Abstract
Osteoblast differentiation of mesenchymal stem cells is regulated by both soluble factor (e.g., bone morphogenetic proteins (BMP)) and mechanically transduced signaling, but the mechanisms have only been partially elucidated. In this study, physical association of BMP Receptor I (BMPRI) with integrin β1 sub-unit (Iβ1) was hypothesized to mediate osteoblast differentiation of rat bone marrow-derived mesenchymal stem cells (MSCs) on bone-like substrates. The effects of substrate modulus on osteoblast differentiation of MSCs were investigated for 2D poly(ester urethane) films with moduli varying from 5 – 266 MPa, which spans the range from collagen fibrils to trabecular bone. SMAD1/5 and p44/42 MAPK signaling, expression of markers of osteoblast differentiation, and matrix mineralization increased with increasing substrate modulus. The effects of substrate modulus on osteoblast differentiation were mediated by Iβ1, which was also expressed at higher levels on increasingly rigid substrates. Förster resonance energy transfer (FRET) and immunoprecipitation (IP) experiments showed that physical association of Iβ1 and BMP Receptor I (BMRPRI) increased with substrate modulus, resulting in activation of the BMP signaling pathway. Thus, these studies showed that integrin and BMP signaling converge to regulate osteoblast differentiation of MSCs, which may potentially guide the design of scaffolds and rhBMP-2 delivery systems for bone regeneration.
Graphical Abstract
Introduction
The design of biomaterials for treatment of bone defects resulting from trauma or disease has attracted considerable attention due to the high incidence of complications for severe bone injuries1. Autogenous bone is the current standard of care, but is limited by availability and donor site morbidity2. Because of their tunable chemical and physical properties3, polymer scaffolds have been extensively investigated for treatment of bone defects. In addition to providing a temporary scaffold for growth of new bone, these biomaterials can also be augmented with biological cues to enhance the host tissue response and improve healing4, 5. Cell therapy has also been considered a promising approach for bone regeneration due to the potential of self-renewal and enhancement of osteoblast differentiation, especially for patients who experience fracture nonunion and metabolic bone diseases, such as osteogenesis imperfecta and hypophosphatasia6, 7. However, without specific biological signals, the delivered mesenchymal stem cells do not substantially improve bone repair by direct differentiation to osteoblasts. While the transplanted regenerative cells can enhance bone repair by the recruitment and differentiation of tissue-specific stem cells through secretion of soluble factors, the secreted soluble factors must be in sufficient amounts to function at the wound site, which is a concern due to possible migration of MSCs to other anatomical sites8.
Bone morphogenetic proteins (BMPs) are a subfamily of transforming growth factor beta (TGF-β) that are closely associated with the growth, maturation, and regulation of bone tissues9. BMP signaling is required for endochondral ossification, maintenance of the adult skeleton, and bone regeneration, and up-regulation of the BMP signaling pathway is essential for bone regeneration and osteogenesis 1. Among BMPs, previous studies have shown that BMP2 plays an essential role in bone regeneration10. Local delivery of recombinant human BMP-2 from a bovine collagen sponge carrier has been approved by the US Food and Drug Administration (FDA) for clinical application11. However, multiple adverse events, complications, and concerns associated with rhBMP2 usage have also been reported recently8, 12. Consequently, improving the safety of rhBMP2 treatment by reducing the dose of rhBMP2 is emerging as an important area of investigation.
Osteoblasts express α5β1 integrin, a cell surface receptor for fibronectin, and Integin α5 sub-unit is required for osteoblast differentiation of MSCs13. Thus, osteoblast differentiation is also regulated by mechanically transduced signaling, but the precise mechanisms have only been partially elucidated. Previous studies investigating the cellular response to substrate modulus have utilized substrates with moduli less than 100 MPa14, and only a limited number of studies have investigated the effects of modulus on osteogenic differentiation using rigid substrates approximating trabecular bone (100 – 400 MPa15). Expression of the osteogenic transcription factor Runx2 and the osteoblast marker Alkaline Phosphatase (ALP) increased with 2D substrate rigidity when MC3T3-E1 pre-osteoblasts were cultured on PEG-diacrylate hydrogels (0.6 MPa) or tissue culture polystyrene (2000 MPa)16. RhoA activity increased on stiffer substrates, thereby promoting increased cellular contractility through ROCK. A later study reported that for 2D acrylate films with moduli ranging from 5–850 MPa, the composition of the polymer had a more significant effect on osteoblast differentiation than substrate modulus, prompting the authors to challenge the notion that cells can sense rigidity in the range of trabecular bone15. We recently reported that osteoblast differentiation and mineralization of rat MSCs cultured on 3D scaffolds increased with decreasing pore size and increasing substrate modulus over the range 5 – 300 MPa17, but the mechanism was not investigated.
To initiate the activation of RhoA/ROCK signaling, integrin-mediated cell-matrix interactions generate an adhesion molecule-integrin-actomyosin complex that can be shifted between inactive and signaling states by activation of myosin II or matrix rigidity18. Cells have been reported to sense matrix rigidity based on whether a critical force is generated by displacing the matrix a distance of 100 – 150 nm19–21. However, it has been suggested that cells interacting with matrices with substrate moduli >100 kPa are in a state of isometric contraction22 and cannot displace the matrix. Thus, the previously reported correlations of MSC proliferation and differentiation with substrate modulus over ranges comparable to mineralized bone (100 – 400 MPa) cannot be explained by uniform displacements of the matrix. These observations raise further questions regarding the mechanisms by which matrix rigidity regulates osteoblast differentiation of MSCs.
These previous studies suggest that integrin-mediated signaling regulates osteoblast differentiation on bone-like substrates by mechanisms other than the uniform displacements of the matrix conventionally associated with mechanotransduction. Elucidating this mechanism is anticipated to guide the design of scaffolds and delivery systems for osteobiologics. A previous study has reported that physical association of integrin β3 sub-unit (Iβ3) and TGF-β Receptor type II (TGF-β RII) drives expression of bone-metastatic genes by tumor cells on bone-like substrates through stimulation of MAP Kinases (MAPKs)23. However, the effects of substrate modulus on the association of integrins with soluble factor (e.g., BMP) receptors in MSCs has not been investigated. In the present study, we hypothesized that on bone-like substrates, BMP Receptor I (BMPRI) physically associates with Iβ1, which has been associated with increased migration of MSCs through FAK and p44/42 MAPK24, to enhance osteoblast differentiation of MSCs. We used a 2D poly(ester urethane) (PUR) film monoculture system to design substrates with moduli ranging from that of collagen fibrils (20 MPa25) to trabecular bone (100 – 400 MPa15). Rat bone marrow-derived MSCs were cultured on 2D films, and expression of genes associated with osteoblast differentiation and matrix mineralization were assessed. Integrin β1 sub-unit and BMP pathways were inhibited or stimulated to investigate how crosstalk between solid-state and soluble factor signaling regulates osteoblast differentiation of rat bone marrow-derived MSCs.
Experimental
Materials
DMEM (1.0 g/L glucose = 1.0 g/L) and fetal bovine serum (FBS) were purchased from Thermo Scientific. Penicillin/streptomycin (P/S), trypsin EDTA, and Amphotericin B were obtained from Corning Cellgro. Glycolide and D,L-lactide were purchased from Polysciences (Warrington, PA). Hexamethylene diisocyanate trimer (HDIt) was supplied by Bayer Material Science (Pittsburgh, PA). Iron acetylacetonate (FeAA) catalyst and Dorsomorphin was supplied by Sigma-Aldrich. ε-caprolactone (Sigma-Aldrich) was dried over anhydrous MgSO4, and all other materials were used as received.
Fabrication and characterization of 2D PUR films
Polyester triols (300 g/mol, 450 g/mol, 720 g/mol or 3000 g/mol) were synthesized from a glycerol starter and a backbone comprising ε-caprolactone, glycolide, and D,L-lactide as described previously26. Poly(ester urethane) (PUR) films were synthesized by reaction of HDIt with a hardener component comprising the polyester triol and iron catalyst (5% iron acetylacetonate (FeAA) in dipropylene glycol). The reactants were poured into tissue culture plates immediately after mixing and cured at 60°C overnight. The bulk moduli of the 2D films were measured under compression by MTS as described previously23.
Cell culture and osteoblast induction
All animal studies were performed in compliance with the Vanderbilt University Institutional Animal Care and Use Committee (IACUC) and the National Institutes of Health. Primary rat bone marrow-derived mesenchymal stem cells (MSCs) were generated from pooled bone marrow from femurs of Sprague-Dawley rats (n=4). MSCs were maintained in DMEM with 10% FBS, 1% P/S, and 0.1% Amphotericin B (Sigma). Cells were detached at sub-confluency by trypsin EDTA (0.25%) and re-suspended at 2 × 105/mL in complete medium and cultured on 2D PUR films. To facilitate cell attachment, PUR films were incubated in fibronectin solution (4 μg/ml) in a cell culture incubator for 24 h prior to seeding23. Measurements of cell area were performed by immunofluorescence staining23. Cells were cultured on 5- or 266-MPa films for 48 h, fixed in 10% formaldehyde, blocked in 3% BSA for 1 h, and labeled with an anti-Focal Adhesion Kinase (FAK) antibody (1:800, Cell Signaling) overnight at 4°C. FAK was imaged using an Alexa Fluor® 546-tagged secondary antibody. Cell (bright-field) and FAK areas were measured by microscopy (40 and 100X) using ImageJ analysis software. For conditioned medium experiments, MSCs were cultured on 5- or 266-MPa films for 24 h, at which time the medium was harvested and transferred to MSCs cultured on tissue culture plates. For osteogenic differentiation experiments, cells were cultured in complete medium in 12-well plates until confluent and then changed to osteoinductive (Os+) medium to stimulate osteoblast differentiation (10 nM dexamethasone, 50 μg/ml ascorbic acid, and 0.1 mM β-glycerophosphate) with or without rhBMP-2. Cells were detached by trypsin EDTA (0.25%) at D4, D7, and D14, and total RNA was isolated from the harvested cell pellets by RNeasy mini Kit (Qiagen). cDNA synthesis was carried out from purified total RNA using iScript™ Reverse Transcription Supermix (Biorad). RT-PCR amplified for osteogenesis genes were measured to compare the differentiation of osteoblast. The primer used for RT-PCR amplification was listed in Table 1.
Table 1.
Primer sequence of osteoblast genes
| Gene | Forward 5′ to 3′ | Reverse 5′ to 3′ |
|---|---|---|
| Runx2 | TCCAGACCAGCAGCACTCC | GTTATGAAAAACCAAGTAGCCAGGT |
| Osx | GGAGGTTTCACTCCATTCCA | TAGAAGGAGCAGGGGACAGA |
| Opn | AGTGGTTTGCCTTTGCCTGTT | TCAGCCAAGTGGCTACAGCAT |
| IB1 | GAGAGAGATTACTTCAGAC | AGCAGTCGTGTTACATTC |
| Alp | AAGGACATCGCATATCAG | TCTCATCCAGTTCGTATTC |
| GAPDH | GACTTCAACAGCAACTCC | GCCATATTCATTGTCATACCA |
| BMPR1A | GGCCATTGCTTTGCCATTATAG | CTTTCGGTGAATCCTTGCATTG |
Western Blotting
PUR films with varying substrate moduli were synthesized in 6-well tissue culture plates as described above and incubated in fibronectin solution (4 μg/ml) overnight to facilitate cell attachment. Rat bone marrow-derived MSCs were plated on PUR films and cultured with or without osteogenic induction. Cells were detached by trypsin EDTA (0.25%) and total protein extracted from cell pellets by RIPA buffer (Thermo Scientific) containing protease inhibitors and phosphatase inhibitors (Thermo Scientific) on ice for 15 min. Protein concentration was measured by Pierce BCA Protein Assay Kit (Thermo Scientific). Cell lysates were then centrifuged at full speed for 15 min to remove cellular debris at 4°C. Equal amounts of total protein were loaded onto SDS-PAGE gels, separated by Bio-rad 2-D Electrophoresis units, and transferred to PVDF membranes. The membrane was blocked in LI-COR preformed blocking buffer for 1h at room temperature and then incubated with primary antibodies in blocking buffer at 4°C with gentle shaking overnight. Proper HRP-conjugated secondary antibodies were then applied to the membranes after harshly wash and signals were detected by Western Lightning Chemiluminescent (PerkinElmer). Primary and secondary antibodies utilized in this study is listed in Table 2
Table 2.
Antibodies applied
| Antibody | Species | Supplier |
|---|---|---|
| pMAPK | Rabbit | Cell signaling |
| MAPK | Rabbit | Cell signaling |
| pSMAD1,5 | Rabbit | Cell signaling |
| SMAD1,5 | Rabbit | Santa cruz |
| pFAK | Rabbit | Cell signaling |
| FAK | Rabbit | Cell signaling |
| ITGB1 | Rabbit | Santa cruz |
| GAPDH | Goat | Santa cruz |
| BMPR1 | Rabbit | Santa cruz |
| Anti-rabbit | Goat | Santa cruz |
| Anti-goat | Donkey | Santa cruz |
Immunoprecipitation
Cells were cultured and treated as described above. A Pierce Crosslink IP Kit was utilized to conduct the immunoprecipitation process and eliminate interference of IgG from the precipitation antibody23. Immunoprecipitation was performed according to the instructions provided by the kit. Briefly, harvested cells were lysed with the mild lysis buffer (provided by the kit) at low temperature, and equal amounts of total proteins were diluted into equal volumes. The same small proportion were taken out as loading controls and the rest were incubated with BMPR1 antibody-coated beads to precipitate BMPRI and any other cell components that were physically associated with the receptor. Beads without antibody coating were run as a negative control. The precipitated proteins were detached using the mild elution buffer, denatured and loaded onto SDS-PAGE gels, separated by Bio-rad 2-D Electrophoresis units, and transferred to PVDF membranes. Detection of proteins bound to BMPRI was performed as described above for Western blotting.
Nuclear fractionation
Cells were plated and cultured on PUR films for designated times and then detached by trypsin/EDTA, and cell number for each sample was counted and recorded. An NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Scientific) was utilized for nuclear fractionation following the kit’s manual. Briefly, cell pellets were first lysed by Cytoplasmic Extraction Reagent to collect cytoplasmic proteins, and then lysed by Nuclear Extraction Reagent to recover the nucleus protein compartment. After fractionation, protein concentrations for each sample were measured by BCA protein kit, and Western blotting was performed as described above.
Förster resonance energy transfer (FRET)
To investigate the association of BMPR1 and Iβ1, we performed Förster Resonance Energy Transfer (FRET)23, 27. Briefly, the donor antibody (anti Iβ1 (SantaCruz)) was labeled with Alexa Fluor® 488 Carboxylic Acid, Succinimidyl Ester (Life Technologies) (Iβ1-488) and the acceptor antibody (anti TGF-β RII (SantaCruz)) was labeled with Alexa Fluor® 546 Carboxylic Acid, Succinimidyl Ester (Life Technologies) (BMPRI-546) in a 2.25:1 molar ratio of antibody:dye overnight at 4°C. Labelled antibody was purified with size exclusion chromatography using PD-10 Desalting Columns (GE Healthcare). Rat MSCs were plated on pre-soaked compliant (5 MPa) and rigid (266 MPa) PUR films. After 24 hours of culture, cells were fixed with 10% Formaldehyde in PBS and stained overnight at 4° with Iβ1-488 and Iβ1-488 + BMPRI-546 (1 ug/1×106 cells). FRET experiments were performed on a BioTek Synergy 2 plate reader using excitation filter 485/20 and emission filter 530/35.
Iβ1 Transduction and knockdown by siRNA treatment
The amplified IB1 coding sequence was cloned and transferred into the pCMV3-C-FLAG vector by in vitro recombination using Lipofectamine 3000 (Life Technology) following the vendor’s instructions28. An siRNA encoding sequence for rat cells were purchased from Santa Cruz and Lipofectamine RNAiMAX was applied for knockdown treatment23.
Statistics
For the study investigating the effects of rhBMP-2 dose and substrate modulus on osteogenic differentiation, statistical significance between experimental groups was determined by a two-factor ANOVA. A one-factor ANOVA or Student’s t test was used to determine statistical significance for all other studies. Unless otherwise noted, graphs show mean ± standard error of the mean (SEM), and p ≤ 0.05 was considered statistically significant.
Results
Osteoblast differentiation of rat bone marrow-derived mesenchymal stem cells (MSCs) correlates with substrate modulus on 2D bone-like substrates
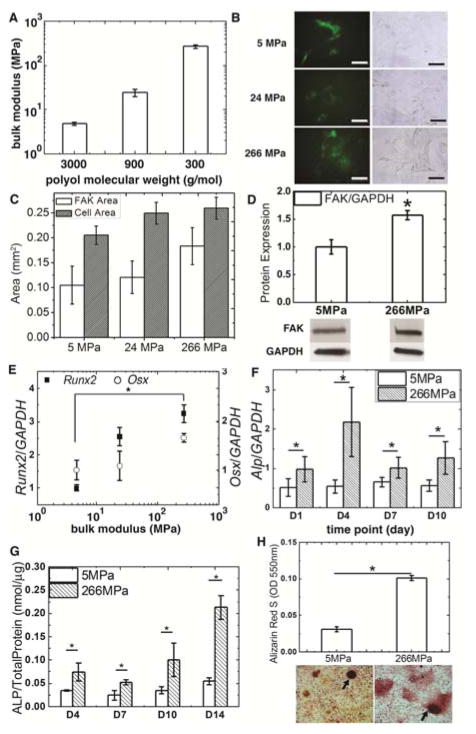
PUR films were cast in tissue culture plates as substrates for MSC culture. We varied the molecular weight of the polyester triol component from 3000 to 300 g mol−1 to synthesize films with moduli ranging from 5 – 266 MPa (Fig. 1A) while maintaining a relatively constant contact angle of 60 – 65° 17. Rat bone marrow-derived MSCs were plated on PUR films, which were pre-incubated in a solution of fibronectin (4 ug/mL) to facilitate cell attachment. Osteogenic (Os+) medium was added to MSC culture after the cells reached confluence in complete cell culture medium. Cell area increased with substrate modulus, but the effects were not statistically significant (Fig. 1B–C). However, expression of FAK assessed by Western blot was significantly higher on the rigid substrates (Fig. 1D). Gene expression of the transcription factors Runx2 and Osx was evaluated 3 days after addition of Os+ medium. Expression of Runx2 and Osx increased monotonically with the substrate modulus of the PUR film (Fig. 1E). Since Runx229 and Osx30 are essential for osteogenic differentiation and bone formation, the dose-response data in Fig. 1E suggest that the substrate regulates differentiation of MSCs to osteoblasts as the modulus increases from 5 MPa (comparable to collagen25) to 266 MPa (comparable to trabecular bone15). Consequently, we performed subsequent studies using the compliant, collagen-like (5 MPa) and rigid, bone-like (266 MPa) PUR films to investigate the mechanisms by which osteogenic differentiation is enhanced on bone-like substrates. Both Alp gene expression (Fig. 1F) and ALP activity (Fig. 1G) increased significantly on the rigid films up to D14. After osteogenic induction for 21 days, cells cultured on the films were fixed and stained with Alizarin Red S. Increased mineral deposition was observed on the rigid films and the mineral nodules were significantly larger (Fig. 1H). The dye was then washed from the substrates and the light absorbance of the resulting solution read at 550 nm. Significantly higher absorbance was measured for the rigid substrates (Fig. 1H), which shows increased mineralization of mature osteoblasts.
Figure 1.
Osteoblast differentiation of rat bone marrow-derived MSCs increased with substrate modulus. (A) Substrate modulus of 2D PUR films increased with decreasing polyol molecular weight (error bars denote standard deviation). (B) FAK (left) and bright-field (right) images of MSCs cultured on PUR films. (C) Cell area and (D) FAK protein expression increased with substrate modulus. (E) Expression of Runx2 and Osx increased with substrate rigidity. (F) Alp gene expression and (G) ALP enzyme activity increased with substrate rigidity from D4 to D10. (H) Alizarin S staining of mineral nodules (black arrows) formed by MSCs on PUR films at D21 showed increased mineralization on rigid films. * p < 0.05.
p44/42 MAPK and SMAD1/5 mediate osteoblast differentiation of MSCs induced by bone-like substrates
The effects of substrate modulus on osteoblast differentiation of MSCs requires the activity of p44/42 MAPK for moduli ranging from 0.001 – 0.1 MPa16, 31, 32. Thus, we investigated the same kinase signaling pathway to see if MSCs were able to respond to substrate modulus in Os+ medium through the same signaling cascade. Activation of p44/42 MAPK was evaluated by the measurement of phosphorylated p44/42 MAPK (p-p44/42 MAPK) by western blotting. As early as 16 h after seeding MSCs, activation of the kinase was upregulated on the rigid (266 MPa) films (Fig. 2A–C). After 4 days culture, expression of p-p44/42 MAPK between the compliant and rigid substrates increased further (Fig. 2A–C), indicating the continuing up-regulation of osteogenesis on the rigid films. To further understand this outside-in pathway, we also evaluated SMAD1/5 protein activation, which was previously shown to crosstalk with MAPK pathways and is activated by the BMP signaling pathway33–36. Both expression of SMAD1/5 protein and p-SMAD1/5 were analyzed by Western blotting (Fig. 2D). Semi-quantification of the blots (Fig. 2E) showed that p-SMAD1/5 normalized by GAPDH was significantly higher on the rigid films on day 3, suggesting that the modulus mediates both p44/42 MAPK and SMAD1/5 signaling on bone-like substrates.
Figure 2.
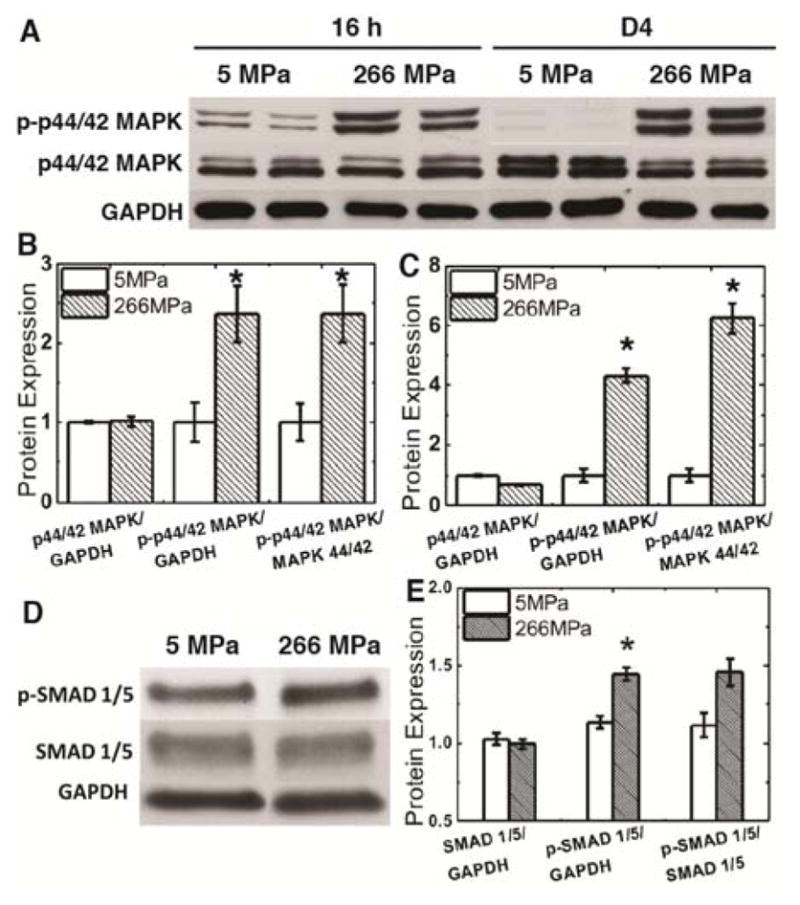
Expression and activities of mechanotransduction related-kinases increased with substrate modulus. (A) Time-course activation of p-p44/42 MAPK in response to substrate modulus. Two sets of blots are shown from independent experiments. (B–C) Quantification of the blots in (A) shows that p-p44/42 MAPK normalized to GAPDH or p44/42 MAPK increased with substrate modulus at (B) 16 h and (C) D4. (D) Activation of p-SMAD1/5 increased with substrate modulus at D3. (E) Quantification of the blots in (D) shows that p-SMAD1/5 normalized to GAPDH or SMAD1/5 increased with substrate modulus. *p < 0.05.
Iβ1 mediates osteoblast differentiation of MSCs by physical interactions between Iβ1 and BMPRI
Conditioned medium treatment was first investigated to examine whether the effects of substrate modulus were caused by secreted factors. Conditioned medium was harvested from MSCs cultured on compliant and rigid 2D films and then transferred to MSCs cultured on tissue culture plates. No significant difference in expression of Runx2, Osx, or Opn was observed between the two groups (Fig. 3A), which points to a mechanotransduction mechanism requiring physical contact of the MSCs with a rigid, bone-like substrate.
Figure 3.
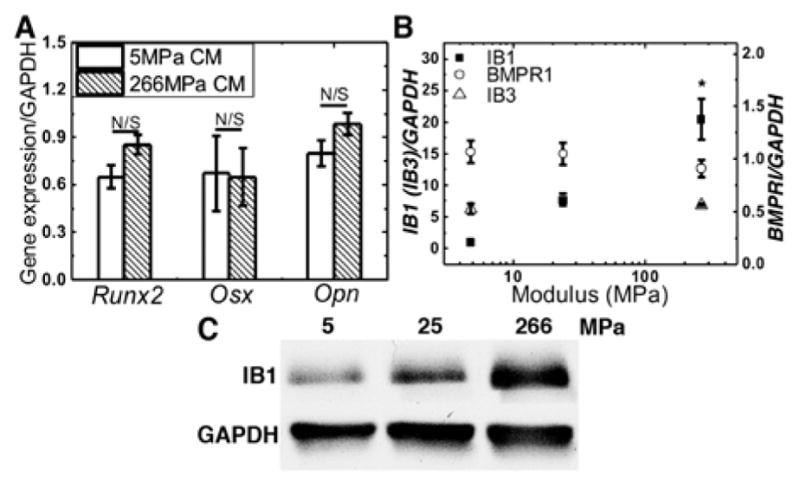
(A) MSCs cultured on tissue culture plates were treated with conditioned medium from MSCs cultured on compliant or rigid PUR films. (B) Iβ1 gene expression increased significantly with substrate modulus, while Iβ3 and BMPRI did not. (C) Iβ1 protein expression increased with substrate modulus.
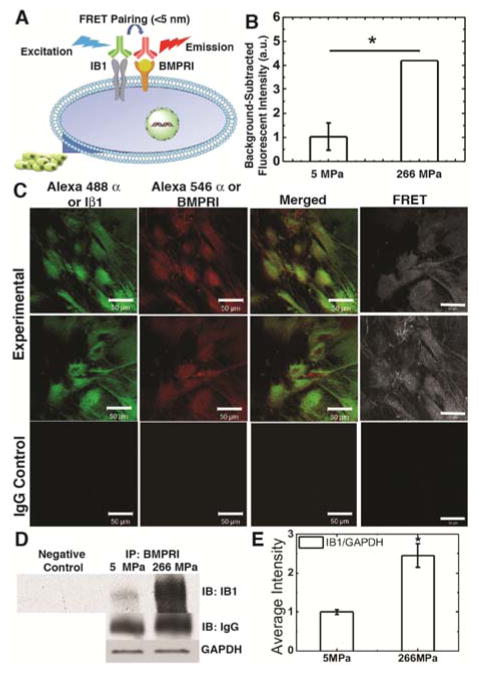
Considering previous studies reporting that integrin β1 sub-unit (Iβ1) is required for mechanically transduced osteoblast differentiation of MSCs37, we hypothesized that Iβ1 mediates the cellular response to substrate modulus. Gene expression of Iβ1 significantly increased more than 10-fold as the substrate modulus increased from 5 to 266 MPa (Fig. 3B). Similarly, Iβ1 protein expression measured by Western blotting also increased with substrate modulus (Fig. 3C). However, gene expression of Type I BMP receptor (BMPRI) and Integrin β3 sub-unit (Iβ3) were independent of substrate modulus (Fig. 3B). To further investigate the mechanism of crosstalk between the Iβ1 and BMP signaling pathways, we performed Förster resonance energy transfer (FRET) and immunoprecipitation (IP) experiments. The FRET signal was detected with the labeled Iβ1 (donor in the FRET system) and BMPRI (receptor in the FRET system) without penetrating the cell membrane, which suggests that the receptors physically associated in the plasma membrane (Fig. 4A). The FRET signal was integrated using a fluorescence plate reader and was significantly higher when MSCs were cultured on rigid compared to compliant films (Fig. 4B). Representative confocal images of MSCs cultured on glass (to improve the quality of the image) show Iβ1 (labelled with green Alexa 488α), BMPRI (labeled with red Alexa 546α), the merged images showing both receptors, and the FRET image (Fig. 4C). No FRET signal was observed for the IgG control group. To confirm that physical association of the two receptors increased with substrate modulus, immunoprecipitation was performed (Fig. 4D–E). Physical association of BMPRI and Iβ1 was significantly higher on rigid (266 MPa) compared to compliant (5 MPa) substrates. Taken together, the data in Figs. 2 – 4 suggest that substrate modulus activates the BMP signaling pathway through physical association of BMPRI and Iβ1 in the cell membrane.
Figure 4.
Iβ1 and BMPRI physically interact on rigid but not compliant substrates. (A) Physical association of Iβ1 and BMPRI generated a FRET signal. (B) The FRET signal was significantly higher on rigid compared to compliant substrates. (C) Confocal images of Iβ1 labeled with Alexa 488α, BMPRI labelled with Alexa 546α, merged images, and the FRET signal for MSCs cultured on on glass. (D) Immunoprecipitation of Iβ1 physically bound to BMPRI. Cells were cultured on compliant (5 MPa) or rigid (266 MPa) substrates. Similar amounts of protein (confirmed by GAPDH loading control) were incubated and selected with BMPRI-bonded beads and the collected proteins were blotted with and anti-Iβ1 antibody. (E) Association of BRMPRI and Iβ1 was higher on rigid substrates. * p < 0.05.
Iβ1 expression regulates osteoblast gene expression by MSCs
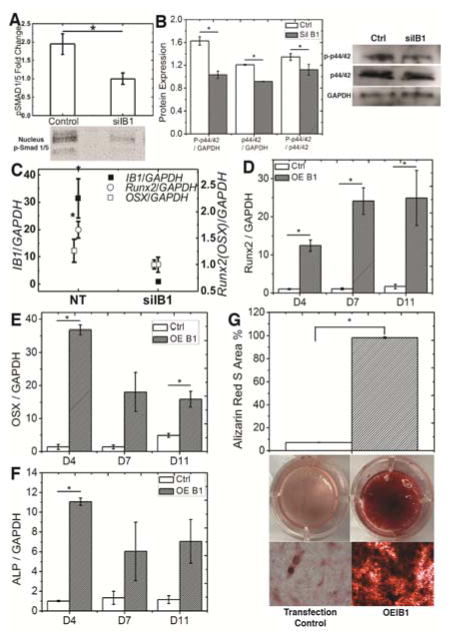
To confirm the role of Iβ1 in substrate-mediated osteoblast differentiation, we used siRNA to inhibit Iβ1 expression to <10% of its normal level. Since the siRNA transfections were transient, we assessed the effects of Iβ1 inhibition on intracellular signaling and expression of transcription factors at short time points (<5 days). MSCs were cultured on rigid tissue culture well plates (without PUR film) to confluency and then treated with osteogenic medium. Trafficking of p-SMAD1/5 to the nucleus (Fig. 5A) and phosphorylation of p44/42 MAPK (Fig. 5B) were significantly decreased in siIβ1 cells compared to control (no treatment) cells. Expression of Runx2 evaluated after 5 days of induction was significantly inhibited in siIβ1 compared to control cells, while the reduction in Osx expression was not significant (Fig. 5C). MSCs were also transduced with Iβ1 plasmid, resulting in a stable 2.5-fold increase in Iβ1 expression, and the resulting OEIβ1 cells were cultured on compliant (5 MPa) PUR films and treated with Os+ medium for up to 18 days. Significantly increased Runx2 (Fig. 5D), Osx (D4 and D11, Fig. 5E), and Alp (D4, Fig. 5F) expression was observed in OEIβ1 cells compared to the control cells. As assessed by Alizarin Red staining on D18, mineralization of OEIβ1 significantly exceeded that of control cells. These results indicate that increased Iβ1 expression stimulated osteoblast differentiation of MSCs.
Figure 5.
Osteoblast differentiation of rat bone marrow-derived MSCs is regulated by Iβ1. (A) Trafficking of p-SMAD1/5 to the nucleus was higher in control cells compared to shIβ1 cells. (B) Phosphorylation of p44/42 MAPK was higher in control cells compared to siIβ1 cells. (C) siRNA knockdown of Iβ1 reduced Runx2 and Osx expression. (D–F) Expression of (D) Runx2, (E) Osx, and (F) Alp was higher in OEIβ1 compared to control cells cultured on compliant (5 MPa) substrates. (F) Mineralization of OEIβ1 cells exceeded that of control cells cultured on compliant substrates on D18. *p < 0.5.
Inhibiting the activity of BMPRI hindered MSC osteogenesis in response to substrate modulus
We utilized a BMPRI kinase inhibitor (dorsormorphin)38 to further investigate the effects of interactions between Iβ1 and BMPRI on osteoblast differentiation. Treatment of MSCs cultured in a 48-well plate with dorsormorphin significantly inhibited expression of Runx2 and the osteoblast marker Osteopontin (Opn) in a dose-responsive manner (Fig. 6A). MSCs were also plated on both compliant and rigid 2D PUR films and treated with 1 μM dorsormorphin. After 7 days of culture in Os+ medium, there was no difference in Runx2 expression between the compliant and rigid susbstrates. These data suggest that BMRPI is required for Iβ1-mediated osteoblast differentiation even in the absence of exogenous BMP ligands.
Figure 6.
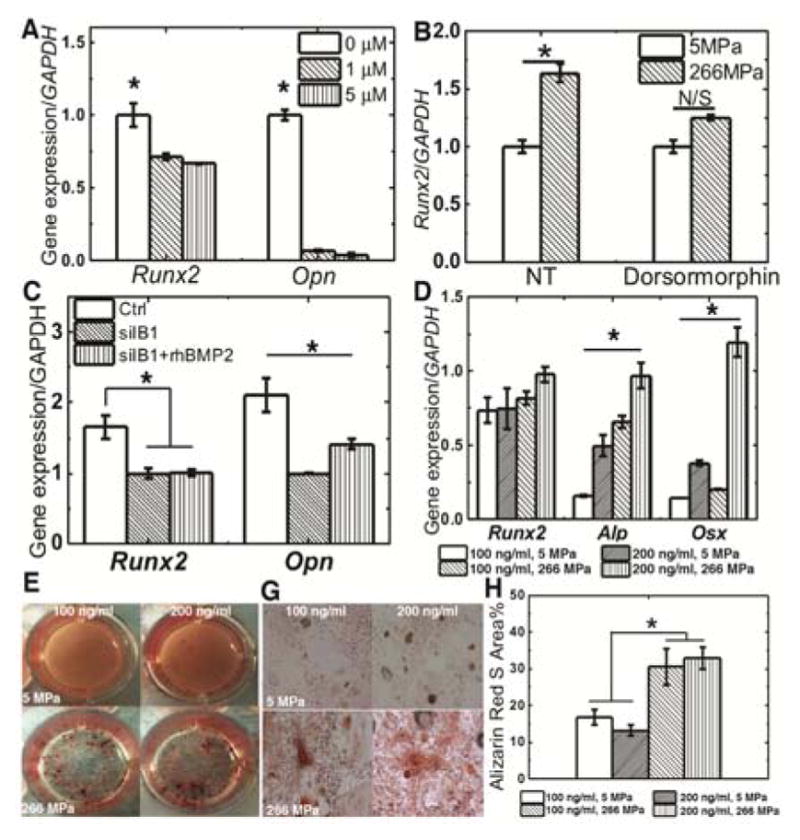
The role of the BMP signaling pathway in substrate-mediated osteoblast differentiation. (A) Expression of Runx2 and Opn significantly decreased with increasing dorsomorphin dose on rigid well plates. (B) Dorsomorphin (1 μM) reduced Runx2 expression on rigid substrates. (C) Silencing Iβ1 inhibited expression of Runx2 and Opn, which was only partially recovered by treatment with exogenous rhBMP-2 (100 ng/ml). (D) Expression of Alp and Osx significantly increased with both substrate modulus and exogenous rhBMP-2 concentration (increases in Runx2 expression were not significant) (E–H) Mineralization increased significantly with substrate modulus but not rhBMP-2 concentration. * p < 0.05.
Substrate modulus and BMP signaling converge to regulate osteoblast differentiation of MSCs
To determine whether Iβ1 contributes to osteogenesis through the BMP signaling pathway, siRNA was utilized to silence expression of Iβ1 and rhBMP-2 ligand was added to cell culture. When Iβ1 was silenced, expression of Runx2 and Opn were significantly reduced (Fig. 6C). Addition of rhBMP-2 only partially rescued osteoblast differentiation, as evidenced by the negligible increase in Runx2 expression and a modest (but significant) increase in Opn expression (Fig. 6C). As further confirmation that Iβ1 and BMPRI enhance osteogenic differentiation through the same pathway, rat MSCs were cultured on compliant or rigid PUR films and treated with 100 or 200 ng rhBMP-2/ml. Expression of Osx and Alp significantly increased with both exogenous rhBMP-2 concentration and substrate modulus (Fig. 6D), while the increases in Runx2 expression were not significant. Mineralization of MSCs was assessed by Alizarin Red staining on day 21 (Fig. 6E–G). The area% Alizarin Red staining increased significantly with substrate modulus (Fig. 6H), while the effects of rhBMP-2 concentration on mineralization were not significant. Taken together, these findings suggest Iβ1 and BMP signaling converge to stimulate osteoblast differentiation.
Discussion
Although it has been reported that integrins are key factors mediating mechanotransduction in MSCs, the mechanism by which integrins mediate osteoblast differentiation in response to substrate rigidity has remained unclear39, 40. Integrins crosstalk with multiple signaling pathways, including BMP signaling through FAK phosphorylation41. However, other than the conventional “outside-in” signaling in which a series of an integrin-bound kinases are activated by conformational changes of the integrin, the physical interaction of BMP receptors with Iβ1 sub-unit has not been reported. In this study, we utilized PUR substrates with substrate moduli ranging from that of collagen (<20 MPa) to trabecular bone (100 – 400 MPa15) to investigate the mechanism by which substrate modulus and BMP signaling regulate osteogenic differentiation. We observed increased expression of Iβ1 and the transcription factors Runx2 and Osx, increased Alp activity, and increased mineralization with increasing substrate modulus. Furthermore, both p-p44/42 MAPK and p-SMAD1/5 were activated on rigid, bone-like substrates, which points to direct crosstalk between integrins and BMP receptors similar to the recently reported physical interaction of integrins with growth factor receptors in bone-metastatic tumor cells23, 27. Using FRET and immunoprecipitation, we discovered that Iβ1 and BMPRI physically interact in rat MSCs on rigid, bone-like substrates, which stimulates BMP signaling through SMAD1/5 and p44/42 MAPK and the transcription factors Runx2 and Osx.
Previous studies using hydrogels have reported that osteoblast differentiation of MSCs increases with substrate modulus up to 0.1 MPa42–44. A limited number of studies have investigated the effects of substrate modulus on osteoblast differentiation using rigid substrates comparable to trabecular bone. A recent study reported that for acrylate networks with moduli ranging from 5–850 MPa, the composition of the polymer had a more significant effect on differentiation than the modulus, prompting the authors to challenge the notion that cells can sense rigidity in the range of trabecular bone15. This observation is consistent with reports that cells are in a state of isometric contraction and cannot sense changes in matrix rigidity above 0.1 MPa19, 22. However, in a recent study, we found that osteoblast differentiation and mineralization of osteoprogenitor cells cultured on 3D scaffolds increased with substrate rigidity over the range 20 – 300 MPa17. These findings are in agreement with a previous study reporting that expression of Runx2 and Alp increased with 2D substrate modulus when MC3T3-E1 pre-osteoblasts were cultured on PEG-diacrylate hydrogels (0.6 MPa) or tissue culture polystyrene (2000 MPa)16, 45. Crosstalk between RhoA-ROCK and p44/42 MAPK stimulated Runx2 and markers of osteoblast differentiation. Integrins were hypothesized to catalyze RhoA activation and the activation of other signals in cooperation with soluble factor receptors, but the mechanism by which soluble factor receptors crosstalk with integrins was not identified.
The mineralized extracellular matrix differentiates bone from other tissues. Interactions between cells and the extracellular matrix result in a complex of an adhesion molecule, integrin, and actomyosin that can be shifted between active and inactive states in response to the rigidity of the matrix18. These changes in gene expression are driven by uniform displacements of the matrix in the range of 100 – 150 nm19–21. Thus, the notion that contractility mediates osteoblast differentiation on bone-like substrates is inconsistent with recent findings that cells cannot generate displacements > 100 nm on substrates more rigid than 0.01 – 0.1 MPa19. We hypothesized that enhanced osteoblast differentiation of MSCs on bone-like substrates is mediated by physical association of Iβ1 and BMPRI. TGF-β Receptor type II (TGF-β RII) interacts physically with Iβ3 to enhance TGF-β-mediated stimulation of MAP-kinases (MAPKs) during epithelial-mesenchymal transition (EMT) of mammary epithelial cells27. Furthermore, we have recently reported that physical interactions between Iβ3 and TGF-β RII in bone-metastatic tumor cells increased with substrate modulus, resulting in up-regulation of bone-metastatic genes23. However, the role of integrin-soluble factor interactions in osteoblast differentiation of MSCs in response to substrate modulus have not been previously investigated.
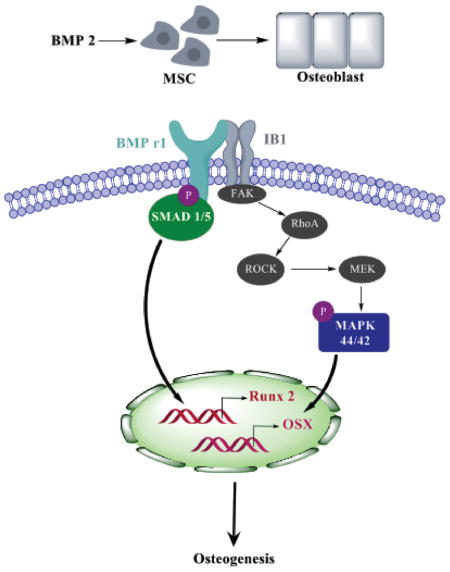
To identify the relative contributions of Iβ1 and BMPRI to modulus-mediated osteogenesis, we inhibited and/or over-expressed each factor individually. Since the expression of Iβ1 on the PUR films correlated with expression of genes associated with osteogenesis, MSCs transfected with siIβ1 were cultured on rigid (2 GPa) tissue culture plates to inhibit the expression of Iβ1. Inhibition of Iβ1 resulted in reduced SMAD1/5 and p44/42 MAPK activation (Fig. 5A–B) and Runx2 and Osx expression (Fig. 5C). Similarly, overexpression of Iβ1 (plasmid) promoted increased Runx2, Osx, and Alp expression and mineralization in MSCs cultured on compliant PUR substrates. Since the mechanism of BMPs in osteoblast differentiation is well established, we selected a BMPRI kinase inhibitor, which inhibits the BMP type I receptors ALK2, ALK3 and ALK6 and blocks BMP-mediated SMAD1/5 phosphorylation38, to determine the relative contribution of the BMP signaling pathway to Iβ1-mediated osteoblast differentiation. No soluble ligand stimuli (BMPs) were added, and thus the decreased expression of osteoblast genes induced by dorsomorphin treatment points to the direct involvement of BMPRI in substrate-mediated osteoblast differentiation. Based on our finding that physical interactions between Iβ1 and BMPRI increase with substrate modulus, we propose the signaling pathway in Fig. 7 as a mechanism by which substrate modulus regulates osteoblast differentiation. With increasing substrate modulus, both expression of Iβ1 and also physical association of Iβ1 and BMPRI increase, resulting in activation of SMAD1/5 and p44/42 MAPK. A previous study has reported that activation of FAK on bone-like substrates activates p44/42 MAPK through RhoA/ROCK16, 45 (shown in grey in Fig. 7). Phosphorylation of p-44/42 MAPK and SMAD1/5 up-regulates expression of the transcription factors Runx2 and Osx, resulting in enhanced osteoblast differentiation and mineralization on rigid, bone-like substrates compared to compliant, collagen-like substrates.
Figure 7.
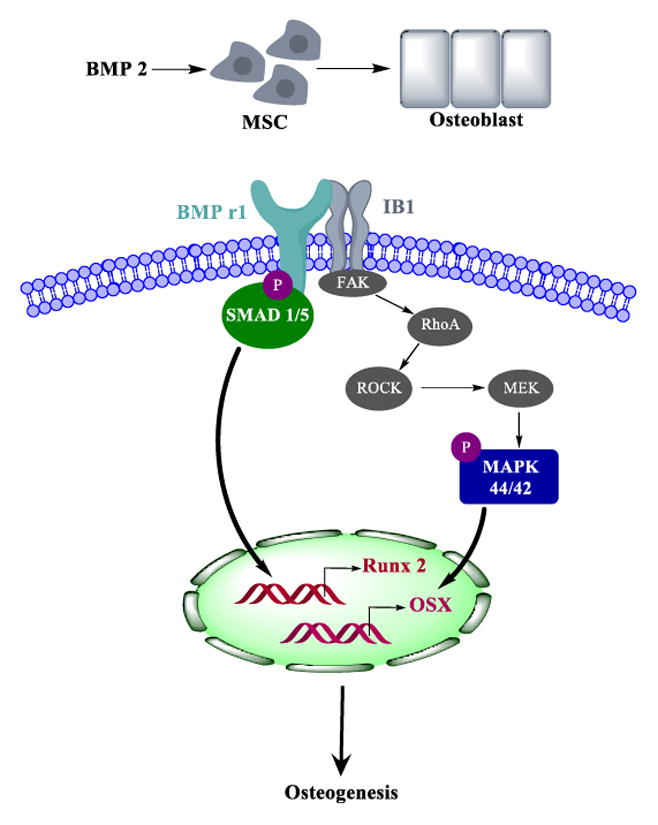
Model for regulation of osteoblast differentiation through Iβ1 and BMPRI signaling. Physical association of Iβ1 and BMPRI on rigid, bone-like substrates activates SMAD1/5 and p44/42 MAPK and expression of the transcription factors Rux2 and Osx that stimulate osteoblast differentiation. Factors in grey have been reported to stimulate MAPK 44/42 through FAK activation16, 45 and are shown to improve clarity.
The observed crosstalk between Iβ1 and BMPRI suggests that the mechanically transduced and soluble factor signaling converge on the same pathway. To test this hypothesis, we assessed the effects of exogenous rhBMP-2 ligand and substrate modulus on osteoblast differentiation and mineralization of MSCs. Expression of the transcription factor Osx and the osteoblast marker Alp increased significantly with both substrate modulus and rhBMP-2 concentration, while mineralization increased significantly with substrate modulus (Fig. 6D–G). Local delivery of rhBMP-2 from osteoconductive scaffolds has been extensively investigated as a strategy for healing critical-size bone defects. However, recent studies have reported that off-label use of rhBMP-2 can result in complications due to a bolus release of high concentrations of the drug46. Sustained release of rhBMP-2 for up to 4 weeks is an effective strategy for reducing the required dose of rhBMP-2 to achieve bone healing47, 48. Taken together, our findings point to local delivery of rhBMP-2 from grafts with bone-like substrate modulus as an alternative strategy for reducing the dose of rhBMP-2 and potentially reducing the frequency of complications associated with its use.
Conclusions
The effects of substrate modulus on osteoblast differentiation of bone marrow-derived MSCs were investigated for 2D poly(ester urethane) films with moduli varying from 5 – 266 MPa, which spans the range from collagen fibrils to trabecular bone. Expression of markers of osteoblast differentiation and matrix mineralization increased with increasing substrate modulus, which was mediated by physical association of Iβ1 sub-unit and BMPRI through SMAD1/5 and p44/42 MAPK signaling. Thus, integrin and BMP signaling converge to regulate osteoblast differentiation of MSCs.
Acknowledgments
Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under award number R01CA163499 and by the National Science Foundation under Grant No. NSF DMR-0847711. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Rosset P, Deschaseaux F, Layrolle P. Orthopaedics & traumatology, surgery & research : OTSR. 2014;100:S107–112. doi: 10.1016/j.otsr.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P. J Bone Joint Surg Am. 2002;84-A:716–720. doi: 10.2106/00004623-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Giannoudis PV, Dinopoulos H, Tsiridis E. Injury. 2005;36(Suppl 3):S20–27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Vo TN, Kasper FK, Mikos AG. Adv Drug Deliv Rev. 2012;64:1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi W, Yuan W, Yan J, Wang H. Journal of Materials Chemistry B. 2014;2:5461–5467. doi: 10.1039/c4tb00856a. [DOI] [PubMed] [Google Scholar]
- 6.Undale AH, Westendorf JJ, Yaszemski MJ, Khosla S. Mayo Clin Proc. 2009;84:893–902. doi: 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XX, Allen RJ, Jr, Tutela JP, Sailon A, Allori AC, Davidson EH, Paek GK, Saadeh PB, McCarthy JG, Warren SM. Plast Reconstr Surg. 2011;128:395–405. doi: 10.1097/PRS.0b013e31821e6e10. [DOI] [PubMed] [Google Scholar]
- 8.Tannoury CA, An HS. Spine J. 2014;14:552–559. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 9.Blackwood KA, Bock N, Dargaville TR, Woodruff MA. Int J Polym Sci. 2012;2012:174942. [Google Scholar]
- 10.Rosen V. Cytokine Growth Factor Rev. 2009;20:475–480. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Valentin-Opran A, Wozney J, Csimma C, Lilly L, Riedel GE. Clin Orthop Relat Res. 2002:110–120. doi: 10.1097/00003086-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Boraiah S, Paul O, Hawkes D, Wickham M, Lorich DG. Clin Orthop Relat Res. 2009;467:3257–3262. doi: 10.1007/s11999-009-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamidouche Z, Fromigué O, Ringe J, Häupl T, Vaudin P, Pagès J, Srouji S, Livne E, Marie P. Proc Natl Acad Sci U S A. 2009;106:18587–18591. doi: 10.1073/pnas.0812334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. J Bone Miner Res. 2011;26:730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- 15.Smith KE, Hyzy SL, Sunwoo M, Gall KA, Schwartz Z, Boyan BD. Biomaterials. 2010;31:6131–6141. doi: 10.1016/j.biomaterials.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khatiwala CB, Peyton SR, Metzke M, Putnam AJ. J Cellular Physiology. 2007;211:661–672. doi: 10.1002/jcp.20974. [DOI] [PubMed] [Google Scholar]
- 17.Guo R, Lu S, Page JM, Merkel AR, Basu S, Sterling JA, Guelcher SA. Advanced healthcare materials. 2015;4:1826–1832. doi: 10.1002/adhm.201500099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boettiger D. Curr Opin Cell Biol. 2012;24:592–599. doi: 10.1016/j.ceb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Moore SW, Roca-Cusachs P, Sheetz MP. Dev Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Biophys J. 2006;90:1804–1809. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saez A, Buguin A, Silberzan P, Ladoux B. Biophys J. 2005;89:L52–54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Discher DE, Janmey P, Wang YL. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 23.Page JM, Merkel AR, Ruppender NS, Guo R, Dadwal UC, Cannonier SA, Basu S, Guelcher SA, Sterling JA. Biomaterials. 2015;64:33–44. doi: 10.1016/j.biomaterials.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou C, Luo Q, Qin J, Shi Y, Yang L, Ju B, Song G. Cell Biochem Biophys. 2013;65:455–462. doi: 10.1007/s12013-012-9449-8. [DOI] [PubMed] [Google Scholar]
- 25.Graham JS, Vomund AN, Phillips CL, Grandbois M. Experimental cell research. 2004;299:335–342. doi: 10.1016/j.yexcr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Guelcher SA, Patel V, Gallagher KM, Connolly S, Didier JE, Doctor JS, Hollinger JO. Tissue Engineering. 2006;12:1247–1259. doi: 10.1089/ten.2006.12.1247. [DOI] [PubMed] [Google Scholar]
- 27.Galliher AJ, Schiemann WP. Breast cancer research : BCR. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rust A, Hassan HH, Sedelnikova S, Niranjan D, Hautbergue G, Abbas SA, Partridge L, Rice D, Binz T, Davletov B. Sci Rep. 2015;5:12444. doi: 10.1038/srep12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roca H, Phimphilai M, Gopalakrishnan R, Xiao G, Franceschi RT. J Biol Chem. 2005;280:30845–30855. doi: 10.1074/jbc.M503942200. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Zhou Z, de Crombrugghe B, Nakashima K, Guan H, Duan X, Jia SF, Kleinerman ES. Cancer Res. 2005;65:1124–1128. doi: 10.1158/0008-5472.CAN-04-2128. [DOI] [PubMed] [Google Scholar]
- 31.Huang C, Ogawa R. FASEB J. 2010;24:3625–3632. doi: 10.1096/fj.10-157370. [DOI] [PubMed] [Google Scholar]
- 32.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 33.Wang YK, Yu X, Cohen DM, Wozniak MA, Yang MT, Gao L, Eyckmans J, Chen CS. Stem Cells Dev. 2012;21:1176–1186. doi: 10.1089/scd.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song B, Estrada KD, Lyons KM. Cytokine Growth Factor Rev. 2009;20:379–388. doi: 10.1016/j.cytogfr.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rath B, Nam J, Deschner J, Schaumburger J, Tingart M, Grassel S, Grifka J, Agarwal S. Biorheology. 2011;48:37–48. doi: 10.3233/BIR-2011-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beederman M, Lamplot JD, Nan N, Wang J, Liu X, Yin L, Li R, Shui W, Zhang H, Kim SH, Zhang W, Zhang J, Kong Y, Denduluri S, Rogers MR, Pratt P, Haydon RC, Luu HH, Angeles J, Shi LL, He TC. J Biomed Sci Eng. 2013;6:32–52. doi: 10.4236/jbise.2013.68A1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Zong C, Li B, Shen D, Tang Z, Chen J, Zheng Q, Tong X, Gao C, Wang J. J Tissue Eng Regen Med. 2014;8:85–96. doi: 10.1002/term.1498. [DOI] [PubMed] [Google Scholar]
- 38.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harburger DS, Calderwood DA. Journal of cell science. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eliceiri BP. Circ Res. 2001;89:1104–1110. doi: 10.1161/hh2401.101084. [DOI] [PubMed] [Google Scholar]
- 41.Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Experimental cell research. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 43.Guvendiren M, Burdick JA. Nature communications. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 44.Rowlands AS, George PA, Cooper-White JJ. Am J Physiol Cell Physiol. 2008;295:C1037–1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 45.Khatiwala CB, Kim PD, Peyton SR, Putnam AJ. J Bone Miner Res. 2009;24:886–898. doi: 10.1359/JBMR.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wenke JC, Guelcher SA. Expert opinion on drug delivery. 2011:1–15. doi: 10.1517/17425247.2011.628655. [DOI] [PubMed] [Google Scholar]
- 47.Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC. Tissue engineering Part A. 2011;17:1735–1746. doi: 10.1089/ten.TEA.2010.0446. [DOI] [PubMed] [Google Scholar]
- 48.Boerckel JD, Kolambkar YM, Dupont KM, Uhrig BA, Phelps EA, Stevens HY, Garcia AJ, Guldberg RE. Biomaterials. 2011;32:5241–5251. doi: 10.1016/j.biomaterials.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]