In a typical day in Korle-Bu Teaching Hospital, Ghana, (and in most teaching and referral hospitals in Sub-Saharan Africa), physicians are often confronted with challenging cases not based on medical diagnosis but on the ability to offer appropriate medical care. Here, we describe a case that typifies the ethical dilemma facing nephrologists in low resource countries. Ama (name changed for privacy), age 15, developed oedema a few months prior to presentation. At admission, she had significant pulmonary congestion and was diagnosed with end stage renal disease (ESRD) documented by ultrasound with small kidneys bilaterally. She received urgent in-hospital haemodialysis for pulmonary oedema but her family struggled to raise the $50 USD needed for each dialysis session and could not cope with the burden of life-long therapy with dialysis or kidney transplant. Ama was abandoned by her family and spent her last months hospitalized, receiving dialysis only when her pulmonary oedema was severe. Acute dialysis was paid through a small hospital fund; however, this could not support long-term dialysis. Ama died in the hospital nine months after diagnosis. Sadly, this is often the fate of a large number of individuals in Sub-Saharan Africa who develop ESRD and even more worrisome is the rising burden of kidney disease throughout Africa without additional healthcare resources. During a one-month period in the Renal ward at Korle-Bu Teaching Hospital, 29 patients with ESRD were admitted but only 18 (62.1%) could afford dialysis. The situation is no different across the continent, for example, at University hospitals in Nairobi, Kenya and Ibadan, Nigeria, fewer than half the patients can afford dialysis. The situation is even worse in Ethiopia where chronic kidney disease (CKD) and ESRD patients are managed only in private hospitals (Table 1).
Table 1.
Estimates of prevalence of kidney disease in 4 countries participating in H3 Africa Kidney Disease Network and world development indicators
| Country | Population (million) |
Medical admissions for CKD per month** |
ESRD treated with dialysis per month** |
Estimated Prevalence of CKD † |
Life expectancy at Birth (years)‡ |
Gross national income (GNI) per capita‡ |
Gross Domestic Product (GDP) |
Health expenditure per capita (% GDP) ‡ |
|---|---|---|---|---|---|---|---|---|
| Ethiopia * |
97.0 | - | - | - | 64 | $550 | 54.8 | 3.8 |
| Ghana | 26.8 | 17.2% (42/244) |
62.1 % (18/29) |
17.0% | 61 | $1600 | 38.7 | 5.4 |
| Kenya | 44.9. | 13.9 % (25/180) |
60 % (15/25) |
4% | 62 | $1,290 | 60.9 | 4.5 |
| Nigeria | 177.5 | 15.2 % (32/211) |
69.2% (18/26) |
17.6% | 52 | $2970 | 568.5 | 3.5 |
No hospital data available for Addis Ababa University in Ethiopia
Medical admissions per month was obtained from data collected during March and April 2015 in Korle-Bu Teaching hospital, Ghana, and the average admission per month on the medical ward over a year in Kenyatta National Hospital, Nairobi, Kenya; and University College Hospital, Ibadan, Nigeria; The proportion of medical admissions for CKD per month was calculated with total number of CKD patients admitted by total admissions on the medical ward; The proportion of ESRD on dialysis per month was calculated from the total number of ESRD admissions within the calendar month who required dialysis and could afford it.
The prevalence data on CKD for each country was obtained from a systematic review by Stanifer et al (2)
All data on development indicators are from worldbank.org (2014) except health expenditure per capita were obtained from http://www.who.int/countries (2013)
Non-communicable diseases, such as cardiovascular disease, diabetes, hypertension and respiratory disease, are increasingly common in Africa and contribute substantially to morbidity and mortality (1). The burden of CKD is often forgotten, despite being a common complication of hypertension, diabetes and HIV infection. CKD affects an estimated 13·9% (95% CI 12·2–15·7) of adults in Sub-Saharan Africa but precise estimates are lacking (2). If only 5–10% of patients with CKD reach ESRD, this will lead to a continent-wide health care burden, which cannot be handled by the current government healthcare systems. Kidney disease imposes incalculable human suffering and a catastrophic economic burden on the African continent in several respects. The age of onset of ESRD is approximately 20 years younger in African populations compared to developed Western countries (40–45 years vs. 63 years). Africa is also experiencing an accelerated increased incidence of hypertension and type 2 diabetes mellitus. Current dialysis treatment or transplant options in Sub-Saharan Africa are severely limited because of inadequate healthcare resources. Given the limited health expenditure across the continent, none of the 54 countries in Sub-Saharan Africa will be able to afford the medical costs associated with predialysis CKD care or renal replacement therapy (3, 4). Even more out of reach is the annual cost of dialysis treatment, approximately $10,000 to $20,000 USD per person each year in Sub-Saharan Africa. This means that most children and adults will only receive palliative care or limited dialysis treatments of 1–2 per week (5). These distressing facts elevate the urgent need for research to mitigate the incidence and severity of kidney disease for the Sub-Saharan African population. Scientific research in Africa has primarily focused on infectious diseases with recently emerging clinical trial networks and innovations in diagnostic testing. In contrast, noncommunicable diseases, specifically kidney diseases, have not been systematically screened for among general populations in Sub-Saharan countries. Although CKD and the nephrotic syndrome are reportedly common in Africa, little is known about the prevalence of disease or possible genetic contributions to these disorders.
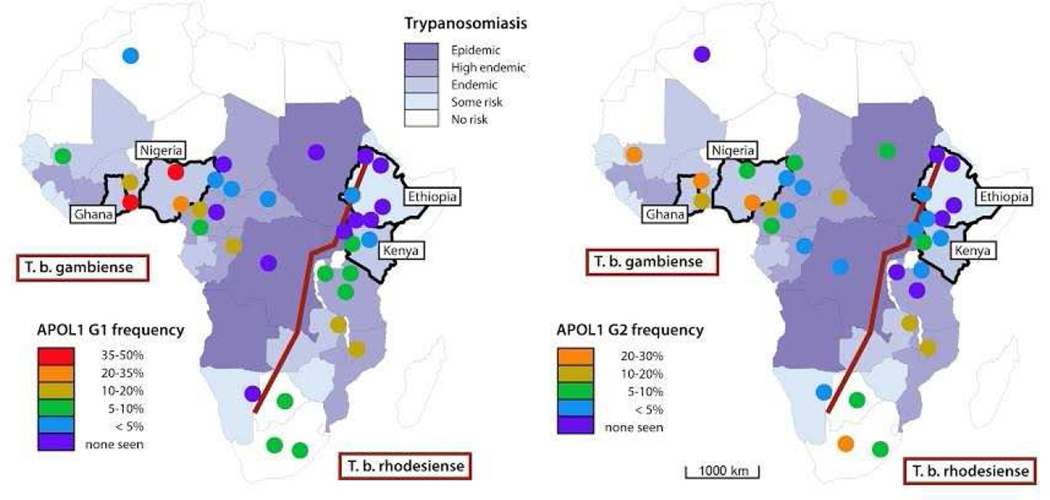
The recent discovery of a major genetic risk factor among African Americans highlights the potential genetic contribution to kidney disease especially among those of West African ancestry. Persons who are homozygous or compound heterozygous for the apolipoprotein L1 (APOL1) G1 or G2 alleles have about a two to three times increased risk of CKD compared to low-risk carriers across the spectrum of kidney diseases including glomerular disease, human immunodeficiency virus (HIV) associated nephropathy, sickle cell disease and non-diabetic CKD (6). Persons harboring one or two copies of the APOL1 risk alleles are also protected against some species of trypanosomal infection (African sleeping sickness), suggesting that selection pressures from an infectious agent lead to a high prevalence of high-risk alleles. The story is more complicated, though, as the trypanosomal infections from Trypanosoma brucei gambiense species in West Africa are not protected by APOL1 high-risk carrier status, unlike Trypanosoma brucei rhodesiense which is more prevalent in East Africa where APOL1 high-risk GI and G2 haplotype prevalence is very low (Figure 1). It is unclear whether sub-Saharan Africans share the same pernicious proclivity seen in African Americans for CKD based on genetic risk and the contribution of environmental factors such as infection, hypertension, diabetes and cardiovascular disease.
Figure 1.
Map of Africa showing the distribution of APOL1 risk alleles and of Trypanosoma brucei subspecies.
The Human Hereditary and Health in Africa (H3 Africa) consortium was established in June 2010 to facilitate a contemporary research approach to the study of genomics and environmental determinants of common diseases with the goal of improving the health of Africans (7). The consortium, funded by both the U.S. National Institutes of Health (NIH) and Wellcome Trust (United Kingdom), supports studies throughout Sub-Saharan Africa. The H3Africa Kidney Disease Research Network (H3AKDRN) is a collaborative research network involving ten institutions in five African countries with an overall goal to increase the capacity for study of kidney disease, and specifically address genetic risk for kidney disease in Sub-Saharan Africa. Conducting studies in Africa is important as the populations harbor the greatest genetic diversity among mankind and could lead to novel genetic discoveries. Additionally, understanding selection across the continent will provide insights into possible migrations, infectious exposures, or admixture of populations.
The design of the H3 Africa Kidney Disease Study is a multinational case-control approach with approximately 4,000 participants with the specified kidney disease diagnoses who meet the inclusion criteria to be enrolled over 36 months. Approximately 4,000 control participants are also being recruited for cross-sectional assessment. Cases will be followed longitudinally to understand risk factors for progression. The network consists of two coordinating centers in Accra, Ghana and Michigan, US, nine clinical sites which enroll participants from university teaching hospitals located in four African countries (Ethiopia, Ghana, Kenya and Nigeria), and one bioinformatics core (South Africa). The overall aims of the studies include: (1) perform comprehensive phenotyping of 8000 patients and controls and 50 families from four African countries (Ethiopia, Ghana, Kenya, and Nigeria); (2) train clinical research personnel and genomic investigators for Africa based genomic research; (3) establish genomic research laboratories in West Africa using sustainable, low-capital intensity laboratory technologies; and (4) conduct international level quality genetic and translational research projects in CKD with more detailed methods previously described (8)
We are conducting comprehensive baseline evaluation of clinical characteristics, risk factors, treatment and outcomes of the kidney disease cohorts with specific clinically defined diseases such as hypertension, HIV, diabetes, sickle cell disease and glomerular disorders. There are a few studies in Africa that have evaluated the genetic components of kidney disease occurrence but none has studied the genetic components of kidney disease progression (9). The identification of the genetic determinants for kidney disease progression phenotype is of great significance in Sub-Saharan Africa as the onset of ESRD portends imminent demise for the majority who progress. Therefore, we will leverage the case-control study for future longitudinal cohort and translational studies of kidney disease. Genotyping will use a new array platform to be developed among the H3Africa Consortium to cover rare variants among those of African ancestry. Genetic analyses will conduct both unbiased and hypothesis driven approaches when studying each specific cause of kidney disease and CKD progression. We will study the association of APOL1 with subtypes of kidney diseases using a case-control design and the association with CKD progression using a prospective cohort design. We will also conduct genetic discovery using the entire group.
An essential component of the activities of the Kidney Disease Network is the development of scientific capacity within the continent to undertake further independent, large-scale research into genetic and environmental factors underlying the substantial disease burden on the continent that is attributable to kidney disease. Rapid advances and the decreasing costs of sequencing technology have resulted in growing engagement with human health genetics and genomics research on the African continent. Key areas for capacity development include development of research skills, laboratory facilities to enable genetic analysis and bioinformatics capacity. Development of this infrastructure and expertise will empower African investigators to independently analyze and store clinical, genomic and transcriptomic data within the continent. These aims are in keeping with the principles underpinning the H3Africa initiative (7). To date, five doctors have completed a 2-year training program on Clinical Research Methods and Biostatistics, five biomedical scientists are training in laboratory genomics, one MPhil student is training in biochemistry, three scientists are training in bioinformatics and two research administrators are training in NIH program management.
There are social and ethical implications for conducting a study of this magnitude across four countries in nine different cities with many logistical, political and cultural differences. The pressures on the economies in Sub-Saharan Africa limits the ability to afford chronic dialysis care. Studying kidney disease addresses access to costly therapy such as dialysis or transplantation and highlights the burden of disease to legislative bodies in each country. Additionally, collaboration with local community leaders will also facilitate further change and education. The overall project has significant merit for training clinicians and scientists throughout Africa and advancing scientific questions needed to address the burden of chronic disease across West and East Africa. The international collaborative project provides the infrastructure, network and support to continue to build and innovate in clinical research.
The goal of the H3 Africa Kidney Disease Research Network is to leave a footprint on the continent to study kidney disease with the ambitious goals of improving the care of patients and ultimately saving lives.
Acknowledgments
Sources of Support: National Institute of Health, National Human Genome Research Institute
We are grateful to the participants of the H3Africa Kidney Disease Research Network who have agreed to participate in the study and to the research teams that make this study possible. The H3Kidney Disease Research Network and investigators are funded under a cooperative agreement from the National Human Genome Research Institute (1U54HG006939-01). (http://h3africa.org/). This project is also in part funded with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We would like to thank the participants, families and research staff for taking part in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Alleyne G, Binagwaho A, Haines A, Jahan S, Nugent R, Rojhani A, et al. Embedding non-communicable diseases in the post-2015 development agenda. Lancet. 2013;381(9866):566–574. doi: 10.1016/S0140-6736(12)61806-6. [DOI] [PubMed] [Google Scholar]
- 2.Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(3):e174–e181. doi: 10.1016/S2214-109X(14)70002-6. [DOI] [PubMed] [Google Scholar]
- 3.Naicker S. Burden of end-stage renal disease in sub-Saharan Africa. Clin Nephrol. 2010;74(Suppl 1):S13–S16. doi: 10.5414/cnp74s013. [DOI] [PubMed] [Google Scholar]
- 4.Arogundade FA, Barsoum RS. CKD prevention in Sub-Saharan Africa: a call for governmental, nongovernmental, and community support. Am J Kidney Dis. 2008;51(3):515–523. doi: 10.1053/j.ajkd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Ulasi II, Ijoma CK. The enormity of chronic kidney disease in Nigeria: the situation in a teaching hospital in South-East Nigeria. Journal of tropical medicine. 2010;2010:501957. doi: 10.1155/2010/501957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotimi C, Abayomi A, Abimiku A, Adabayeri VM, Adebamowo C, Adebiyi E, et al. Research capacity. Enabling the genomic revolution in Africa. Science. 2014;344(6190):1346–1348. doi: 10.1126/science.1251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osafo C, Raji YR, Burke D, Tayo BO, Tiffin N, Moxey-Mims MM, et al. Human Heredity and Health (H3) in Africa Kidney Disease Research Network: A Focus on Methods in Sub-Saharan Africa. Clin J Am Soc Nephrol. 2015 doi: 10.2215/CJN.11951214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulasi II, Tzur S, Wasser WG, Shemer R, Kruzel E, Feigin E, et al. High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract. 2013;123(1–2):123–128. doi: 10.1159/000353223. [DOI] [PubMed] [Google Scholar]