Abstract
Tea, a popular beverage made from leaves of the plant Camellia sinensis, has been shown to reduce body weight, alleviate metabolic syndrome, and prevent diabetes and cardiovascular diseases in animal models and humans. Such beneficial effects have generally been observed in most human studies when the level of tea consumption was 3 to 4 cups (600–900 mg tea catechins) or more per day. Green tea is more effective than black tea. In spite of numerous studies, the fundamental mechanisms for these actions still remain unclear. From a review of the literature, we propose that the two major mechanisms are: 1) decreasing absorption of lipids and proteins by tea constituents in the intestine, thus reducing calorie intake; and 2) activating AMPK by tea polyphenols that are bioavailable in the liver, skeletal muscle, and adipose tissues. The relative importance of these two mechanisms depends on the types of tea and diet consumed by individuals. The activated AMPK would decrease gluconeogenesis and fatty acid synthesis and increase catabolism, leading to body weight reduction and MetS alleviation. Other mechanisms and the health relevance of these beneficial effects of tea consumption remain to be further investigated.
Keywords: AMPK, diabetes, EGCG, obesity, tea
1. Introduction
Tea, made from leaves of the plant Camellia sinensis, has been used in ancient days for medicinal purposes and now as a popular beverage. Cultivated in more than 30 countries, more than 4 million metric tons of tea are produced annually. Depending on the processing of the tea leaves, tea is classified into two major types: green tea and black tea. While black tea is the major type of tea produced and consumed worldwide, green tea is more popular than black tea in China and Japan.
For the past three decades, tea has been studied for its beneficial health effects. These include the reduction of body weight, alleviation of metabolic syndrome (MetS), prevention of cardiovascular diseases (CVDs) and cancer, and protection against neurodegeneration (reviewed in [1]). Overweight, obesity and diabetes are emerging as major global health issues, and the closely related MetS also predisposes individuals to CVDs. If tea could prevent or delay the development of these diseases, then the public health implications would be tremendous. Many laboratory and epidemiological studies have shown that tea and tea polyphenols can decrease body weight, markers for MetS, and risk for diabetes and CVDs (reviewed in [1–4]). However, such beneficial effects have been observed only in some human studies, when the level of tea consumption was 3 to 4 cups or higher per day. Most of the observed beneficial effects are believed to be due to the polyphenols in tea, although caffeine also contributes to some of the effects. In spite of the many published studies, the underlying molecular mechanisms are still not fully understood. The dose-response relationship between tea consumption and health effects remains to be further substantiated.
This article reviews the evidence and discusses the molecular mechanisms for the mitigation of overweight and MetS, as well as related prevention of diabetes and CVDs by different types of tea. Based on this information, the possible human relevance of the published health effects will be evaluated. Because of the large number of publications on this topic, information from recently published meta-analyses and systematic reviews is used to help assess the relative strengths of the existing publications. It is hoped that this article will enhance our understanding of the health effects of tea consumption and stimulate future research.
2. Chemistry and Biological Actions of Tea Constituents
2.1 Major constituents of different types of tea
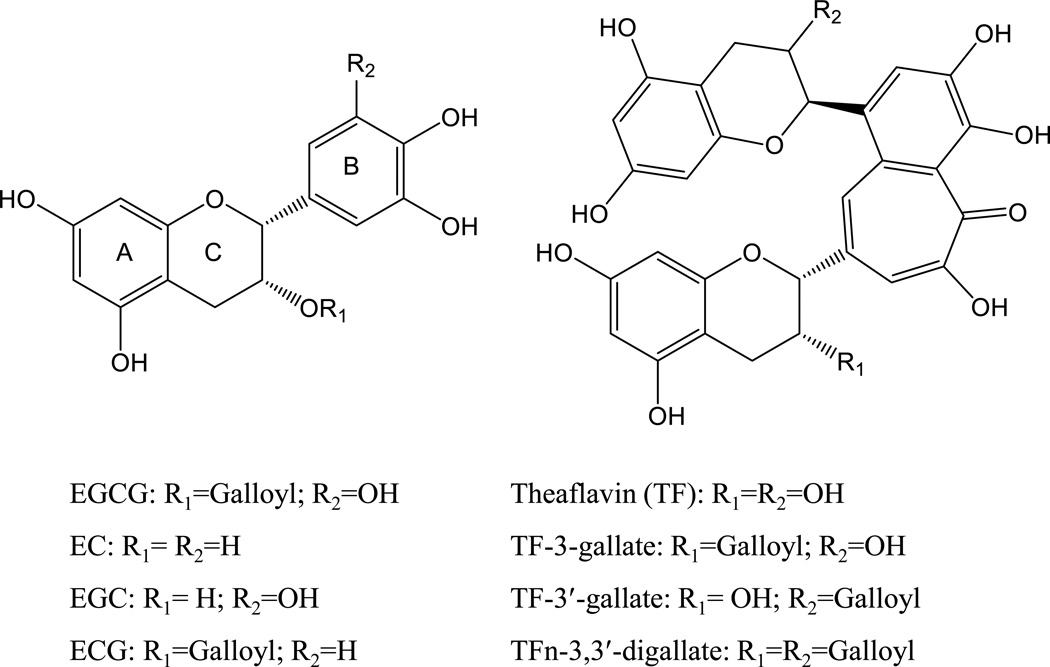
In the manufacturing of green tea, the tea leaves are heated to inactivate the enzymes, rolled and then dried. This process prevents the autolysis of the leaves and the oxidation of the constituents. The drying of the tea leaves also helps to stabilize the tea constituents during storage. Green tea contains characteristic polyphenolic compounds known as catechins, which include: (−)-epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), (−)-epigallocatechin, and (−)-epicatechin (Figure 1). Catechins account for about 30–42% of the dry weight of brewed green tea, and EGCG is the major form of tea catechin. Tea leaves also contain lower quantities of other polyphenols, such as quercetin, kaempferol, and myricetin as well as alkaloids, such as caffeine and theobromine. A typical brewed green tea beverage (e.g., 2.5 g tea leaves in 250 ml of hot water) contains 240–320 mg of catechins, of which 60–65% is EGCG, and 20–50 mg of caffeine [5, 6].
Figure 1.
Chemical structures of major tea polyphenols: catechins and theaflavins. Abbreviations are: EGCG, (−)-epigallocatechin-3-gallate; EGC, (−)-epigallocatechin; ECG, (−)-epicatechin-3-gallate; EC, (−)-epicatechin.
Black tea is commonly referred to as “fermented tea”, but it does not involve fermentation by microorganisms. In its manufacturing, the tea leaves are withered, crushed and allowed to undergo enzyme-mediated oxidation. During this process, most of the catechins are oxidized, dimerized to form theaflavins and polymerized to form thearubigins [5, 6]. Theaflavins are produced from the dimerization of two catechin molecules and exist in four major forms (theaflavin, theaflavin-3 gallate, theaflavin-3′-gallate, and theaflavin-3,3′-digallate), which contribute to the red-orange color and characteristic taste of black tea. Thearubigins are mixtures of heterogeneous polymers with red-brown color and the structures are poorly understood [6]. In brewed black tea, catechins, theaflavins, and thearubigins each account for 3–10%, 2–6%, and >20% of the dry weight, respectively. The caffeine contents in black tea are the same as those in green tea.
Oolong tea is manufactured by mildly crushing the leaves and limiting fermentation to a specified period of time to produce specific flavors and tastes of the tea. Generally, Oolong tea contains catechins, theaflavins, and thearubigins as well as some characteristic components such as epigallocatechin esters, theasinensins, dimeric catechins, and dimeric proanthocyanidins [6]. Two specialized teas, “white tea” and “dark tea”, have also received much recent attention for their possible beneficial health effects [7–10]. White tea is usually made from younger leaves with less extensive processing than green tea, but its composition is probably within the range of different varieties of green tea. Dark tea (such as Pu-erh tea) is made by fermentation with microorganisms, which converts catechins to many new compounds. The overall composition is not well characterized and varies in different brands and preparations of dark tea.
2.2 Biochemical properties and bioavailability of tea polyphenols
The health effects of tea depend on the biochemical properties and bioavailability of the constituents in tea. Tea catechins, especially EGCG, have received most of the attention. It is commonly recognized that tea catechins are strong antioxidants, efficiently scavenging free radicals and also preventing the formation of reactive oxygen species (ROS) by chelating metal ions (reviewed in [6]). In vivo, EGCG (and perhaps other catechins) may also cause the formation of ROS in the mitochondria [11, 12]. The ROS may activate nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant and other cytoprotective enzymes [13–15], and this may be referred to as an “indirect antioxidant” effect. EGCG is also known to undergo superoxide-catalyzed auto-oxidation to produce ROS [16]. Nevertheless, such auto-oxidation of EGCG is unlikely to occur in internal organs because of the lower oxygen partial pressure (than in solution in vitro) and the presence of antioxidant enzymes in animal tissues. Therefore results on EGCG obtained from cell culture studies need to be interpreted with caution.
The phenolic groups in catechins can be donors for hydrogen bonding; the multiple H-bonds enable tea polyphenols to bind strongly to proteins, lipids and nucleic acids. The binding of EGCG to many proteins, such as 67-kDa laminin receptor (67 LR) [17] and prolyl cis/trans isomerase [18], has been proposed to be a key mechanism for its anti-cancer activities (reviewed in [19]). Black tea polyphenols may bind to biomolecules and biomembranes with even higher affinity than EGCG.
The bioavailabilities of tea polyphenols follow the prediction of “Lipinski's Rule of 5” [20] (reviewed in [21, 22]). Both human and animal studies have shown that the bioavailabilities of epicatechin and catechin (molecular weight 290 and 5 phenolic groups) are higher than that of EGCG (molecular weight 458 and 8 phenolic groups). The plasma bioavailability of EGCG in mice following intragastric (i.g.) administration of EGCG (75 mg/kg) was low, with more than 50% of EGCG existing as glucuronide conjugates. Levels of EGCG in the small intestine and colon were 20.6 and 3.6 ng/g, respectively [21]. In humans, following oral administration of the equivalent of two or three cups of green tea, the peak plasma levels of tea catechins (including the conjugated forms) were usually 0.2–0.3 µM [22]. With high pharmacological oral doses of EGCG, peak plasma concentrations of 2–9 µM and 7.5 µM were reported in mice and humans, respectively [21]. On the other hand, theaflavin and theaflavin-3,3′-digallate (molecular weights of 564 and 868 and containing 9 and 14 phenolic groups, respectively) have extremely low or no bioavailabilities when administered orally [6, 23]. It was reported that following consumption of 700 mg of pure theaflavins mixture, equivalent to about 30 cups of black tea by an individual, the maximum concentrations of theaflavin in plasma and urine were only 1 µg/L (1.8 nM) and 4.2 µg/L, respectively, [23]. Apparently, gallated theaflavins were not detected. The bioavailability of many polyphenolic compounds is regulated by active efflux. The multidrug resistance-associated protein 2, located on the apical surface of the intestine and liver, mediates the transport of some polyphenolic compounds to the lumen and bile, respectively. Therefore, EGCG is predominantly effluxed from the enterocytes into the intestinal lumen, or to be effluxed from the liver to the bile and excreted in the feces, with little or no EGCG excreted in the urine.
EGCG and other tea catechins undergo extensive biotransformation [6]. They are readily methylated by catechol-O-methyltransferase (COMT), glucuronidated by UDP-glucuronosyltransferases, and sulfated by sulfotransferases [6]. Tea catechins can be degraded in the intestinal tract by microorganisms to form ring fission metabolites 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone, 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone and 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone, which can be found in urine and plasma samples [6, 24]. These compounds can undergo further degradation to phenylacetic and phenylpropionic acids. The biological activities of these metabolites are lower than their parent compounds.
3. Reduction of Body Weight, Alleviation of Metabolic Syndrome and Prevention of Related Diseases
Overweight, obesity and type 2 diabetes are emerging as major health issues in the U.S. and many other countries. MetS is a complex of symptoms that include elevated waist circumference and two or more of the following: elevated serum triglyceride, dysglycemia, elevated blood pressure and reduced high-density lipoprotein-associated cholesterol [25]. Therefore, beneficial effects of tea consumption on body weight reduction and MetS alleviation could have huge public health implications.
3.1. Decrease of body weight and alleviation of metabolic syndrome and fatty liver in animal models
The effects of tea and tea polyphenols on body weight and MetS have been studied extensively in animal models (reviewed in [1–3]). Most of the studies showed that the consumption of green tea extracts (GTE) or EGCG significantly reduced the gaining of body weight and/or adipose tissue weight, lowered blood glucose or insulin levels, and increased insulin sensitivity or glucose tolerance. These studies used rodents on high-fat diets or genetically obese/diabetic animal models. For example, in mice fed with a high-fat (60% of the calories) diet, we found that dietary EGCG treatment (0.32 % in diet) for 16 weeks significantly reduced body weight gain, body fat and visceral fat weight compared to mice without EGCG treatment [26]. These results were also reproduced in a second study using a high-fat/Western style diet [27]. EGCG treatment also attenuated insulin resistance, plasma cholesterol and monocyte chemoattractant protein concentrations in mice on the high-fat diet [26, 27]. Similar results were also observed in several recent studies [28–30]. For example, treatment of male Swiss mice with green tea extract (GTE, 50 mg/kg, i.g., daily) for 8 weeks decreased body weight and white adipose tissue weight [28]. In another study, EGCG administration (20 mg/kg, i.p., 3 times weekly) to C57BL/6b mice that were fed a high-fat diet significantly reduced body weight and liver fat accumulation at 42 and 66 weeks [29]. In a genetic diabetes model, the db/db mice, dietary supplementation of EGCG (1% in diet) prevented the progression of glucose intolerance and reduced the number of pathologically altered islets of Langerhans [30]. A recent study in diabetic mice showed that both GTE and black tea extract (BTE), administered at 0.01% in drinking water, lowered blood glucose levels, and GTE was more efficacious [31]. GTE was also more effective in lowering body weight gain and histological liver deterioration, but only BTE significantly elevated serum insulin levels [31]. The doses of GTE and BTE used in this study were very low and the mechanism of BTE action is unknown.
Green tea polyphenols also alleviate MetS in other insulin-resistant animals. For example, in insulin-resistant rats, treatment with green tea polyphenols significantly decreased blood glucose, insulin, triglycerides, total cholesterol, LDL-cholesterol and free fatty acids [32]. In insulin-resistant beagle dogs, oral administration of GTE (80 mg/kg daily, before the daily meal) for 12 weeks also markedly increased insulin sensitivity index [33].
Diet-induced liver steatosis, which predisposes to liver cancer, is becoming a common disease, and its possible prevention by tea consumption warrants more investigation. We have shown that EGCG treatment reduces the incidence of hepatic steatosis, liver size (48% decrease), liver triglycerides (52% decrease), plasma alanine aminotransferase concentration (67% decrease) and liver pathology in mice fed with a high-fat diet [26, 27]. Tea catechins have been reported to also reduce hepatic steatosis and liver toxicity in rodents treated with ethanol, tamoxifen or endotoxins, or rodents with liver ischemia/reperfusion injury (reviewed in [4]). These findings may have potential for practical applications.
3.2. Human studies on weight reduction and mitigation of metabolic syndrome
The effects of tea consumption on body weight and biomarkers of MetS have been studied in many short-term randomized controlled trials (RCTs) during the past decade. Systematic reviews and meta-analysis covering more than 26 earlier RCTs indicated the beneficial effects of tea consumption in reducing body weight and alleviating MetS [34, 35]. Most of these studies used green tea or green tea extracts with caffeine, in studies for 8–12 weeks, on normal weight or overweight subjects. Some of the more recent RCTs also showed that daily consumption of 458–886 mg of green tea catechins by moderately overweight Chinese subjects for 90 days reduced body fat [36], and that intake of Polyphenon E capsules (containing 400 or 800 mg EGCG and lower amounts of other catechins, but negligible amounts of caffeine) daily for 2 months by postmenopausal women in the U.S. decreased blood levels of LDL-cholesterol, glucose and insulin [37]. In another study, GTE supplementation (379 mg per day) to obese patients for 3 months decreased body weight and waist circumference [38]. Improvements in lipid profiles, including the decrease in levels of total cholesterol, LDL-cholesterol, and triglycerides were also observed in this study [38]. However, a recent RCT with obese Caucasian women, after an energy-restricted diet intervention, showed that supplementation with EGCG (200 mg daily) for 12 weeks did not cause changes in body weight, fat mass, energy and fat metabolism, insulin resistance, total or LDL-cholesterol levels and other biomarkers [39]. The lack of beneficial effects of the supplement is possibly due to the relatively low dose of EGCG used, especially in subjects who were previously treated for weight reduction.
A recent metabolomic study with healthy male subjects demonstrated that green tea extract supplementation (1200 mg catechins and 240 mg caffeine daily) for 7 days increased lipolysis, fat oxidation and citric acid cycle activity under resting conditions without enhancing adrenergic stimulation [40]. The role of caffeine in these studies was inconsistent among the different studies. A meta-analysis of metabolic studies showed that both a catechin-caffeine mixture and caffeine alone dose-dependently stimulated daily energy expenditure, but only the catechin-caffeine combination significantly increased fat oxidation [41].
In contrast to the above described beneficial effects, a recent study in English adults showed that daily supplementation with green tea (>560 mg EGCG) plus caffeine (0.28–0.45 mg) for 12 weeks had no effects on fat absorption, resting energy expenditure and body composition [42]. The reasons for these contradictory results are not known. The different populations, with different physiological and dietary conditions, could be contributing factors. The authors also discussed a time factor; that is, the increase in energy expenditure was observed in short-term (7–10 days) studies with catechins, but not in long term studies [42]. This may be related to our previous observations in mice that the plasma levels of EGCG increased in the first several days of daily catechin administration, but the EGCG levels decreased gradually to ~13% of the maximal levels [43]. More research is needed on the time-dependent changes in bioavailability and health beneficial effects of catechins.
Two recent epidemiological studies also suggested the beneficial effects of green tea consumption on MetS [44, 45]. One study on elderly Taiwanese males in a rural community indicated that tea drinking, especially for individuals who drank 240 ml or more tea daily, was inversely associated with incidence of MetS [44]. The second, a cross-sectional study of U.S. adults, showed that intake of hot (brewed) tea, but not iced tea, was inversely associated with obesity and biomarkers of MetS and CVDs [45]. These results are exciting and need confirmation from additional studies. On the other hand, two recent cross-sectional studies in Japan did not find a preventive effect of green tea consumption against MetS [46, 47], which further support the idea that more studies on this topic are needed.
3.3. Lowering risk of diabetes in humans
The lowering of body weight and alleviation of MetS by tea should lead to the reduction of type 2 diabetes. Such an association was found in some, but not all, human studies (reviewed in [1–4]). For example, a prospective cross-sectional study with U.S. women aged 45 years and older showed that consumption of more than 4 cups of tea per day was associated with a 30% lower risk of developing type 2 diabetes, whereas the intake of total flavonoids or flavonoid-rich foods was not associated with reduced risk [48]. A retrospective cohort study of 17,413 Japanese adults aged 40–65 years indicated that daily consumption of more than 6 cups of green tea (but not Oolong or black tea) lowered the risk of diabetes by 33% [49]. The effects of caffeine in these epidemiological studies are unclear. A meta-analysis based on 7 studies (286,701 total participants) showed that individuals who drank 3–4 or more cups of tea per day had lower risks of type 2 diabetes than those consuming no tea [50]. A more recent RCT in patients with obesity-related hypertension showed that consumption of GTE (379 mg daily) for 3 months reduced fasting serum glucose and insulin levels [51]. Several clinical intervention studies have yielded inconclusive results concerning the effects of tea on insulin resistance and blood glucose control, but there are reported changes in certain biomarkers such as an increase in satiety, a reduction of hemoglobin A1c, and a decrease in diastolic blood pressure [52–55].
3.4. Lowering of blood cholesterol, blood pressure and incidence of cardiovascular diseases in humans
The alleviation of MetS by tea logically leads to the reduction of the risks for CVDs (reviewed in [56–58]). The lowering of plasma cholesterol levels and blood pressure as well as improvement of insulin sensitivity and endothelial function by green tea have been reported by many investigators [58]. In a review of 11 RCTs, both green and black teas decreased LDL-cholesterol and blood pressure [59]. The strongest evidence for the reduction of CVD risk by the consumption of green tea is provided by large cohort studies in Japan. In the Ohsaki National Health Insurance Cohort Study (n = 40,530), deaths due to CVDs were decreased dose-dependently by tea consumption at quantities of 1 to > 5 cups of tea per day [60]. In another study with 76,979 Japanese adults, the consumption of green tea was also associated with decreased CVD mortality, but daily consumption of > 6 cups of tea was needed to manifest the effect [61]. In a recent study in Japan, consumption of 3–4 cups of tea was associated with a decreased incidence of stroke [62]. A case-control study in China also showed a correlation between the consumption of green (or Oolong) tea and a decreased risk of ischemic stroke [63]. A meta-analysis of 14 prospective studies, covering 513,804 participants with a median follow-up of 11.5 years, found an inverse association between tea consumption and risk of stroke, and the protective effect of green tea appeared to be stronger than that of black tea [64].
Many, but not all, studies in the U.S. and Europe demonstrated an inverse association between black tea consumption and CVD risk (reviewed in [56]). For example, in the Determinants of Myocardial Infarction Onset Study, black tea consumption (> 2 cups daily) was associated with reduced CVD mortality and lower prevalence of ventricular arrhythmia for myocardial infarction during a follow-up of 3.8 years [65]. In a Dutch cohort study involving 37,514 healthy men and women followed for 13 years, consumption of black tea (3–6 cups daily) was associated with a decreased risk for CVD mortality [66]. In this study, however, tea consumption was also associated with a healthy lifestyle. The quantity and types of tea consumed are also key factors in affecting the results of these studies. For example, in the Women’s Health Study [67], the level of tea consumption was rather low (only a small percentage of women drank more than 4 cups of black tea per day), and only a trend in the prevention of CVD was observed. In a previous meta-analysis, green and black tea drinkers were shown to have lower incidences of myocardial infarction and ischemic stroke [68]. A subsequent meta-analysis, including 6 case-control and 12 cohort studies (5 measured green tea and 13 measured black tea as the exposure), found a reduced risk of coronary artery disease by 28% via green tea consumption (10% decrease in risk with an increment in consumption of 1 cup/day). However, there was no significant protective effect from black tea [69].
4. Molecular Mechanisms of Action of Tea Constituents in Body Weight Reduction and Metabolic Syndrome Mitigation
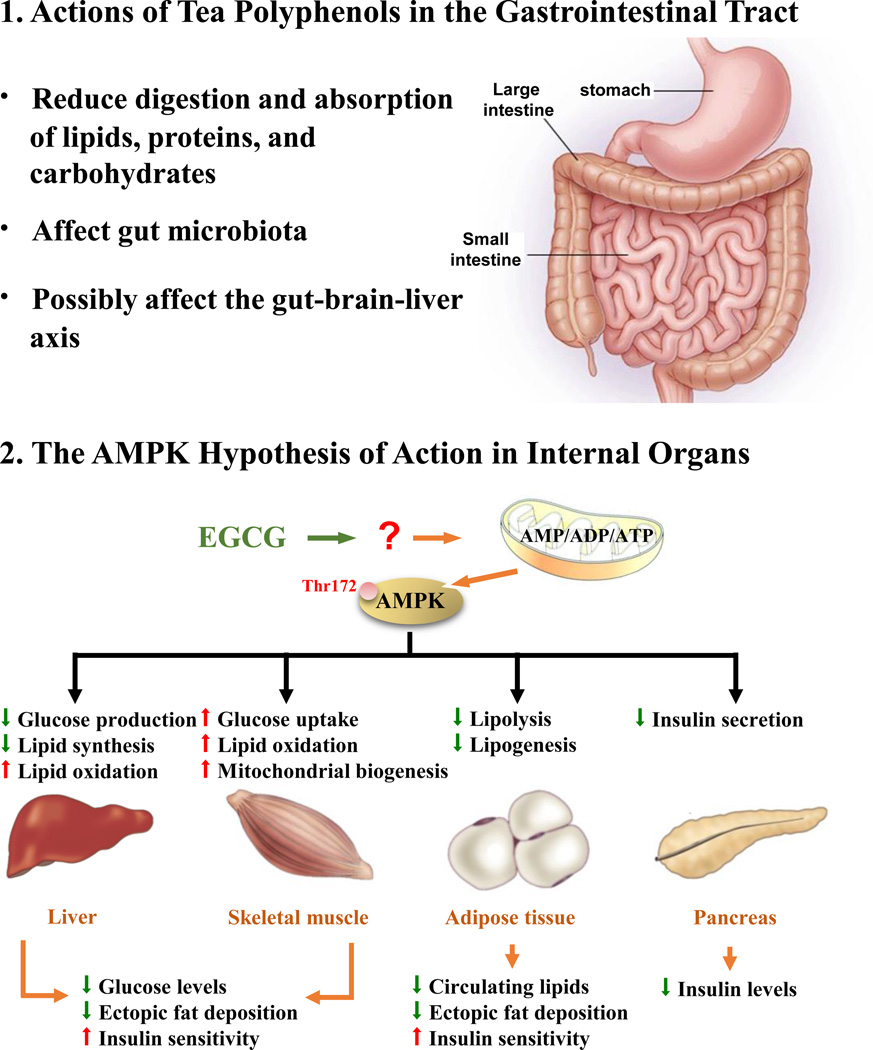
In the above reviewed publications, many possible mechanisms have been suggested. They can be summarized into two major types of actions as shown in Figure 2. One is the action of tea constituents in the gastrointestinal tract in decreasing digestion and absorption of macronutrients, or by altering the gut microbiota. The second type of action is produced by tea constituents after systemic absorption in the inhibition of anabolism and stimulation of catabolism in liver, muscle, adipose and other tissues. The combined effects would reduce body weight, alleviate MetS and reduce the risk of diabetes and CVDs. These mechanisms of action of tea constituents are discussed below.
Figure 2.
Proposed mechanisms for the actions of tea constituents in lowering body weight and alleviating metabolic syndrome: 1. Actions of tea polyphenols in the gastrointestinal tract. 2. A hypothesis on the central role of AMPK in metabolic regulation by EGCG. EGCG is proposed to active AMPK through affecting the ratios of AMP/ADP/ATP. The activated (phosphorylated) AMPK regulates metabolism in different organs toward the direction of reducing (↓) gluconeogenesis, fatty acid synthesis, insulin secretion and ectopic fat deposition in muscle and liver. These are accompanied by increased (↑) insulin sensitively and the oxidation of glucose and fatty acids. The lower part of the figure was modified from [84].
4.1. Actions in the gastrointestinal tract
4.1.1. Reduction of macronutrient digestion and absorption
Ingestion of green tea polyphenols has been shown to increase fecal lipid and total nitrogen contents, suggesting that polyphenols can decrease digestion and absorption of lipids and proteins [1, 2, 26, 27, 70]. For example, in mice fed a high-fat diet, EGCG dose-dependently decreased food digestibility and increased the fecal mass; with a 13C-triglycerides enriched diet, EGCG supplementation increased 13C levels in the feces [70].
After ingestion, lipids are emulsified, hydrolyzed and absorbed in the small intestine. Lipid transporters on the apical surface of the intestine facilitate the transfer of fatty acid and cholesterol into the enterocytes. The absorbed lipids are packaged into chylomicrons and secreted into the lymphatic system. Many in vitro studies have demonstrated that tea polyphenols can interfere with emulsification and inhibit pancreatic lipase and phospholipases (reviewed in [2]). Such actions have been used to explain results from animal studies. For example, the lowering of intestinal absorption of cholesterol in rats was attributed to decreased micellar solubility of cholesterol by black tea polyphenols [71]. EGCG was shown to inhibit pancreatic lipase and reduce body weight gain in mice fed a high-fat diet [72]. The possible inhibition of lipid transporters has also been suggested [73].
The mechanisms for the inhibition of digestion and absorption of proteins by tea catechins have not been studied extensively. Tight binding to ingested proteins and digestive proteinases by catechins through H-bonding and hydrophobic interactions would lead to decreased digestion.
The inhibitory activities of catechins against digestive enzymes, α-amylase and glucosidases, as well as glucose transporters have been demonstrated in many studies in vitro (reviewed in [2]). Gallated tea catechins such as EGCG (10 mg/kg, i.v.), but not non-gallated tea catechins, have shown inhibitory effects on glucose absorption in intestinal cells due to the competitive inhibition of Na-glucose co-transporter 1 [74]. When EGCG was co-administered with common corn starch to mice, the decrease of digestion and absorption of glucose was proposed to be due to the inhibition of α-amylase by EGCG [75].
In summary, decreased digestion and absorption of nutrients could play a significant role in lowering body weight and alleviating MetS by green tea polyphenols. We propose that such a mechanism of action is even more important for the action of black tea polyphenols (theaflavins and thearubigins), which have very low or no bioavailability.
4.1.2. Possible effects on intestinal microbiota
The possibility that tea may affect gut microbiome has been studied in mice. For example, green tea powder and Lactobacillus plantarum feeding affected gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice [76]. Green tea reduced the body fat content and hepatic triglyceride and cholesterol accumulation, and the reduction was correlated with the amount of Akkermansia and/or the total amount of bacteria in the small intestine [76]. The abundance of Akkermansia muciniphila has been shown previously to be increased in prebiotic-treated ob/ob mice, which had lower fat mass compared to the control ob/ob mice [77]. Changing gut microbiota, for example by the administration of Saccharomyces boulardii, has also been shown to reduce hepatic steatosis, low grade inflammation, and fat mass in obese and type 2 diabetic db/db mice [78]. In humans, green tea consumption has been reported to increase the proportion of the Bifidobacterium species in fecal samples [79]. Increase of intestinal Bifidobacteria by a prebiotic (oligofructose) has been shown to decrease biomarkers for diabetes in mice [80]. These results suggest the possibility that tea may alleviate MetS by enriching the probiotic population in the intestine. However, more studies with green and black tea preparations are needed to assess this possibility.
4.1.3. Possible actions on the gut-brain-liver axis
Recently, evidence for a gut-brain-liver axis mechanism has been provided for the acute glucose lowering effect by intraduodenal infused metformin in diabetes rat models [81]. According to this mechanism, metformin activates AMP-activated protein kinase (AMPK) in duodenal mucosa, and subsequently lowers hepatic glucose production (without involving hepatic AMPK) through a neuronal mediated gut-brain-liver pathway. As will be discussed in the following section, hepatic AMPK is proposed as a major mediator of EGCG in chronic glucose lowering effects. The possibility that the EGCG activates the gut AMPK-brain-liver pathway, intestinal endocrine systems or intestinal receptors has not been assessed and warrants investigation.
4.2. Actions of tea constituents after systemic absorption
The anti-obesity and anti-diabetes effects of tea polyphenols have been studied in different experimental animal models and systems in vitro (reviewed in [1–4, 82]). However, the relevance of some in vitro studies is uncertain because the concentrations of catechins used (20–100 µM or higher) were much higher than their blood or tissue levels observed after tea ingestion. This is especially a concern with black tea polyphenols that have very low or no bioavailability [23]. Therefore, we will weigh studies in animal models heavily and try to distinguish between direct effects of tea constituents and indirect effects due to the reduction of body weight. In addition to decreasing the absorption of nutrients as discussed above, tea constituents can increase energy expenditure. In an experiment with indirect calorimetry, a single dosage of EGCG (200 mg/kg body weight, i.g.) to mice was shown to significantly increase oxygen consumption and fat oxidation in mice during the first 3 hours following administration [83].
4.2.1. In general energy metabolism
The observations in many studies that ingestion of tea catechins suppressed gluconeogenesis and lipogenesis and enhanced lipolysis in a coordinated manner [reviewed in [1–4]] suggest that these actions of tea catechins are mediated by energy sensing molecules, such as AMPK. In response to falling energy status, AMPK is activated to inhibit energy-consuming processes and promote catabolism to produce ATP [84–86]. In addition to maintaining cellular energy homeostasis, AMPK also responds to different hormone signals to maintain whole body energy balance [86]. We propose that the activation of AMPK is the main mechanism for EGCG and other catechins to influence energy metabolism and to alleviate MetS, and this proposal will be referred to as the “AMPK hypothesis.”
AMPK is activated by phosphorylation of Thr172 in the activation loop within the kinase domain of the α-subunit. AMP binding to the regulatory domain of the γ-subunit causes allosteric activation, which promotes the phosphorylation by the upstream kinase, such as liver kinase B1 (LKB1), and protects AMPK from dephosphorylation [86]. It was also demonstrated that ADP binding to one of the 2 exchangeable AXP (AMP/ADP/ATP) binding sites on the γ-subunit regulatory domain protects the enzyme from dephosphorylation, and the binding affinity of ADP is much higher than that of Mg-ATP. This observation explains how AMPK is regulated under physiological conditions where the concentration of Mg-ATP is higher than that of ADP and much higher than that of AMP [87]. It appears that AMPK activation is regulated by both ADP/ATP and AMP/ATP ratios. The formation of AMP is catalyzed by adenylate kinase in the following reversible reaction, when the cells are in need of ATP.
The possible mediation of the action of EGCG by AMPK in the liver, skeletal muscle, adipose tissue and pancreas is shown in Figure 2. The activation of AMPK by EGCG and different types of teas has been demonstrated in vivo and in vitro [8, 83, 88–93]. It has been shown that at 1, 3 and 6 h after i.g. administration of GTE (100 mg/kg body weight) to mice, the phosphorylation of AMPK and its upstream kinase, LKB1, increased in the liver (by 2–3 and 1.5–2 fold, respectively) [88]. However, black tea extract was ineffective. Nevertheless, there are also reports indicating that AMPK was activated in adipose tissues and skeletal muscle by black tea, as well as by Oolong and Pu-erh teas [8, 93]. The detailed mechanism by which EGCG activates AMPK is still unclear, although the involvement of ROS has been suggested based on studies in vitro [90]. The situation in vivo could be different due to the lack of auto-oxidation of EGCG as discussed in Section 2.2. It has been suggested that EGCG blocks mitochondrial electron transport to produce ROS [12]. ROS may further decrease ATP levels, because glutathione and NADPH are required for the detoxification of ROS in the mitochondria and mitochondrial proton gradient is required to drive the transhydrogenase to reduce NADP+ (by NADH) to form NADPH [94]. EGCG has also been reported to inhibit mitochondrial oxidative phosphorylation to decrease ATP levels [95]. Another possibility is that EGCG may serve as an uncoupler of oxidative phosphorylation. All these actions would result in an increase of ADP/ATP ratio to activate AMPK, but these possibilities remain to be investigated.
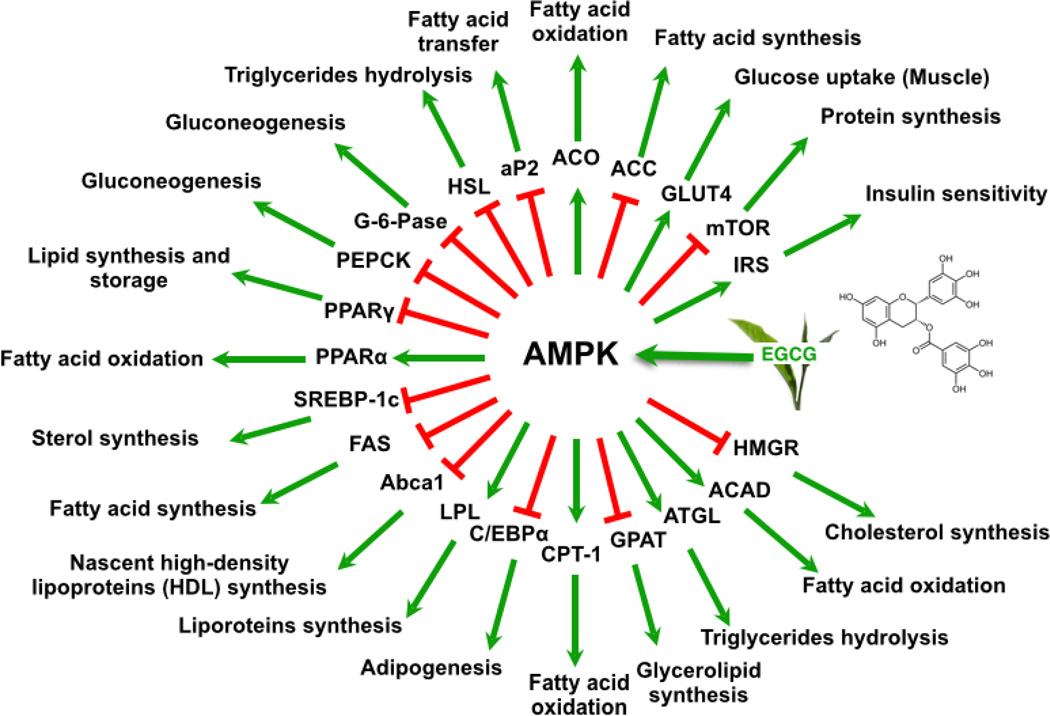
Activated AMPK has been shown to decrease the expressions or activities of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) (for gluconeogenesis), acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) (for fatty acid synthesis), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) (for cholesterol synthesis), glycerol phosphate acyltransferase (GPAT) (for glycerolipid synthesis), and homolog of target of rapamycin (mTOR) (for protein synthesis) [84–86]. It also activates glucose transporter type 4 (GLUT4), carnitine palmitoyltransferase-1 (CPT-1) and acyl-CoA dehydrogenase (ACAD) to increase the catabolism of glucose and fatty acids. All these actions have also been demonstrated by EGCG as summarized in Figure 3 and discussed in the following sections. We propose that most of the reported effects of EGCG or green tea on metabolism can be explained by the AMPK hypothesis (Figures 2 and 3). The bioavailability of EGCG and its action on AMPK in different organs determine most of the biological effects. In addition, the direct inhibitory action of EGCG on enzymes with low inhibitory constants may play a role.
Figure 3.
EGCG induces changes in proteins and genes that are mediated by AMPK. Abbreviations are IRS, insulin receptor substrate; mTOR, homolog of target of rapamycin; GLUT4, glucose transporter type 4; ACC, acetyl-CoA carboxylase; ACO, acyl-CoA oxidase; aP2, adipocyte protein 2; HSL, hormone sensitive lipase; G-6-Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate carboxykinase; PPARγ, peroxisome proliferator-activated receptor γ; PPARα, peroxisome proliferator-activated receptor α; SREBP-1c, sterol regulatory element-binding protein-1c; FAS, fatty acid synthase; Abca1, ATP-binding cassette superfamily of transporter proteins 1; LPL, lipoprotein lipase; C/EBPα, CCAAT enhancer binding protein α; CPT-1, carnitine palmitoyltransferase-1; GPAT, glycerol phosphate acyltransferase; ATGL, adipose triglyceride lipase; ACAD, acyl-CoA dehydrogenase; HMGR, 3-hydroxy-3-methylglutaryl-CoA reductase.
4.2.2. In the liver, skeletal muscle and adipose tissues
The liver is a vital organ for metabolic processing of different nutrients and is the major organ for lipogenesis and gluconeogenesis. Muscle is the major site of ATP production and energy consumption; the uptake and oxidation of glucose and fatty acids are key molecular events. White adipose tissue is the major site of fatty acid storage, and some fatty acids can be synthesized from glucose. Most of the reported molecular changes caused by EGCG as described below may be mediated by the activation of AMPK as illustrated in Figure 3.
The downregulation of the two key enzymes in gluconeogenesis, PEPCK and G-6-Pase, and associated decrease of glucose production in the liver by EGCG has been shown to be mediated by AMPK activation [90]. The activated form, p-AMPK, is also known to phosphorylate and inactivate ACC, the rate limiting enzyme of fatty acid synthesis. The resulting lowered levels of malonyl-CoA can activate CPT-1, which facilitates long-chain fatty acyl CoA transport into mitochondria for β-oxidation [84].
In ovariectomized rats that were fed fructose, green tea treatment significantly downregulated the hepatic expression of sterol regulatory element-binding protein-1c (SREBP-1c) and its target genes coding for FAS and stearoyl-CoA desaturase 1 (SCD1) as well as the expression of HMGR and efflux proteins (ATP binding cassette superfamily of transporter proteins 1, Abca1) [96]. A series of investigations in high-fat diet-induced obese mice, rats or chickens have shown that green tea and catechins downregulate the expression of genes coding for fat synthesis (malic enzyme, G-6-P also dehydrogenase and SCD1), and upregulate the mRNA levels of enzymes for fatty acid β-oxidation (CPT-1, acyl-CoA oxidase (ACO), ACAD and PPARα) in the liver (reviewed in [2, 3]).
In studies with rats on a high-fructose diet, green tea administration for 6 weeks increased GLUT4 and increased insulin receptor substrate (IRS) mRNA levels in the liver and muscle [97]. In insulin-resistant rats, green tea polyphenol treatment also increased the cardiac mRNA levels of IRS1, IRS2, GLUT1, GLUT4 and glycogen synthase 1 and decreased pro-inflammatory cytokines [32]. In insulin-resistant dogs, tea extract treatment upregulated PPARα and lipoprotein lipase in the skeletal muscle and induced GLUT4 translocation into the plasma membrane in muscle cells [33]. The enhanced GLUT4 translocation and activation of AMPK (together with activation of PI3K/AKT) in skeletal muscle have been observed in mice treated with Oolong, black, or Pu-erh tea (2% extracts as drinking fluid) for 7 days [93]. The active constituents of these teas are probably EGCG (and other catechins) and caffeine. The induction of AMPK in skeletal muscle by caffeine has been reported [98].
Treatment with extracts of Oolong, black or Pu-erh tea (in drinking fluid, similar to the experiment described above) was shown to activate AMPK in white adipose tissues [8]. Dietary supplementation of EGCG (0.2 or 0.5% in high-fat diet) to mice for 8 weeks significantly decreased the mRNA levels of adipogenic genes, such as those for PPARγ, C/EBPα, SREBP-1c, lipoprotein lipase and FAS in the epididymal white adipose tissues. This was accompanied by the increase of mRNA levels of lipolytic and catabolic genes such as hormone sensitive lipase (HSL) adipose triglyceride lipase, and CPT-1 (Figure 3) [99]. Oral gavage of green tea extract (400 mg/kg) for 8 weeks significantly reduced body weight and adipose tissue gain and this was associated with increased HSL, 1-acylglycerol-3-phosphate O-acyltransferase, and perilipin in mesenteric adipose tissues [100]. Green tea (catechins) has also been proposed to prevent obesity or reduce fat deposits in mice or rats on a high-fat diet by increasing the insulin-like growth factor binding protein-1 in adipose tissues [101], or by activating the ERK1/2-PPARγ-adiponectin pathway [102]. It has also been suggested that EGCG improves insulin signaling in adipose tissues of rats maintained on a high-fat diet by attenuating inflammation, decreasing macrophage content and inhibiting toll-like receptor 4 activity [103].
Green tea or catechins have been shown, in white adipose tissue or in adipocytes in vitro, to suppress the mRNA levels of lipogenesis, adipogenesis and fatty acid uptake genes (reviewed in [2]). The possible antimitogenic and differentiation effects of EGCG on preadipocytes and the involvement of 67R have been discussed [104, 105] primarily based on cell line studies with rather high EGCG concentrations (50 µM, 100 µM or higher). Whether such effects can be exerted by EGCG after tea ingestion by animals remains to be determined.
4.2.3. In the cardiovascular system
As discussed above, reduction of body weight and alleviation of MetS by tea consumption would decrease the stress to the cardiovascular system. Beneficial effects of tea catechins in lowering plasma cholesterol levels, preventing hypertension and improving endothelial function contribute to the prevention of CVDs. The cholesterol lowering effect is likely due to the decrease of cholesterol absorption or reabsorption by catechins as well as the decrease of cholesterol synthesis via the inhibition of HMGR (mediated by the activation of AMPK). Enhanced nitric oxide signaling has been suggested as a common mechanism for catechins to decrease blood pressure and the severity of myocardial infarction [58]. Several studies showed that green tea or black tea polyphenols increased endothelial nitric oxide synthase (eNOS) activity in bovine aortic endothelial cells and rat aortic rings [106–108], possibly mediated by AMPK and other signal pathways. Tea catechins may suppress the expression of caveolin-1, a negative regulator of eNOS [109]. Similarly, EGCG has also been shown to lower the expression of endothelin-1, possibly through the activation of AMPK, and this reduces vasoconstrictor tone and may directly increase bioavailability of nitric oxide to improve endothelial function [110]. AMPK has also been suggested to regulate the antioxidant status [111] and to mediate the anti-inflammatory activity of EGCG in endothelial cells [112]. EGCG has also been shown to induce the expression of heme oxygenase 1 in aortic endothelial cells [113], and this may increase anti-inflammatory activity to benefit the cardiovascular system. While moderate doses of EGCG have yielded beneficial effects, a very high dose (1% in diet) has been shown to promote, rather than to attenuate, vascular inflammation in hyperglycemic mice [114].
4.2.4. In neuroendocrine system
There have been a few studies on the effects of EGCG administration on endocrine systems (reviewed in [104]). The thorough study in rats by Kao et al. [115] is rather intriguing. The effective concentration of EGCG used, 85 mg/kg intraperitoneally, is rather high. The molecular basis for the significant suppression of appetite, body and organ (prostate, uterus and ovary) weights, and levels of several hormones is not known. Since this dose is beyond human EGCG exposure from consumption of tea, the relevance of these interesting observations is unknown.
Epinephrine and norepinephrine, two hormones produced in adrenal medulla in animals under stress conditions, increase delivery of oxygen to muscle and ATP production. Both hormones are inactivated by a methylation reaction catalyzed by COMT. EGCG and other catechins may prolong the effectiveness of these hormones, because they have been shown to be inhibitors of COMT [116]. This is the mechanism suggested by Dulloo et al. to explain their observations that tea catechins increased energy expenditure and fat oxidation in humans [117]. Caffeine, an important constituent in tea, has been well documented to increase energy expenditure [118].
4.2.5. Antioxidant effects
Tea polyphenols are generally recognized as strong antioxidants, and their antioxidant action has been suggested to contribute to the alleviation of MetS. The antioxidant activities of tea and tea polyphenols have been studied extensively in animals and humans. A recent meta-analysis of 31 intervention studies on this topic, however, indicated that there was limited evidence that regular consumption of green tea increased plasma antioxidant capacity and reduced oxidation of LDL cholesterol [119]. In recent studies in subjects with MetS or obesity [38, 120], however, green tea supplementation has been shown to increase the antioxidant capacity. It appears that the antioxidant effect of tea is more apparent in animals and humans under higher oxidative stress and when rather high doses of tea catechins are used. It remains to be determined whether the observed “antioxidant” activity is due to the direct antioxidant effects of tea polyphenols or due to its “indirect antioxidant” effect through activation of Nrf2-medication antioxidant enzymes [14, 15].
5. Concluding Remarks
As discussed in the earlier part of this article, tea consumption at the levels of 3–4 cups (600–900 mg tea catechins) or more a day has shown to reduce body weight gain, alleviate MetS and reduce the risk for diabetes and CVDs. These results are supported by laboratory studies in animal models. We proposed the hypothesis that most of these beneficial effects can be explained by the decreased absorption of macronutrients plus the systemic effects of tea catechins in metabolic regulation, which are mediated mostly by the activation of AMPK. We further hypothesize that the relative importance of these two types of action depends on the types and amounts of tea consumed as well as the dietary conditions. For example, with black tea, the decrease of nutrient absorption, especially with a high-fat diet, may play a more important role than its systemic effects, because of the low bioavailability of theaflavins and thearubigins. Another possibility is that these large molecular weight black tea polyphenols are degraded to form smaller molecular weight metabolites, which can be absorbed and exhibit biological effects on internal organs. Experimental evidence for this proposal is still lacking.
Even though our “AMPK hypothesis” proposes that AMPK plays a major role in mediating the actions of EGCG on gluconeogensis, fatty acid synthesis, and catabolism of fat and glucose; actions that are independent of AMPK could also be involved. Some of these actions are described in this article, and some have been discussed in reviews by Wang et al. [3] and by Kim et al. [92]. It is puzzling why EGCG, a single molecule, can have so many diverse activities, and it is likely that some of these reported activities are secondary events.
Recent studies have shown that administration of moderate doses of EGCG to mice can produce ROS, which activates the Nrf-2-mediated induction of antioxidant and other cytoprotective enzymes [14, 15]. ROS has also been proposed to activate AMPK [90]. Considering the effective doses of tea catechins in animal studies and in human consumption in alleviating MetS, an interesting question is whether the beneficial effects are due to the pro-oxidant activity of EGCG or due to other catechins. Further research is needed to determine whether EGCG and other catechins exert their health beneficial effects by their classical antioxidant activity in quenching ROS, by their prooxidant activities, by inhibiting digestive or other enzymes, or by their actions as inhibitors or uncouplers of oxidative phosphorylation to decrease cellular ATP levels and activate AMPK.
From a public health point of view, consumption of tea at a level of 3–4 cups a day is achievable in some individuals, but not in other individuals. Green tea, especially when consumed in large amounts on an empty stomach, is known to cause irritation in the gastrointestinal system. Black tea is considered to be milder (less irritating), but the beneficial health effects are weaker. Dark tea is also considered milder, and its composition and health effects need to be systematically studied. Caution should be applied in using dietary supplements that contain high concentrations of tea extracts, since liver toxicity has been reported (reviewed in [1]). In addition to tea catechins, many other dietary substances have been shown to activate AMPK, reduce body weight gain or alleviate MetS. These include resveratrol, curcumin, capsaicin and garlic constituents (reviewed in [3, 85]). An interesting possibility, which remains to be explored, is that the effects of these dietary materials can work additively to reduce body weight gain and alleviate MetS.
Acknowledgments
This work was supported by US NIH grants CA122474 and CA133021, Anhui Major Demonstration Project for Leading Talent Team on Tea Chemistry and Health, and Tea Project of Anhui Provincial Agriculture Committee. We thank Ms. Dorothy Wong for her capable assistance in the preparation of this manuscript.
Abbreviations
- ACAD
acyl-CoA dehydrogenase
- ACC
acetyl-CoA carboxylase
- ACO
acyl-CoA oxidase
- AMPK
AMP-activated protein kinase
- BTE
black tea extract
- CPT-1
carnitine palmitoyltransferase-1
- COMT
catechol-O-methyltransferase
- CVDs
cardiovascular diseases
- ECG
(−)-epicatechin gallate
- EGCG
(−)-epigallocatechin gallate
- eNOS
endothelial nitric oxide synthase
- FAS
fatty acid synthase
- G-6-Pase
glucose-6-phosphatase
- GLUT4
glucose transporter type 4
- GPAT
glycerol phosphate acyltransferase
- GTE
green tea extract
- HMGR
3-hydroxy-3-methylglutaryl-CoA reductase
- HSL
hormone sensitive lipase
- IRS
insulin receptor substrate
- 67 LR
67-kDa laminin receptor
- LKB1
liver kinase B1
- MetS
metabolic syndrome
- Nrf2
nuclear factor erythroid 2-related factor 2
- PEPCK
phosphoenolpyruvate carboxykinase
- PPAR
peroxisome proliferator-activated receptor
- RCTs
randomized controlled trials
- ROS
reactive oxygen species
- SCD1
stearoyl-CoA desaturase 1
- SREBP-1c
sterol regulatory element-binding protein-1c
References
- 1.Yang CS, Hong J. Prevention of chronic diseases by tea: possible mechanisms and human relevance. Annu Rev Nutr. 2013;33:161–181. doi: 10.1146/annurev-nutr-071811-150717. [DOI] [PubMed] [Google Scholar]
- 2.Huang J, Wang Y, Xie Z, Zhou Y, et al. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr. 2014;68:1075–1087. doi: 10.1038/ejcn.2014.143. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Moustaid-Moussa N, Chen L, Mo H, et al. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sae-tan S, Grove KA, Lambert JD. Weight control and prevention of metabolic syndrome by green tea. Pharmacol Res. 2011;64:146–154. doi: 10.1016/j.phrs.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 6.Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Alves MG, Martins AD, Teixeira NF, Rato L, et al. White tea consumption improves cardiac glycolytic and oxidative profile of prediabetic rats. J Functional Foods. 2015;14:102–110. [Google Scholar]
- 8.Yamashita Y, Wang L, Wang L, Tanaka Y, et al. Oolong, black and pu-erh tea suppresses adiposity in mice via activation of AMP-activated protein kinase. Food Funct. 2014;5:2420–2429. doi: 10.1039/c4fo00095a. [DOI] [PubMed] [Google Scholar]
- 9.Yang TY, Chou JI, Ueng KC, Chou MY, et al. Weight reduction effect of Puerh tea in male patients with metabolic syndrome. Phytother Res. 2014;28:1096–1101. doi: 10.1002/ptr.5111. [DOI] [PubMed] [Google Scholar]
- 10.Du WH, Peng SM, Liu ZH, Shi L, et al. Hypoglycemic effect of the water extract of Pu-erh tea. J Agric Food Chem. 2012;60:10126–10132. doi: 10.1021/jf302426w. [DOI] [PubMed] [Google Scholar]
- 11.Li GX, Chen YK, Hou Z, Xiao H, et al. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;31:902–910. doi: 10.1093/carcin/bgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao L, Forester SC, Lambert JD. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (−)-epigallocatechin-3-gallate, in oral cells. Mol Nutr Food Res. 2014;58:665–676. doi: 10.1002/mnfr.201300427. [DOI] [PubMed] [Google Scholar]
- 13.Shen G, Xu C, Hu R, Jain MR, et al. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;22:1805–1820. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Wang Y, Wan X, Yang CS, Zhang J. Green tea polyphenol (−)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol Appl Pharmacol. 2015;283:65–74. doi: 10.1016/j.taap.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 15.James KD, Forester SC, Lambert JD. Dietary pretreatment with green tea polyphenol, (−)-epigallocatechin-3-gallate reduces the bioavailability and hepatotoxicity of subsequent oral bolus doses of (−)-epigallocatechin-3-gallate. Food Chem Toxicol. 2015;76:103–108. doi: 10.1016/j.fct.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou Z, Sang S, You H, Lee MJ, et al. Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 17.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 18.Urusova DV, Shim JH, Kim DJ, Jung SK, et al. Epigallocatechin-gallate suppresses tumorigenesis by directly targeting Pin1. Cancer prevention research. 2011;4:1366–1377. doi: 10.1158/1940-6207.CAPR-11-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 21.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl 1):S139–S151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 22.Chow HH, Hakim IA. Pharmacokinetic and chemoprevention studies on tea in humans. Pharmacol Res. 2011;64:105–112. doi: 10.1016/j.phrs.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulder TP, van Platerink CJ, Wijnand Schuyl PJ, van Amelsvoort JM. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;760:271–279. doi: 10.1016/s0378-4347(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Lee MJ, Sheng S, Meng X, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chemical Res Toxicol. 2000;13:177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- 25.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 26.Bose M, Lambert JD, Ju J, Reuhl KR, et al. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YK, Cheung C, Reuhl KR, Liu AB, et al. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J Agric Food Chem. 2011;59:11862–11871. doi: 10.1021/jf2029016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuda MH, Zemdegs JC, de Santana AA, Santamarina AB, et al. Green tea extract improves high fat diet-induced hypothalamic inflammation, without affecting the serotoninergic system. J Nutr Biochem. 2014;25:1084–1089. doi: 10.1016/j.jnutbio.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Byun JK, Yoon BY, Jhun JY, Oh HJ, et al. Epigallocatechin-3-gallate ameliorates both obesity and autoinflammatory arthritis aggravated by obesity by altering the balance among CD4+ T-cell subsets. Immunol Lett. 2014;157:51–59. doi: 10.1016/j.imlet.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Ortsater H, Grankvist N, Wolfram S, Kuehn N, Sjoholm A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr Metab (Lond) 2012;9:11. doi: 10.1186/1743-7075-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang W, Li S, Liu Y, Huang M-T, Ho C-T. Anti-diabetic activity of chemically profiled green tea and black tea extracts in a type 2 diabetes mice model via different mechanisms. J Functional Foods. 2013;5:1784–1793. [Google Scholar]
- 32.Qin B, Polansky MM, Harry D, Anderson RA. Green tea polyphenols improve cardiac muscle mRNA and protein levels of signal pathways related to insulin and lipid metabolism and inflammation in insulin-resistant rats. Mol Nutr Food Res. 2010;54(Suppl 1):S14–S23. doi: 10.1002/mnfr.200900306. [DOI] [PubMed] [Google Scholar]
- 33.Serisier S, Leray V, Poudroux W, Magot T, et al. Effects of green tea on insulin sensitivity, lipid profile and expression of PPARalpha and PPARgamma and their target genes in obese dogs. Br J Nutr. 2008;99:1208–1216. doi: 10.1017/S0007114507862386. [DOI] [PubMed] [Google Scholar]
- 34.Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes. 2009;33:956–961. doi: 10.1038/ijo.2009.135. [DOI] [PubMed] [Google Scholar]
- 35.Phung OJ, Baker WL, Matthews LJ, Lanosa M, et al. Effect of green tea catechins with or without caffeine on anthropometric measures: a systematic review and meta-analysis. J Clin Nutr. 2010;91:73–81. doi: 10.3945/ajcn.2009.28157. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Wen Y, Du Y, Yan X, et al. Effects of catechin enriched green tea on body composition. Obesity. 2010;18:773–779. doi: 10.1038/oby.2009.256. [DOI] [PubMed] [Google Scholar]
- 37.Wu AH, Spicer D, Stanczyk FZ, Tseng CC, et al. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res (Phila) 2012;5:393–402. doi: 10.1158/1940-6207.CAPR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suliburska J, Bogdanski P, Szulinska M, Stepien M, et al. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. 2012;149:315–322. doi: 10.1007/s12011-012-9448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mielgo-Ayuso J, Barrenechea L, Alcorta P, Larrarte E, et al. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br J Nutr. 2014;111:1263–1271. doi: 10.1017/S0007114513003784. [DOI] [PubMed] [Google Scholar]
- 40.Hodgson AB, Randell RK, Boon N, Garczarek U, et al. Metabolic response to green tea extract during rest and moderate-intensity exercise. J Nutr Biochem. 2013;24:325–334. doi: 10.1016/j.jnutbio.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Hursel R, Viechtbauer W, Dulloo AG, Tremblay A, et al. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obesity Rev. 2011;12:e573–e581. doi: 10.1111/j.1467-789X.2011.00862.x. [DOI] [PubMed] [Google Scholar]
- 42.Janssens PL, Hursel R, Westerterp-Plantenga MS. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J Nutr. 2015;145:864–870. doi: 10.3945/jn.114.207829. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Lee MJ, Hong J, Li C, et al. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr Cancer. 2000;37:41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- 44.Chang CS, Chang YF, Liu PY, Chen CY, et al. Smoking, habitual tea drinking and metabolic syndrome in elderly men living in rural community: the Tianliao old people (TOP) study 02. PloS One. 2012;7:e38874. doi: 10.1371/journal.pone.0038874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vernarelli JA, Lambert JD. Tea consumption is inversely associated with weight status and other markers for metabolic syndrome in US adults. Eur. J. Nutr. 2013;52:1039–1048. doi: 10.1007/s00394-012-0410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takami H, Nakamoto M, Uemura H, Katsuura S, et al. Inverse correlation between coffee consumption and prevalence of metabolic syndrome: baseline survey of the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study in Tokushima, Japan. J Epidemiol. 2013;23:12–20. doi: 10.2188/jea.JE20120053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pham NM, Nanri A, Kochi T, Kuwahara K, et al. Coffee and green tea consumption is associated with insulin resistance in Japanese adults. Metabolism. 2014;63:400–408. doi: 10.1016/j.metabol.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr. 2005;24:376–384. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- 49.Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554–562. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]
- 50.Huxley R, Lee CM, Barzi F, Timmermeister L, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 51.Bogdanski P, Suliburska J, Szulinska M, Stepien M, et al. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32:421–427. doi: 10.1016/j.nutres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Hsu CH, Liao YL, Lin SC, Tsai TH, et al. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. J Clin Therapeutic. 2011;16:157–163. [PubMed] [Google Scholar]
- 53.Fukino Y, Ikeda A, Maruyama K, Aoki N, et al. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur Clin Nutr. 2008;62:953–960. doi: 10.1038/sj.ejcn.1602806. [DOI] [PubMed] [Google Scholar]
- 54.Josic J, Olsson AT, Wickeberg J, Lindstedt S, Hlebowicz J. Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: a randomized controlled tria. Nutr J. 2010;30:63. doi: 10.1186/1475-2891-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown AL, Lane J, Coverly J, Stocks J, et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr. 2009;101:886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deka A, Vita JA. Tea and cardiovascular disease. Pharmacol Res. 2011;64:136–145. doi: 10.1016/j.phrs.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Castelnuovo A, di Giuseppe R, Iacoviello L, de Gaetano G. Consumption of cocoa, tea and coffee and risk of cardiovascular disease. Eur J Intern Med. 2012;23:15–25. doi: 10.1016/j.ejim.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 58.Munir KM, Chandrasekaran S, Gao F, Quon MJ. Mechanisms for food polyphenols to ameliorate insulin resistance and endothelial dysfunction: therapeutic implications for diabetes and its cardiovascular complications. Am J Physiol Endocrinol Metab. 2013;305:E679–E686. doi: 10.1152/ajpendo.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartley L, Flowers N, Holmes J, Clarke A, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;6:CD009934. doi: 10.1002/14651858.CD009934.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. Jama. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 61.Mineharu Y, Koizumi A, Wada Y, Iso H, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health. 2011;65:230–240. doi: 10.1136/jech.2009.097311. [DOI] [PubMed] [Google Scholar]
- 62.Kokubo Y, Iso H, Saito I, Yamagishi K, et al. The impact of green tea and coffee consumption on the reduced risk of stroke incidence in Japanese population: the Japan public health center-based study cohort. Stroke. 2013;44:1369–1374. doi: 10.1161/STROKEAHA.111.677500. [DOI] [PubMed] [Google Scholar]
- 63.Liang W, Lee AH, Binns CW, Huang R, et al. Tea consumption and ischemic stroke risk: a case-control study in southern China. Stroke. 2009;40:2480–2485. doi: 10.1161/STROKEAHA.109.548586. [DOI] [PubMed] [Google Scholar]
- 64.Shen L, Song LG, Ma H, Jin CN, et al. Tea consumption and risk of stroke: a dose-response meta-analysis of prospective studies. J Zhejiang Univ Sci B. 2012;13:652–662. doi: 10.1631/jzus.B1201001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukamal KJ, Alert M, Maclure M, Muller JE, Mittleman MA. Tea consumption and infarct-related ventricular arrhythmias: the determinants of myocardial infarction onset study. J Am Coll Nutr. 2006;25:472–479. doi: 10.1080/07315724.2006.10719561. [DOI] [PubMed] [Google Scholar]
- 66.de Koning Gans JM, Uiterwaal CS, van der Schouw YT, Boer JM, et al. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler Thromb Vasc Biol. 2010;30:1665–1671. doi: 10.1161/ATVBAHA.109.201939. [DOI] [PubMed] [Google Scholar]
- 67.Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr. 2003;77:1400–1408. doi: 10.1093/ajcn/77.6.1400. [DOI] [PubMed] [Google Scholar]
- 68.Arab L, Liu W, Elashoff D. Green and black tea consumption and risk of stroke: a meta-analysis. Stroke. 2009;40:1786–1792. doi: 10.1161/STROKEAHA.108.538470. [DOI] [PubMed] [Google Scholar]
- 69.Wang ZM, Zhou B, Wang YS, Gong QY, et al. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. J Clin Nutr. 2011;93:506–515. doi: 10.3945/ajcn.110.005363. [DOI] [PubMed] [Google Scholar]
- 70.Friedrich M, Petzke KJ, Raederstorff D, Wolfram S, Klaus S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int J Obes (Lond) 2012;36:735–743. doi: 10.1038/ijo.2011.136. [DOI] [PubMed] [Google Scholar]
- 71.Ikeda I, Yamahira T, Kato M, Ishikawa A. Black-tea polyphenols decrease micellar solubility of cholesterol in vitro and intestinal absorption of cholesterol in rats. J Agric Food Chem. 2010;58:8591–8595. doi: 10.1021/jf1015285. [DOI] [PubMed] [Google Scholar]
- 72.Grove KA, Sae-tan S, Kennett MJ, Lambert JD. (−)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity (Silver Spring) 2012;20:2311–2313. doi: 10.1038/oby.2011.139. [DOI] [PubMed] [Google Scholar]
- 73.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–183. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park JH, Jin JY, Baek WK, Park SH, et al. Ambivalent role of gallated catechins in glucose tolerance in humans: a novel insight into non-absorbable gallated catechin-derived inhibitors of glucose absorption. J Physiol Pharmacol. 2009;60:101–109. [PubMed] [Google Scholar]
- 75.Forester SC, Gu Y, Lambert JD. Inhibition of starch digestion by the green tea polyphenol, (−)-epigallocatechin-3-gallate. Mol Nutr Food Res. 2012;56:1647–1654. doi: 10.1002/mnfr.201200206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Axling U, Olsson C, Xu J, Fernandez C, et al. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 2012;9:105. doi: 10.1186/1743-7075-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Everard A, Lazarevic V, Derrien M, Girard M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Everard A, Matamoros S, Geurts L, Delzenne NM, Cani PD. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio. 2014;5:e01011–e01014. doi: 10.1128/mBio.01011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin JS, Touyama M, Hisada T, Benno Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol Immunol. 2012;56:729–739. doi: 10.1111/j.1348-0421.2012.00502.x. [DOI] [PubMed] [Google Scholar]
- 80.Cani PD, Neyrinck AM, Fava F, Knauf C, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 81.Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21:506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011;22:1–7. doi: 10.1016/j.jnutbio.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Murase T, Misawa K, Haramizu S, Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem Pharmacol. 2009;78:78–84. doi: 10.1016/j.bcp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 84.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 87.Xiao B, Sanders MJ, Underwood E, Heath R, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banerjee S, Ghoshal S, Porter TD. Phosphorylation of hepatic AMP-activated protein kinase and liver kinase B1 is increased after a single oral dose of green tea extract to mice. Nutr Res. 2012;32:985–990. doi: 10.1016/j.nutres.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou J, Farah BL, Sinha RA, Wu Y, et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS One. 2014;9:e87161. doi: 10.1371/journal.pone.0087161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Collins QF, Liu HY, Pi J, Liu Z, et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5'-AMP-activated protein kinase. J Biol Chem. 2007;282:30143–30149. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serrano JC, Gonzalo-Benito H, Jove M, Fourcade S, et al. Dietary intake of green tea polyphenols regulates insulin sensitivity with an increase in AMP-activated protein kinase alpha content and changes in mitochondrial respiratory complexes. Mol Nutr Food Res. 2013;57:459–470. doi: 10.1002/mnfr.201200513. [DOI] [PubMed] [Google Scholar]
- 92.Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties, lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamashita Y, Wang L, Tinshun Z, Nakamura T, Ashida H. Fermented tea improves glucose intolerance in mice by enhancing translocation of glucose transporter 4 in skeletal muscle. J Agric Food Chem. 2012;60:11366–11371. doi: 10.1021/jf303597c. [DOI] [PubMed] [Google Scholar]
- 94.Leung JH, Schurig-Briccio LA, Yamaguchi M, Moeller A, et al. Structural biology. Division of labor in transhydrogenase by alternating proton translocation and hydride transfer. Science. 2015;347:178–181. doi: 10.1126/science.1260451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valenti D, de Bari L, Manente GA, Rossi L, et al. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochim Biophys Acta. 2013;1832:2085–2096. doi: 10.1016/j.bbadis.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 96.Shrestha S, Ehlers SJ, Lee JY, Fernandez ML, Koo SI. Dietary green tea extract lowers plasma and hepatic triglycerides and decreases the expression of sterol regulatory element-binding protein-1c mRNA and its responsive genes in fructose-fed, ovariectomized rats. J Nutr. 2009;139:640–645. doi: 10.3945/jn.108.103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao H, Hininger-Favier I, Kelly MA, Benaraba R, et al. Green tea polyphenol extract regulates the expression of genes involved in glucose uptake and insulin signaling in rats fed a high fructose diet. J Agric Food Chem. 2007;55:6372–6378. doi: 10.1021/jf070695o. [DOI] [PubMed] [Google Scholar]
- 98.Egawa T, Hamada T, Ma X, Karaike K, et al. Caffeine activates preferentially alpha1-isoform of 5'AMP-activated protein kinase in rat skeletal muscle. Acta Physiol (Oxf) 2011;201:227–238. doi: 10.1111/j.1748-1716.2010.02169.x. [DOI] [PubMed] [Google Scholar]
- 99.Lee MS, Kim CT, Kim Y. Green tea (−)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann Nutr Metab. 2009;54:151–157. doi: 10.1159/000214834. [DOI] [PubMed] [Google Scholar]
- 100.Cunha CA, Lira FS, Rosa Neto JC, Pimentel GD, et al. Green tea extract supplementation induces the lipolytic pathway, attenuates obesity, and reduces low-grade inflammation in mice fed a high-fat diet. Mediators Inflamm. 2013;2013:635470. doi: 10.1155/2013/635470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ueda M, Ashida H. Green tea prevents obesity by increasing expression of insulin-like growth factor binding protein-1 in adipose tissue of high-fat diet-fed mice. J Agric Food Chem. 2012;60:8917–8923. doi: 10.1021/jf2053788. [DOI] [PubMed] [Google Scholar]
- 102.Tian C, Ye X, Zhang R, Long J, et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via ERK1/2-PPARgamma-adiponectin pathway. PLoS One. 2013;8:e53796. doi: 10.1371/journal.pone.0053796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bao S, Cao Y, Fan C, Fan Y, et al. Epigallocatechin gallate improves insulin signaling by decreasing toll-like receptor 4 (TLR4) activity in adipose tissues of high-fat diet rats. Mol Nutr Food Res. 2014;58:677–686. doi: 10.1002/mnfr.201300335. [DOI] [PubMed] [Google Scholar]
- 104.Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res. 2006;50:188–210. doi: 10.1002/mnfr.200500109. [DOI] [PubMed] [Google Scholar]
- 105.Ku HC, Liu HS, Hung PF, Chen CL, et al. Green tea (−)-epigallocatechin gallate inhibits IGF-I and IGF-II stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor, but not AMP-activated protein kinase pathway. Mol Nutr Food Res. 2012;56:580–592. doi: 10.1002/mnfr.201100438. [DOI] [PubMed] [Google Scholar]
- 106.Jochmann N, Lorenz M, Krosigk A, Martus P, et al. The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br J Nutr. 2008;99:863–868. doi: 10.1017/S0007114507838992. [DOI] [PubMed] [Google Scholar]
- 107.Lorenz M, Wessler S, Follmann E, Michaelis W, et al. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 108.Aggio A, Grassi D, Onori E, D'Alessandro A, et al. Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur J Nutr. 2013;52:263–272. doi: 10.1007/s00394-012-0320-x. [DOI] [PubMed] [Google Scholar]
- 109.Li Y, Ying C, Zuo X, Yi H, et al. Green tea polyphenols down-regulate caveolin-1 expression via ERK1/2 and p38MAPK in endothelial cells. J Nutr Biochem. 2009;20:1021–1027. doi: 10.1016/j.jnutbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 110.Akiyama S, Katsumata S, Suzuki K, Nakaya Y, et al. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci Biotechnol Biochem. 2009;73:2779–2782. doi: 10.1271/bbb.90576. [DOI] [PubMed] [Google Scholar]
- 111.Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421:163–169. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- 112.Wu J, Xu X, Li Y, Kou J, et al. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur J Pharmacol. 2014;745:59–68. doi: 10.1016/j.ejphar.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 113.Pullikotil P, Chen H, Muniyappa R, Greenberg CC, et al. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-alpha. J Nutr Biochem. 2012;23:1134–1145. doi: 10.1016/j.jnutbio.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pae M, Ren Z, Meydani M, Shang F, et al. Dietary supplementation with high dose of epigallocatechin-3-gallate promotes inflammatory response in mice. J Nutr Biochem. 2012;23:526–531. doi: 10.1016/j.jnutbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 115.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 116.Chen D, Wang CY, Lambert JD, Ai N, et al. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol. 2005;69:1523–1531. doi: 10.1016/j.bcp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 117.Dulloo AG, Duret C, Rohrer D, Girardier L, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70:1040–1045. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 118.Gonzalez de Mejia E, Ramirez-Mares MV. Impact of caffeine and coffee on our health. Trends Endocrinol Metab. 2014;25:489–492. doi: 10.1016/j.tem.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Ellinger S, Muller N, Stehle P, Ulrich-Merzenich G. Consumption of green tea or green tea products: is there an evidence for antioxidant effects from controlled interventional studies? Phytomedicine. 2011;18:903–915. doi: 10.1016/j.phymed.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 120.Basu A, Betts NM, Mulugeta A, Tong C, et al. Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr Res. 2013;33:180–187. doi: 10.1016/j.nutres.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]