Abstract
In this study, novel fermented chickpea milk with high γ -aminobutyric acid (GABA) content and potential neuroprotective activity was developed. Fermentation starter that can produce GABA was selected from 377 strains of lactic acid bacteria isolated from traditional Chinese fermented foods. Among the screened strains, strain M-6 showed the highest GABA-producing capacity in De Man–Rogosa and Sharp (MRS) broth and chickpea milk. M-6 was identified as Lactobacillus plantarum based on Gram staining, API carbohydrate fermentation pattern testing, and 16s rDNA sequencing. The complete gene encoding glutamate decarboxylase was cloned to confirm the presence of the gene in L. plantarum M-6. The fermentation condition was optimized by response surface methodology. Results demonstrated that L. plantarum M-6 produced the highest GABA content of 537.23 mg/L. The optimal condition included an inoculum concentration of 7%, presence of 0.2% (m/v) monosodium glutamate and 55 µ M pyridoxal-5-phosphate, incubation temperature of 39 °C and fermentation time of 48 h . GABA-enriched chickpea milk exerted protective effects on PC12 cells against MnCl2 -induced injury. GABA-enriched chickpea milk improved cell viability and markedly attenuated the release of lactate dehydrogenase compared with the impaired cells.
Keywords: Chickpea, γ-aminobutyric acid (GABA), Lactobacillus plantarum, Neuroprotective effect
Introduction
Gamma-aminobutyric acid (GABA) is a non-protein amino acid that is naturally present in microorganisms, plants, and animals. GABA acts as the major inhibitory neurotransmitter in the mammalian central nervous system (Li & Cao, 2010). This amino acid also exhibits several physiological functions, such as inducing insulin secretion (Adeghate & Ponery, 2002), regulating the rate of protein synthesis in the brain (Tujioka et al., 2009), and reducing blood pressure (Inoue et al., 2003). GABA deficiency in the brain is related to neurological disorders, such as Parkinson’s and Huntington’s diseases (Cho, Chang & Chang, 2007). In addition, GABA can improve the function of visual cortex cells and thus can be used to alleviate decline in sensory, motor, and cognitive skills, which occur with aging (Leventhal et al., 2003). In this regard, researchers have focused on developing functional foods containing GABA. However, direct addition of GABA to food is considered unsafe and unnatural (Seok et al., 2008). Utilization of GABA-rich foods produced by natural techniques, such as fermentation, is thus preferred.
Lactic acid bacteria (LAB) are widely used in fermented food and generally regarded as safe (Molina et al., 2012). Some LAB strains can produce GABA by catalyzing the α-decarboxylation of L-glutamic acid or monosodium glutamate (MSG) via the enzyme glutamate decarboxylase (GAD); this enzyme is dependent on pyridoxal 5′-phosphate (PLP) or vitamin B6 (Diana et al., 2014). GABA is generated by LAB during fermentation of different kinds of food, such as bovine milk (Inoue et al., 2003), black raspberry juice (Kim et al., 2009), and black soybean milk (Ko, Lin & Tsai, 2013). Chickpea, the third most important pulse crop, is extensively produced in several regions worldwide (Roy, Boye & Simpson, 2010). Chickpea is rich in proteins, vitamins, and glutathione and low in fat (Xiao et al., 2014); this legume is also used in Uygur folk medicine in China. Chickpea is a good source of α-galactooligosaccharides (5%–10%) (Xiang et al., 2008); these polysaccharides facilitate the growth of LAB and are suitable for GABA production.
This work aims to develop GABA-rich chickpea milk through fermentation using an LAB strain with high capacity to produce GABA. Various LAB strains were screened based on their capacity to produce GABA. The fermentation conditions of the chickpea milk were then optimized. The protective effects of fermented chickpea milk extracts (FCE) were also assessed based on MnCl2-induced toxicity in PC12 cells to illustrate the functional properties of the fermented product.
Materials and Methods
Materials
Chickpeas (Kabuli) were purchased from a supermarket in Xinjiang Uygur Autonomous Region, China. The rat pheochromocytoma cell line (PC12 cells) was purchased from KeyGEN BioTECH (Nanjing, China). GABA, 3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and the solvents used for HPLC were acquired from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were supplied by Gibco Ltd. (Grand Island, NY, USA). All other chemicals used were of analytical grade and purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China).
Bacterial strains and culture conditions
LAB strains with high GABA production capacity were screened from 377 strains isolated from traditional fermented food. LAB was activated for two successive transfers in De Man–Rogosa and Sharp (MRS) broth (Merck, Darmstadt, Germany) at 37 °C for 14–16 h prior to use; the strain was then cultured in MRS containing 1% (w/v) MSG at 37 °C for 48 h.
GABA assay using pre-staining paper chromatography
GABA levels were determined qualitatively using the method described by Li et al. (2009) with slight modifications. The culture broth was centrifuged at 10,000 ×g for 10 min, and 1 µL of the supernatant was spotted onto chromatography paper. The paper was developed using n-butanol–acetic acid–water (2:1:1) containing 0.8% ninhydrin. The paper was then dried in a convection oven at 80 °C for 30 min for color development.
GABA and MSG assay by high-performance liquid chromatography (HPLC)
GABA and MSG in the supernatant were determined by HPLC using the method of Ko, Lin & Tsai (2013) with minor modifications. HPLC analysis was performed with a ZORBAX Eclipse Plus C18 reversed-phase analytical column (4.60 mm × 250 mm, 5 µm, Agilent). Briefly, 100 µL of the sample was added into 500 µL of boric acid buffer solution (0.4 M, pH 10.4) and 100 µL of derivatization reagent (containing 10 mg of ortho-phthalaldehyde (OPA) and 10 µL of 2-mercaptoethanol in 2.5 mL of acetonitrile). The mixture was vortexed for 30 s and reacted at room temperature for 5 min. Each sample (20 µL) was injected and monitored at 334 nm wavelength by using a DAD detector (G1315 B). The elution solvent system consisted of (A) 0.02 M ammonium acetate buffer (pH 7.3) and (B) HPLC-grade acetonitrile. The program was set at 20% of B for 10 min, ramped at 100% for 20 min, then at 20% of B for 25 min. A flow rate of 0.6 mL/min was used. The temperature of the column oven was set at 25 °C. The calibration curve was obtained based on six levels (100, 200, 300, 400, 500, and 600 mg/L) of the GABA standard.
Liquid chromatography–mass spectrophotometry (LC–MS)
The molecular weight of the OPA derivative of GABA was determined through LC–MS (Agilent 1200 and 6410 triple quadrupole mass spectrometry series; Agilent, Palo Alto, CA, USA). The Triple Quad mass spectrometer was equipped with an ESI and operated in positive-ion mode. The identification conditions were as follows: HV voltage, 3.5 kV; capillary, 4 nA; nebulizer, 30 psi; gas temperature, 300 °C; gas flow, 10 L/min; and scan range, m/z 50–400 units.
Preparation of fermented chickpea milk by using LAB
Chickpeas were washed and soaked in distilled water for 12 h at room temperature. Water was decanted, and the soaked chickpeas were boiled with distilled water (w/v) 10 times for 15 min by using an electric pot (C21-SH808, Shandong Joyoung Small Household Electrical Appliance Co., Ltd., Jinan, China). Chickpeas were wet-milled continuously for 5 min by using a homogenizer (BE601AB, Midea, China) and filtered through double-layered cheesecloth. The filtrate of chickpea milk was added with 0.2% MSG and sterilized at 108 °C for 15 min. Fermentation was started by inoculation of LAB to achieve an initial count of 107 cfu/mL, followed by incubation at 37 °C for 48 h. The fermented chickpea milk was stored at 4 °C for 12 h after maturation.
Sensory analysis
Sensory analysis was performed in accordance with the method of Lawless & Heymann (1999) with minor modifications. Ten trained panelists familiar with basic sensory evaluation skills were asked to assess the appearance, aroma, texture, flavor, taste, and overall acceptability of the fermented chickpea milk by using a five-point scale (1 = dislike very much; 2 = dislike; 3 = acceptable; 4 = like; 5 = like very much).
Identification of LAB
Carbohydrate fermentation patterns of the strain M-6 were determined using the API 50 CHL kit (bioMérieux, France). The kit contains 50 biochemical experiments for examining the carbohydrate fermentation pattern of LAB strains, thereby enabling the identification of the strain at the species level. The strain was further identified by 16S rDNA sequencing (Chin et al., 2006).
Cloning of the L. plantarum M-6 GAD gene and sequence analysis
Genomic DNA was extracted according to the method of Van der Meulen et al. (2007) and used as template for subsequent experiments. DNA encoding GAD was amplified by using the forward primer 5′-ATGGCAATGTTATACGGTAAACAC-3′ and the reverse primer 5′- TCAGTGTGTGAATAGGTATTTCTTAGGT-3′, which were designed using Primer 5 software based on the GAD sequences collected from NCBI. The reaction program was set as follows: 5 min at 94 °C; followed by 25 cycles of 30 s at 94 °C, 30 s at 55 °C, and 180 s at 68 °C; and an additional extension at 68 °C for 5 min. After agarose gel electrophoresis, the amplified product was purified using BiosPure Gel Extraction Kit (Hangzhou Biosci Biotech Co. Lid, Hangzhou, Zhejiang, China), ligated into pMD19T (simple) by T4 DNA ligase (Thermo Scientific, Waltham, USA), and transformed into E. coli JM109. Positive transformants were selected on Luria–Bertani plates containing ampicillin and confirmed through colony PCR; the validated positive clone was verified by sequencing (Sangon Biotech, Shang Hai, China). The GAD sequence obtained in this study was subjected to similarity search in the GenBank database.
Determination of LAB counts and pH
Viable count was determined using MRS agar plates. An aliquot of 1 mL of the sample was extracted from the flasks and diluted by 10-fold in sterile physiological saline (0.85%, w/v). The diluted sample was inoculated onto MRS agar plates and incubated at 37 °C under 5% CO2 for 48 h. Viable colonies were counted and expressed as log (cfu/mL). pH was measured using a pH meter (Lab850, Schott, Germany).
Response surface methodology (RSM) experimental design
A three-level, three-factor factorial Box–Behnken design (BBD) of response surface methodology (RSM) was performed. Inoculum concentration (A), temperature (B), and PLP (C) were selected as independent variables, and GABA content was chosen as dependent variable. The independent variables were studied at three different levels (−1, 0, + 1), and a set of 17 experiments were performed (Table 1). Design Expert software v7.0.0 (StaEase Corp., Minneapolis, MN, USA) was used to analyze the experimental data.
Table 1. Box–Behnken design and responses to GABA yield in fermented chickpea milk.
| Trials | A Inoculum concentration (%) | B Temperature (°C) | C PLP (µM) | GABA(mg/L) | |
|---|---|---|---|---|---|
| Predicted | Observed | ||||
| 1 | −1(3) | 0(37) | −1(20) | 325.57 | 326.54 ± 3.77 |
| 2 | 0(5) | 0 | 0(50) | 467.53 | 495.53 ± 5.11 |
| 3 | 0 | 1(42) | −1 | 356.08 | 346.62 ± 8.48 |
| 4 | −1 | 1 | 0 | 311.12 | 319.61 ± 8.02 |
| 5 | 1(7) | 0 | 1(80) | 453.05 | 452.08 ± 2.90 |
| 6 | 0 | 0 | 0 | 467.53 | 479.85 ± 3.57 |
| 7 | 0 | 1 | 1 | 398.02 | 395.05 ± 5.27 |
| 8 | -1 | 0 | 1 | 335.07 | 329.54 ± 5.11 |
| 9 | 0 | 0 | 0 | 467.53 | 412.97 ± 7.12 |
| 10 | 0 | −1(32) | 1 | 111.39 | 120.85 ± 1.60 |
| 11 | 0 | −1 | −1 | 112.57 | 115.54 ± 0.87 |
| 12 | 1 | 0 | −1 | 421.79 | 427.32 ± 3.50 |
| 13 | −1 | −1 | 0 | 108.30 | 104.36 ± 1.25 |
| 14 | 1 | −1 | 0 | 153.15 | 144.65 ± 9.21 |
| 15 | 0 | 0 | 0 | 467.53 | 479.26 ± 7.75 |
| 16 | 0 | 0 | 0 | 467.53 | 470.06 ± 4.99 |
| 17 | 1 | 1 | 0 | 480.47 | 484.41 ± 3.79 |
Protective effects of FCE on manganese-induced PC12 cell death
Determination of cell viability by MTT assays
MTT assays were used to evaluate the protective effect of FCE on manganese -induced PC12 cell death by measuring cell viability (Lee, Yoon & Park, 2008). PC12 cells were cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin. The cell suspension was seeded into 96-well plates at a concentration of 2 × 105/well and incubated at 37 °C for 24 h. The cells were preincubated with different concentrations of FCE/unfermented chickpea milk extract (UCE) and 40 µg/mL GABA for 30 min. The cells were then treated with MnCl2 at 37 °C in 5% CO2for another 24 h. FCE/UCE was prepared. The samples were centrifuged at 10,000× g for 10 min, and the supernatant was filtered through a 0.22 µm filter. The resulting filtrate was freeze dried. The freeze-dried extract was dissolved in DMEM and diluted to different concentrations.
After 24 h, the mixture was collected. Each well was added with 100 µL of the MTT solution (1 mg/mL) and incubated for another 4 h. Finally, the supernatant was discarded and 150 µL of dimethyl sulfoxide (DMSO) was added into each well to dissolve the formazan. After 10 min, absorbance was determined at 570 nm by using a microplate reader (BioTek Synergy2, Vermont, USA). Cells cultured without FCE/UCE and MnCl2 were used as control. Cell viability was calculated as follows: cell viability (%) = (As/Ac) × 100, where As is the absorbance in the presence of the sample and/or MnCl2 and Ac is the absorbance of the control in the absence of the sample and MnCl2.
Observation of morphological changes
PC12 cells cultured normally, treated with 750 µM MnCl2 only, and protected with 2,000 µg/mL FCE, 2,000 µg/mL UCE, and 40 µg/mL GABA were observed with an inverted microscope (ECLIPSE TE2000-S, Nikon, Japan).
Determination of LDH activity (lactate dehydrogenase)
PC12 cells were exposed to MnCl2 in the presence or absence of different concentrations of FCE/UCE or 40 µg/mL of GABA for 24 h. The culture medium was collected, and a commercially available assay kit (Nanjing Jiancheng, China) was used to determine LDH activity according to the manufacturer’s protocol. Absorbance was determined at 450 nm.
Statistical analysis
Each sample was analyzed in triplicate, and mean values were calculated. Analysis of variance (ANOVA) and Duncan’s multiple comparison tests were used to determine significant differences among means (P < 0.05) by using IBM SPSS Statistics.
Results and Discussion
Isolation and screening of GABA-producing LAB
LAB strains isolated from traditional fermented foods, such as paocai, were screened for their GABA-producing ability in MRS broth supplemented with 1% MSG. Paper chromatography analysis showed that 17 of the 377 strains produced GABA (representative results are shown in Fig. S1); of these strains, 10 strains produced GABA at concentrations higher than 100 mg/L as confirmed by HPLC analysis (Table 2). Strain M-6 produced the highest GABA content; ca. 545.33 and 282.12 mg/L GABA were detected after 48 h of fermentation with MRS (fortified with 1% MSG) and chickpea milk (fortified with 0.2% MSG), respectively. Chickpea milk fermented by M-6 also had good overall acceptance based on sensory evaluation results (Table S1).
Table 2. Screening of GABA-producing LAB in MRS broth and chickpea milk containing 1% and 0.2 % MSG, respectively.
| Strains | MRS with 1% MSG | Chickpea milk containing 0.2 % MSG | ||
|---|---|---|---|---|
| GABA (mg/L) | pH | GABA (mg/L) | pH | |
| M-9 | 529.23 ± 4.12b | 4.31 | 252.56 ± 4.12c | 4.25 |
| M-6 | 545.33 ± 5.43a | 4.42 | 282.12 ± 4.03a | 4.32 |
| M-7 | 541.52 ± 4.24a | 4.48 | 265.32 ± 3.01b | 4.28 |
| M-5 | 333.68 ± 4.44c | 4.29 | 205.59 ± 3.03e | 4.21 |
| 22 | 232.80 ± 5.28e | 4.22 | 186.34 ± 2.91f | 4.01 |
| W-21 | 240.46 ± 5.56e | 4.20 | 153.71 ± 2.21g | 4.05 |
| P-1 | 270.24 ± 4.67d | 4.21 | 232.27 ± 4.33d | 4.18 |
| 5 | 126.46 ± 4.15f | 4.15 | 115.58 ± 2.42h | 4.05 |
| M-8 | 129.26 ± 3.12f | 4.14 | 104.36 ± 3.42j | 4.10 |
| E–5 | 123.24 ± 3.36f | 4.16 | 122.25 ± 2.52i | 4.08 |
Notes.
Data are expressed as mean ± SD from triplicate experiments. Different superscripts within the same column indicate significant difference (P < 0.05).
GABA detected by paper chromatography and HPLC analyses were characterized through LC–MS. The molecular weight of the derivative is 279.35 g/mol. Mass spectrometry identification of the OPA derivative of the GABA standard and the culture supernatant of chickpea milk revealed that this compound had an m/z value of 280.2000 [M + H]+ (Fig. S2). Thus, strain M-6 was confirmed to convert MSG to GABA in chickpea milk.
Identification and DNA sequencing of strain M-6
Physiological and biochemical tests were conducted to determine the identity of the M-6 strain. The bacterium was non-spore forming, rod-type, hetero-fermentative, Gram positive, and negative for catalase and motility. M-6 also did not produce gas and ammonia from glucose and arginine. Thus, the strain was identified as Lactobacillus. API carbohydrate fermentation pattern testing showed that the strain was L. plantarum. The 16S rDNA sequence of the strain M-6 was 1,477 bp, which showed 99% homology with that of L. plantarum strains JNB25 and LP-1. The API identification result was confirmed by 16S rDNA sequencing, and the strain was identified as L. plantarum M-6. The sequence of L. plantarum M-6 strain was deposited in NCBI Genbank under the accession number KU214638.
Characterization of glutamate decarboxylase (GAD) gene from L. plantarum M-6
The full length of GAD gene from L. plantarum M-6 was successfully amplified and sequenced (Fig. 1). The gene was 1,410-bp long, encoding a protein of 469 amino acid residues (Fig. 2) with a predicted molecular weight of 53.7 kDa and pI of 5.58. Similarly, the full-length GAD gene was also amplified from other LAB strains, such as L. plantarum Taj-Apis362 (Tajabadi et al., 2015), L. paracasei (Komatsuzaki et al., 2008), and L. brevis OPK-3 (Park & Oh, 2007). The amino acid sequence of L. plantarum M-6-derived GAD showed a similarity of 99%, 99%, 94%, 94%, and 68% with the other GAD proteins from L. plantarum GB 01–21, L. plantarum Taj-Apis362, L. delbrueckii subsp. bulgaricus ATCC 11842, L. brevis OPK-3, and Lactococcus lactis, respectively. Among the analyzed GAD proteins, a similar sequence encoding amino acids SGHKY, including lysine, was found and identified as essential to the binding of PLP (a cofactor of GAD) (Park & Oh, 2007). Thus, GAD gene was confirmed to be present in L. plantarum M-6. The GAD gene of L. plantarum M-6 was submitted to GenBank under the accession number KU214639.
Figure 1. Electrophoresis analysis of GAD gene.
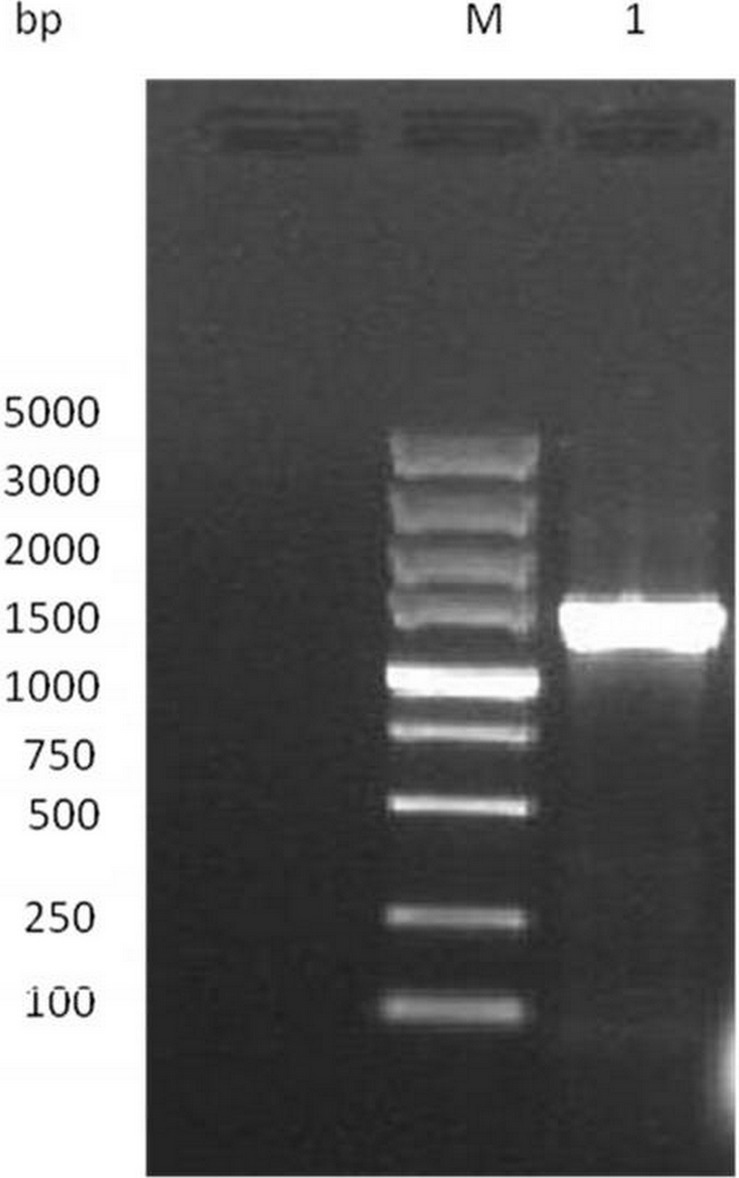
Lane M, DL 2000 DNA Marker; lane 1, GAD gene of the strain.
Figure 2. Multiple alignment of GAD from Lactobacillus plantarum M-6 and other GAD proteins.
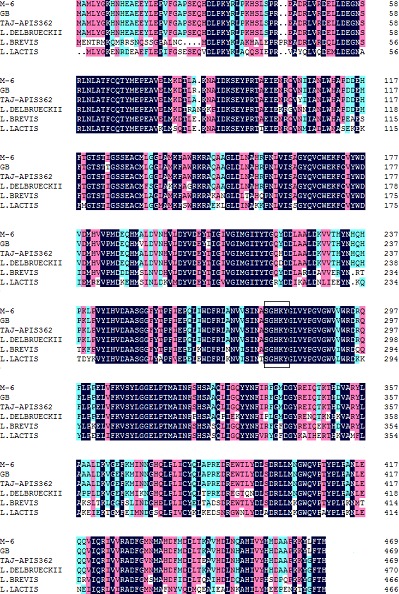
Amino acid sequences of GAD from L. plantarum M-6 (M-6), L. plantarum GB 01-21 (GB, GenBank accession no. AEL29212), L. plantarum Taj-Apis362 (Taj-Apis362, AHG59384), L. delbrueckii subsp. bulgaricus ATCC 11842 (L. delbrueckii, AHX56283), L. brevis OPK-3 (L. brevis, AAZ95185), and Lactococcus lactis (L. lactis, WP_023164198) were analyzed using ClustalW (1.81). The box represents the PLP binding site.
Determination of the optimum culture conditions for GABA production in chickpea milk by L. plantarum M-6
Figure 3 shows the effect of MSG concentration, inoculum concentration, culture temperature, and PLP concentration on GABA production, LAB populations, and pH level of fermented chickpea milk. The GABA concentration increased with MSG supplementation at low levels (0%–0.2%); further increase in the MSG concentration to 0.6% did not significantly affect GABA production but resulted in lower conversion yield (9%) than that of 0.2% MSG supplementation (26%). MSG could be a minor nutritional element to promote the growth of M-6. The pH of the fermented chickpea milk increased with increasing MSG concentration possibly because of the presence of alkaline MSG remnants. Considering the results for taste, GABA concentration, and conversion rate, 0.2% MSG was selected as the optimal MSG concentration. Moreover, the yield of GABA initially increased with increasing inoculum concentration (v/v), until the concentration reached 5% and yield started to decrease. pH level decreased when the inoculum concentration was increased from 1% to 5%. Inoculum concentration minimally influenced LAB counts and pH (P > 0.05). However, temperature strongly influenced both microbial growth and GABA production. An elevated temperature increased the reaction rate and influenced the final yield of GABA. The optimal temperature for GABA production and LAB growth was 37 °C. Temperatures higher than 37 °C cause enzyme inactivation and cell aging, leading to decrease in GABA production and microbial growth. As a cofactor of the enzyme, PLP plays an important role in stimulating GAD activity (Tong et al., 2002). GABA production reached its highest when PLP concentration increased from 0 µM to 50 µM; higher PLP concentration resulted in no obvious effect on GABA concentration (P > 0.05). This trend may be due to that the amount of PLP at 50 µM is enough for promoting GAD activity. Addition of PLP had little effect on LAB growth and pH value (P > 0.05), suggesting that PLP cannot be used as a nutritional factor for M-6 growth. The optimal concentration of PLP was established as 50 µM, at which GABA production is increased by 24.48% relative to that of the control (442.39 mg/L).
Figure 3. Changes in GABA concentrations, LAB counts, and pH values in chickpea milk fermented for 48 h containing different concentrations of MSG (A), under different inoculum concentrations (B), at different fermentation temperatures (C), and containing different concentrations of PLP (D).
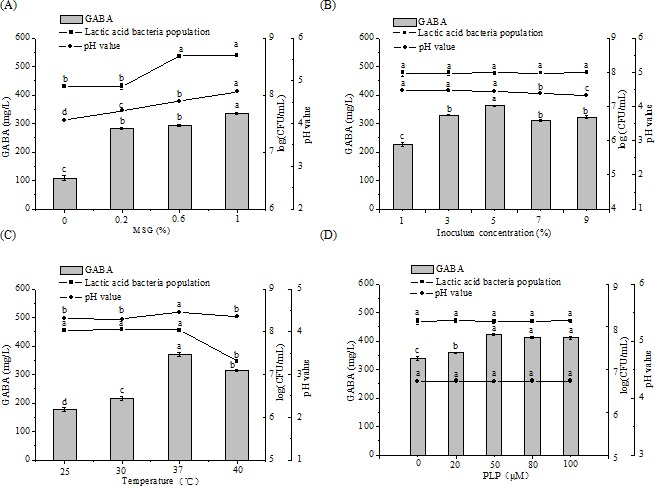
Data are expressed as mean ± SD from triplicate experiments. Different letters at the top of the bars indicate significant difference (P < 0.05).
Based on the results of the single-factor test, chickpea milk fortified with 0.2% MSG was chosen as the culture substrate for GABA production. The three variables applied in BBD were inoculum concentration, temperature, and PLP concentration. Table 1 shows the 17 various combination sets produced and the corresponding GABA yields for both the predicted and actual values. Table 3 presents the regression coefficients and the ANOVA results for BBD. An F-value of 54.88 indicated that the model was significant (P < 0.0001). The value of the determination coefficient (R2) was 0.9860, indicating that the experimental data fitted well with the model. was 0.9895, which is close to R2, confirming that the model was highly significant. The model also possessed non-significant lack-of-fit values (F = 0.14, P = 0.9290). The results above proved the validity of the experimental model, which can be used to describe the real relationship between the variables and the yield of GABA. Values of P value less than 0.05 indicate that the model terms are significant, so the independent variables, A and B, the quadratic terms of A, B, and C, and the interaction between A and B had significant effects on GABA production.
Table 3. Analysis of variance (ANOVA) for the fitted quadratic polynomial model.
| Source | Sum of squares | df | Mean Square | F Value | P value | Significance |
|---|---|---|---|---|---|---|
| Model | 3.169E+005 | 9 | 35211.12 | 54.88 | <0.0001 | ** |
| A | 22941.89 | 1 | 22941.89 | 35.75 | 0.0006 | ** |
| B | 1.405E+005 | 1 | 1.405E+005 | 219.01 | <0.0001 | ** |
| C | 830.28 | 1 | 830.28 | 1.29 | 0.2928 | |
| AB | 3875.69 | 1 | 3875.69 | 6.04 | 0.0436 | * |
| AC | 118.37 | 1 | 118.37 | 0.18 | 0.6805 | |
| BC | 464.83 | 1 | 464.83 | 0.72 | 0.4229 | |
| A2 | 4436.63 | 1 | 4436.63 | 6.91 | 0.0339 | * |
| B2 | 1.243E+005 | 1 | 1.243E+005 | 193.71 | <0.0001 | ** |
| C2 | 11039.04 | 1 | 11039.04 | 17.20 | 0.0043 | ** |
| Residual | 4491.62 | 7 | 641.66 | |||
| Lack of fit | 435.05 | 3 | 145.02 | 0.14 | 0.9290 | |
| Pure error | 4056.57 | 4 | 1014.14 | |||
| Cor total | 3.214E+005 | 16 | ||||
| R2 = 0.9860 |
Notes.
Significant at 0.05 level.
Significant at 0.01 level.
The contour and response surface plots in Fig. 4A shows steep surfaces, indicating the effects of inoculum concentration and temperature interaction on GABA production were highly significant, with P value of 0.0436. On the contrary, the response surface and contour plots in Figs. 4B and 4C shows smoother surfaces, indicating the effects of inoculum concentration and PLP interaction, and PLP and temperature interaction on GABA production were not significant, with a P value of 0.6805 and 0.4229, respectively.
Figure 4. Contour plots and response surface plots showing the effect of inoculum concentration, temperature, and PLP concentration on GABA yield.
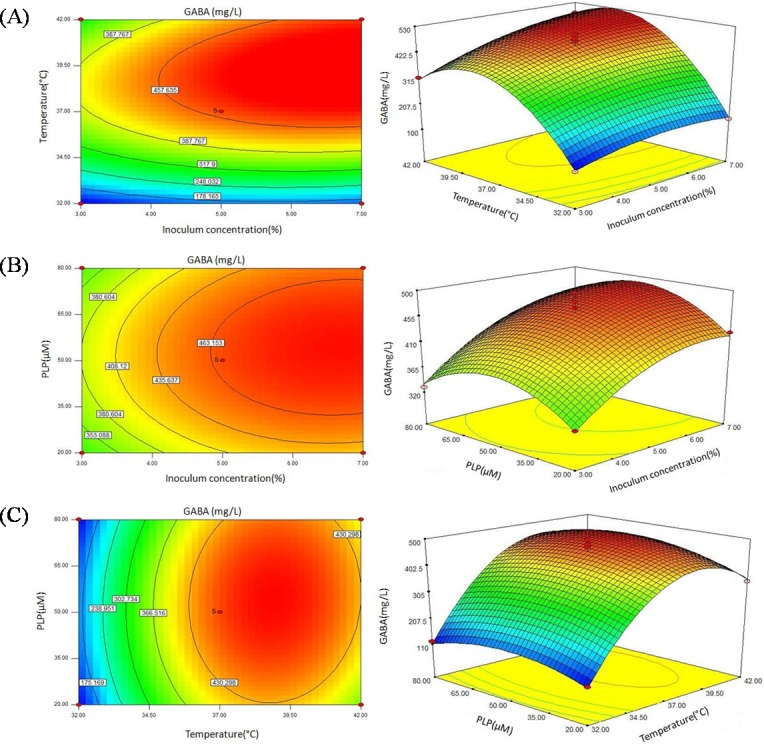
According to RSM results, the optimal culture conditions for GABA production were obtained as follows: inoculum concentration of 7%, culture temperature of 39.42 °C, and PLP concentration of 56.10 µM. Under these conditions, the maximum GABA production was predicted to be 529.71 mg/L. Considering the conditions for actual production, the optimal conditions were modified as follows: inoculum concentration of 7%, culture temperature of 39 °C, and PLP concentration of 55 µM. Under these conditions, a mean value of 527.26 ± 2.53 mg/L (n = 3) was obtained from actual experiments, which is close to the predicted value of 529.71 (P > 0.05). The results demonstrated that the response model was adequate for the optimization of GABA production.
Time course synthesis of GABA during fermentation
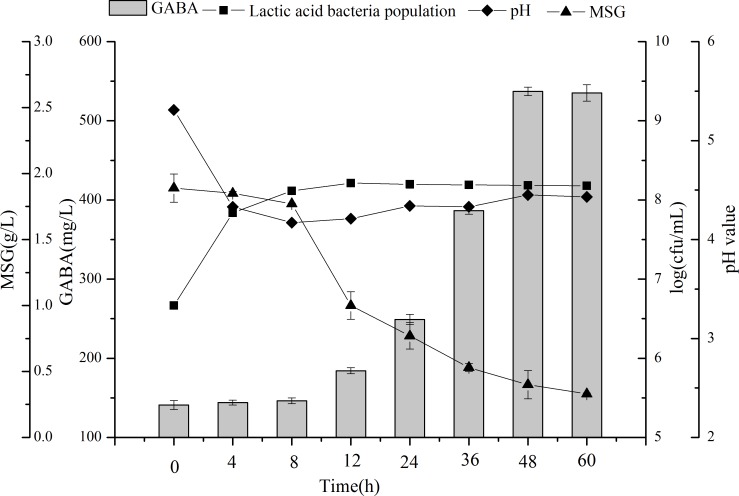
Figure 5 shows GABA content, MSG content, LAB count, and pH value at different fermentation time points and under the determined optimal conditions. The strain grew rapidly after inoculation and reached the stationary phase after 8 h of incubation, with a LAB population of 8.11 log (cfu/mL), which is 1.44 log cycles greater than the value obtained at the beginning of incubation. To exert the therapeutic benefits in vivo, bacterial populations in probiotic foodstuff reportedly should be at least 106 cfu/mL (Ghosh, Chattoraj & Chattopadhyay, 2013). The LAB count in our study was similar to that obtained from soy milk fermented by Enterococcus faecium strains, which had a cell density value ranging from 8.06–8.96 log (cfu/mL) after 16 h of fermentation (Cristina et al., 2012). The value obtained in the current study was slightly higher than that obtained from black soybean milk fermented by L. brevis FPA3709, which had a bacterial count of 1.2 × 108 cfu/mL after fermentation at 37 °C for 24 h (Ko, Lin & Tsai, 2013). Thus, chickpea milk is suitable for LAB growth and can be used as a good, functional food carrier.
Figure 5. Changes in LAB counts, pH value, GABA, and MSG concentrations during fermentation with L. plantarum M-6. Data are expressed as mean ± SD from triplicate experiments.
Different letters at the top of the bars indicate significant differences (P < 0.05).
GABA content increased slowly in the first eight hours of fermentation, then rapidly to reach a maximum of 537.23 mg/L at 48 h. Past that time point GABA production reached a plateau (P > 0.05). With the increment of GABA, MSG was consumed, and resulted in a residual concentration of 0.33 g/L. Thus, GABA production does not appear to be growth-associated, and instead occurs in the stationary phase of bacterial growth. GABA was shown to be produced by LAB fermentation from many kinds of legume, including black soybean milk and lentil (Ko, Lin & Tsai, 2013; Torino et al., 2013). To the best of our knowledge, the present study is the first to demonstrate the production of GABA from chickpea milk by LAB fermentation.
The pH level sharply decreased from 5.31 to 4.17 during the first 8 h probably because of the production of organic acids by LAB during fermentation (Schindler et al., 2012). The result is consistent with the LAB counts during the first 8 h, in which L. plantarum M-6 had a rapid growth. Subsequently, the pH value increased slightly to 4.45 at 48 h. These results agree with a previous study by Villegas et al. (2016). The probable reason for the increment of pH value follows. First, GAD activity allows the producing bacteria to overcome the low-pH stress of fermented food. When GABA is produced, glutamate is taken in by a specific transporter, then an intracellular proton is removed during glutamate decarboxylation. Meanwhile, via an antiporter, GABA is exported from the cell, thus increasing the cytoplasmic pH, and also slightly increasing the extracellular pH (Cotter & Hil, 2003).
Neuroprotective effect of FCE on manganese-induced PC12 cell death
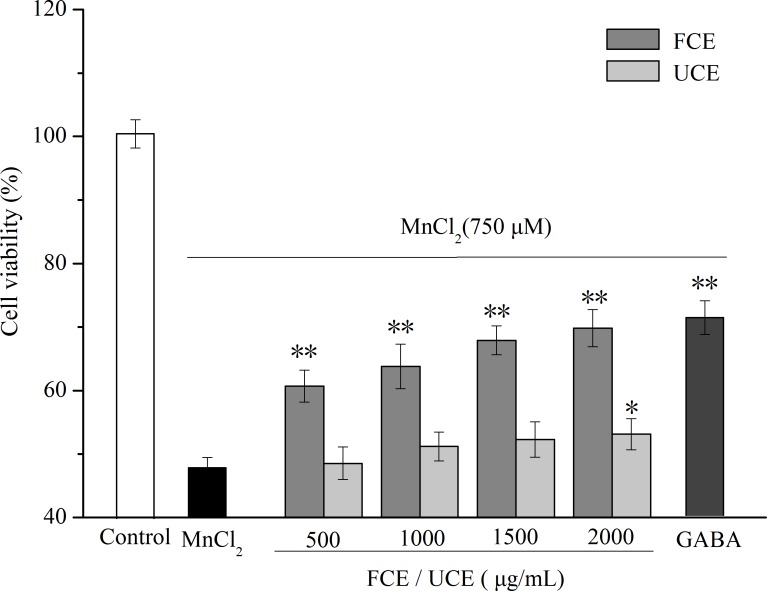
Effect of FCE on cell viability and cell morphology
The amount of bioactive component in GABA as contained in FCE is 17.78 mg/g. To determine the effect of GABA on cell viability, 40 µg/mL of GABA was chosen as positive control, comparable to the content of GABA in 2,000 µg/mL of FCE. The survival of the PC12 cells was determined by MTT assay. As shown in Fig. 6, MnCl2 treatment reduced cell survival. A concentration of 750 µM MnCl2 resulted in a cell viability rate of 47.87%. After pretreatment with different concentrations of FCE (500–2,000 µg/mL) or GABA, cell viability significantly increased by 26.80%–48.80%. Thus, FCE protected PC12 cells from injury in a dose-dependent manner, whereas UCE only showed little protective effect at its highest concentration. The morphological changes of PC12 cells under different conditions were also observed. Untreated cells were exuberant and fibriform, showed a distinct boundary and were associated with the cells (Fig. 7A). By contrast, when treated with MnCl2, the number of PC12 cells decreased and most of the cells crimpled to a spherical point and assembled; moreover, the links among the cells were absent (Fig. 7B). After pretreatment with UCE, not much changes were apparent (Fig. 7C). In FCE or GABA pretreatment, some PC12 cells regained their inherent morphology, that is, a fibriform structure and a distinct boundary (Figs. 7D–7E).
Figure 6. Effect of fermented chickpea milk extracts (FCE) and unfermented chickpea milk extracts (UCE) on manganese-induced PC12 cell death.
GABA (40 µg/mL) was used as positive control. All data are reported as mean ± SD (n = 3), ∗ indicates statistical difference (P < 0.05) with MnCl2 treated group, ∗∗ indicates statistical difference (P < 0.01) with MnCl2 treated group.
Figure 7. Morphology of PC12 cells observed by inverted microscope.
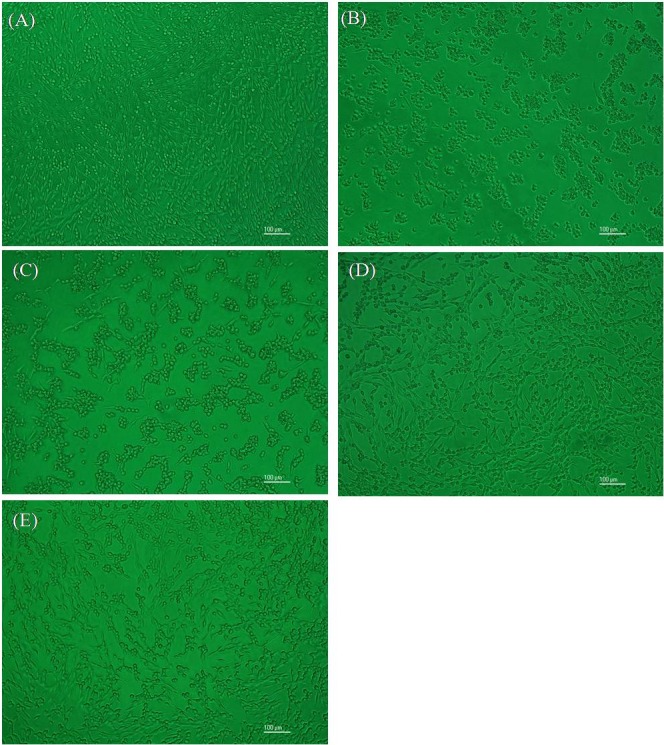
(A) Normal cells without MnCl2 or samples, (B) cells injured by MnCl2 (750 µM), cells treated with (C) UCE (2,000 µg/mL) + MnCl2(750 µM), (D) FCE (2,000 µg/mL) + MnCl2 (750 µM), and (E) GABA (40 µg/mL) + MnCl2 (750 µM).
Manganese at elevated concentrations is toxic and can cause irreversible damage to the central nervous system. Occupational exposure to manganese results in a syndrome that resembles Parkinson’s disease (Pal, Samii & Calne, 1999). PC12, a clonal cell line of rat pheochromocytoma, has similar physiological characteristics to neuronal cells, and is used for neuronal investigations (Jiang et al., 2003). Manganese has also been reported to induce apoptosis in PC12 cells (Hirata, 2002). Thus, PC12 cells can be employed as a model to evaluate the protection of different samples on manganese-induced injury. Based on the results above, FCE, which has an enhanced level of GABA, is confirmed to confer a neuroprotective effect against manganese-induced PC12 cell death, probably because of the higher levels of GABA after fermentation. Results agree with a previous investigation in which treatment of PC12 cells with L. buchneri MS strain culture medium, with enhanced levels of GABA, conferred protection from cytotoxic MnCl2 of 100–500 µM concentration (Cho, Chang & Chang, 2007).
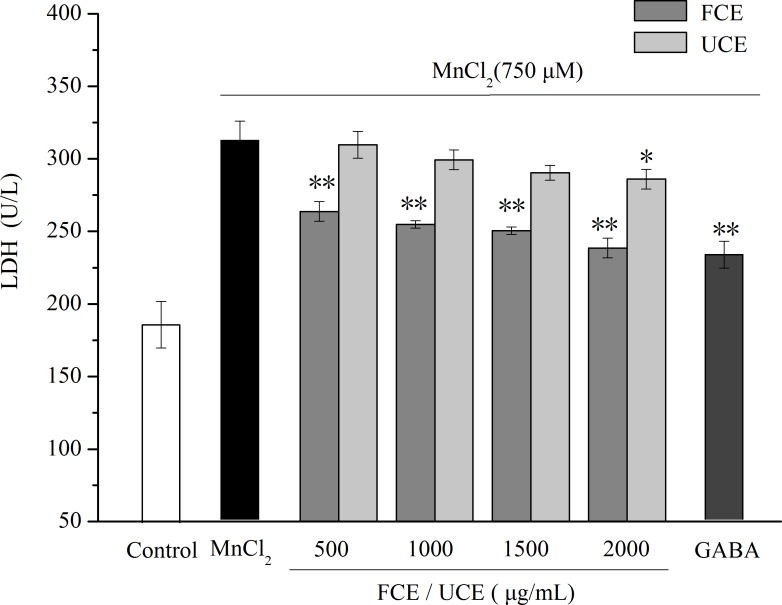
Effect of FCE on LDH activity
When the cell membrane is damaged, LDH is released from inside the cell to the culture medium; thus, LDH activity can be used as an indicator for cell membrane damage. The effect of FCE/UCE on LDH activity in MnCl2-treated PC12 cells is shown in Fig. 8. After exposure to MnCl2, LDH activity significantly increased in the culture medium of PC12 cells (P < 0.01), suggesting serious membrane damage caused by MnCl2. FCE or GABA treatment markedly reduced LDH activity in MnCl2-treated PC12 cells (P < 0.01), whereas UCE showed a slightly reduced LDH activity only at 2,000 µg/mL (P < 0.05). The results in LDH activity were in agreement with the morphological assessment and the MTT assay, which all confirm that GABA-enriched FCE can attenuate MnCl2-induced injury in PC12 cells. In summary, GABA-enriched FCE protects PC12 cells partially by retaining the integrity of membrane, which is necessary for the viability of cells.
Figure 8. Determination of the LDH activity in PC12 cells.
GABA (40 µg/mL) was used as positive control. All data are reported as mean ± SD (n = 3), ∗ indicates statistical difference (P < 0.05) with MnCl2 treated group, ∗∗ indicates statistical difference (P < 0.01) with MnCl2 treated group.
Conclusion
This study obtained chickpea milk with high GABA content through fermentation by L. plantarum M-6, a strain isolated from traditional fermented foods. Optimization by single-factor test and RSM indicated that the fermented chickpea milk contained high amounts of GABA (537.23 mg/L) when fortified with 0.2% MSG and 55 µM PLP and incubated at 39 °C for 48 h. The extract of GABA-enriched fermented chickpea milk demonstrated a neuroprotective effect on manganese-induced PC12 cell death. Thus, the fermented chickpea milk exhibits potential health benefits. Further study is suggested to test whether fermented chickpea milk exerts physiological effect in vivo, and to determine the molecular mechanisms underlying the protective effects of FCE against manganese-induced toxicity.
Supplemental Information
Lane G, GABA standard (2 g/L); lane GM, GABA and MSG standards (2 g/L); lane 1, MRS with 1% MSG (blank control); lanes 2–5, 7–10, 13–15, LAB isolates without capacity for GABA production; lane 6, M-6; lane 11, M-5; lane 12, M-7; lane 16, M-9.
Data are expressed as mean ± SD from triplicate experiments. 1 = dislike very much; 2 = dislike; 3 = acceptable; 4 = like; 5 = like very much.
Funding Statement
This work was financed by National Natural Science Foundation of China (No. 31371807). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Wen li conceived and designed the experiments, performed the experiments, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Mingming Wei performed the experiments.
Junjun Wu conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Xin Rui contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Mingsheng Dong conceived and designed the experiments, reviewed drafts of the paper.
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as Data S1.
References
- Adeghate & Ponery (2002).Adeghate E, Ponery AS. GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell. 2002;34:1–6. doi: 10.1054/tice.2002.0217. [DOI] [PubMed] [Google Scholar]
- Chin et al. (2006).Chin HS, Breidt F, Fleming HP, Shin WC, Yoon SS. Identification of predominant bacterial isolates from the fermenting kimchi using ITS-PCR and partial 16S rDNA sequence analyses. Journal of Microbiology and Biotechnology. 2006;18:68–76. [Google Scholar]
- Cho, Chang & Chang (2007).Cho YR, Chang JY, Chang HC. Production of gamma aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. Jounal of Microbiology and Biotechnology. 2007;17:104–109. [PubMed] [Google Scholar]
- Cotter & Hil (2003).Cotter PD, Hil C. Surviving the acid test: response of gram-positve bacteria to low pH. Microbiology and Molecular Biology Reviews. 2003;67:429–453. doi: 10.1128/MMBR.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristina et al. (2012).Cristina MV, Maria IT, Martin V, Rebeca A, Patricia GM, Isabel EP, Concepcion VV, Juan MR, Juana F. Multifunctional properties of soy milk fermented by Enterococcus faecium strains isolated from raw soy milk. Journal of Agricultual and Food Chemistry. 2012;60:10235–10244. doi: 10.1021/jf302751m. [DOI] [PubMed] [Google Scholar]
- Diana et al. (2014).Diana M, Tres A, Quílez J, Llombart M, Rafecas M. Spanish cheese screening and selection of lactic acid bacteria with high gamma-aminobutyric acid production. LWT—Food Science and Technology. 2014;56:351–355. doi: 10.1016/j.lwt.2013.11.027. [DOI] [Google Scholar]
- Ghosh, Chattoraj & Chattopadhyay (2013).Ghosh D, Chattoraj DK, Chattopadhyay P. Studies on changes in microstructure and proteolysis in cow and soy milk curd during fermentation using lactic cultures for improving protein bioavailability. Journal of Food Science and Technology. 2013;50:979–985. doi: 10.1007/s13197-011-0421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata (2002).Hirata Y. Manganese-induced apoptosis in PC12 cells. Neurotoxicology and Teratology. 2002;24:639–653. doi: 10.1016/S0892-0362(02)00215-5. [DOI] [PubMed] [Google Scholar]
- Inoue et al. (2003).Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H. Blood-pressure-lowering effect of a novel fermented milk containing gaminobutyric acid (GABA) in mild hypertensives. European Journal of Clinical Nutrition. 2003;57:490–495. doi: 10.1038/sj.ejcn.1601555. [DOI] [PubMed] [Google Scholar]
- Jiang et al. (2003).Jiang B, Liu JH, Bao YM, An LJ. Hydrogen peroxide-induced apoptosis in PC12 cells and the protective effect of puerarin. Cell Biology International. 2003;27:1025–1031. doi: 10.1016/j.cellbi.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2009).Kim JY, Lee MY, Ji GE, Lee YS, Hwang KT. Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. International Journal of Food Microbiology. 2009;130:12–16. doi: 10.1016/j.ijfoodmicro.2008.12.028. [DOI] [PubMed] [Google Scholar]
- Ko, Lin & Tsai (2013).Ko CY, Lin H, Tsai GJ. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochemistry. 2013;48:559–568. doi: 10.1016/j.procbio.2013.02.021. [DOI] [Google Scholar]
- Komatsuzaki et al. (2008).Komatsuzaki N, Nakamura T, Kimura T, Shima J. Characterization of glutamate decarboxylase from a high gamma-aminobutyric acid (GABA)-producer, Lactobacillus paracasei. Bioscience, Biotechnology, and Biochemistry. 2008;72:278–285. doi: 10.1271/bbb.70163. [DOI] [PubMed] [Google Scholar]
- Lawless & Heymann (1999).Lawless HT, Heymann H. Sensory evaluation of food: principles and practice. Aspen Publishers Inc; New York: 1999. [Google Scholar]
- Lee, Yoon & Park (2008).Lee SM, Yoon MY, Park HR. Protective effects of paeonia lactiflora pall on hydrogen peroxide-induced apoptosis in PC12 cells. Bioscience Biotechnology and Biochemistry. 2008;72:1272–1277. doi: 10.1271/bbb.70756. [DOI] [PubMed] [Google Scholar]
- Leventhal et al. (2003).Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonist improved visual cortical function in senescent monkey. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Li & Cao (2010).Li H, Cao Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids. 2010;39:1107–1116. doi: 10.1007/s00726-010-0582-7. [DOI] [PubMed] [Google Scholar]
- Li et al. (2009).Li H, Qiu T, Cao Y, Yang J, Huang Z. Pre-staining paper chromatography method for quantification of gamma-aminobutyric acid. Journal of Chromatography A. 2009;1216:5057–5060. doi: 10.1016/j.chroma.2009.04.044. [DOI] [PubMed] [Google Scholar]
- Molina et al. (2012).Molina V, Medici M, De Valdez GF, Taranto MP. Soybean-based functional food with vitamin B-12-producing lactic acid bacteria. Journal of Functional Foods. 2012;4:831–836. doi: 10.1016/j.jff.2012.05.011. [DOI] [Google Scholar]
- Pal, Samii & Calne (1999).Pal PK, Samii A, Calne DB. Manganese neurotoxicity:a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Park & Oh (2007).Park KB, Oh SH. Cloning, sequencing and expression of a novel glutamate decarboxylase gene from a newly isolated lactic acid bacterium, Lactobacillus brevis OPK-3. Bioresource Technology. 2007;98:312–319. doi: 10.1016/j.biortech.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Roy, Boye & Simpson (2010).Roy F, Boye JI, Simpson BK. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Research International. 2010;43:432–442. doi: 10.1016/j.foodres.2009.09.002. [DOI] [Google Scholar]
- Schindler et al. (2012).Schindler S, Zelena K, Krings U, Bez J, Eisner P, Berger RG. Improvement of the aroma of pea (Pisum sativum) protein extracts by lactic acid fermentation. Food Biotechnology. 2012;26:58–74. doi: 10.1080/08905436.2011.645939. [DOI] [Google Scholar]
- Seok et al. (2008).Seok JH, Park KB, Kim YH, Bae MO, Lee MK, Oh SH. Production and characterization of kimchi with enhanced levels of gamma-aminobutyric acid. Food Science and Biotechnology. 2008;17:940–946. [Google Scholar]
- Tajabadi et al. (2015).Tajabadi N, Baradaran A, Ebrahimpour A, Rahim RA, Bakar FA, Manap MYA, Mohammed AS, Nazamid S. Overexpression and optimization of glutamate decarboxylase in Lactobacillus plantarum Taj-Apis362 for high gamma-aminobutyric acid production. Microbial Biotechnology. 2015;8:623–632. doi: 10.1111/1751-7915.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong et al. (2002).Tong JC, Mackay IR, Chin J, Law RHP, Fayad K, Rowley MJ. Enzymatic characterization of a recombinant isoform hybrid of glutamic acid decarboxylase (rGAD67/65) expressed in yeast. Journal of Biotechnology. 2002;97:183–190. doi: 10.1016/S0168-1656(02)00060-3. [DOI] [PubMed] [Google Scholar]
- Torino et al. (2013).Torino MI, Limón RI, Martínez-Villaluenga C, Mäkinen S, Pihlanto A, VidalValverde C, Frias J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chemistry. 2013;136:1030–1037. doi: 10.1016/j.foodchem.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Tujioka et al. (2009).Tujioka K, Ohsumi M, Horie K, Kim M, Hayase K, Yokogoshi H. Dietary gamma-aminobutyric acid affects brain protein synthesis rate in ovariectomized female rats. Journal of Nutritional Science and Vitaminology. 2009;55:75–80. doi: 10.3177/jnsv.55.75. [DOI] [PubMed] [Google Scholar]
- Van der Meulen et al. (2007).Van der Meulen RV, Scheirlinck I, Schoor AV, Huys G, Vancanneyt M, Vandamme P, De Vuyst L. Population dynamics and metabolite target analysis of lactic acid bacteria during laboratory fermentations of wheat and spelt sourdoughs. Applied and Environmental Microbiology. 2007;73:4741–4750. doi: 10.1128/AEM.00315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas et al. (2016).Villegas JM, Brown L, Giori G, Hebert EM. Optimization of batch culture conditions for GABA production by Lactobacillus brevis CRL 1942, isolated from quinoa sourdough. LWT—Food Science and Technology. 2016;67:22–26. doi: 10.1016/j.lwt.2015.11.027. [DOI] [Google Scholar]
- Xiang et al. (2008).Xiang XL, Yang LY, Hua S, Li W, Sun Y, Ma H, Zhang J, Zeng XX. Determination of oligosaccharide contents in 19 cultivars of chickpea (Cicer arietinumL) seeds by high performance liquid chromatograph. Food Chemistry. 2008;111:215–219. doi: 10.1016/j.foodchem.2008.03.039. [DOI] [Google Scholar]
- Xiao et al. (2014).Xiao Y, Xing GL, Rui X, Li W, Chen XH, Jiang M, Dong MS. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. Journal of Functional Foods. 2014;10:210–222. doi: 10.1016/j.jff.2014.06.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lane G, GABA standard (2 g/L); lane GM, GABA and MSG standards (2 g/L); lane 1, MRS with 1% MSG (blank control); lanes 2–5, 7–10, 13–15, LAB isolates without capacity for GABA production; lane 6, M-6; lane 11, M-5; lane 12, M-7; lane 16, M-9.
Data are expressed as mean ± SD from triplicate experiments. 1 = dislike very much; 2 = dislike; 3 = acceptable; 4 = like; 5 = like very much.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as Data S1.