Abstract
Prenatal opioid agonist therapy with methadone or buprenorphine prevents maternal illicit opioid use and withdrawal and improves pregnancy outcomes compared to heroin use alone. Historically, methadone has been the first-line opioid agonist therapy for pregnant opioid dependent women; in recent years buprenorphine has become first-line treatment for some opioid dependent pregnant women. While there is some evidence of better outcomes in neonates exposed to buprenorphine vs. methadone, the effect of confounding from differences in women who use buprenorphine and methadone has not been carefully examined in most studies. This review explores mechanisms by which confounding can arise in measuring associations between prenatal buprenorphine vs. methadone exposure on neonatal outcomes using a graphical approach, directed acyclic graphs. The goal of this paper is to facilitate better understanding of the factors influencing neonatal abstinence syndrome and accurate assessment of the comparative safety of opioid agonist therapies on the neonate.
Keywords: Confounding, Buprenorphine, Methadone, Pregnancy, Neonatal abstinence syndrome
Background
There has been a striking increase in rates of opioid use in pregnant women and in neonatal abstinence syndrome (NAS) in their infants in the United States and Canada [1,2]. NAS is a drug withdrawal syndrome that most commonly manifests from in utero opioid exposure and affects the neonate’s postnatal life adaptation in critical areas of sleep, feeding, and autonomic function [3,4]. NAS incidence rose from 1.20 to 3.39 per 1,000 American live-births from 2000 to 2009 [5], and from 0.28 to 4.29 per 1000 Canadian live-births from 1992 to 2011[2].Total hospital charges for NAS grew from $190 to $720 million United States during this period [5]. Significant increases in the rate of neonatal intensive care unit admissions for NAS, the median length of neonatal hospitalization for NAS, and neonatal receipt of pharmacotherapy for NAS in the Unites States from 2004 to 2014 were recently observed [1].
Studies have attributed this increase in NAS to rising rates of opioid addiction in pregnant women [1,2].Some cohort studies and randomized controlled trials (RCTs) have observed decreased NAS severity [6–9], lower risk of NAS treatment [10], and higher gestational age at birth [8], birth weight [8,11], body length [11] and head circumference [11,12] in buprenorphine vs. methadone exposed neonates. Evidence, however, is subject to bias from study drop out in RCTs and by confounding in cohort studies [8–17]. A meta-analysis showed that confounding may account for some of the protective effect of buprenorphine vs. methadone on NAS severity, and that limited data on confounding of the comparative safety of buprenorphine vs. methadone on NAS are available [18].
In epidemiologic studies that aim to assess the effect of treatment A vs. treatment B on the risk of illness, a central problem is the need to consider extraneous factors that might explain, partially or totally, the association (or lack of) between treatment and the risk of illness [19]. When the extraneous factor affects both the choice of treatment (i.e., is an indication for treatment) and the risk of the illness, this is called confounding by indication. With respect to the effect of buprenorphine vs. methadone on NAS, the central problem of confounding by indication has not been carefully considered in many studies [18]. Confounding by indication is a concern because buprenorphine and methadone are not only pharmacologically different, but in the United States, each treatment is delivered differently. Buprenorphine generally is provided in an office-based setting where women fill a prescription and take buprenorphine on their own, while methadone is given through observed daily dosing at a methadone clinic. As a result, pregnant women treated with methadone (vs. buprenorphine) tend to have poorer patient profiles including a longer duration of opioid dependence, a higher prevalence of hepatitis B infection, and more severe addiction [8,10,14–16]. And of course, women with poorer clinical profiles are more likely to have neonates with worse NAS and birth outcomes than women with better clinical profiles. Consequently, when the regression model is unadjusted for an important confounder – such as an important difference in clinical profile—the estimated measure of effect (i.e., risk ratio, mean difference) is a mix of the effect of prenatal opioid agonist exposure on NAS and the confounder effect on NAS. Confounding can attenuate, increase, or reverse the true effect of prenatal opioid exposure to buprenorphine vs. methadone on neonatal outcomes. The impact of confounding depends on the strength of the relationships between the confounder and the exposure, the confounder and the outcome, and the prevalence of the confounder.
A clear understanding of potential confounders is essential to designing a study that will minimize confounding in the study’s estimated measure of effect of the exposure on the outcome. We undertook the present review paper to explore how confounding might arise in the use of buprenorphine vs. methadone in pregnancy to inform study design, and ultimately to improve study evidence.
Approach
We previously conducted a systematic review and meta-analysis of the association of prenatal buprenorphine vs. methadone exposure on the neonate using the published literature through October 2013 [18]; 14 published comparative studies were identified. For the present article the computerized search of PubMed, the Cochrane Central Register of Controlled Trials and the Cochrane Database using the key words buprenorphine, methadone, opioid agonist therapy, pregnancy, infant, and neonatal abstinence syndrome was updated through October 2015. Variables considered as confounders in the identified articles were abstracted and used to guide our discussion.
We used diagrams known as directed acyclic graphs (DAGs) [20] to illustrate the relationships between potential confounders, prenatal opioid agonist therapy, and NAS severity. DAGs link these different variables (i.e., confounders, treatments, outcomes) by arrows that represent direct causal effects (protective or increasing risk) of one variable on another. DAGs are acyclic because the arrows never point from a given variable to any other variable in its past (i.e., causes precede their effects). The absence of an arrow between two variables indicates there is no proposed direct effect of one variable on the other. We build upon previous publications in which investigators used DAGs [21] to show how confounding might arise in assessing the comparative safety of buprenorphine vs. methadone on the neonate under several proposed scenarios. We considered NAS severity as our outcome for the purpose of illustration.
Results
Our updated search identified 16 published studies of prenatal buprenorphine vs. methadone exposure on the neonate. Fifteen of the studies used the Subutex formulation of buprenorphine, which contains only buprenorphine and is preferred in pregnancy [7]; one study used the Suboxone formulation of buprenorphine, which contains naloxone and may have potential harm in pregnancy [22–24]. Of the 16 studies, five (31%) adjusted for one or more variable that the investigators had identified as a potential confounder (Table 1). As shown, a variety of factors were considered. The proposed confounders can be broadly classified as maternal characteristics measured prior to the initiation of opioid agonist therapy (i.e., age, years of opioid dependence, other concomitant medication use at enrollment, number of prenatal visits at enrollment, cigarette use at enrollment, other drug use in the month before pregnancy confirmation) and maternal and neonatal characteristics measured after the initiation of opioid agonist therapy (i.e., heroin and other drug use late in pregnancy or at delivery, gestational age at birth). Variables from these two broad groups are included in our DAGs for further discussion of confounding; we selected years of opioid dependence as our potential measured confounder. Some studies also adjusted for the dose of buprenorphine or methadone at delivery and the number of days of buprenorphine or methadone at delivery. These variables, however, are alternate classifications of prenatal exposure and not confounders.
Table 1.
Covariates considered as potential confounders in 5 of 16 identified studies of the comparative safety of prenatal buprenorphine vs. methadone exposure on the neonate.
| Study | Participants | Location | Years of Birth | Covariates |
|---|---|---|---|---|
| Kakko, et al. [15] | Prospective (2001–2006) and retrospective (1982–2006) cohort of 83 mother-neonate pairs at an antenatal clinic | Stockholm, Sweden | 2001 to 2006 buprenorphine exposed, 1982 to 2006 methadone exposed | Maternal age |
| Jones, et al. [7] | Randomized controlled trial of 175 mother- neonate pairs | Six sites in the US, Canada and Austria | 2005 to 2008 | Number of days of buprenorphine or methadone exposure, cigarette use at enrollment, selective serotonin reuptake inhibitor use at enrollment, percent of urine tests positive for cocaine in pregnancy, number of prenatal visits at enrollment, gestational age at delivery |
| Lacroix, et al. [10] | Prospective multisite cohort of 135 mother-neonate pairs (125 resulted in live births) | France | 1998 to 2006 | Heroin use late in pregnancy |
| Welle Strand, et al. [11] | Cohort of 139 mother-neonate pairs in opioid maintenance treatment | Norway | 1996 to 2009 | Maternal age, years of opioid dependence before treatment, dose of buprenorphine or methadone at delivery, other drug use (opiates, benzodiazepines, amphetamines, cannabis) one month before pregnancy confirmation, cigarette use and other drug use one month before delivery |
| Wiegand, et al. [25] | Retrospective cohort of 62 mother-neonate pairs | Chapel Hill, North Carolina, US | 2001 to 2013 | Gestational age at birth, maternal indication for opioids |
The DAG in Figure 1 is the simplest representation of a confounder relationship. Here, as shown by the directions of the arrows, years of opioid dependence is causally related to the choice of opioid agonist therapy with buprenorphine or methadone and to the severity of NAS. There is some evidence to suggest that duration of opioid dependence is predictive of treatment outcome in opioid dependent adults [26,27], and it is plausible that duration of dependence could also affect choice of treatment and NAS. If the DAG depicts the correct relationships, and assuming, as shown, there are no unmeasured confounders, adjusting for the duration of opioid dependence prior to treatment initiation will remove the confounding effect of years of opioid dependence from the estimated association of buprenorphine vs. methadone on NAS severity. The same would hold true for the other variables that that were associated with both the choice of prenatal opioid agonist therapy and NAS severity (e.g., prenatal selective serotonin reuptake inhibitors). Most published studies, however, have been unadjusted for such potential confounders (Table 1).
Figure 1.
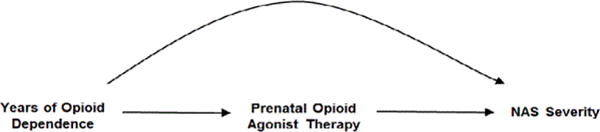
Possible relationship between a measured confounder (years of opioid dependence), prenatal opioid agonist therapy and neonatal abstinence syndrome (NAS) severity.
The DAG in Figure 2 expands on the scenario of Figure 1 and includes relapse to heroin use as an effect of prenatal agonist therapy exposure and a cause of NAS severity. While we cannot be certain this DAG depicts the correct relationship, there are some data to support these relationships. As previously discussed, fewer pregnant women treated with buprenorphine vs. methadone had opioids detected late in pregnancy [18] and NAS severity may be lower in buprenorphine exposed neonates although the results may be subject to confounding. As well, RCTs show different treatment retention rates in adults and pregnant women randomized to certain doses of methadone vs. buprenorphine [7,28]. If the DAG does depict correct relationships, adjusting for heroin use will remove part of the effect of the agonist therapy on the neonate. The total effect of prenatal buprenorphine or methadone treatment on NAS includes the pharmacologic effect of therapy on NAS in addition to any effect of illicit drugs following initiation of buprenorphine or methadone. Failure of agonist therapy to prevent heroin relapse is indeed part of the effect of agonist therapy on the neonate. Further, because heroin use is an effect of the agonist therapy and not a cause of it, it cannot be a confounder and does not need to be adjusted for.
Figure 2.
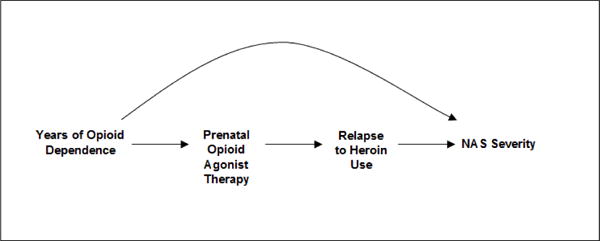
Possible relationship between a measured confounder (years of opioid dependence), prenatal opioid agonist therapy, relapse to heroin use and neonatal abstinence syndrome (NAS) severity.
In perinatal research of in utero exposures on birth outcomes, there is a natural tendency to want to adjust for gestational age at birth [29]. Returning to Figure 2, the relationship with gestational age can be envisioned by replacing heroin relapse with gestational age at birth. While data suggest that preterm infants are less likely to be treated for NAS, it remains unclear whether this is due to NAS severity or physiological immaturity [30,31] and whether opioid agonist therapy is causally related to preterm birth [7,18]. If the DAG is correct, the arguments above negating the need to adjust for a consequence of exposure also apply to gestational age at birth. If we assume that prenatal opioid agonist therapy itself is not causally related to gestational age at birth and it is duration of opioid dependence that plays a causal role, we can instead draw the DAG in Figure 3 to depict the relationships. In this scenario, gestational age at birth does not need to be adjusted for because it is not causally related to prenatal opioid agonist therapy. Indeed, adjustment for duration of opioid dependence would remove the effect of gestational age at birth on the estimated association between prenatal opioid agonist therapy and NAS severity.
Figure 3.
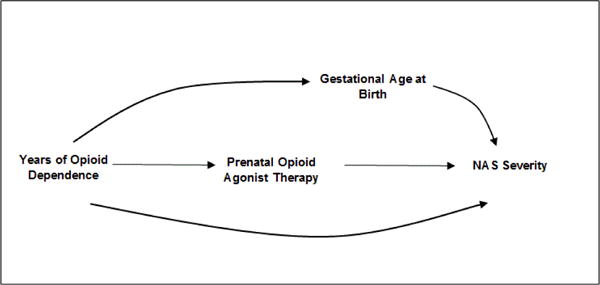
Possible relationship between a measured confounder (years of opioid dependence), prenatal opioid agonist therapy, gestational age at birth and neonatal abstinence syndrome (NAS) severity.
The DAG in Figure 4 expands on Figure 3. Here, duration of prenatal agonist therapy is causally related to gestational age at birth in addition to the covariate, risky behaviour. As drawn, there is no causal relationship between prenatal opioid agonist therapy and risky behaviour. Assuming the DAG depicts the correct relationships among the covariates, there is no need to adjust for risky behaviour or gestational age at birth. Neither variable is a predictor of prenatal opioid agonist therapy and therefore cannot cause confounding. In fact, adjusting for gestational age is likely to bias the estimated direct causal effect of prenatal agonist therapy on NAS severity. In this scenario, gestational at birth is a variable called a collider [20]. As such, adjustment for gestational age at birth creates an association between NAS severity and prenatal agonist therapy exposure through risky behaviour. This will bias the assessment of the effect of prenatal opioid agonist therapy on NAS.
Figure 4.
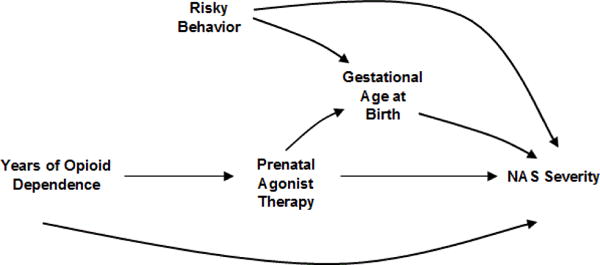
Possible relationship between a measured confounder (years of opioid dependence), prenatal opioid agonist therapy, risky behaviour, gestational age at birth and neonatal abstinence syndrome (NAS) severity.
Discussion
Appropriate adjustment for confounders is necessary to estimate the true causal effects of opioid agonist therapies on infant outcomes, and to carefully guide pregnant women and providers in their treatment choices. Yet, few epidemiologic studies of the comparative safety of buprenorphine vs. methadone on the neonate have adjusted for confounding. Further, characteristics of the exposure, such as duration of agonist therapy use, which are not confounders, were adjusted for in some studies. We illustrate the use of causal diagrams to provide a conceptual framework to evaluate confounding, and to demonstrate that confounders are correctly conceptualized as a common cause of the choice of prenatal agonist therapy and of NAS severity.
In the published studies that have considered confounders, some variables that have been adjusted for cannot be confounders because they do not meet the criteria of being antecedent to both treatment and NAS. For example, gestational age at birth is not a confounder of the effect of prenatal exposure to buprenorphine vs. methadone on NAS outcomes. Adjusting for gestational age, which is potentially affected by prenatal opioid agonist therapy, can introduce selection bias and distort the estimated measure of association between agonist therapy and NAS. Similar comments extend to heroin relapse following treatment initiation. An extreme example of the harm of adjusting for birth weight is the birth weight paradox, where it was suggested that maternal smoking had a beneficial effect on mortality among low birth weight infants [32]. It was subsequently shown through the use of causal diagrams that this apparent paradox could be conceptualized as selection bias due to stratification on a variable (birth weight) that was affected by the exposure of interest (smoking) and that shared common causes with the outcome (infant mortality) [21]. Another less extreme example is a study that observed higher neonatal withdrawal rates in methadone vs. buprenorphine exposed babies, which did not persist after adjusting for heroin use late in pregnancy [10]. Here, heroin relapse cannot be a confounder because it is not an antecedent to treatment; rather it may be in the causal pathway (i.e., an intermediate, Figure 2). Assuming heroin relapse is an intermediate, the implications of this finding could be that the increased risk of NAS from methadone vs. buprenorphine exposure was primarily through a lower effect of methadone vs. buprenorphine on prenatal heroin use. The underlying causal scenario for the exposure and outcome in a study thus needs to be specified for the clinical implications to accurately follow.
A limitation of our work is that our examples may not correctly depict the causal relationships among the exposure, covariates, and outcome. We did, however, use the existing literature to develop relationships between variables. Importantly, the DAGs serve to illustrate the concept of confounding in studying the comparative effect of prenatal opioid agonist therapy exposure on NAS. Specification of the assumptions regarding the causal structure is a prerequisite for the analytical approach and essential to guide clinical practice. Additional research is needed to delineate the relationships between prenatal opioid agonist therapy exposure, other covariates and NAS outcomes.
In sum, researchers are encouraged to use their expert knowledge and available data to propose scenarios that show how prenatal opioid agonist therapy with buprenorphine or methadone, confounders and NAS outcomes are causally related [21]. Adjustment for characteristics that determine the choice of prenatal opioid agonist therapy treatment and are associated with NAS (e.g., smoking, concomitant medications and comorbidities) is necessary to adjust for confounding. This pertains to observational studies, including case-control and cohort designs, and to RCTs; although RCTs are designed to balance confounders among treatment groups, high rates of study drop out have been reported in this population [7]. Adjusting for factors that are potentially affected by prenatal opioid agonist therapy (e.g., heroin use near delivery, gestational age) is not justified to estimate the total effect and can introduce selection bias. The magnitude of the bias and the interpretation depends on each specific study and should be considered in interpreting study evidence. More accurate quantification of the risks associated with prenatal treatment for opioid dependence is essential to guide health care providers and pregnant women in their treatment choices, and to improve the health of this rapidly growing population.
Acknowledgments
This publication was supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development under grant number 1R21HD081271-01 REVISED (S. Brogly). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372:2118–2126. doi: 10.1056/NEJMsa1500439. [DOI] [PubMed] [Google Scholar]
- 2.Turner S, Gomes T, Camacho X. Neonatal Withdrawal and Antenal Opioid Prescribing. Can Med Assoc J Open. 2015;3:E55–E61. doi: 10.9778/cmajo.20140065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudak M, Tan R. Committee on Drugs, Committee on the Fetus and the Newborn. Neonatal drug withdrawal. Pediatr. 2012;129:540–560. [Google Scholar]
- 4.Hayes MJ, Brown MS. Epidemic of prescription opiate abuse and neonatal abstinence. JAMA. 2012;307:1974–1975. doi: 10.1001/jama.2012.4526. [DOI] [PubMed] [Google Scholar]
- 5.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, et al. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307:1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 6.Wachman EM, Newby PK, Vreeland J, Byun J, Bonganzi A, et al. The relationship between maternal opioid agonists and psychiatric medications on length of hospitalization for neonatal abstinence syndrome. J Addict Med. 2011;5:293–299. doi: 10.1097/ADM.0b013e3182266a3a. [DOI] [PubMed] [Google Scholar]
- 7.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metz V, Jagsch R, Ebner N, Würzl J, Pribasnig A, et al. Impact of treatment approach on maternal and neonatal outcome in pregnant opioid-maintained women. Hum Psychopharmacol. 2011;26:412–421. doi: 10.1002/hup.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones HE, Johnson RE, Jasinski DR. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Lacroix I, Berrebi A, Garipuy D, Schmitt L, Hammou Y, et al. Buprenorphine versus methadone in pregnant opioid-dependent women: a prospective multicenter study. Eur J Clin Pharmacol. 2011;67:1053–1059. doi: 10.1007/s00228-011-1049-9. [DOI] [PubMed] [Google Scholar]
- 11.Welle-Strand GK, Skurtveit S, Jones HE, Waal H, Bakstad B, et al. Neonatal outcomes following in utero exposure to methadone or buprenorphine: a National Cohort Study of opioid-agonist treatment of Pregnant Women in Norway from 1996 to 2009. Drug Alcohol Depend. 2013;127:200–206. doi: 10.1016/j.drugalcdep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Pritham UA, Paul JA, Hayes MJ. Opioid dependency in pregnancy and length of stay for neonatal abstinence syndrome. J Obstet Gynecol Neonatal Nurs. 2012;41:180–190. doi: 10.1111/j.1552-6909.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101:275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 14.Lejeune C, Simmat-Durand L, Gourarier L, Aubisson S, Groupe d’Etudes Grossesse et Addictions (GEGA) Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenophine substitution. Drug Alcohol Depend. 2006;82:250–257. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96:69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Bakstad B, Sarfi M, Welle Strand GK, Ravndal E. Opioid maintenance treatment during pregnancy: occurrence and severity of neonatal abstinence syndrome. A national prospective study. Eur Addict Res. 2009;15:128–134. doi: 10.1159/000210042. [DOI] [PubMed] [Google Scholar]
- 17.Binder T, Vavrinkova B. Prospective randomised comparative study of the effect of buprenorphine, methadone and heroin on the course of pregnancy, birthweight of newborns, early postpartum adaptation and course of the neonatal abstinence syndrome (NAS) in women followed up in the outpatient department. Neuro Endocrinol Lett. 2008;29:80–86. [PubMed] [Google Scholar]
- 18.Brogly SB, Saia KA, Walley AY, Du HM, Sebastiani P. Prenatal buprenorphine versus methadone exposure and neonatal outcomes: systematic review and meta-analysis. Am J Epidemiol. 2014;180:673–686. doi: 10.1093/aje/kwu190. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen OS, Cook EF. Confounding: essence and detection. Am J Epidemiol. 1981;114:593–603. doi: 10.1093/oxfordjournals.aje.a113225. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 21.Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164:1115–1120. doi: 10.1093/aje/kwj275. [DOI] [PubMed] [Google Scholar]
- 22.Jurand A. The interference of naloxone hydrochloride in the teratogenic activity of opiates. Teratology. 1985;31:235–240. doi: 10.1002/tera.1420310208. [DOI] [PubMed] [Google Scholar]
- 23.Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, et al. Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. J Neurosci. 2005;25:5117–5126. doi: 10.1523/JNEUROSCI.0866-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas AJ, Meddle SL, Toschi N, Bosch OJ, Neumann ID. Reduced activity of the noradrenergic system in the paraventricular nucleus at the end of pregnancy: implications for stress hyporesponsiveness. J Neuroendocrinol. 2005;17:40–48. doi: 10.1111/j.1365-2826.2005.01272.x. [DOI] [PubMed] [Google Scholar]
- 25.Wiegand SL, Stringer EM, Stuebe AM, Jones H, Seashore C, et al. Buprenorphine and naloxone compared with methadone treatment in pregnancy. Obstet Gynecol. 2015;125:363–368. doi: 10.1097/AOG.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 26.Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13–20. doi: 10.1151/spp021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- 28.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–1068. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruwanpathirana R, Abdel-Latif ME, Burns L, Chen J, Craig F, et al. Prematurity reduces the severity and need for treatment of neonatal abstinence syndrome. Acta Paediatr. 2015;104:e188–194. doi: 10.1111/apa.12910. [DOI] [PubMed] [Google Scholar]
- 31.Dysart K, Hsieh HC, Kaltenbach K, Greenspan JS. Sequela of preterm versus term infants born to mothers on a methadone maintenance program: differential course of neonatal abstinence syndrome. J Perinat Med. 2007;35:344–346. doi: 10.1515/JPM.2007.063. [DOI] [PubMed] [Google Scholar]
- 32.Yerushalmy J. The relationship of parents’ cigarette smoking to outcome of pregnancy–implications as to the problem of inferring causation from observed associations. Am J Epidemiol. 1971;93:443–456. doi: 10.1093/oxfordjournals.aje.a121278. [DOI] [PubMed] [Google Scholar]


