Abstract
Previous studies have demonstrated that β2-adrenergic receptors (β2ARs) can be phosphorylated by G protein-coupled receptor kinases (GRKs) and protein kinase A (PKA), affecting β2AR internalization and desensitization. However, the exact physiological function of β2ARs in cardiomyocytes is unknown. In this study, we showed that neonatal mouse cardiomyocytes had different contraction and internalization responses to sustained or repeated, transient agonist stimulation. Specifically, short-time stimulation (10 min) with epinephrine or norepinephrine increased the cardiomyocyte contraction rate, reaching a maximum at 5 min, followed by a slow decline. When the agonist was re-added after a 60-min wash-out period, the increase in the cardiomyocyte contraction rate was similar to the initial response. In contrast, when cardiomyocytes were exposed continuously to epinephrine or norepinephrine for 60 min, the second agonist stimulation did not increase the contraction response. These results indicated that continuous β2AR stimulation caused functional desensitization. Phosphorylation of β2ARs at serine (Ser)355/356 GRK phosphorylation sites, but not at Ser345/346 PKA phosphorylation sites increased with continuous epinephrine stimulation for 60 min. Accordingly, β2AR internalization increased. Interestingly, β2AR internalization was blocked by mutations at the GRK phosphorylation sites, but not by mutations at the PKA phosphorylation sites. Furthermore, inhibition of β2AR dephosphorylation by okadaic acid, a phosphatase 2A inhibitor, impaired the recovery of internalized β2ARs and reduced the cardiomyocyte contraction rate in response to epinephrine. Finally, epinephrine treatment induced the physical interaction of β-arrestin with internalized β2ARs in cardiomyocytes. Together, these data revealed the essential role of the Ser355/356 phosphorylation status of β2ARs in regulating receptor internalization and physiological resensitization in neonatal cardiomyocytes to contraction functions.
Introduction
β2-Adrenergic receptors (β2ARs) belong to the G protein-coupled receptor (GPCR) superfamily and play significant roles in regulating cardiovascular and airway functions. Dysregulation of β2ARs is associated with heart failure, asthma, and other diseases [1–3]. Activation of β2ARs induces receptor binding to G proteins followed by dissociation of the Gα and Gβγ subunits. β2ARs couple to both Gs and Gi proteins that regulate adenylyl cyclase generating cAMP to activate protein kinase A (PKA), which phosphorylates downstream proteins, thus mediating various physiological functions [4].
Activation of β2ARs leads to rapid receptor phosphorylation by PKA and G protein-coupled receptor kinases (GRKs), which leads to receptor binding to β-arrestin, terminates G protein-mediated signaling, and facilitates receptor endocytosis resulting in receptor desensitization and internalization [5]. This is one of the reasons why prolonged or repeated use of β2 agonists leads to a loss of their effect. Once β2ARs are dephosphorylated through protein phosphatase 2A (PP2A), the receptor becomes resensitized to agonist stimulation. β2ARs can be phosphorylated on their C termini and intracellular loops, such as Ser261/262/345/346 by PKA and Ser355/356/360/364 by GRKs, and the distinct phosphorylation sites mediate different intracellular signaling pathways and physiological functions [6].
Much of the work on β2AR phosphorylation, internalization, and resensitization has been performed in transiently transfected cells. There are few data from primary cells or animal models, which limits our understanding of the physiology of β2AR phosphorylation. At the same time, although the mechanism of β2AR desensitization is well characterized, less is known about β2AR resensitization, particularly the role of β2AR dephosphorylation in functional resensitization and internalization in primary cardiomyocytes. Recent studies have revealed that resensitization should be considered equally important as desensitization for the regulation of β2AR functions [7].
Phosphorylation of Ser261/262 in cardiomyocytes has been previously demonstrated, and was shown to be involved in regulating the contraction rate response and Gi signaling coupling [7]. At the same time, we could not confirm phosphorylation of Ser360/364 in cardiomyocytes owing to the lack of phosphor-specific antibodies for these sites. Therefore, in the current study, we focused on Ser345/346 and Ser355/356. We found that the Ser355/356 phosphorylation sites on β2ARs have a crucial role in regulating β2AR internalization and resensitization in neonatal mouse cardiomyocytes.
Materials and procedures
Isolation and culture of neonatal cardiac myocytes
All animal study protocols were approved by the Institutional Animal Care and Use Committee of the Wenzhou Medical University and complied with the regulations of the Ministry of Health, China, and the USA National Institutes of Health Guidelines for the use and care of laboratory animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
β1/β2AR double knockout (DKO) mice (Adrb1tm1BkkAdrb2tm1Bkk/J) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) [8]. β1AR knockout chimeric mice were crossed originally with C57BL/6J × DBA/2 F1hybrid mice [9]. Neonatal mice (less than 12 h old) were anesthetized with isoflurane and decapitated, and the hearts were excised quickly. Cardiac myocytes were isolated and cultured as described previously [10]. Briefly, each isolated heart was cut into pieces and incubated two times with papain (Sigma-Aldrich, St. Louis, MO, USA) with shaking at 37°C for 5 min. The tissue was then pipetted vigorously to disperse it into single cells. After removing the digestion solution, the cells were resuspended in Dulbecco’s modified Eagle’s medium and plated onto a gelatin-coated dish after filtering through a cell strainer. After 1 h, the myocytes were collected and placed onto new dishes.
Site-directed mutagenesis and recombinant adenoviruses
To determine the role of β2AR phosphorylation at the GRK phosphorylation sites Ser355 and Ser356, a pcDNA 3.1-FLAG-tagged murine β2AR was used as a template for mutagenesis with the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Both serines were replaced with alanines according to the manufacturer’s instructions. In addition, the PKA phosphorylation sites (Ser345 and Ser346) on β2AR were mutated by replacing the two serines with alanines. The mutant plasmids were named GRK2A and PKA2A, respectively. Mutant β2AR constructs were confirmed by sequencing and transformed into adenovectors to produce adenoviruses using the AdEasy Adenoviral Vector System (Agilent Technologies, Santa Clara, CA). Virus titers were assessed by determining the receptor expression levels by both western blot and ligand binding assays, as described previously [11].
Neonatal cardiac myocyte contraction assay
Measurements of the spontaneous contraction of cardiac myocytes were performed as described previously with modification [11]. Briefly, after isolation of the cardiomyocytes from neonatal heart tissues and a 1-h pre-culture in dishes, the cells were collected by centrifugation. The cells from each heart sample were resuspended in 30 μL of medium, and 10 μL of the concentrated cell suspension (approximately 3 × 105 cells) was placed in the center of 35-mm dishes and cultured for 24 h. Then, the medium was changed and the cells were cultured for another 24 h to obtain a uniformly beating syncytium. The cells were equilibrated in a chamber on the stage of an inverted microscope at 37°C for 10 min before monitoring of the contraction rate. For the desensitization/resensitization assay, the contraction rates of the syncytia were recorded every 2 min for 10 min after the addition of epinephrine (Epi, 10 μM) or norepinephrine (NE, 10 μM) (Sigma-Aldrich). Then, the medium was changed or the cells were kept in the medium containing stimulator for 60 min, after which the contraction rates were recorded for another 10 min following a second stimulation. Data were recorded using the software on the computer. For measurements of the effect of repeated stimulation with agonist Epi or NE, the contraction response of mouse neonatal cardiomyocytes was recorded in real-time for 40 min.
β2AR internalization and recycling assay
Neonatal cardiac myocytes isolated from DKO mice were transfected with plasmids encoding wild-type or phosphorylation-site mutant β2ARs at a multiplicity of infection (MOI) of 100 for 24 h. After serum starvation for 2 h, the myocytes were stimulated with 10 μM Epi for different periods. For receptor recycling, the cells were stimulated with Epi for 10 min, rinsed, and refed with serum-free medium for different periods after removal of Epi for 1 h. The cells were then fixed, permeabilized, incubated with anti-FLAG antibody, and visualized using an Alexa 488-conjugated goat anti-mouse antibody (Invitrogen, Carlsbad, CA, USA). Fluorescence images were taken with a camera on a Zeiss Axioplan 2 microscope and analyzed with Metamorph software (Molecular Devices, Sunnyvale, CA, USA). To quantify the surface receptor level, a fluorescence-linked immunosorbent assay (FLISA) was applied as described previously [12]. Briefly, neonatal cardiomyocytes cultured in poly-lysine-coated 12-well plates were transfected with FLAG-β2AR adenovirus at a MOI of 100 for 24 h, stimulated with Epi under the indicated conditions, and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS). Without permeabilization, the cells were blocked directly with 2.5% goat serum in PBS, and then stained with Alexa 488-conjugated M1 antibody (Sigma) at a concentration of 1 μg/mL for 30 min at room temperature. The unbound antibody was removed by washing four times with PBS. The cells were harvested with 1% SDS in PBS, and the intensity of Alexa 488 emission (495 to 580 nm) was measured using a spectrofluorometer with an excitation wavelength of 485 nm and an integration time of 0.3 s/nm. The fluorescence intensity was normalized by subtracting the background from cells without M1 antibody.
Beta2AR phosphorylation assay by western blot analysis
Neonatal cardiac myocytes were transfected with FLAG-tagged wild-type or mutant β2ARs at a MOI of 100 for 48 h. Then, the cells were serum-starved for 2 h and stimulated with 10 μM Epi for the indicated times. The cells were lysed in radioimmunoprecipitation assay lysis buffer (Thermo Scientific, Rockford, IL, USA) at 4°C. Samples were clarified by centrifugation at 16,100 × g for 10 min, and the protein concentration of the supernatant was measured by the bicinchoninic acid assay (Thermo Scientific) according to the manufacturer's instructions. Samples were resolved by SDS-PAGE for western blotting. Phosphorylation of β2AR by GRK at Ser355/356 or by PKA at Ser345/346 was detected with phospho-specific AR antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). IRDye 800CW-conjugated donkey anti-rabbit antibody (LI-COR, Lincoln, NE, USA) was used as a secondary antibody. Western blots were visualized with an Odyssey CLx infrared imager (LI-COR) and quantified after normalization against baseline levels.
Immunofluorescence and confocal microscopy
Cardiac myocytes were cultured on coverslips in 6-well plates and transfected with wild-type or mutant β2AR recombinant adenoviruses at a MOI of 100 for 24 h. The cells were serum-starved for 2 h before stimulation with 10 μM Epi for the indicated times. Then, the cells were fixed with 4% paraformaldehyde for 20 min at room temperature, permeabilized, and incubated with an anti-FLAG antibody overnight at 4°C. After washing with PBS, the cells were incubated with an Alexa 488-conjugated goat anti-mouse secondary antibody for 1 h at room temperature. β2AR recycling was assessed by washing out the agonists after 10 min and allowing receptor recovery for an additional 60 min. Images were obtained using a Zeiss Axioplan 2 microscope with Metamorph software, or a Zeiss 710 confocal microscope.
Statistical analysis
Curve-fitting and statistical analyses were performed using Prism (GraphPad Software, San Diego, CA, USA). All experiments were performed independently at least three times. Data are presented as the mean ± standard deviation (SD). Statistical significance was determined using ANOVA or Student’s t-test; a value of P < 0.05 was considered significant.
Results
Neonatal cardiac myocyte contraction response to Epi or NE under different stimulation conditions
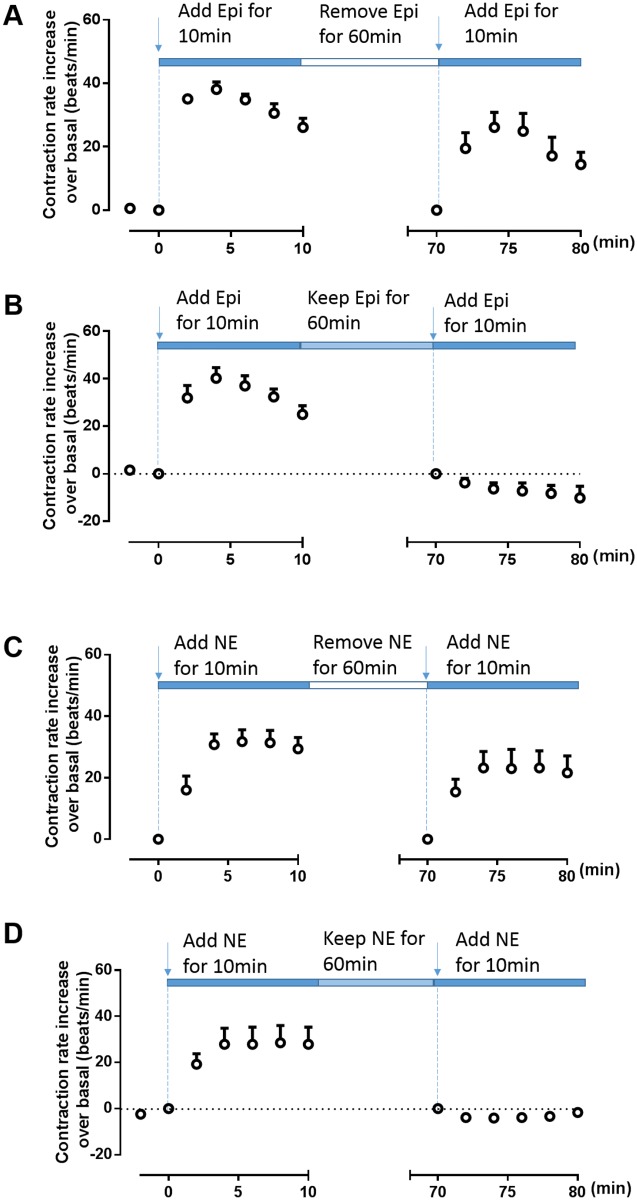
Short-time stimulation (10 min) with Epi or NE rapidly increased the neonatal cardiomyocyte contraction rate, reaching a maximum at 5 min, followed by a slow decline. When the agonist was removed for 60 min and then re-added at the same concentration, the increase in the cardiomyocyte contraction rate was similar to the initial response (Fig 1A and 1C). However, when the cells were exposed continuously to Epi or NE for 60 min beyond the initial 10-min stimulation, a second agonist stimulation did not increase the contraction response and, in fact, weakened the spontaneous contractions to below the basal level (Fig 1B and 1D). These results suggested that continuous β2AR stimulation caused functional desensitization of cardiac myocytes, which prevented contraction rate increases in response to a second agonist challenge. In contrast, removal of the β2AR agonist after 10 min allowed recovery of the contraction response, indicating functional resensitization of the receptor. This suggested that these treatment scenarios provided excellent in vitro cell models for the β2AR desensitization/resensitization response in cardiac myocytes.
Fig 1. Cardiomyocyte contraction response to epinephrine or norepinephrine under different stimulation conditions.
Cardiac myocytes isolated from β1AR knockout mice that express endogenous β2AR were stimulated with 10 μM epinephrine (Epi) or norepinephrine (NE) for 10 min and then re-challenged with (B, D) or without (A, C) Epi or NE for 60 min. The Epi- (A, B) or NE- (F, H) induced contraction rates were recorded. Data are shown as means ± SDs from three independent experiments.
Epi-induced internalization and recycling of neonatal cardiomyocyte β2AR under different stimulation conditions
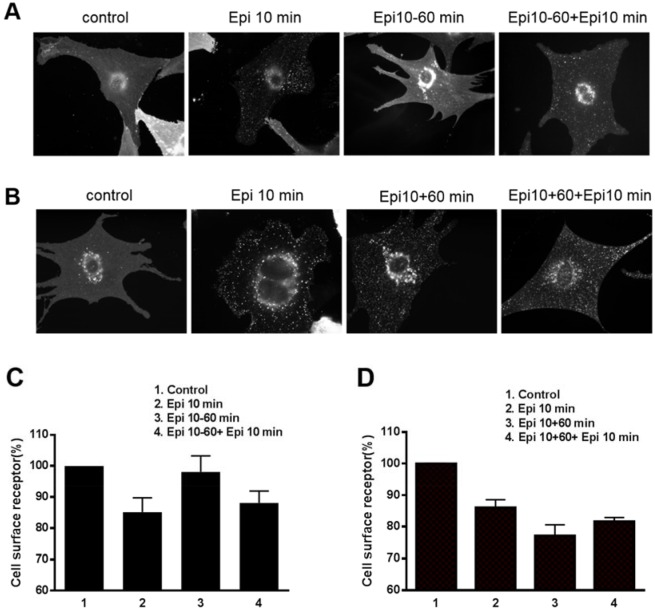
The desensitization/resensitization of β2ARs is related to receptor internalization and recycling. We assessed these processes in our in vitro cardiac myocyte model using the same contraction response conditions as for Epi stimulation. DKO cardiac myocytes transfected with FLAG-β2AR adenovirus were treated with Epi for 10 min. Internalization of FLAG-β2ARs was observed clearly as punctate intracellular staining (Fig 2). Interestingly, the intracellular punctate staining disappeared after Epi withdrawal for 60 min, suggesting that the receptors were recycled back to the cell surface. When the myocytes were stimulated with Epi a second time, the β2ARs were again rapidly (10 min) internalized (Fig 2A and 2C). In contrast, in cells exposed to Epi for 60 min, the internalized FLAG-β2ARs aggregated within the cytoplasm rather than recycling back to the cell surface. When these cells were stimulated with Epi a second time, the receptors remained in the cytoplasm (Fig 2B and 2D). This finding confirmed that hyper-stimulation by a β2AR agonist led to receptor desensitization.
Fig 2. Epinephrine-induced internalization and recycling of β2ARs under different stimulation conditions in cardiomyocytes.
Neonatal cardiomyocytes isolated from β1β2AR double-knockout mice were transfected with FLAG-tagged wild-type β2AR at a multiplicity of infection of 100 for 24 h. After serum starvation for 2 h, the cardiac myocytes were stimulated with 10 μM epinephrine (Epi). Epi was then removed (A) or retained (B) for 60 min followed by Epi re-stimulation for another 10 min. Cardiomyocytes were fixed and stained with anti-FLAG and Alexa 488-conjugated goat anti-mouse antibodies. (A) Punctate intracellular staining of FLAG-tagged β2ARs was observed after 10 min of Epi stimulation. The Epi-activated FLAG-β2AR efficiently recycled back to the cell surface after removal of the drug. Epi-activated FLAG-β2AR was rapidly internalized after secondary stimulation. (B) When Epi was present continually, the receptor was not recycled but remained internalized. Photographs representative of 3 different preparations of cardiomyocytes are shown. The cell surface receptors were quantified by a fluorescence-linked immunosorbent assay (FLISA) with Epi treatment for 10 min and then Epi was removed (C) or retained (D) for 60 min followed by Epi re-stimulation for another 10 min.
Mutation at the β2AR Ser355/356 sites impaired internalization of β2ARs in neonatal cardiac myocytes
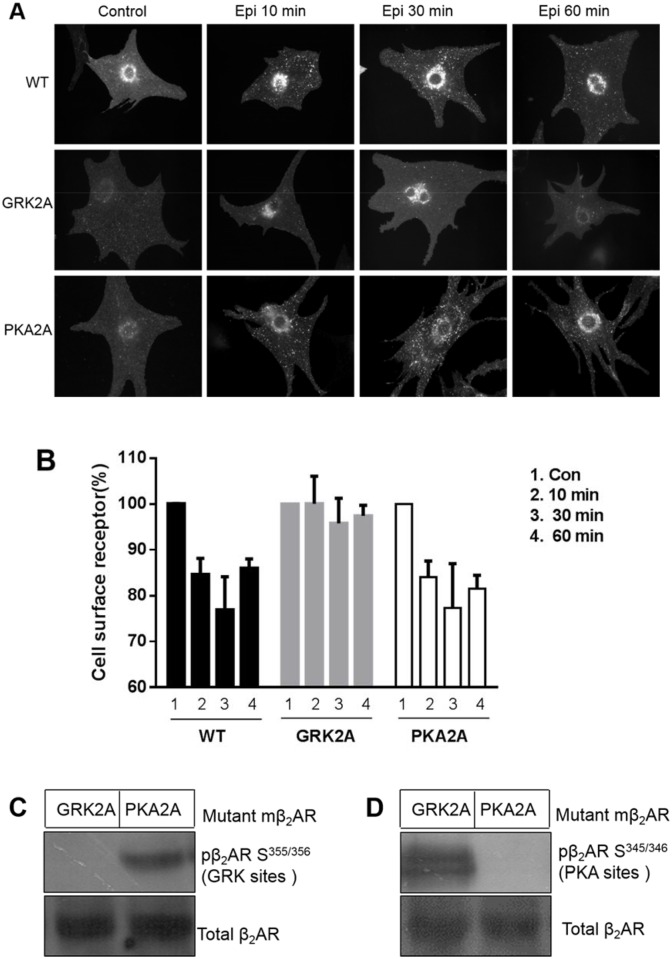
To further assess the importance of β2AR phosphorylation for receptor internalization, we mutated Ser355/356 (GRK2A) or Ser345/346 (PKA2A) to alanines to generate β2ARs with defective phosphorylation sites. When these mutant β2ARs were expressed in DKO cardiac myocytes and stimulated with Epi, the PKA2A mutant receptors generated punctate intracellular staining similar to that in wild-type cells. In contrast, the GRK2A mutant receptors showed impaired internalization (Fig 3, middle panel). In addition, we confirmed that mutation at the Ser355/356 sites of β2AR completely blocked the Ser355/356 phosphorylation after exposure to Epi, while the PKA2A mutant showed no phosphorylation effects (Fig 3B). Thus, PKA2A but not GRK2A mutations impair β2AR phosphorylation upon Epi treatment (Fig 3C). These results confirmed that the phosphorylation status of Ser355/356 was essential for β2AR internalization.
Fig 3. Mutation of β2AR S355/356 sites impaired internalization of β2ARs.
Wild-type and two mutant forms of β2AR (GRK2A and PKA2A) were expressed in neonatal cardiomyocytes isolated from β1β2AR double-knockout mice by adenovirus transfection for 2 days. (A) The cells were stimulated with Epi (10 μM) for 10, 30, or 60 min. Compared to wild-type, GRK2A β2AR (mutation at S355/356), but not PKA2A β2AR (mutation at S345/346), impaired receptor internalization. Photographs representative of 3 different preparations of cardiomyocytes are shown. (B) The cell surface receptors of wild-type or mutant β2AR were quantified by FLISA upon Epi stimulation for the indicated times. The quantitative data represent the means ± SDs of at least 3 different experiments. (C) Cardiomyocytes from β1β2AR double-knockout mice were transfected with GRK2A and PKA2A mutant β2ARs for two days and then lysed with radioimmunoprecipitation assay buffer. The lysates were subjected to SDS-PAGE followed by immunoblot analysis using a polyclonal anti-phosphoserine (355, 356)-specific or anti-phosphoserine (345, 346)-specific β2AR antibody. Phospho-β2AR Ser355/356 (GRK sites) was not observed in GRK2A (mutation of β-AR at Ser355/356), but was present in PKA2A (mutation of β-AR at Ser345/346). (D) In contrast, Phospho-β2AR Ser345/346 was not observed in PKA2A (mutation of β-AR at Ser345/346), but was present in GRK2A (mutation of β-AR at Ser355/356).
Epi-induced β2AR phosphorylation at Ser355/356 but not at Ser345/346 was related to receptor internalization in neonatal cardiac myocytes
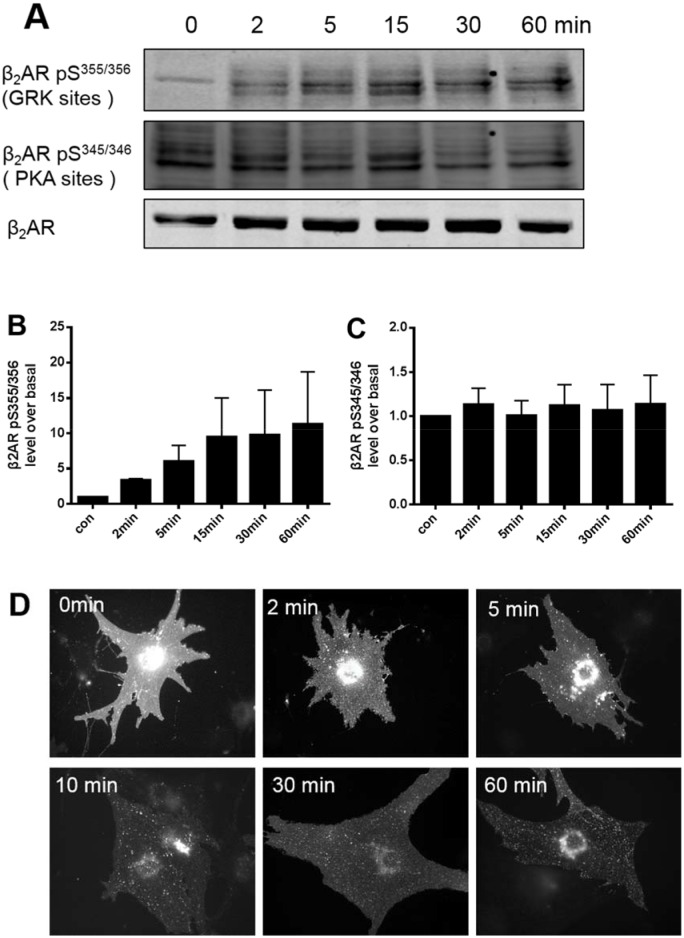
To further assess whether phosphorylation of β2ARs correlated with desensitization and resensitization, cardiac myocytes from neonatal DKO mice were transfected with β2ARs and stimulated with Epi. There was increased β2AR phosphorylation at Ser355/356 but not at Ser345/346 following 2 to 60 min of continuous stimulation with Epi (Fig 4A–4C). Correspondingly, the internalized β2ARs accumulated time-dependently within the cytoplasm during long, uninterrupted stimulation (Fig 4D). This suggested that Epi promotes GRK phosphorylation at Ser355/356, which was vital for receptor internalization in cardiomyocytes.
Fig 4. Epinephrine-induced β2AR phosphorylation at S355/356 (GRK) but not at S345/346 (PKA) is related to receptor internalization in cardiac myocytes.
(A) Neonatal cardiomyocytes from β1β2AR double-knockout mice were transfected with the β2AR adenovirus for 2 days and then stimulated with epinephrine (Epi; 10 μM) for 2, 5, 15, 30, or 60 min. Cell lysates were subjected to SDS-PAGE followed by immunoblot analysis using polyclonal anti-phosphoserine-(355, 356) or -(345, 346)-specific β2AR antibodies. The blots were stripped and probed with an anti-C-terminal β2AR antibody to visualize total β2ARs. (B) and (C) Relative quantitative analysis of the levels of phosphor-β2AR was performed by normalizing the phosphor-β2AR signal against the total β2AR. (D) Punctate intracellular staining of FLAG-β2ARs was observed at all time points of Epi stimulation, indicating that Epi induced receptor internalization.
Blocking phosphorylation of β2ARs at Ser355/356 sites impaired their internalization and the contraction response
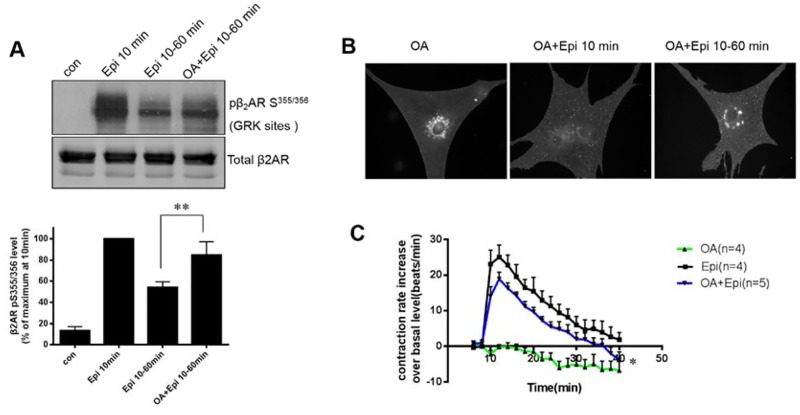
To confirm whether phosphorylation of β2ARs at Ser355/356 was critical to receptor desensitization and resensitization, cardiomyocytes overexpressing β2ARs were pretreated with okadaic acid (OA), a PP2A inhibitor, prior to Epi stimulation. Epi induced rapid (10 min) receptor phosphorylation at Ser355/356 (Fig 5A). After removal of the drug, β2ARs underwent dephosphorylation over 60 min. Pretreatment with OA attenuated β2AR dephosphorylation (Fig 5A). In addition, the blockade of β2AR dephosphorylation by OA resulted in the accumulation of phosphorylated β2ARs in the cytoplasm after withdrawal of Epi for 60 min (Fig 5B). Interestingly, OA treatment not only reduced receptor recycling to the cell surface but also diminished the contraction response to Epi treatment (Fig 5C).
Fig 5. Blocking of phosphorylation of the β2AR S355/356 sites inhibits the contraction response and impairs internalization of β2AR.
Cardiomyocytes from β1β2AR double-knockout mice were transfected with the β2AR adenovirus for 2 days and then stimulated with epinephrine (Epi) (10 μM) for 10 min with or without okadaic acid (OA) pretreatment. Epi was then removed for 60 min. (A) Phosphorylation of the β2ARs at Ser355,356 and (B) internalization of the β2ARs were examined. (C) Cardiac myocytes isolated from β1AR knockout mice were used for the contraction rate assay. Cells were stimulated with Epi with or without OA pre-treatment. The contraction response curves represent the means ± SDs of n beating dishes from at least 3 different neonatal cardiomyocyte preparations. *p < 0.05 vs. Epi-treated group (two-way ANOVA). **p < 0.05 vs. group without OA treatment (Student’s t-test).
Epinephrine induced an interaction between β2ARs and β-arrestin 2
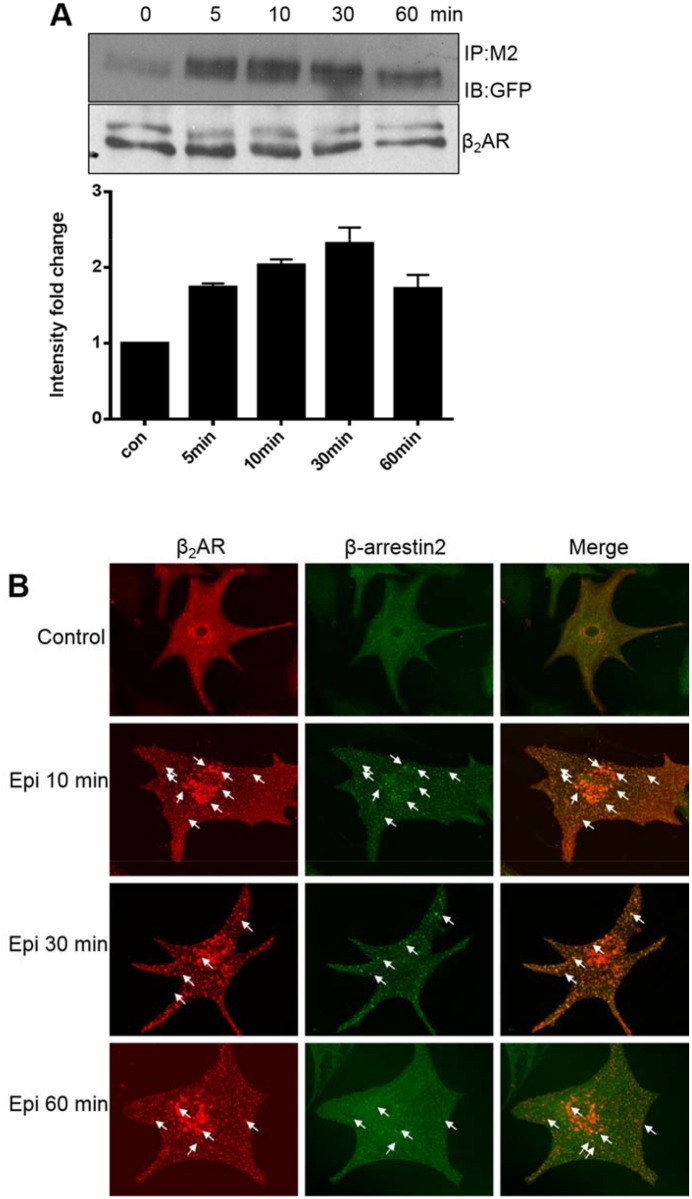
To determine the interaction between β2ARs and the cytoplasmic protein β-arrestin 2 after internalization, we constructed two specifically labeled plasmids that expressed β2ARs and β-arrestin 2. Two days after transfection, DKO cardiac myocytes that highly expressed FLAG-mβ2AR and β-arrestin-GFP were stimulated with Epi for 5 to 60 min. Co-immunoprecipitation demonstrated that phosphorylated β2ARs underwent internalization and interacted with β-arrestin 2 (Fig 6A). Furthermore, immunofluorescence experiments verified that β2ARs interacted and co-located with β-arrestin 2, and that the interacted proteins time-dependently assembled in the cytoplasm (Fig 6B). This suggested that β-arrestin 2 played a significant role in the process of receptor dephosphorylation.
Fig 6. Epinephrine induces interactions between β2ARs and β-arrestin 2.
Cardiac myocytes from β1β2AR double-knockout mice were transfected to express FLAP-β2AR and β-arrestin-GFP for 2 days, and then stimulated with epinephrine (Epi) for 5, 10, 30, or 60 min. (A) Cell lysates were immunoprecipitated with an anti-Flag-m2 antibody. Immunoblot analyses were performed using anti-GFP or β-arrestin antibodies. (B) Cells were fixed for immunofluorescence staining. Intracellular localization of Flag-mβ2AR and β-arrestin-GFP was observed at the indicated times of Epi stimulation. The results presented are representative of at least 3 repeated experiments. Arrows show co-localization of internalized β2ARs and β-arrestin.
Discussion
We report the functional resensitization of β2ARs as assessed by a neonatal cardiomyocyte contraction assay in vitro, as well as the receptor internalization response under repeated agonist stimulation. These responses involved β2AR phosphorylation on the Ser355/356 sites. Desensitization of GPCRs is an important physiological feedback mechanism that protects cells against acute and chronic receptor overstimulation [11]. Indeed, we found that the continuous presence of Epi for 60 min caused β2AR functional desensitization by showing that a second β2AR stimulation did not increase the cardiomyocyte contraction rate after continuous stimulation (Fig 1B). In contrast, early withdrawal of Epi (10 min) caused functional resensitization of β2AR as shown by a similar contraction response when compared to the initial stimulation with Epi (Fig 1A).
Sustained stimulation of β2ARs with Epi promoted receptor internalization and their retention in the cytoplasm (Fig 2B), while withdrawal of Epi helped the receptors recycle back to the cell surface and re-internalize with a second stimulation (Fig 2A). Importantly, we found that phosphorylation of β2ARs at Ser355/356 sites was critical to this physiological resensitization and internalization. Specifically, elimination of β2AR phosphorylation at Ser355/356 but not Ser345/346 by mutation to alanine completely blocked receptor internalization, even with sustained Epi stimulation (Fig 3A). At the same time, inhibiting dephosphorylation of β2AR at Ser355/356 by OA treatment decreased the contraction response in cardiomyocytes (Fig 5C).
In HEK293 cells, Ser355/356 plays a pivotal role in desensitization and internalization of β2ARs [13]. In the present study, we confirmed that phosphorylation of β2ARs at Ser355/356 contributed to receptor internalization in primary neonatal cardiomyocytes. This finding is consistent with previous data from cardiomyocytes [11]. GRK2 and GRK5 are major GRKs expressed in the heart, while GRK6 is usually expressed at low levels in this tissue [12]. We, and others, have reported that GRK2 could phosphorylate β2AR at Ser355/356 affecting β2AR trafficking and the contraction rate response in cardiomyocytes [14]. It has been suggested that GRK6 promotes phosphorylation of β2AR at Ser355/356, while GRK2 inhibits phosphorylation at these sites in HEK293 cells stimulated with isoproterenol [6]. Furthermore, these GRK6 sites were primarily responsible for β2AR desensitization and β-arrestin-mediated ERK activation, but not for internalization.
Previous observations suggested that β2ARs could be phosphorylated by different GRKs in various cells or tissues, thereby mediating distinct physiological functions [15,16]. When we eliminated the Ser345/346 PKA phosphorylation sites, the internalization of β2ARs did not change, indicating that these phosphorylation sites are not necessary for receptor internalization in cardiomyocytes. With Epi continually present in the medium for 60 min, the phosphorylation state of β2AR at Ser355/356 in cardiomyocytes was sustained, and the receptor was retained in the cytoplasm rather than recycled (Fig 4D). In this condition, Ser345/346 sites were not phosphorylated, confirming that phosphorylation of β2ARs at these sites might not be involved in receptor internalization.
Although 13 β2AR phosphorylation sites have been identified in HEK293 cells using isoproterenol treatment [6], the phosphorylation pattern of β2ARs in cardiomyocytes following Epi stimulation was unknown. In addition, whether all 13 phosphorylation sites were essential for receptor internalization and resensitization remained to be elucidated. In the present work, we found that dephosphorylation at Ser355/356 sites 60 min after agonist removal allowed a second functional response, including receptor re-internalization and an increased contraction rate. Therefore, while the total phosphorylation pattern was unclear in cardiac β2ARs with Epi stimulation, the β2AR Ser355/356 phosphorylation state definitely contributed to receptor desensitization and resensitization.
Our results also indicated that elimination of sustained agonist stimulation promoted receptor dephosphorylation at Ser355/356 and recovery of the receptor and cell functions in response to a second agonist challenge. PP2A is important for regulating the dephosphorylation of β2AR in cardiomyocytes [17]. The balance between GRK and PP2A determines the phosphorylation status of β2AR and affects different functions including internalization and resensitization. For example, β2AR resensitization is defective in severe asthma [18]. In this case, PP2A activity is reduced, possibly due to decreased β2AR dephosphorylation at Ser355/356, leading to β2AR dysfunction. The molecular mechanisms of βAR resensitization have been reported to also involve the inhibition of phosphoinositide 3-kinase-γ in the heart [7]. This inhibition increased PP2A activity and effectively dephosphorylated βARs. β2AR dephosphorylation at Ser355/356 was involved in this process, which is consistent with our observations. In addition, some scaffolding A-kinase-anchoring proteins may regulate βAR resensitization by targeting the phosphatase-receptor complex [19,20]. However, additional details of the resensitization mechanism of βARs in cardiomyocytes need to be elucidated.
It is believed that β2AR internalization is required for resensitization. Once internalized, receptors undergo dephosphorylation in the early endosomes by PP2A [17] before they recycle back to the plasma membrane [3]. β-Arrestin plays a central role during this receptor internalization (sequestration) process that contributes to normal β2AR dephosphorylation and resensitization [21]. β-Arrestin- and clathrin-coated vesicles appear to be needed for β2AR internalization, which targets receptors to the endosome for dephosphorylation and resensitization [21]. We confirmed that β-arrestin physically interacted with Ser355/356 phosphorylated β2ARs in cardiomyocytes. Additionally, we found that β-arrestin was bound to β2ARs for a longer time in the continuous presence of the agonist, slowing the dephosphorylation process, which might block the functional resensitization of the receptors.
Together, these data revealed that the Ser355/356 phosphorylation status of β2ARs in neonatal cardiomyocytes is critical for receptor internalization and functional resensitization. This finding suggested that the physiological function of β2ARs not only depends on agonist stimulation, but also on specific conditions in cells or tissues, as well as the current receptor phosphorylation modification status.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81270321, 81200576) and the Natural Science Foundation of Zhejiang Province (LY16H010005). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81270321, 81200576) and Natural Science Foundation of Zhejiang Province (LY16H010005). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McGraw DW, Liggett SB. Molecular mechanisms of β2-adrenergic receptor function and regulation. Proc Am Thoracic Soc. 2005;2: 292–296; discussion 311–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of β2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem. 2002;277: 34280–34286. [DOI] [PubMed] [Google Scholar]
- 3.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415: 206–212. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: Biasing seven-transmembrane receptors. Nature Rev Drug Discov. 2010;9: 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through β-arrestin. Sci STKE. 2005;2005: cm10 [DOI] [PubMed] [Google Scholar]
- 6.Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, et al. Distinct phosphorylation sites on the β2-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal. 2011;4: ra51 10.1126/scisignal.2001707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasudevan NT, Mohan ML, Goswami SK, Naga Prasad SV. Regulation of β-adrenergic receptor function: An emphasis on receptor resensitization. Cell Cycle. 2011;10: 3684–3691. 10.4161/cc.10.21.18042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu R, Ramani B, Soto D, De Arcangelis V, Xiang Y. Agonist dose-dependent phosphorylation by protein kinase A and G protein-coupled receptor kinase regulates β2 adrenoceptor coupling to Gi proteins in cardiomyocytes. J Biol Chem. 2009;284: 32279–32287. 10.1074/jbc.M109.021428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J Biol Chem. 1999;274: 16701–16708. [DOI] [PubMed] [Google Scholar]
- 10.Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP Jr., et al. Targeted disruption of the mouse β1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci U S A. 1996;93: 7375–7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devic E, Xiang Y, Gould D, Kobilka B. β-Adrenergic receptor subtype-specific signaling in cardiac myocytes from β1 and β2 adrenoreceptor knockout mice. Mol Pharmacol. 2001;60: 577–583 [PubMed] [Google Scholar]
- 12.Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. Sequential binding of agonists to the β2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem. 2004;279: 686–691. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan DJ, Millman EE, Godines V, Friedman J, Tran TM, Dai W, et al. Role of the G protein-coupled receptor kinase site serine cluster in β2-adrenergic receptor internalization, desensitization, and β-arrestin translocation. J Biol Chem. 2006;281: 7684–7692. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, De Arcangelis V, Gao X, Ramani B, Jung YS, Xiang Y. Norepinephrine- and epinephrine-induced distinct β2-adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. 2008;283: 1799–1807. [DOI] [PubMed] [Google Scholar]
- 15.Tobin AB, Butcher AJ, Kong KC. Location, location, location. Site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29: 413–420. 10.1016/j.tips.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobin AB. G-protein-coupled receptor phosphorylation: Where, when and by whom. Br J Pharmacol. 2008;153 Suppl 1: S167–176. 10.1038/sj.bjp.0707662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. The role of sequestration in G protein-coupled receptor resensitization. Regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem. 1997;272: 5–8. [DOI] [PubMed] [Google Scholar]
- 18.Gupta MK, Asosingh K, Aronica M, Comhair S, Cao G, Erzurum S, et al. Defective resensitization in human airway smooth muscle cells evokes β-adrenergic receptor dysfunction in severe asthma. PloS One. 2015;10: e0125803 10.1371/journal.pone.0125803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao J, Wang HY, Malbon CC. Protein kinase a regulates AKAP250 (gravin) scaffold binding to the β2-adrenergic receptor. EMBO J. 2003;22: 6419–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cong M, Perry SJ, Lin FT, Fraser ID, Hu LA, Chen W, et al. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J Biol Chem. 2001;276: 15192–15199. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Barak LS, Winkler KE, Caron MG, Ferguson SS. A central role for β-arrestins and clathrin-coated vesicle-mediated endocytosis in β2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem. 1997;272: 27005–27014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.