Abstract
The grey zone (GZ; 45–54 CGG repeats in the FMR1 gene) is considered a normal allele, however several studies have found a high frequency of GZ in movement disordered populations. Here, we describe neurological features of FXTAS in two carriers of GZ alleles, although FXTAS has been defined as occurring only in premutation carriers (55–200 CGG repeats). Both patients had family members who had the premutation and were diagnosed with FXTAS. The presence of relatively high GZ alleles with elevated FMR1-mRNA combined with a family history of FXTAS that may represent a facilitating genetic background for FXTAS are the factors that led to the presence of FXTAS in these individuals with a GZ allele. Further research into clinical involvement of GZ alleles is recommended and the definition of FXTAS may requires revision.
Keywords: ataxia, FMR1-mRNA, FXTAS, grey zone, premutation, tremor
INTRODUCTION
In 2001 the first 5 cases of the fragile X-associated tremor ataxia syndrome (FXTAS) were described and subsequent studies have validated the clinical features of intention tremor, cerebellar ataxia, autonomic dysfunction, neuropathy, and cognitive decline1,2. The cause of FXTAS is thought to be due to the elevated fragile X mental retardation 1 mRNA (FMR1-mRNA) levels observed in all premutation carriers (55–200 CGG repeats in the FMR1 gene) leading to RNA toxicity in the neural cells3.
The grey zone (GZ, 45–54 CGG repeats) allele is considered normal. However, several researchers have studied involvement in the GZ. Loesch et al reported a more than two-fold increased incidence of the GZ allele in patients with parkinsonism, one of the features in FXTAS and suggested a potential role for the GZ in neurodegeneration4. A slightly elevated frequency of GZ alleles was also found in male MSA patients in European MSA populations5. Kenneson et al. found reduced fragile X mental retardation protein (FMRP) levels in GZ alleles6, while Loesch et al observed increased FMR1-mRNA levels for alleles as low as 39 CGG repeats in size7. More recently, Hall et al reported 3 patients with FMR1 GZ alleles who met diagnostic criteria for FXTAS8. The consequence of these findings is the possibility of the development of FXTAS or related neurological symptoms in some GZ carriers. The neurodegenerative changes including FXTAS would likely be associated with the toxic gain-of-function mechanism of raised FMR1-mRNA. Here, we report 2 carriers of GZ alleles exhibiting FXTAS.
Clinical presentation of grey zone with FXTAS
Patient 1 is a 58-year-old male with a GZ of 52 CGG repeats and his FMR1-mRNA level is 1.83±0.08 fold above normal. He noticed tremor at age 55 when holding a coffee cup. At age 56, he noticed balance problems and handwriting difficulties. He had numbness and tingling in his feet. He has also had erectile dysfunction. He has noticed some weakness in his hands including a weak grip. His past medical history includes sleeping problems, type II diabetes, hypertension, migraine headaches, and eye surgery related to his diabetes.
Neurological examination: Finger to nose touching shows a bilateral intention tremor with a significant terminal tremor. His motor tone is increased on the left more than on the right. He has great difficulty with tandem walking. Deep tendon reflexes are 1+ in the upper extremities, 2+ at the knees, and absent at the ankles. Vibration sense is decreased on the left side. He has a dyskinesia with the right hand and a positive pull test. Brain MRI demonstrated mild brain atrophy and some scattered white matter disease involving the pons but not the middle cerebellar peduncles (MCPs). (Figure 1).
Figure 1. T1 and T2 weighted images of case 1 and 2 and normal control.
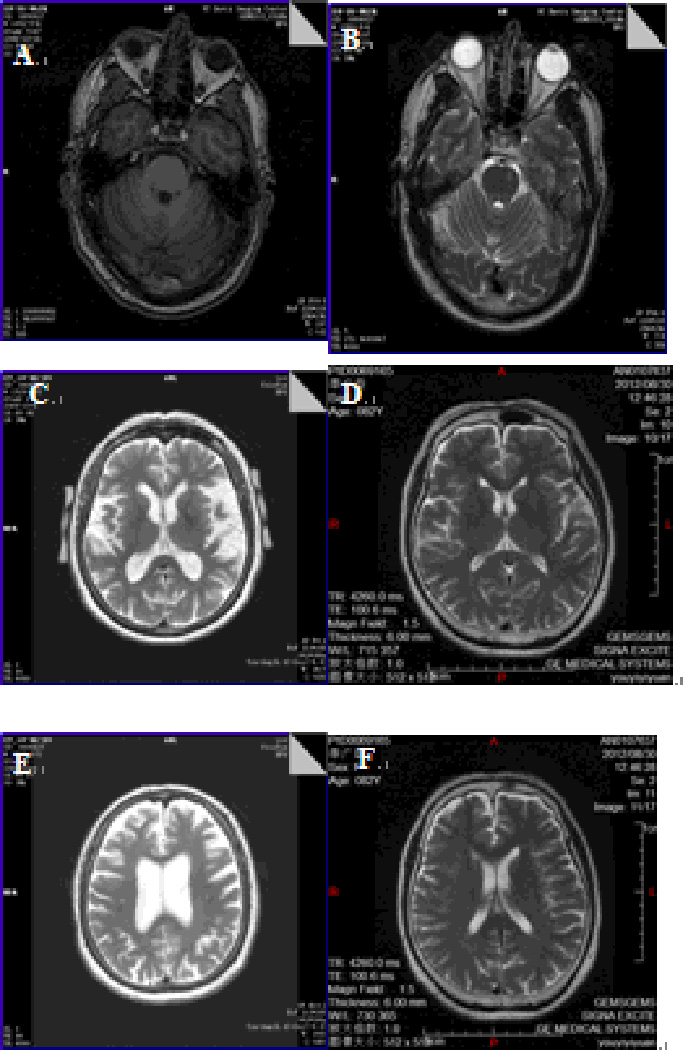
A) showing mild atrophy of the cerebellum in case 1.
B) showing increased signal intensity in the left of the pons in case 1.
C) showing atrophy of the temporal lobe in case 2.
D) showing normal T2 weighted images of normal control (female 83 years old).
E) showing atrophy of the frontal lobe and enlargement of the ventricules in case 2.
F) showing normal T2 weighted images of normal control (female 83 years old).
Family history: His brother (II:3) has the premutation and a GZ allele (52,68 CGG repeats; mRNA 2.62±0.34) and has been diagnosed with FXTAS and he has been previously reported (Chonchiaya et al 2009 JDBP). His brother has three daughters, one (III:2) with GZ (56 repeats) and symptoms of anxiety and two are premutation carriers (56 repeats (III:6) and 67 repeats (III:7)). The daughter with GZ has two sons (IV:1 and IV:2) with the premutation (61 and 69 CGG repeats) and autism. Another daughter (III:6) has a daughter (IV:4) with the premutation (69 repeats) and she has symptoms of anxiety, ADHD and shyness which are typically seen in premutation carriers (Figure 2).
Figure 2. Pedigree of the family of patient 1 (II:1, indicate with arrow).
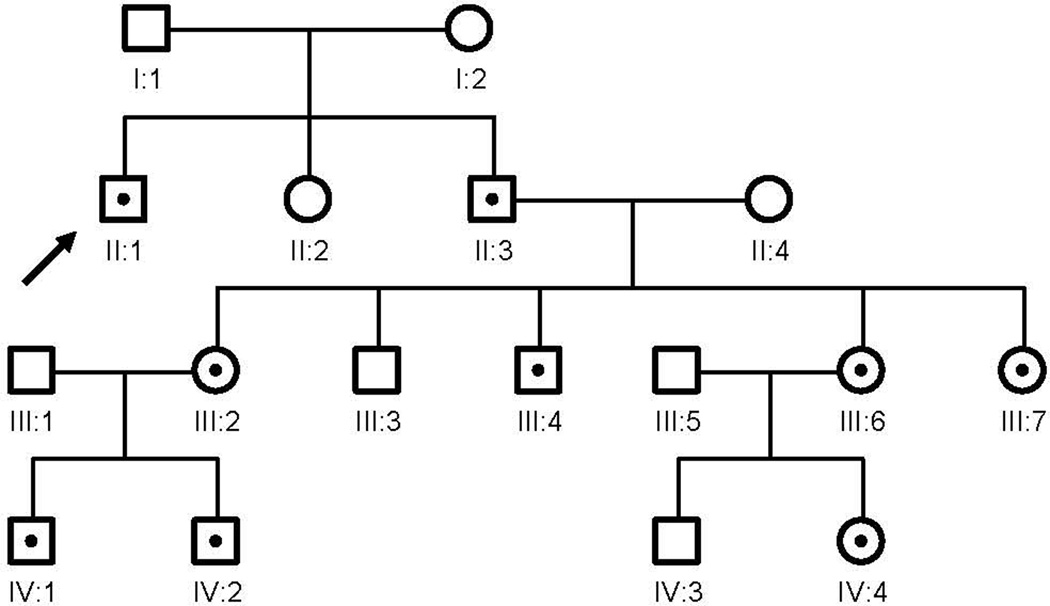
His brother (II:3), has the premutation and GZ allele and has been diagnosed with FXTAS. His brother has three daughters, one with a GZ (III:2) and two are premutation carriers (III:6 and III:7). The daughter with the GZ had two sons with the premutation and autism (IV:1 and IV:2) and III:6 has a daughter with the premutation allele (IV:4).
Patient 2 is an 80-year old female with a GZ of 53 CGG repeats and her FMR1-mRNA levels was 1.35±0.07 fold above normal. Her neurological symptoms began at age 52 with an intermittent intention tremor in her right hand. It began to involve her left side within 2 years. At age 75 she developed balance problems. At age 79, she began using a cane on a regular basis. She had burning and numbness in her lower legs. She has had autonomic dysfunction including urinary frequency since age 55 and she has also been anorgasmic throughout her life. Other medical problems include migraine headaches, osteoarthritis, anxiety problems, and uterine and cervical cancer.
Her family history is significant in that her father and uncle had and FXTAS tremor and ataxia consistent with FXTAS. Her uncle was seen before his death and his neuropathology studies documented the inclusions of FXTAS in both neurons and astrocytes and his MRI had the MCP sign. Her uncle has three daughters and all three of his daughters have children diagnosed with fragile X syndrome (Figure 3).
Figure 3. Pedigree of the family of patient 2 (III:1, indicate with arrow).
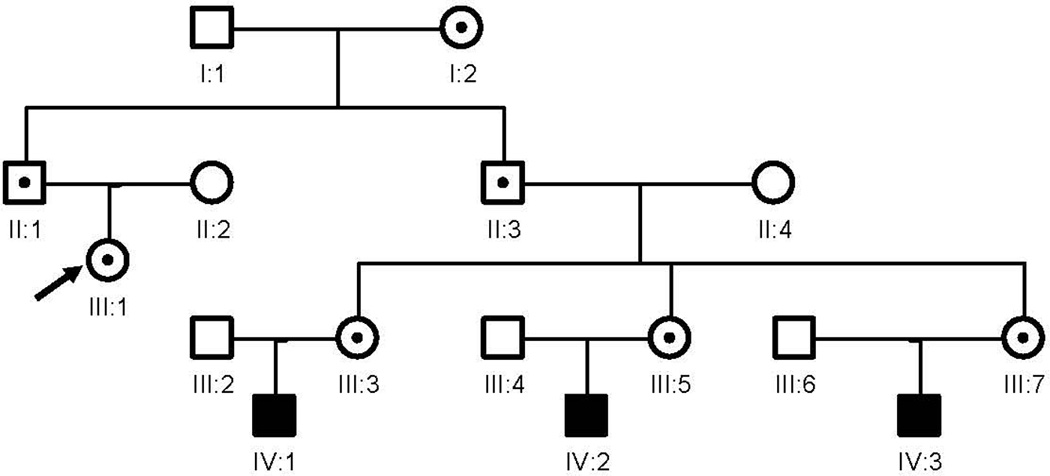
Her father (II:1) and her uncle (II:3) are premutation carriers and both have been diagnosed FXTAS. Her uncle (II:3) has three daughters (III:3; III:5; and III:7) who have premutation alleles and all of them have children who had been diagnosed fragile X syndrome (IV:1; IV:2; and IV:3).
Upon examination, a “no-no” head tremor is persistent throughout the exam. She has an intermittent resting tremor in her hands and an intention tremor bilaterally. She is ataxic, cannot tandem walk, and uses a cane. Deep tendon reflexes are 1+ and symmetrical in the upper extremities and her knees, absent at ankles. Her vibration sense is decreased in both feet. Brain MRI showed moderate to severe loss of the brain volume, enlargement of the ventricles, and limited white matter disease (Figure 1). Significant atrophy can also occur in individuals who are 80 years old and it is likely that a combination of atrophy from age and FXTAS are present in this case, although her MRI demonstrates far more atrophy than a control of her age (Figure 1).
DISCUSSION
FXTAS is defined as a premutation disorder2,9. Patient 1 and 2 have most of the neurological features of FXTAS but not a repeat number in the premutation range as delineated by American College of Human Genetics (ACHG)10. The lower cut off for the premutation at 55 repeats was set by ACHG related to a concern for the risk of expansion to the full mutation in one generation, but this delineation of CGG repeat number does not take into account possible clinical involvement of premutation or GZ carriers related to elevation of FMR1-mRNA. The cause of FXTAS is thought to be the elevated FMR1-mRNA, which is typically 2 – 8 fold in premutation carriers11. However, elevations in FMR1-mRNA typically occur on a continuum that starts in the GZ range7. Loesch et al4 also observed a 2 fold increase of GZ alleles in males with Parkinsonism.4. More recently, increased levels of the antisense (ASFMR1) transcript were described in a cohort of subjects with GZ and low premutation alleles and parkinsonian phenotype suggesting a potential cytotoxic effect of elevated levels of both the FMR1 and ASFMR1 genes leading to mitochondrial dysfunction and neurodegenerative disorders12.
From the onset of the intention tremor, delay of onset of ataxia was 1 year in patient 1 and, 23 years in patient 2. A longitudinal study of FXTAS showed that from the onset of intention tremor, median delay of onset of ataxia was 2 years13. When compared to the progression in the above study, patient 2 had a much slower progression, which is seen in many females with FXTAS. The X-inactivation with a diluting effect of the second normal X chromosome may have contributed to her slow progression. It is also possible that FXTAS, when it occasionally occurs in GZ carriers, may exhibit a slower deterioration in the GZ than that in the premutation.
Family members of both our patients had the premutation and were earlier diagnosed with FXTAS; thus it is likely that these families have a facilitating genetic background acting in concert with the premutation or GZ to produce FXTAS. In both patients, the alleles were in the upper GZ range with elevated FMR1-mRNA combined with a family background that could lead to the observed symptoms of FXTAS. FXTAS is the late outcome of an RNA-associated pathogenic process that in mouse models actually begins early in life, which may also be true in humans14. However, not all premutation carriers develop FXTAS so there may be additional genetic or environmental factors that facilitate the development of FXTAS. Here we propose that FXTAS may also be present in some GZ carriers with the addition of facilitating genetic and environmental factors.
Our report draws attention to the need for larger studies in GZ carriers who may have a clinical picture similar to FXTAS. Further neuropathological studies are needed in GZ carriers who die of neurological problems to clarify if indeed this is truly FXTAS with the characteristic neuropathological features including inclusions and spongiform changes in the white matter15. Although our patients did not have the classic MCP sign seen in 60% of males and 13% of females with FXTAS16, their features are otherwise classic for FXTAS and they meet criteria for probable FXTAS2. We therefore recommend that the definition of FXTAS should not be limited to just the 55–200 repeat range, but instead this definition should be broadened to include the GZ, at least in the upper GZ allele CGG repeat range and with facilitating genetic background.
Acknowledgments
Dr. Hagerman’s work has been funded by the NIH. Other funding has been received for clinical trials from Seaside Therapeutics, Roche, Forest, Curemark, and Novartis.
Dr. Zhang’s work has been funded by the NIH. Other funding has been received for clinical trials from Boehringer-Ingelheim, and Allergan. Dr. Zhang serves as consultant and/or adviser for Allergan, Dysport, Teva Neuroscience, and Merz. Dr. Zhang serves as speakers for Allergan, Novartis, and Teva Neuroscience.
Dr. Ying Liu’s work has been funded by Teva Neuroscience, and Allergan.
This research was supported by the NIH grants HD036071 and HD02274, Neurotherapeutic Research Institute (NTRI) grants DE019583 and DA02484, National Institute on Aging grants AG032119 and AG032115, the National Center for Research Resources UL1 RR024146, and the Health and Human Services Administration of Developmental Disabilities grant 90DD05969. The authors thank Patrick Adams for his providing the brain MRI images.
Footnotes
Conflict of Interest:
There are no other conflicts of interest from the authors.
REFERENCES
- 1.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 2.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72(4):869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raske C, Hagerman PJ. Molecular pathogenesis of fragile X-associated tremor/ataxia syndrome. J Investig Med. 2009;57(8):825–829. doi: 10.231/JIM.0b013e3181be329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loesch DZ, Khaniani MS, Slater HR, et al. Small CGG repeat expansion alleles of FMR1 gene are associated with parkinsonism. Clin Genet. 2009;76(5):471–476. doi: 10.1111/j.1399-0004.2009.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamm C, Healy DG, Quinn NP, et al. The fragile X tremor ataxia syndrome in the differential diagnosis of multiple system atrophy: data from the EMSA Study Group. Brain. 2005;128(Pt 8):1855–1860. doi: 10.1093/brain/awh535. [DOI] [PubMed] [Google Scholar]
- 6.Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10(14):1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 7.Loesch DZ, Bui QM, Huggins RM, Mitchell RJ, Hagerman RJ, Tassone F. Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised, and correlate with the number of CGG repeats. J Med Genet. 2007;44(3):200–204. doi: 10.1136/jmg.2006.043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall D, Tassone F, Klepitskaya O, Leehey M. Fragile X–associated tremor ataxia syndrome in FMR1 gray zone allele carriers. Movement Disorders. 2012;27(2):297–301. doi: 10.1002/mds.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagerman RJ, Leavitt BR, Farzin F, et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004;74(5):1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddalena A, Richards CS, McGinniss MJ, et al. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001;3(3):200–205. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, et al. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13(4):555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loesch DZ, Godler DE, Evans A, et al. Evidence for the toxicity of bidirectional transcripts and mitochondrial dysfunction in blood associated with small CGG expansions in the FMR1 gene in patients with parkinsonism. Genet Med. 2011;13(5):392–399. doi: 10.1097/GIM.0b013e3182064362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leehey MA, Berry-Kravis E, Min SJ, et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22(2):203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Tassone F, Berman RF, et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19(1):196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greco C, Tassone F, Jacquemont S, et al. Intranuclear neuronal inclusions in two female carriers of the fragile X premutation. Am J Hum Genetics. 2003;73((suppl)(5)):A2452–A2586. 53rd Annual Meeting, Los Angeles, CA. [Google Scholar]
- 16.Adams JS, Adams PE, Nguyen D, et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69(9):851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]


