doré LC, Chlon TM, Brown CD, White KP, Crispino JD. Chromatin occupancy analysis reveals genome-wide GATA factor switching during hematopoiesis. Blood. 2012;119:3724–3733. doi: 10.1182/blood-2011-09-380634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. GATA switches as developmental drivers. J Biol Chem. 2010;285:31087–31093. doi: 10.1074/jbc.R110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grass JA, Boyer ME, Pal S, Wu J, Weiss MJ, Bresnick EH. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc Natl Acad Sci USA. 2003;100:8811–8816. doi: 10.1073/pnas.1432147100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martowicz ML, Grass JA, Boyer ME, Guend H, Bresnick EH. Dynamic GATA factor interplay at a multicomponent regulatory region of the GATA-2 locus. J Biol Chem. 2005;280:1724–1732. doi: 10.1074/jbc.M406038200. [DOI] [PubMed] [Google Scholar]
- 26.Grass JA, Jing H, Kim SI, Martowicz ML, Pal S, Blobel GA, Bresnick EH. Distinct functions of dispersed GATA factor complexes at an endogenous gene locus. Mol Cell Biol. 2006;26:7056–7067. doi: 10.1128/MCB.01033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 28.Lee RC, Feinbaum RL, Ambros V. The C. elegans hetero chronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Huang Z, Xue H, Jin C, Ju XL, Han JD, Chen YG. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. 2008;111:588–595. doi: 10.1182/blood-2007-05-092718. [DOI] [PubMed] [Google Scholar]
- 30.Fu YF, Du TT, Dong M, Zhu KY, Jing CB, Zhang Y, Wang L, Fan HB, Chen Y, Jin Y, et al. Mir-144 selectively regulates embryonic alpha-hemoglobin synthesis during primitive erythropoiesis. Blood. 2009;113:1340–1349. doi: 10.1182/blood-2008-08-174854. [DOI] [PubMed] [Google Scholar]
- 31.Patrick DM, Zhang CC, Tao Y, Yao H, Qi X, Schwartz RJ, Jun-Shen Huang L, Olson EN. Defective erythroid differentiation in miR-451 mutant mice mediated by 14-3-3zeta. Genes Dev. 2010;24:1614–1619. doi: 10.1101/gad.1942810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen KD, Simmini S, Abreu-Goodger C, Bartonicek N, Di Giacomo M, Bilbao-Cortes D, Horos R, Von Lindern M, Enright AJ, O'Carroll D. The miR-144/451 locus is required for erythroid homeostasis. J Exp Med. 2010;207:1351–1358. doi: 10.1084/jem.20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu D, dos Santos CO, Zhao G, Jiang J, Amigo JD, Khandros E, Dore LC, Yao Y, D'Souza J, Zhang Z, et al. miR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes Dev. 2010;24:1620–1633. doi: 10.1101/gad.1942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sankaran VG, Menne TF, Šćepanović D, Vergilio JA, Ji P, Kim J, Thiru P, Orkin SH, Lander ES, Lodish HF. MicroRNA-15a and -16-1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13. Proc Natl Acad Sci USA. 2011;108:1519–1524. doi: 10.1073/pnas.1018384108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Flygare J, Wong P, Lim B, Lodish HF. miR-191 regulates mouse erythroblast enucleation by down-regulating Riok3 and Mxi1. Genes Dev. 2011;25:119–124. doi: 10.1101/gad.1998711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgantas RW, III, Hildreth R, Morisot S, Alder J, Liu CG, Heimfeld S, Calin GA, Croce CM, Civin CI. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci USA. 2007;104:2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norfo R, Zini R, Pennucci V, Bianchi E, Salati S, Guglielmelli P, Bogani C, Fanelli T, Mannarelli C, Rosti V, et al. Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators: miRNA-mRNA integrative analysis in primary myelofibrosis CD34+ cells: role of miR-155/JARID2 axis in abnormal megakaryopoiesis. Blood. 2014;124:e21–e32. doi: 10.1182/blood-2013-12-544197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crispino JD, Lodish MB, MacKay JP, Orkin SH. Use of altered specificity mutants to probe a specific protein-protein interaction in differentiation: the GATA-1:FOG complex. Mol Cell. 1999;3:219–228. doi: 10.1016/S1097-2765(00)80312-3. [DOI] [PubMed] [Google Scholar]
- 39.Jelkmann W. Molecular biology of erythropoietin. Intern Med. 2004;43:649–659. doi: 10.2169/internalmedicine.43.649. [DOI] [PubMed] [Google Scholar]
- 40.Fried W. Erythropoietin and erythropoiesis. Exp Hematol. 2009;37:1007–1015. doi: 10.1016/j.exphem.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Zhang FL, Shen GM, Liu XL, Wang F, Zhao YZ, Zhang JW. Hypoxia-inducible factor 1-mediated human GATA1 induction promotes erythroid differentiation under hypoxic conditions. J Cell Mol Med. 2012;16:1889–1899. doi: 10.1111/j.1582-4934.2011.01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss MJ, Orkin SH. GATA transcription factors: key regulators of hematopoiesis. Exp Hematol. 1995;23:99–107. [PubMed] [Google Scholar]
- 43.Orkin SH. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80:575–581. [PubMed] [Google Scholar]
- 44.Moriguchi T, Yamamoto M. Network regulation of Gata1 and Gata2 gene-dynamics underlies erythroid differentiation. Rinsho Ketsueki. 2014;55:633–642. In Japanese. [PubMed] [Google Scholar]
- 45.Yang Y, Ma W, Wu D, Huang Y, Li H, Zou J, Zhang Y, Feng M, Luo J. MiR-17 partly promotes hematopoietic cell expansion through augmenting HIF-1α in osteoblasts. PLoS One. 2013;8:e70232. doi: 10.1371/journal.pone.0070232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bianchi N, Zuccato C, Lampronti I, Borgatti M, Gambari R. Expression of miR-210 during erythroid differentiation and induction of gamma-globin gene expression. BMB Rep. 2009;42:493–499. doi: 10.5483/BMBRep.2009.42.8.493. [DOI] [PubMed] [Google Scholar]
- 47.Fabbri E, Manicardi A, Tedeschi T, Sforza S, Bianchi N, Brognara E, Finotti A, Breveglieri G, Borgatti M, Corradini R, et al. Modulation of the biological activity of microRNA-210 with peptide nucleic acids (PNAs) Chem Med Chem. 2011;6:2192–2202. doi: 10.1002/cmdc.201100270. [DOI] [PubMed] [Google Scholar]