Abstract
The “scaly-tailed squirrels” of the rodent family Anomaluridae have a long evolutionary history in Africa, and are now represented by two gliding genera (Anomalurus and Idiurus) and a rare and obscure genus (Zenkerella) that has never been observed alive by mammalogists. Zenkerella shows no anatomical adaptations for gliding, but has traditionally been grouped with the glider Idiurus on the basis of craniodental similarities, implying that either the Zenkerella lineage lost its gliding adaptations, or that Anomalurus and Idiurus evolved theirs independently. Here we present the first nuclear and mitochondrial DNA sequences of Zenkerella, based on recently recovered whole-body specimens from Bioko Island (Equatorial Guinea), which show unambiguously that Zenkerella is the sister taxon of Anomalurus and Idiurus. These data indicate that gliding likely evolved only once within Anomaluridae, and that there were no subsequent evolutionary reversals. We combine this new molecular evidence with morphological data from living and extinct anomaluromorph rodents and estimate that the lineage leading to Zenkerella has been evolving independently in Africa since the early Eocene, approximately 49 million years ago. Recently discovered fossils further attest to the antiquity of the lineage leading to Zenkerella, which can now be recognized as a classic example of a “living fossil,” about which we know remarkably little. The osteological markers of gliding are estimated to have evolved along the stem lineage of the Anomalurus–Idiurus clade by the early Oligocene, potentially indicating that this adaptation evolved in response to climatic perturbations at the Eocene–Oligocene boundary (∼34 million years ago).
Keywords: Anomaluridae, Mammals, Phylogeny, Bioko Island, Equatorial Guinea, Patagium, Gliding, Eocene, Oligocene
Introduction
Current taxonomy recognizes the rodent family Anomaluridae with seven extant species across three genera (Anomalurus, Idiurus and Zenkerella) (Dieterlen, 2005; Kingdon, 2013). Living anomalurids are endemic to western and central Africa and until recently were commonly referred to as “scaly-tailed squirrels.” As the name suggests, the group’s hallmark feature is a set of scales situated on the ventral surface of the proximal tail which reportedly provide support and traction when climbing trees (Kingdon, 2013; Nowak, 1999). However, despite the vernacular, anomalurids are not closely related to the true squirrels of the rodent family Sciuridae (Fabre et al., 2012). A switch to the common name “anomalures” avoids an implied anomalurid-sciurid alliance and this term has become increasingly popular in recent years. With the exception of Zenkerella, living anomalurids are equipped with patagial membranes that allow them to move through forests by gliding. This adaptation evolved independently from the two other extant lineages of gliding placental mammals (i.e., dermopterans and pteromyin sciurid rodents) (Jackson & Thorington, 2012). The evolutionary pattern by which Zenkerella came to be differentially adapted from other anomalurids is poorly understood. In fact, Zenkerella is among the least studied of all mammals; virtually nothing is known of its behavior, ecology or life-history, and scarce distribution reports make it difficult to assess its conservation status. Although first described in 1898, only eleven Zenkerella specimens are curated in world collections (Perez del Val, Juste & Castroviejo, 1995) and to our knowledge, Zenkerella has never been observed alive by trained mammalogists.
Molecular phylogenetic studies recover Anomaluridae as the sister clade of the monotypic family Pedetidae (Pedetes, springhares) and together these families comprise the rodent suborder Anomaluromorpha (Blanga-Kanfi et al., 2009; Churakov et al., 2010; Fabre et al., 2012; Meredith et al., 2011). Like anomalurids, the two living species of Pedetes are African endemics. However, by contrast, springhares are adapted as burrowing terrestrial hoppers and are anatomically quite different from anomalures. Specialized details of middle ear anatomy (Meng, 1990; Ruf, Frahnert & Maier, 2009) and cranial arterial patterns (Bugge, 1974; Bugge, 1985; George, 1981) are among the few shared morphological features that support an anomalurid-pedetid clade—whereas the microstructure of incisor tooth enamel, a character classically used to affiliate rodent groups, is fundamentally different (Martin, 1993). The disparate morphotypes and molecular distances separating these families, taken in combination with the Eocene fossil record of anomalurid evolution in Africa (Marivaux et al., 2015; Sallam, Seiffert & Simons, 2010; Sallam et al., 2010), seemingly indicate an ancient divergence of anomaluromorph lineages on that continent.
Extant anomalurids are arranged in two subfamilies. Anomalurinae includes four species placed in the genus Anomalurus—although craniodental distinctions have prompted some authors to advocate the transfer of Anomalurus beecrofti into a different genus, Anomalurops (Kingdon, 2013). Both species of Idiurus and the monotypic Zenkerella (Zenkerella insignis) are grouped in the subfamily Zenkerellinae (Dieterlen, 2005). Previous authors have applied the synonym Idiurinae to ally these two genera (Dieterlen, 2005; Nowak, 1999). Characters common to zenkerelline taxa include an origin of the lower maxillary process close to the incisors (cf. closer to the cheek teeth in Anomalurus), a relatively deep dorsoventral dimension of the premaxilla (cf. relatively shallow in Anomalurus) and a notch on the lingual surface of the upper incisors (cf. unnotched in Anomalurus) (Kingdon, 2013).
If this taxonomic arrangement reflects phylogenetic descent, there are implications for how to interpret the evolution of patagial gliding. Anomalurus and Idiurus are unique among mammalian gliders in having an attachment of the patagium to a cartilaginous support rod that extends from the elbow (Coster et al., 2015). By contrast, pteromyin sciurids have a support cartilage that extends from the wrist (Jackson & Schouten, 2012). In gliding anomalurids, the structure is approximately the length of the forearm and projects anterolaterally from a bony expansion of the ulnar olecranon process. Zenkerella lacks this osteological feature and the patagia and cartilaginous struts are absent. At least three evolutionary scenarios are plausible (Coster et al., 2015). Gliding could have evolved prior to the common ancestor of living anomalurids and was subsequently lost in the Zenkerella lineage. Alternatively, from a non-gliding ancestor, Anomalurus and Idiurus could be convergently adapted while Zenkerella has retained the ancestral condition. A third scenario is that Anomalurus and Idiurus are more closely related to each other than either is to Zenkerella, in which case patagial gliding is more likely to have evolved once, along the stem lineage of the Anomalurus–Idiurus clade. This third scenario, however, is incongruent with the current classification and phylogenetic hypotheses. A robust phylogenetic framework is required to evaluate these competing hypotheses, but the paucity and preservation of available specimens have been limiting factors for incorporating Zenkerella into molecular phylogenetic analyses.
As part of an ongoing biodiversity monitoring and conservation program on Bioko Island, Equatorial Guinea, we have recently recovered three additional Z. insignis specimens. With newly generated nuclear and mitochondrial sequences from two of these individuals, here we test the proposed phylogenetic relationships of Zenkerella using a Bayesian approach. After combining the molecular data with morphological data from living and fossil taxa, we then use Bayesian “tip-dating” methods to estimate divergence dates within Anomaluromorpha. With these results, we reassess the taxonomic placement of Zenkerella and suggest a scenario for the evolution of gliding in anomaluromorph rodents.
Methods
Specimens
The three new Z. insignis specimens are whole animals deposited at the Duke Lemur Center Division of Fossil Primates at Duke University (Durham, NC, USA). Specimen voucher numbers are DPC 91000–91002. The animals were caught in ground snares by local trappers in the proximity of Ureca village near the southern tip of Bioko Island, Equatorial Guinea. Specimens were collected and exported under permits issued to the Bioko Biodiversity Protection Program (BBPP) (field permit 154/2015 from the Equatorial Guinea Department of Culture and Tourism; export permit 169/2015 from the National University of Equatorial Guinea). DPC 91000 (male) was collected July 2014 at GPS coordinates ∼N3.25469 E8.58544; DPC 91001 (male) and DPC 91002 (female) were collected in September 2015 and October 2015 at ∼N3.24999 E8.56825 and ∼N3.25627 E8.58408, but trappers did not note sex upon recovery and were unable to associate these individuals to specific sites. While in Bioko, the earlier specimen was preserved in formalin for 11 weeks and then transferred to 70% ethanol. The more recently recovered specimens were preserved in 70% ethanol upon collection and later transferred to a molecular biology grade 70% ethanol which is certified DNase, RNase and protease free.
DNA extraction and purification
Total DNA was extracted from DPC 91001 and DPC 91002 using a Qiagen DNEasy Blood and Tissue Kit and protocol. Cheek swabs were used to collect buccal epithelial cells as starting genetic material. Specimens were handled using sterile gloves and instruments. For the lysis step, we elected to use 40 µl of Proteinase K, 360 µl of buffer ATL and to incubate at 56 °C for 24 h. For the purification steps, we followed kit protocol and eluted the spin column membrane twice to increase total yield. A negative control was processed in parallel following identical protocol but starting with a sterile swab.
Amplification and sequencing
Two nuclear (IRBP, VWF) and three mitochondrial (12S, COX1, CYTB) loci were targeted for PCR amplification. Oligonucleotide primers were newly designed using a comparative sample of existing anomaluromorph and other rodent sequences retrieved from GenBank. Amplifications were performed using Promega GoTaq G2 Hot Start Master Mix and protocol for 25 µl reactions. PCR was run for 40 cycles with each cycle consisting of denaturation (1 min, 95 °C), annealing (1 min, variable temperature) and extension (variable time, 72 °C). The first cycle was preceded by an initial denaturation (5 min, 95 °C) and the last cycle was followed by a final extension (5 min, 72 °C). Primer sequences, annealing temperatures and extension times are reported in Data S1. PCR products were electrophoresed on a 1% agarose gel to screen for expected lengths and non-specifics. All negative control lanes were blank. DNA bands in excised gel slices were purified and sequenced twice at Eurofins MWG Operon (Louisville, KY, USA) using an Applied Biosystems 3730xl DNA Analyzer. To screen for possible contamination, Zenkerella sequence results were compared to available orthologs from Anomalurus, Idiurus, Pedetes and all mammalian taxa that have been previously processed in the lab. In all cases, Zenkerella sequences were unique and shared a higher percent identity with other anomaluromorphs than with non-anomaluromorph sequences. Zenkerella sequences are deposited at GenBank under accession numbers KU900240–KU900249.
DNA dataset
Our DNA dataset comprises five gene segments across 67 rodent and 11 outgroup taxa. All rodent suborders and families are represented. To improve data completeness, 14 non-anomaluromorph operational taxa combine congeneric sequences. Accession numbers for sequences retrieved from GenBank and identifications of chimeric operational taxa are in Data S2. Alignments were performed using the Geneious (v7.1.7) (Kearse et al., 2012) multiple alignment tool with default settings. For the four amino acid coding sequences, some positions in the alignments were manually adjusted using the translation frame as a guide. Translated sequences contained no stop codons. For the rRNA sequence, regions of alignment ambiguity were manually removed. Individual gene alignments were concatenated using Geneious yielding a 5,906 base pair alignment which is available in Data S3.
Phylogenetic analysis
PartitionFinder (v1.1.1) (Lanfear et al., 2012) was used to select a subset scheme and substitution models as assessed by the Bayesian Information Criterion (BIC). Input data blocks were defined by gene and codon positions. Settings specified exploration of all models available in MrBayes and all subset schemes given the data blocks. The best-fit scheme proposed eight subsets: (12S, CYTB-pos1) (COX1-pos1) (COX1-pos2, CYTB-pos2) (COX1-pos3) (CYTB-pos3) (IRBP-pos1, VWF-pos1) (IRBP-pos2, VWF-pos2) (IRBP-pos3, VWF-pos3). The best-fit model for subsets 1–7 was GTR+I+G and for subset 8 was GTR+G.
MrBayes (v3.2.6) (Ronquist et al., 2012b) was used to infer phylogenetic relationships. Partitions and models were set according to PartitionFinder results and model parameters were unlinked. Two MCMCMC runs were called with four chains each (three hot, one cold) using sample frequency 1000 over 50 million generations. Heating temperature was set to 0.05 and relative burn-in to 0.25. We assessed adequate run length using three diagnostics. (1) Minimum effective sample sizes (ESS) for all parameters were >200 with a mean of 11,254. (2) The average standard deviation of split frequencies (ASDSF) was 0.000954. (3) Across all parameters, the mean potential scale reduction factor (PSRF) was 1.000487 with a maximum |1-PSRF| of 0.006835. We interpret these values as indication that parameter space was sufficiently sampled and the runs had converged.
In addition to Bayesian inference, we used RAxML (v8.2.7) (Stamatakis, 2014) to perform maximum likelihood (ML) analyses using the concatenated alignment and each individual marker. Appropriate subset and model choices were discerned with PartitionFinder. Full methodological details of ML runs are in Data S4.
Clock analysis
To estimate divergence times, we used a total-evidence Bayesian “tip-dating” approach (Ronquist et al., 2012a; Ronquist et al., 2012b) implemented in MrBayes. With the exception of Pedetes surdaster, all anomaluromorph species in the molecular dataset are scored for morphological characters in the matrix of Marivaux et al. (2015). That character matrix also includes fossil data for three anomalurid and four nementchamyid / nonanomalurid taxa which bear on the chronology of the suborder. We expanded the fossil sample by using the same character descriptions and interpretations of crest homologies to newly score Zenkerella wintoni (Lavocat, 1973), Prozenkerella saharaensis (Coster et al., 2015) and Propedetes efeldensis (Mein & Pickford, 2008). Our version of the morphological matrix includes 15 anomaluromorph taxa and 150 (mostly dental) characters. The molecular and morphological datasets were concatenated using Mesquite (v3.04) (Maddison & Maddison, 2015) and the combined evidence matrix is available in Data S5.
A gamma distributed Markov k model was assigned to the morphological partition using default priors and variable coding. Following Marivaux et al. (2015), 95 morphological characters were treated as ordered. Model settings for the molecular partition were identical to those used in the non-clock analysis. For fossil “tip” calibrations, age uncertainty was accommodated by employing uniform priors based on (when possible) the currently recognized upper and lower bounds of magnetochrons in which species might reasonably be placed. Data S6 reports the chronology and provenance of included fossil taxa. Using the molecular tree branching pattern, hard topological constraints were applied to all taxa outside the anomaluromorph suborder. For anomaluromorphs, partial constraints were applied to extant species to serve as a scaffold for the placement of fossils. We selected the independent gamma rates (IGR) model to estimate relaxed clock rate variation and used a uniform distribution for the branch lengths prior.
Non-rodent taxa were excluded from the clock analysis rendering the crown rodent node and tree root as synonymous. Previous cladistic testing has positioned the fossil taxon Paramys within the rodent crown group (Benton et al., 2015; Marivaux, Vianey-Liaud & Jaeger, 2004). The earliest appearances of Paramys in the latest Paleocene signals a minimum age for the crown rodent node (Benton et al., 2015; Rose, 1981). The earliest Paleocene marks the first appearance of fossil taxa definitively positioned within the placental mammal crown group. Included are the laurasiatherian Protungulatum, euarchontan Purgatorius and glirans Mimotona and Heomys (O’Leary et al., 2013). From a paleontological perspective, we find it reasonable to assume that the relatively nested crown rodent node postdates the earliest known crown placentals. Therefore the maximum and minimum of the uniformly distributed tree age prior were set to correspond to the beginning (66 Ma) and end (56 Ma) of the Paleocene epoch. We recognize that many molecular-based estimates for the age of the crown rodent node extend into the Cretaceous. To accommodate this possibility an alternate analysis was run after lifting the K-Pg limit of the tree age prior.
The clock rate prior is an initial estimate for a distribution describing the number of substitutions per site per million years. To derive this estimate, we first used the non-clock molecular tree and the dist.nodes function from the R package APE (v3.4) (Paradis, Claude & Strimmer, 2004) to extract path lengths from each rodent tip to the rodent node. Next, each path length was divided by the center of the tree age prior. The fitdist function from the R package fitdistrplus (v1.0-6) (Delignette-Muller & Dutang, 2015) was then used to fit gamma, log-normal and normal distributions to the set of scaled path lengths. A comparison of BIC scores was used to discern the log-normal distribution as best fit. With this result, we estimated the clock rate prior mean at −2.646972 with a 0.202816 standard deviation. The heating temperature was lowered to 0.02 to promote chain swapping. With the exception of generations, all other MCMCMC settings were identical to the non-clock analysis.
To choose an IGR variance prior, we ran several pilot analyses using exponential distributions with values ranging from 5 to 35 in increments of 5. Pilots were run for 10 million generations applying the stepping-stone method. Marginal likelihoods and select node dates were compared to investigate the effect of the prior value. Tree topologies across all pilots were identical. The range of marginal likelihoods was 1.73. The range of mean age estimates for the crown rodent and crown anomaluromorph nodes were 1.21 and 0.93 million years, respectively. We interpret these findings as negligible variation and suspect that longer, more densely sampled analyses would converge. When applied to our combined evidence dataset, clock analysis seems mostly insensitive to a prior value for exponential IGR variance—at least given the other analysis settings. Therefore, we selected the MrBayes default value of 10.
The final clock analyses (rodent node with and without K-Pg maximum limit) were run for 35 million generations each. Again, adequate run length, sampling and convergence were assessed with three diagnostics. (1) Minimum ESS for all parameters were >200 with means of 8280 and 8488. (2) The ASDSFs were 0.007418 and 0.006440. (3) The parameter mean PSRFs were 1.005415 and 1.001295.
Results
Specimens
We compared preliminary micro-CT images of DPC 91000 to Fig. 9 from Marivaux et al. (2011) and Figs. 2 and 6 from Coster et al. (2015) and confirm that this specimen shares the craniodental and postcranial morphological features that distinguish Zenkerella. Compared to other living anomalures, Zenkerella exhibits a highly derived molar morphology that is characterized by simple loph and lophid patterns. Unlike Anomalurus and Idiurus, the molar occlusal surfaces of Zenkerella are surrounded by a continuous external crest that, in occlusal view, is relatively round in shape (Coster et al., 2015). Zenkerella molars are distinct in exhibiting a single transverse crest that subdivides the occlusal surface into two foveae. Zenkerella is also distinguished from other living anomalures in the relative sizes of the upper cheek teeth. P4 and M3 are similar in size and both are smaller than M1 and M2 (Kingdon, 2013). The rows of scales on the ventral tail (Fig. 1) are diagnostic for anomalurids and the lack of patagia is unique to Zenkerella (Kingdon, 2013). Previous reports of Zenkerella’s geographic distribution include Bioko near Ureca (Perez del Val, Juste & Castroviejo, 1995). Our comparison to the craniodental and postcranial features of mainland specimens (Marivaux et al., 2011; Coster et al., 2015) finds that at present there is no compelling reason to identify the Bioko Island population as a new species.
Figure 1. Photographs of Zenkerella insignis DPC 91001 male.
(A) dorsal view, scale bar 50 mm. (B) lateral view, scale bar 50 mm. (C) palmar view of right hand and plantar view of left foot, scale bar 10 mm. (D) ventral view of proximal tail and genitals, scale bar 25 mm.
Literature accounts of new Z. insignis data are especially rare, prompting our report of basic metrics, description and ecological information. For the three new specimens, head and body lengths are 181–198 mm and tail lengths are 168–184 mm. These measurements are within or slightly larger than previously reported ranges (Kingdon, 2013; Nowak, 1999). We cannot confirm that all specimens are full adults. Body hair is gunmetal (or ashy) grey and tail hair is black and bushy. Hairs of the lateral ankle tufts are black, coarse and spiky. Maximum bilateral vibrissae span is approximately six times the head width. The scaly portion of the tail extends for 20–25% of tail length. Hands and feet have four and five digits respectively and claws are present on all digits. The first toe (pedal digit I) is somewhat divergent. The scrotum is relatively large and CT images indicate that a baculum is present. The female has 4 nipples and our dissection of the abdomen confirms that she is not pregnant. Specimen photographs are in Fig. 1. Entrapment in ground snares indicates that Zenkerella is not exclusively arboreal. When interviewed, villagers in the vicinity of the collection site reported that Zenkerella is infrequently (once or twice a year) caught in forest floor traps and that the species is not desirable for meat. Interviewees also suggested that Zenkerella is nocturnally active and sleeps in tree hollows. Additional details of anatomy and ecology will be part of forthcoming research.
Phylogeny
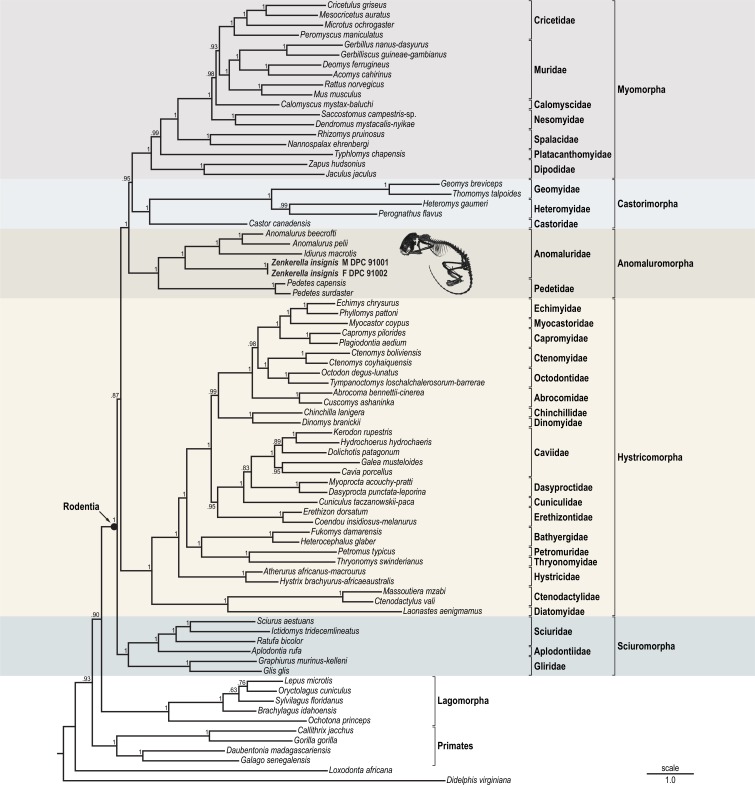
Bayesian analysis of the concatenated nuclear and mitochondrial alignment strongly supports monophyly for each of the five currently recognized rodent suborders and the pattern of basal divergence branches into three major lineages. The suborder Sciuromorpha derives from the earliest split and is positioned as sister to all other living rodents. The remaining two lineages are the suborder Hystricomorpha and its sister-clade which comprises the suborders Myomorpha, Castorimorpha and Anomaluromorpha. This basal divergence pattern among the three lineages is consistent with previous retroposon analyses and very large scale DNA sequence analyses (Churakov et al., 2010; Fabre et al., 2012).
With all anomaluromorph genera and all rodent families represented, our results recover Anomaluridae and Pedetidae as sister taxa; all anomaluromorph splits were inferred with maximum posterior probabilities (Fig. 2). Among anomalurids, the genera Idiurus and Anomalurus are sister taxa to the exclusion of Zenkerella. This finding is incompatible with the current classification of Zenkerella and Idiurus in the subfamily Zenkerellinae, but is consistent with the relationships proposed by Tullberg (1899). A taxonomic revision is discussed below.
Figure 2. Result of Bayesian phylogenetic analysis of the 5,906 base pair concatenated DNA alignment.
Two nuclear (IRBP, VWF) and three mitochondrial (12S, COX1, CYTB) sequences were included. Values at nodes are posterior probability (=clade credibility). Branch lengths are mean values from the posterior distribution representing expected number of substitutions per site. Rodent suborder and family designations follow the current taxonomy of Wilson & Reeder (2005).
ML analysis of the concatenated alignment resulted in a topology which is identical to the Bayesian tree and all anomaluromorph nodes are supported with very strong bootstrap proportions. ML analyses of all individual markers recovered monophyletic anomaluromorph groups to the exclusion of other rodents. Individual marker analyses also inferred relationships among anomaluromorphs that are fully congruent with the concatenation trees. These results detect no conflict in phylogenetic signal between nuclear and mitochondrial sequences. Data S4 includes ML results.
Divergence time
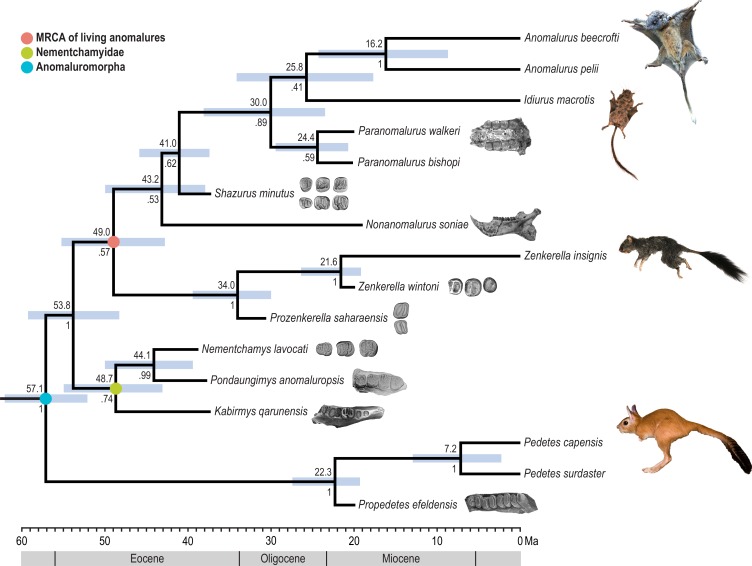
Tip-dating analysis using molecular and morphological data from living and fossil taxa estimates an Eocene date for the most recent common ancestor of extant anomalures (Fig. 3). The mean highest posterior density of this estimate is at 49.0 Ma with a 95% range (55.2 to 42.8 Ma) through the early and early middle Eocene. The node representing the most recent common ancestor of Anomalurus and Idiurus has a mean estimate at 25.6 Ma with a 95% range (34.2 to 17.7 Ma) spanning the Oligocene and into the early Miocene.
Figure 3. Result of Bayesian time-tree analysis using the fossil tip-dating method applied to combined molecular and morphological data.
Time scale is in millions of years. Values above nodes indicate mean highest posterior density age estimates. Bars underlying nodes represent 95% age ranges which accommodate topological uncertainty. Values below nodes are posterior probabilities of splits. Images of fossil specimens are modified after Coster et al. (2015, Prozenkerella saharaensis), Jaeger et al. (1985, Nementchamys lavocati), Lavocat (1973; Zenkerella wintoni), Marivaux et al. (2005, Pondaungimys anomaluropsis), Mein & Pickford (2008; Propedetes efeldensis), Pickford et al. (2013; Nonanomalurus soniae), Sallam, Seiffert & Simons (2010; Shazurus minutus), and Sallam et al. (2010; Kabirmys qarunensis).
For fossil taxa, the topological estimate of our combined-evidence clock analysis is identical or nearly identical to recent parsimony analyses that sample similar taxa and morphological characters (Coster et al., 2015; Marivaux et al., 2011; Marivaux et al., 2015), but the phylogenetic position of Zenkerella (and its putative close relative Prozenkerella) is novel. A comparison is in Data S7. The previous parsimony applications have yielded relatively low clade support for splits among fossil species. While our topology for extant anomaluromorphs is supported by 100% clade credibility at all nodes, posterior probabilities for fossil relationships are suboptimal. However, a major advantage of the Bayesian method is that topological uncertainty is accommodated in age-range estimates. Given the constraints of the tree age prior, estimated divergence time ranges for living species should be robust despite alternate possibilities for relationships among fossils.
We included only undisputed anomaluromorph fossil taxa in the clock analysis and therefore all fossil calibrations were within the suborder. A K-Pg constraint for the maximum age of the crown rodent node served as an external calibration. Results of an alternate analysis which lifted this limit resulted in Cretaceous estimates for crown rodents, crown anomaluromorphs and crown anomalurids. From a paleontological perspective we consider these dates to be unrealistic; the anomaluromorph presence in Africa is almost certainly due to an early Paleogene dispersal across the Tethys Sea, and there are no rodents in the African fossil record before the early-middle Eocene boundary (Marivaux et al., 2015). Methodologically, the absence of an informed constraint for the maximum age of crown rodents may be countered by the inclusion of multiple fossil taxa from other rodent suborders and mammalian orders. As this falls beyond the scope of the current study, we caution that the alternate time-tree modeled without the external reference may be unreliable.
Discussion
These new Z. insignis individuals are the first field reports of the species in over 20 years. Importantly, we can confirm Zenkerella distribution near a BBPP research site, and efforts have been initiated to investigate Zenkerella’s mostly unknown basic biology. The results presented here provide necessary phylogenetic context. The specimen count in world collections is now fourteen. As whole animals originally preserved in ethanol, these may be the only Zenkerella museum specimens that can provide long molecular sequence reads. However, methods to extract archival DNA from older dried and formalin-fixed tissues are improving. Accordingly, we have begun amplifying additional nuclear and mitochondrial targets to be made available in future studies. Furthermore, we are optimistic that additional specimens will be recovered in the future as local trappers have been made aware of their importance for scientific research.
We recommend a taxonomic revision of the family Anomaluridae. The genera Zenkerella and Idiurus were originally grouped in the subfamily Zenkerellinae without explicit phylogenetic testing. This arrangement is inconsistent with our phylogenetic results which recover strong support for an Anomalurus–Idiurus clade that is sister to Zenkerella. We therefore advocate removal of Idiurus from the Zenkerellinae.
Idiurus has previously warranted subfamilial distinction from the Anomalurinae (Matschie, 1898). Our mean estimate for the Anomalurus–Idiurus divergence is late Oligocene. Independent analyses of mammalian phylogenetic chronology have estimated several rodent sister families as diverging around the Oligocene–Miocene transition and some more recently (e.g., Ctenomyidae–Octodontidae, Echimyidae–Myocastoridae, Petromuridae–Thryonomyidae, Muridae–Cricetidae) (Meredith et al., 2011). Therefore, placing Idiurus in the subfamily Anomalurinae is not justifiable. We suggest resurrecting Idiurinae (Miller & Gidley, 1918) as a valid subfamily to include the genus Idiurus.
With an estimated phylogenetic divergence deep in the Eocene, we argue that Zenkerella should be placed in its own family, Zenkerellidae, and that all of the anomalures should be encompassed within a superfamily, Anomaluroidea. All fossil taxa sharing the most recent common ancestor of Z. insignis and A. beecrofti should be considered part of the crown superfamily. Our proposed taxonomic revision of extant anomalures is as follows:
| SUPERFAMILY Anomaluroidea Gervais, 1849 |
| FAMILY Anomaluridae Gervais, 1849 |
| SUBFAMILY Anomalurinae Gervais, 1849 |
| Anomalurus Waterhouse, 1843 |
| Anomalurus beecrofti Fraser, 1853 |
| Anomalurus derbianus (Gray, 1842) |
| Anomalurus pelii (Schlegel & Müller, 1845) |
| Anomalurus pusillus Thomas, 1887 |
| SUBFAMILY Idiurinae Miller & Gidley, 1918 new rank |
| Idiurus Matschie, 1894 |
| Idiurus macrotis Miller, 1898 |
| Idiurus zenkeri Matschie, 1894 |
| FAMILY Zenkerellidae Matschie, 1898 new rank |
| Zenkerella Matschie, 1898 |
| Zenkerella insignis Matschie, 1898 |
The phylogenetic position of Zenkerella as the sister taxon of other living anomalures and the ancient divergence of the lineage leading to Zenkerella, combined with the evidence provided by recently discovered anomaluroid fossils, helps to clarify several aspects of the group’s deep evolutionary history in Africa. First, our results are consistent with a single origin of patagial gliding along the shared stem lineage of Anomalurus and Idiurus. Early Miocene Paranomalurus from fossil sites in Uganda and Kenya already show the characteristic expanded ulnar olecranon process that correlates with the presence of a styliform cartilaginous support for the patagial gliding membrane (Pickford et al., 2013), and our tip-dating analysis estimates that this gliding Anomalurus–Idiurus–Paranomalurus clade originated in the early Oligocene (∼30 Ma). These results open up the possibility that anomalurid gliding adaptations evolved during a particularly turbulent “icehouse” phase in the Earth’s climatic and biotic history that was marked by the rapid onset of global cooling and, in northern Africa, the local extinction of thermophilic strepsirrhine primates (Seiffert, 2007). Fossil members of the Anomalurus–Idiurus–Paranomalurus clade have never been recovered from the well-sampled early Oligocene deposits of the Fayum Depression of Egypt, potentially indicating that the range of this radiation of gliding anomalurids was restricted to more equatorial parts of Africa.
Just as remarkable, our estimate of an early or middle Eocene divergence of crown anomaluroids indicates that the Zenkerella lineage has been independently evolving in Africa for almost 50 million years. The recent discovery of isolated teeth of a ∼31 Ma zenkerellid, Prozenkerella saharaensis (Coster et al., 2015), in Libya provides further support for this scenario, and also reveals that zenkerellids were able to persist through early Oligocene paleoenvironments where gliding anomalurids have never been found. The tooth morphology of P. saharaensis also indicates that zenkerellids’ simple molar structure evolved very early in that clade’s evolutionary history and remained highly conserved for tens of millions of years. The presence of the genus Zenkerella in ∼20 Ma deposits in east Africa (Lavocat, 1973) further indicates that Zenkerella is a true “living fossil” about which we know remarkably little. Analyses in preparation on the skeletal and muscular morphology of Zenkerella will allow us to generate hypotheses about the locomotion and positional behavior of Z. insignis that can be tested through future field studies on Bioko Island.
The early-middle Eocene estimate for the origin of crown Anomaluroidea also indicates that the presumably homologous shared behavioral and anatomical features of extant anomaluroids likely trace back to this ancient phase in Africa’s biotic history. The scales on the ventral surface of anomaluroids’ tails, which reportedly provide traction in arboreal settings, and reports from Bioko trappers that Zenkerella might be nocturnal and sleep in tree hollows like Anomalurus and Idiurus, suggest that the ancestral anomaluroid would have also been a nocturnal, and at least partially arboreal, mammal that utilized trees as sleeping sites during the day. An arboreal and nocturnal lifestyle in early anomaluroids might have allowed members of this clade to avoid direct competition with the presumably largely terrestrial, and possibly largely diurnal, hystricognathous rodents that dispersed into Afro-Arabia in the middle Eocene, and underwent a very successful adaptive radiation during the later Paleogene (Marivaux et al., 2014; Sallam & Seiffert, 2016; Sallam et al., 2009). Anomaluroids are the only mammals aside from anthropoid and strepsirrhine primates (and perhaps some bat lineages) that can, by comparison with living members, be identified as probable occupants of arboreal niches in the Paleogene of Africa.
Supplemental Information
Acknowledgments
We thank the government of Equatorial Guinea, the National University of Equatorial Guinea, Santiago Francisco Engonga Esono and Dr. Maximiliano Fero Meñe for permission to conduct research and export the specimens. Special thanks to Marcelino Bahe, Tonnie Choueiri, Dionisio Eparelele, Bryan Featherstone, Matt Mitchell, David Montgomery, Epifanio Müaleri, Felipe Teaché and Dana Venditti for invaluable logistic support. We greatly appreciate the advice of Gail Hearn and Katy Gonder. Gregg Gunnell facilitated DPC requisition and vouchers. Bob Golding kindly provided his A. beecrofti photo. We’re grateful to C William Kilpatrick and especially Ryan Norris for previews of A. beecrofti mitochondrial sequences. Robert Voss and three anonymous reviewers provided helpful comments that improved the manuscript.
Funding Statement
This research was supported by the US National Science Foundation (BCS-1231288), the Research Foundation of SUNY, and the Turkana Basin Institute. BBPP’s activities in Bioko Island are supported by the ExxonMobil Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Steven Heritage, Email: steven.heritage@stonybrook.edu.
Erik R. Seiffert, Email: erik.seiffert@gmail.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Steven Heritage conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper, performed molecular lab work and built the data matrices.
David Fernández and Drew T. Cronin contributed reagents/materials/analysis tools, reviewed drafts of the paper, collected specimens, organized permits, facilitated transport, and conducted field interviews.
Hesham M. Sallam reviewed drafts of the paper, scored morphological characters for fossils.
José Manuel Esara Echube reviewed drafts of the paper, organized permits, facilitated transport.
Erik R. Seiffert conceived and designed the experiments, analyzed the data, wrote the paper, reviewed drafts of the paper, scored morphological characters for fossils and compiled geologic age ranges for tip calibrations.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All specimens were already dead when collected; no approval was necessary.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Equatorial Guinea Department of Culture and Tourism—154/2015 (Field Permit)
UNGE—Dr. Maximiliano Fero Meñe—169/2015 (Export)
Department of Culture and Tourism—Guillermina Mekuy Mba Obono (Field Permit)
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as Supplemental Files.
References
- Benton et al. (2015).Benton MJ, Donoghue PCJ, Asher RJ, Friedman M, Near TJ, Vinther J. Constraints on the timescale of animal evolutionary history. Palaeontologia Electronica. 2015;18.1.1FC:1–106. [Google Scholar]
- Blanga-Kanfi et al. (2009).Blanga-Kanfi S, Miranda H, Penn O, Pupko T, DeBry RW, Huchon D. Rodent phylogeny revised: analysis of six nuclear genes from all major rodent clades. BMC Evolutionary Biology. 2009;9(1):1–12. doi: 10.1186/1471-2148-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge (1974).Bugge J. The cephalic arterial system in insectivores, primates, rodents and lagomorphs, with special reference to the systematic classification. Acta Anatomica. 1974;87(Suppl. 62):1–160. [PubMed] [Google Scholar]
- Bugge (1985).Bugge J. Systematic value of the carotid arterial pattern in rodents. In: Luckett WP, Hartenberger J-L, editors. Evolutionary relationships among rodents: a multidisciplinary analysis. Plenum Press; New York: 1985. pp. 355–379. [Google Scholar]
- Churakov et al. (2010).Churakov G, Sadasivuni MK, Rosenbloom KR, Huchon D, Brosius J, Schmitz J. Rodent evolution: back to the root. Molecular Biology and Evolution. 2010;27(6):1315–1326. doi: 10.1093/molbev/msq019. [DOI] [PubMed] [Google Scholar]
- Coster et al. (2015).Coster P, Beard KC, Salem M, Chaimanee Y, Jaeger J-J. New fossils from the Paleogene of central Libya illuminate the evolutionary history of endemic African anomaluroid rodents. Frontiers in Earth Science. 2015;3:56. doi: 10.3389/feart.2015.00056. [DOI] [Google Scholar]
- Delignette-Muller & Dutang (2015).Delignette-Muller ML, Dutang C. fitdistrplus: an R package for fitting distributions. Journal of Statistical Software. 2015;64(4):1–34. doi: 10.18637/jss.v064.i04. [DOI] [Google Scholar]
- Dieterlen (2005).Dieterlen F. Family anomaluridae. In: Wilson DE, Reeder DM, editors. Mammal species of the world: a taxonomic and geographic reference. Third edition. Johns Hopkins University Press; Baltimore: 2005. pp. 1532–1534. [Google Scholar]
- Fabre et al. (2012).Fabre P-H, Hautier L, Dimitrov D, P Douzery EJ. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evolutionary Biology. 2012;12(1):1–19. doi: 10.1186/1471-2148-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser (1853).Fraser L. Description of a new species of Anomalurus from Fernando Po. Proceedings of the Zoological Society of London. 1853;1852:16–17. [Google Scholar]
- George (1981).George W. Blood vascular patterns in rodents: contributions to an analysis of rodent family relationships. Zoological Journal of the Linnean Society. 1981;73(4):287–306. doi: 10.1111/j.1096-3642.1981.tb01597.x. [DOI] [Google Scholar]
- Gervais (1849).Gervais FLP. Rongeurs. In: D’Orbigny C, editor. Dictionnaire universel d’Histoire naturelle. Renard, Martinet et Cie; Paris: 1849. [Google Scholar]
- Gray (1842).Gray JE. Description of some new genera and fifty unrecorded species of Mammalia. Annals and Magazine of Natural History. 1842;10:255–267. [Google Scholar]
- Jackson & Schouten (2012).Jackson SM, Schouten P. Gliding mammals of the world. Csiro Publishing; Collingwood: 2012. [Google Scholar]
- Jackson & Thorington (2012).Jackson SM, Thorington R. Gliding mammals: taxonomy of living and extnct species. Smithsonian Contributions to Zoology. 2012;638:1–117. doi: 10.5479/si.00810282.638.1. [DOI] [Google Scholar]
- Kearse et al. (2012).Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon (2013).Kingdon J. Family Anomaluridae: anomalures. In: Happold DCD, editor. Mammals of Africa volume III: rodents, hares and rabbits. Bloomsbury Publishing; London: 2013. pp. 602–617. [Google Scholar]
- Lanfear et al. (2012).Lanfear R, Calcott B, Ho SYW, Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution. 2012;29(6):1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- Lavocat (1973).Lavocat R. Les rongeurs du Miocène d’Afrique Orientale, Miocène inférieur. Memoires et Travaux de l’Institut de Montpellier de l’Ecole Pratique des Hautes Etudes. 1973;1:1–284. [Google Scholar]
- Maddison & Maddison (2015).Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. version 3.04http://mesquiteproject.org 2015
- Marivaux et al. (2011).Marivaux L, Adaci M, Bensalah M, Rodrigues HG, Hautier L, Mahboubi MH, Mebrouk F, Tabuce R, Vianey-Liaud M. Zegdoumyidae (Rodentia, Mammalia), stem anomaluroid rodents from the early to middle Eocene of Algeria (Gour Lazib, Western Sahara): new dental evidence. Journal of Systematic Palaeontology. 2011;9(4):563–588. doi: 10.1080/14772019.2011.562555. [DOI] [Google Scholar]
- Marivaux et al. (2005).Marivaux L, Ducrocq S, Jaeger J-J, Marandat B, Sudre J, Chaimanee Y, Tun ST, Htoon W, Soe AN. New remains o f Pondaungimys anomaluropsis (Rodentia, Anomaluroidea) from the latest middle Eocene Pondaung formation of central Myanmar. Journal of Vertebrate Paleontology. 2005;25(1):214–227. doi: 10.1671/0272-4634(2005)025[0214:NROPAR]2.0.CO;2. [DOI] [Google Scholar]
- Marivaux et al. (2014).Marivaux L, Essid EM, Marzougi W, Khayati Ammar H, Adnet S, Marandat B, Merzeraud G, Tabuce R, Vianey-Liaud M. A new and primitive species of Protophiomys (Rodentia, Hystricognathi) from the late middle Eocene of Djebel el Kébar, Central Tunisia. Palaeo Vertebrata. 2014;38(1):1–17. doi: 10.18563/pv.38.1.e2. [DOI] [Google Scholar]
- Marivaux et al. (2015).Marivaux L, Essid EM, Marzougui W, Khayati Ammar H, Merzeraud G, Tabuce R, Vianey-Liaud M. The early evolutionary history of anomaluroid rodents in Africa: new dental remains of a zegdoumyid (Zegdoumyidae, Anomaluroidea) from the Eocene of Tunisia. Zoologica Scripta. 2015;44(2):117–134. doi: 10.1111/zsc.12095. [DOI] [Google Scholar]
- Marivaux, Vianey-Liaud & Jaeger (2004).Marivaux L, Vianey-Liaud M, Jaeger J-J. High-level phylogeny of early Tertiary rodents: dental evidence. Zoological Journal of the Linnean Society. 2004;142(1):105–134. doi: 10.1111/j.1096-3642.2004.00131.x. [DOI] [Google Scholar]
- Martin (1993).Martin T. Early rodent incisor enamel evolution: phylogenetic implications. Journal of Mammalian Evolution. 1993;1(4):227–254. doi: 10.1007/bf01041665. [DOI] [Google Scholar]
- Matschie (1894).Matschie P. Neue Säugethiere aus den sammlungen der herren Zenker, Neumann, Stuhlmann und Emin. Sitzungs-Berichte der Gesellschraft Naturforschender Freunde zu Berlin. 1894;8:194–202. [Google Scholar]
- Matschie (1898).Matschie P. Eine neue mit Idiurus Mtsch. verwandte Gattung der Nagethiere. Sitzungs-Berichte der Gesellschraft Naturforschender Freunde zu Berlin. 1898;4:23–30. [Google Scholar]
- Mein & Pickford (2008).Mein P, Pickford M. Early Miocene Rodentia from the northern Sperrgebiet, Namibia. Memoir of the Geological Survey of Namibia. 2008;20:235–290. [Google Scholar]
- Meng (1990).Meng J. The auditory region of Reithroparamys delicatissimus (Mammalia, Rodentia) and its systematic implications. American Museum Novitates. 1990;2972:1–35. [Google Scholar]
- Meredith et al. (2011).Meredith RW, Janečka JE, Gatesy J, Ryder OA, Fisher CA, Teeling EC, Goodbla A, Eizirik E, Simão TLL, Stadler T, Rabosky DL, Honeycutt RL, Flynn JJ, Ingram CM, Steiner C, Williams TL, Robinson TJ, Burk-Herrick A, Westerman M, Ayoub NA, Springer MS, Murphy WJ. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science. 2011;334(6055):521–524. doi: 10.1126/science.1211028. [DOI] [PubMed] [Google Scholar]
- Miller (1898).Miller GS. Description of a new rodent of the genus Idiurus. Proceedings of the Biological Society of Washington. 1898;12:73–76. [Google Scholar]
- Miller & Gidley (1918).Miller GS, Gidley JW. Synopsis of the supergeneric groups of rodents. Journal of the Washington Academy of Sciences. 1918;8:431–448. [Google Scholar]
- Nowak (1999).Nowak RM. Walker’s mammals of the world. Johns Hopkins University Press; Baltimore: 1999. [Google Scholar]
- O’Leary et al. (2013).O’Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo Z-X, Meng J, Ni X, Novacek MJ, Perini FA, Randall ZS, Rougier GW, Sargis EJ, Silcox MT, Simmons NB, Spaulding M, Velazco PM, Weksler M, Wible JR, Cirranello AL. The placental mammal ancestor and the post–K-Pg radiation of placentals. Science. 2013;339(6120):662–667. doi: 10.1126/science.1229237. [DOI] [PubMed] [Google Scholar]
- Paradis, Claude & Strimmer (2004).Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20(2):289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Perez del Val, Juste & Castroviejo (1995).Perez del Val JP, Juste J, Castroviejo J. A review of Zenkerella insignis Matschie, 1898 (Rodentia, Anomaluridae) First records in Bioko island (Equatorial Guinea) Mammalia. 1995;59(2):441–443. [Google Scholar]
- Pickford et al. (2013).Pickford M, Senut B, Musalizi S, Musiime E. The osteology of Nonanomalurus soniae, a non-volant arboreal rodent (Mammalia) from the early Miocene of Napak, Uganda. Geo-Pal Uganda. 2013;7:1–33. [Google Scholar]
- Ronquist et al. (2012a).Ronquist F, Klopfstein S, Vilhelmsen L, Schulmeister S, Murray DL, Rasnitsyn AP. A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Systematic Biology. 2012a;61(6):973–999. doi: 10.1093/sysbio/sys058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist et al. (2012b).Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012b;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose (1981).Rose KD. The Clarkforkian land-mammal age and mammalian faunal composition across the Paleocene–Eocene boundary. Museum of Paleontology, University of Michigan; Ann Arbor: 1981. [Google Scholar]
- Ruf, Frahnert & Maier (2009).Ruf I, Frahnert S, Maier W. The chorda tympani and its significance for rodent phylogeny. Mammalian Biology. 2009;74(2):100–113. doi: 10.1016/j.mambio.2008.01.002. [DOI] [Google Scholar]
- Sallam & Seiffert (2016).Sallam HM, Seiffert ER. New phiomorph rodents from the latest Eocene of Egypt, and the impact of Bayesian “clock”-based phylogenetic methods on estimates of basal hystricognath relationships and biochronology. PeerJ. 2016;4:e2320. doi: 10.7717/peerj.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam, Seiffert & Simons (2010).Sallam HM, Seiffert ER, Simons EL. A highly derived anomalurid rodent (Mammalia) from the earliest late Eocene of Egypt. Palaeontology. 2010;53(4):803–813. doi: 10.1111/j.1475-4983.2010.00962.x. [DOI] [Google Scholar]
- Sallam et al. (2010).Sallam HM, Seiffert ER, Simons EL, Brindley C. A large-bodied anomaluroid rodent from the earliest late Eocene of Egypt: phylogenetic and biogeographic implications. Journal of Vertebrate Paleontology. 2010;30(5):1579–1593. doi: 10.1080/02724634.2010.501439. [DOI] [Google Scholar]
- Sallam et al. (2009).Sallam HM, Seiffert ER, Steiper ME, Simons EL. Fossil and molecular evidence constrain scenarios for the early evolutionary and biogeographic history of hystricognathous rodents. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(39):16722–16727. doi: 10.1073/pnas.0908702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel & Müller (1845).Schlegel H, Müller S. Bijdragen tot de Natuurlijke Geschiedenis der Vliegende Eekhorens (Pteromys) In: Temminck CJ, editor. Verhandelingen over de Natuurlijke Geschiedenis der Nederlandische Overzeeche Bezittingen. Natuurkundige Commissie in Oost-Indië; Leiden: 1845. [Google Scholar]
- Seiffert (2007).Seiffert ER. Evolution and extinction of Afro-Arabian primates near the Eocene–Oligocene boundary. Folia Primatologica. 2007;78(5–6):314–327. doi: 10.1159/000105147. [DOI] [PubMed] [Google Scholar]
- Stamatakis (2014).Stamatakis A. RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas (1887).Thomas O. Diagnosis of two new central-African mammalia. Annals and Magazine of Natural History. 1887;20 Article 440. [Google Scholar]
- Tullberg (1899).Tullberg T. Ueber das System der Nagethiere: eine Phylogenetische Studie. Uppsala: Akademische Buchdruckerei; 1899. [Google Scholar]
- Waterhouse (1843).Waterhouse GR. Mr Fraser at Fernando Po. Proceedings of the Zoological Society of London. 1843;10:124–127. [Google Scholar]
- Wilson & Reeder (2005).Wilson DE, Reeder DM, editors. Mammal species of the world: a taxonomic and geographic reference. Baltimore: Johns Hopkins University Press; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as Supplemental Files.