Abstract
Perceptions of disease risk play an important role in motivating people to adopt healthy behaviors. However, little is known about psychosocial factors that influence women’s perceived risk for developing disease. The present study investigated the extent to which individual traits, social influences, objective risk factors, and demographic characteristics were associated with women’s risk perceptions for cardiovascular disease, breast cancer, and lung cancer. Using structural equation modeling, we examined hypothesized associations among 452 younger (ages 18-25 years) and 167 middle-aged (ages 40-64 years) women. A greater number and variety of factors were associated with middle-aged women’s risk perceptions compared to younger women. For both groups, some objective risk factors were associated with risk perceptions; yet, associations also existed between multiple psychosocial variables (optimism, health locus of control, social exposure to disease, perceived stigma) and risk perceptions. Results suggested that women may base their risk estimates on factors beyond those considered important by healthcare providers.
Keywords: risk perception, cancer, cardiovascular disease, women’s health, psychosocial
Introduction
Chronic disease susceptibility is characterized by largely modifiable risk factors such as exercise, obesity, and smoking (American Cancer Society, 2013; Go et al., 2013). Thus, understanding variables that influence people’s likelihood of adopting healthy behaviors is crucial. Theory (Janz & Becker, 1984; Rogers, 1975; Weinstein, 1988; Weinstein & Klein, 1995) and empirical evidence (Dillard et al., 2010; Katapodi et al., 2004; Mosca et al., 2006) have demonstrated that perceptions of risk play a key role in motivating people to adopt healthy behaviors. Although classic work has described that people’s understanding of the characteristics of (e.g., controllability) and affective responses (e.g., dread, worry) to a particular health threat are instrumental in shaping perceptions of risk (Slovic, 1987; Slovic et al., 2005), much remains to be learned about the diverse psychosocial factors that may influence people’s perceived risk of disease (Eibner et al., 2006; Gerend, Aiken, West et al., 2004). Clarifying such mechanistic relationships may reveal opportunities for targeted health messages and interventions aimed at improving the adoption of healthy behaviors. Although many health behavior theoretical models have yet to incorporate psychosocial factors that contribute to people’s understanding of their susceptibility to disease (Aiken et al., 2011; Gerend, Aiken, West et al., 2004), individual traits and social influences may be associated with perceptions of disease risk. Furthermore, the presence of objective risk factors is presumably also related to people’s perceived disease risk. Exemplars of each of these likely correlates of disease risk perceptions are the focus of the present study and are discussed in the following sections.
Individual traits (optimism, health locus of control)
Stable across time and situations, individual traits influence how people understand and interact with the world. One such trait is dispositional optimism, a generalized expectancy for positive rather than negative outcomes (Scheier & Carver, 1985). Optimism is associated with positive psychological and physical outcomes, including reduced likelihood of re-hospitalization after surgery, lower risk of adverse birth outcomes, lower levels of emotional distress, and use of adaptive coping strategies (e.g., Carver et al., 1993; Hamilton & Lobel, 2008; Lobel et al., 2000; Scheier et al., 1999). In addition, people who are more optimistic perceive that they are at lower risk for a variety of disease-related outcomes (Eibner et al., 2006; Gerend, Aiken, & West, 2004; Norman & Brain, 2007).
Health locus of control, a generalized expectancy about whether people can control their health status, also influences people’s beliefs and responses to disease-related issues (Wallston et al., 1978). Health locus of control is conceptualized as having three separate dimensions; an internally-oriented dimension characterized by the belief that one has the ability to influence one’s health, and two externally-oriented dimensions characterized by the belief that either powerful others or chance forces, respectively, control one’s well-being. People who believe that they can control their health may also believe that they are less susceptible to disease. A few studies have supported this prediction, demonstrating that internal health locus of control was associated with lower perceptions of risk for breast cancer and heart disease (Gerend, Aiken, & West, 2004; Rowe et al., 2005), whereas external locus of control was associated with greater perceptions of risk for these diseases (Gerend, Aiken, & West, 2004).
Social influences (social exposure to disease, perceptions of stigma by others)
The social environment is likely to inform people’s understanding of their disease risk. For example, other people could indirectly influence perceptions of risk by providing information about disease symptoms, risk factors, and treatments. Additionally, if people in one’s social network become ill, they may serve as easily-accessible reminders of a disease’s prevalence. Such direct social exposure to disease may increase feelings of vulnerability, possibly through the availability heuristic, a decision-making strategy in which people estimate the probability of an event based on the number of examples they can easily recall (Tversky & Kahneman, 1973, 1974). Greater social exposure to a disease may therefore result in heightened perceptions of risk. Evidence has been mixed regarding this possibility: People’s awareness of myocardial infarction in their social network was unrelated to risk perceptions in one study (Meischke et al., 2000), yet, having friends afflicted with breast cancer, colon cancer, heart disease, or diabetes has also been positively associated with women’s disease risk perceptions (Montgomery et al., 2003).
How others respond to those afflicted with disease may further influence people’s perceptions of risk. In some cases, a specific disease is considered shameful or embarrassing, and people typically hold negative views of those afflicted with the stigmatized condition. Weinstein (1987) proposed that people might be motivated to believe they are less susceptible to diseases that they perceive as being highly stigmatized. Perceiving themselves at decreased risk for such diseases may help protect people from emotional distress. Diseases believed to be highly controllable due to behavioral risk factors, such as sexually-transmitted infections or lung cancer, tend to be most stigmatized (Barth et al., 2002; Kahn et al., 2007; Weiner et al., 1988).
Objective risk factors (family history, age, body mass index, health behaviors)
Objective risk factors directly affect an individual’s susceptibility to disease. Some risk factors, such as family history or age, are beyond personal control. Others, such as obesity or engaging in unhealthy behaviors, are relatively controllable. People seem to be particularly aware of the risk conferred by family history, as awareness of a family history of breast cancer, heart disease, colorectal cancer, diabetes, and osteoporosis has been consistently and positively associated with higher perceptions of personal disease risk (DiLorenzo et al., 2006; Erblich et al., 2000; Gerend, Aiken, West et al., 2004; Katapodi et al., 2004; McQueen et al., 2008; Montgomery et al., 2003; Vassy & Meigs, 2011; Vernon et al., 1993). Although risk for chronic disease reliably increases with age, findings regarding age and perceived risk have been inconsistent; studies have found that older participants perceive greater risks for disease than do younger participants (Meischke et al., 2000; Renner et al., 2000), whereas others have found the opposite association between age and perceptions of disease risk (Gerend, Aiken, West et al., 2004; Harwell et al., 2005; Vernon et al., 1993). Some limited evidence has suggested that people consider their standing on controllable risk factors when estimating their disease risk. For instance, people categorized as overweight based on their body mass index (BMI) had higher perceptions of heart disease risk than did people of normal weight (Renner et al., 2000). Similarly, women smokers were more likely to rate their risk for lung cancer and heart disease as above average, compared to former smokers or women who had never smoked cigarettes (Moran et al., 2003). A lack of physical activity has also been associated with increased perceived colorectal cancer risk among middle-aged men and women (Robb et al., 2004).
Study Overview
In summary, some evidence has suggested that various psychosocial factors are associated with personal disease risk perceptions. However, the relative influence of these different factors on individuals’ perceived risk is unknown. How these factors may operate in the context of various diseases or for those of varying ages (and consequently, chronic disease risk) is also unclear. Thus, the present study used cross-sectional survey data from two different age groups of women to examine associations of psychosocial factors with perceptions of chronic disease risk. Specifically, we investigated perceptions of risk for cardiovascular disease (CVD; the leading cause of death in women; Go et al., 2013), breast cancer (the most prevalent form of cancer among women apart from skin cancer; American Cancer Society, 2013), and lung cancer (the leading cause of women’s cancer deaths; American Cancer Society, 2013) in a sample of younger (ages 18-25 years) and middle-aged (ages 40-64 years) women.
Here we conceptualize perceptions of disease risk as multiply-indicated latent variables comprised of commonly used risk perception measures (see Hamilton & Lobel, 2012). Our past research demonstrated that such measures are reliable indicators of women’s disease-specific risk perceptions, and that women’s disease-specific risk perceptions are partially explained by underlying, generalized beliefs about disease risk (conceptualized as an underlying, higher-order latent factor). Thus, for the present study, we used structural equation modeling (SEM), a powerful multivariate analysis technique that allows for the inclusion of latent variables, to test a hypothesized model of how individual traits (optimism, health locus of control), social influences (social exposure to disease, perceptions of stigma), and objective risk factors (family history, age, BMI, health behaviors) may be simultaneously associated with women’s perceptions of risk for CVD, breast cancer, and lung cancer. We hypothesized that lower optimism, internal health locus of control, perceptions of stigma, and preventive health behaviors, and greater external (chance, powerful others) health locus of control, social exposure to disease, family history, BMI, alcohol and cigarette use, and age would be associated with greater perceptions of overall disease risk (Figure 1). We also explored associations of sociodemographic characteristics, including income and education, with women’s risk perceptions.
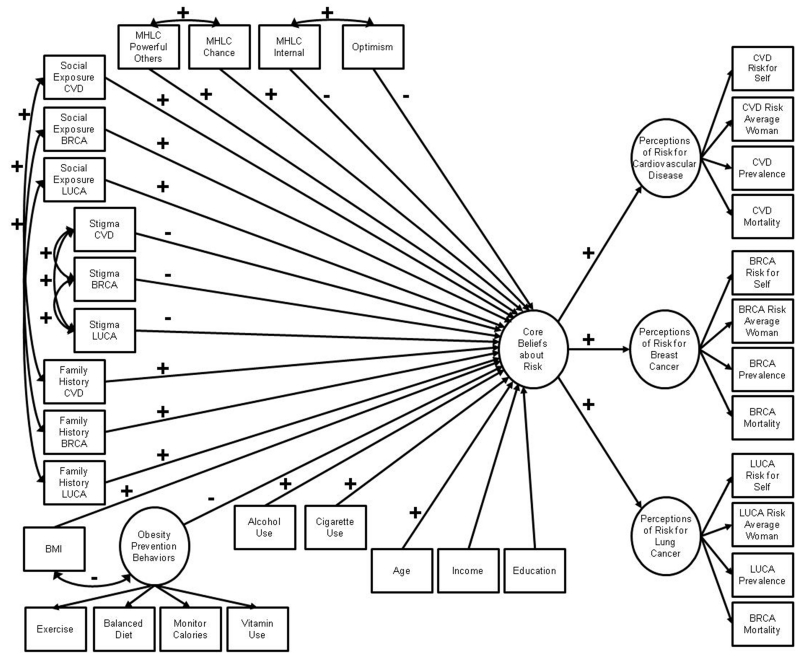
Figure 1.
Hypothesized structural equation model of psychosocial correlates of women’s risk perceptions for chronic disease. Signs above the paths indicate the hypothesized direction of the associations between psychosocial variables and the criterion variable of core beliefs about risk (note that no hypotheses were proposed for associations between the sociodemographic variables of income and education with risk). For simplicity, signs for the direction of the relationships between indicator variables and latent variables have been omitted, as have all error terms. Variable names have been abbreviated (MHLC=Multidimensional Health Locus of Control; CVD=cardiovascular disease; BRCA=breast cancer; BMI=body mass index; LUCA=lung cancer)
Methods
Participants
The Institutional Review Board at our university approved this cross-sectional study with a waiver of documentation of consent. Participants indicated their informed consent by completing the study questionnaire. We reported study procedures in detail elsewhere (Hamilton & Lobel, 2012). Briefly, we recruited younger women between the ages of 18-25 years (n=458) from an undergraduate pool comprised predominantly of students enrolled in an Introductory Psychology course, but also included students from some other courses. Unfortunately, we cannot provide a reliable estimate of the numbers of female students enrolled because the department did not maintain records of the total number of female students eligible to participate in the pool.
Middle-aged women (i.e., women age 40 years or older) were identified by students enrolled in upper-division undergraduate Psychology courses. To our knowledge, younger and middle-aged participants were unrelated. We mailed each woman a packet with a consent letter, questionnaire, and a pre-addressed, stamped envelope in which to return the completed questionnaire. We mailed a total of 360 packets to the identified middle-aged women; 205 of these questionnaires were returned (57% response rate). We did not specify a maximum age while recruiting participants in this subsample, yet most respondents (94%) were ages 40-64 years. The age distribution of this subsample, and the fact that women younger than age 65 years have different morbidity and mortality patterns compared to those older than age 65 years (e.g., Horiuchi et al., 2003), led us to restrict all analyses to women in the 40-64 year age range. We collected the study data from March 2006 through December 2008.
Measures
Questionnaires included both novel and frequently-used measures of perceived risk (items grouped by disease and presented in the order of CVD, breast cancer, and lung cancer), individual traits, social influences, objective risk factors, and sociodemographic characteristics.1
Perceptions of Disease Risk
The questionnaire measured four dimensions of perceived disease risk. Perceptions of disease risk for the self. Each participant estimated her “chance of developing [CVD/breast cancer/lung cancer]” during her lifetime on a 5-point scale ranging from 0 (no chance) to 4 (very high chance). Items similar in phrasing and response-scale format have been used in a number of studies, including the routinely-administered National Cancer Institute’s Health Information National Trends Survey (Nelson et al., 2004). Participants indicated whether they had been previously diagnosed with the disease. Perceptions of disease risk for the average woman. Each participant estimated “the average woman’s chance of developing [specific disease] during her lifetime” on a scale ranging from 0 (no chance) to 4 (very high chance). Perceived prevalence rate of disease. Participants estimated how many women out of 100 would “develop [specific disease] during their lifetime.” Perceived mortality rate of disease. Participants estimated how many women out of 100 would “die from [specific disease] during their lifetime.” For each disease, items assessing these four dimensions of perceived risk demonstrated acceptable reliability (reliability rho ranging from 0.62-0.82; Hamilton & Lobel, 2012).
Individual Traits
Optimism
The 8-item Life Orientation Test is a widely-used measure of dispositional optimism (Scheier & Carver, 1985; sample item: “In uncertain times, I usually expect the best”). Respondents rated items on a scale ranging from 1 (strongly disagree) to 5 (strongly agree); higher summary scores indicate greater optimism. The internal consistency of this scale was high; Cronbach’s α=0.83 among younger women and α=0.84 among middle-aged women.
Health locus of control
The widely-adopted 18-item Multidimensional Health Locus of Control (MHLC) Scale – Form B (Wallston et al., 1978) assesses general expectancies about whether people have control over their health. The MHLC has three subscales: Internal (sample item: “If I become sick, I have the power to make myself well again”), Chance (“When I become ill, it’s a matter of fate”), and Powerful Others (“Other people play a big part in whether I stay healthy or become sick”). Respondents rated items from 1 (strongly disagree) to 6 (strongly agree). Subscale summary scores range from 6 to 36. The MHLC subscales have good internal consistency (α=0.65-0.75; Wallston, 2004); although their performance was somewhat low in the present study (younger women: Internal α=0.61, Chance α=0.62, Powerful Others α=0.53; middle-aged women: Internal α=0.57, Chance α=0.63, Powerful Others α=0.53), we examined the subscales separately to provide a thorough test of the hypothesized associations.
Social Influences
Social exposure to disease
Participants listed “all of the women you personally know who have developed [specific disease].” Participants identified each woman in general terms (e.g., initials, relationship such as mother) to maintain anonymity. The total number of women listed for each disease represented the extent of social exposure to the disease.
Perceptions of stigma by others
Three investigator-designed items assessed the extent to which participants perceived that “people have negative views or attitudes toward women with [specific disease]” on a scale ranging from 0 (people have no negative views) to 3 (people have many negative views).
Objective Risk Factors
Family history of disease
Participants indicated whether they had a family history of CVD, breast cancer, and lung cancer (3 items; yes/no format).
Body mass index (BMI)
Participants reported their height and weight; responses were converted to inches and pounds, respectively, and then to kilograms and meters, respectively, and used to calculate BMI as kg/m2 (National Heart, Lung, and Blood Institute, 2009).
Health behaviors
The questionnaire included six items from a measure of health behaviors validated in pregnant women (Lobel et al., 2008). Items assessed how frequently in the past month participants drank alcohol, smoked cigarettes, exercised for at least 15 minutes, used vitamins, monitored their calorie intake, and consumed a balanced diet. Responses were made on a scale ranging from 0 (never) to 4 (very often). Such self-reported levels of health behaviors have yielded results comparable to data obtained from objective physiological measures (Del Boca & Darkes, 2003; Satia-Abouta et al., 2003; Timperio et al., 2003).
Data Analysis
We examined all data for missing values and violations of statistical normality and conducted separate SEM analyses for younger and middle-aged women using AMOS 18.0 (Arbuckle, 2009). As reported previously (Hamilton & Lobel, 2012), the measures of perceived risk were a good fit for a higher-order model in which a latent factor representing generalized core beliefs about risk contributed to each latent variable representing perceptions of risk for CVD, breast cancer, and lung cancer. This higher-order model was the outcome in all analyses.
All tested models met assumptions necessary for SEM (Kline, 2011). Among younger women, the data violated the assumption of statistical normality. Therefore, bootstrapping (500 bootstrapped samples generated per model) with maximum likelihood estimation was used (Byrne, 2001; Yung & Bentler, 1994). Goodness of fit indices were examined to evaluate the fit of all models, with good model fit defined as a nonsignificant χ2 statistic (because this test overestimates model fit with large samples, other fit indices were also considered), CFI value greater than 0.90, and RMSEA value less than 0.10 with a 90% confidence interval (CI) with the lower limit including 0 and the upper limit below 0.10 (Byrne, 2001). We adopted a model-generating approach (Joreskog, 1993) for evaluation of the hypothesized model (Figure 1). The model-generating approach allows for the development of a model that is consistent with theory, parsimonious, and corresponds well with the sample data (Joreskog, 1993; Kline, 2011). This approach involved first determining the fit of the hypothesized model to the data for each subsample. If the fit was poor, we proceeded in an exploratory manner to modify and re-estimate the model fit. First, we removed all psychosocial variables with nonsignificant paths to the latent core beliefs about risk variable. Next, we systematically added back into the model each of these nonsignificant psychosocial variables, one at a time, beginning with the variable with the lowest p value for the hypothesized path to core beliefs about risk. As each variable was added back into the model, we examined path coefficients, standardized residuals, and modification indices to determine whether conceptually appropriate changes (e.g., correlation of residuals, addition of a direct path from the psychosocial variable to a latent disease-specific risk perception variable or its indicator) would improve model fit. This systematic model-generating approach resulted in a final model for each subsample.
Results
After removal of 10 participants reporting a disease diagnosis, 13 for whom data were missing, and 4 found to be extreme multivariate outliers as indicated by large, significant Mahalanobis d-squared values (Kline, 2011), the analytic subsamples consisted of 452 younger and 167 middle-aged women. The subsample of younger women was racially/ethnically diverse, and the most frequently endorsed income category (36.7%) was “$70,000 or more” (Table 1). The subsample of middle-aged women was diverse in terms of educational attainment, although the majority was White (86.2%) and reported annual household incomes greater than $70,000 (67.7%). The subsamples differed in their psychosocial characteristics (Table 2), with middle-aged women reporting greater optimism, social exposure to disease, family history of CVD and breast cancer, BMI, frequency of eating a balanced diet, monitoring calories, and using vitamins, as well as lower perceptions of stigma for lung cancer (all ps <0.05).
Table 1. Participant Sociodemographic Characteristics.
| Variable (Mean ± SD or %) | Younger women (n = 452) |
Middle-aged women (n = 167) |
|---|---|---|
| Age, years | 19.27 ± 1.62; range: 18-25 |
51.37 ± 5.46; range: 40-64 |
| Annual household income | ||
| Less than $10,000 | 3.5% | 0% |
| $10,000 to $30,000 | 15.3% | 3.6% |
| $30,000 to $50,000 | 21.5% | 13.1% |
| $50,000 to $70,000 | 23.0% | 15.6% |
| $70,000 or more | 36.7% | 67.7% |
| Race or ethnicity | ||
| African-American or Black | 12.2% | 2.4% |
| Asian or Pacific Islander | 31.9% | 4.2% |
| Latina or Hispanic | 10.0% | 5.4% |
| Multiethnic | 4.6% | 1.8% |
| Native American | 0.2% | 0% |
| White or European American | 41.2% | 86.2% |
| Highest level of education completed | ||
| Some high school or less | 0.4% | 1.8% |
| Completed high school | 24.1% | 21.0% |
| Some college/Associate’s degree/Trade school | 71.0% | 38.9% |
| Completed college/Bachelor’s degree | 4.2% | 15.0% |
| Some graduate school | 0% | 3.6% |
| Master’s degree | 0% | 19.2% |
| Ph.D. or M.D. | 0.2% | 0.6% |
Table 2. Participant Psychosocial Characteristics.
| Variable (M ± SD or %) | Younger women (n = 452) |
Middle-aged women (n = 167) |
p a |
|---|---|---|---|
| Optimism (range: 8-40) | 26.88 ± 5.42 | 29.29 ± 5.18 | <0.001 |
| Internal health locus of control (range: 6-36) | 25.65 ± 3.93 | 25.44 ± 4.23 | 0.57 |
| Chancel health locus of control (range: 6-36) | 17.17 ± 4.39 | 16.66 ± 4.94 | 0.21 |
| Powerful others health locus of control (range: 6-36) | 19.23 ± 3.87 | 18.90 ± 4.29 | 0.39 |
| Social exposure to CVD (number of women) | 0.52 ± 0.97 | 1.19 ± 1.42 | <0.001 |
| Social exposure to breast cancer (number of women) | 0.72 ± 0.97 | 2.31 ± 1.75 | <0.001 |
| Social exposure to lung cancer (number of women) | 0.21 ± 0.54 | 0.53 ± 0.78 | <0.001 |
| Perceptions of stigma for CVD (range: 0-3) | 1.01 ± 0.77 | 1.12 ± 0.85 | 0.14 |
| Perceptions of stigma for breast cancer (range: 0-3) | 0.74 ± 0.82 | 0.81 ± 0.80 | 0.29 |
| Perceptions of stigma for lung cancer (range: 0-3) | 1.49 ± 0.86 | 1.31 ± 0.92 | 0.02 |
| Family history of CVD (yes) | 28.5% | 51.5% | <0.001b |
| Family history of breast cancer (yes) | 18.8% | 28.1% | 0.02b |
| Family history of lung cancer (yes) | 16.8% | 16.2% | 0.94b |
| Body mass index | 22.51 ± 4.07 | 26.92 ± 6.32 | <0.001 |
| Exercise (range: 0-4) | 2.24 ± 1.23 | 2.38 ± 1.40 | 0.25 |
| Balanced diet (range: 0-4) | 2.43 ± 0.99 | 2.92 ± 0.85 | <0.001 |
| Monitor calories (range: 0-4) | 1.42 ± 1.32 | 1.87 ± 1.25 | <0.001 |
| Vitamin use (range: 0-4) | 1.30 ± 1.30 | 2.35 ± 1.51 | <0.001 |
| Alcohol use (range: 0-4) | 1.52 ± 1.19 | 1.48 ± 1.14 | 0.72 |
| Cigarette use (yes) | 24.6% | 18.6% | 0.14 |
Note. CVD=cardiovascular disease.
t-tests were used to compute p values unless noted.
Pearson chi-square significance test used to compute p value.
BMI and social exposure to CVD, breast cancer, and lung cancer among younger women, and BMI and social exposure to CVD and lung cancer among middle-aged women were non-normally distributed; thus, we conducted appropriate data transformations (e.g., square root and log transformations). Items assessing exercise, vitamin use, monitoring calories, and diet were indicators of a latent variable labeled obesity prevention behaviors; this measurement model exhibited good fit to the data in younger (χ2(2)=3.66, p=0.16; CFI=0.99; RMSEA=0.04, 90% CI=0.00-0.11) and middle-aged (χ2(2)=0.87, p=0.65; CFI=1.00; RMSEA=0.00, 90% CI=0.00-0.12) women. We treated the remaining health behavior items assessing alcohol use and cigarette use (recoded into a dichotomous variable reflecting “never” versus “ever” smoking due to response frequencies) as observed correlates of risk perceptions.
Correlates of Perceptions of Disease Risk among Younger Women
We examined the hypothesized model (Figure 1) in the subsample of younger women excluding age and education due to the limited range of these variables. We used bootstrapping with maximum likelihood estimation to account for the multivariate non-normality of the data for this subsample. The hypothesized model was a poor fit to the data (χ2(472)=1355.17, p<0.001; CFI=0.80; RMSEA=0.06, 90% CI=0.06-0.07). Perceptions of stigma for breast cancer was the only significant correlate of core beliefs about risk in this initial test of the model. The model-generating approach resulted in a good-fitting model (χ2(149)=371.34, p<0.001; CFI=0.94; RMSEA=0.06, 90% CI=0.05-0.07; see Figure 2).
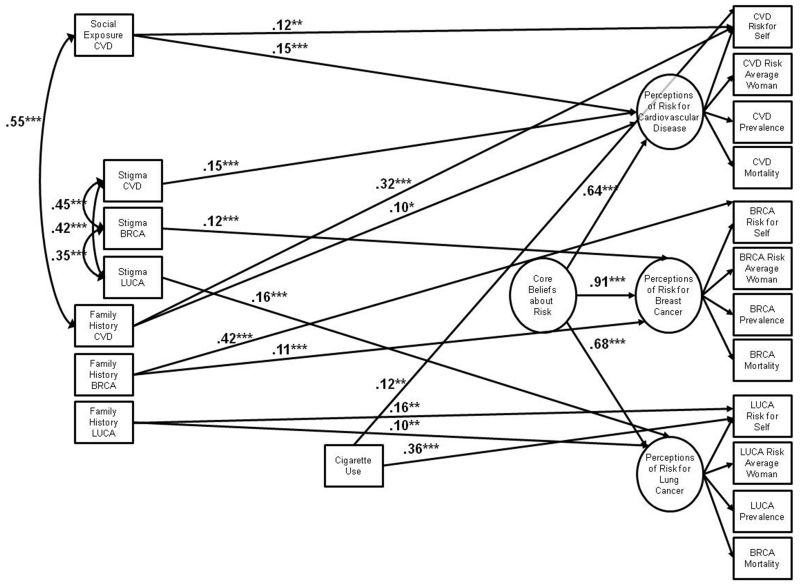
Figure 2.
Final structural equation model of psychosocial correlates of younger women’s risk perceptions for chronic disease. The model was a good fit to the data in the sample of 452 younger women (χ2(149)=371.34, p<0.001; CFI=0.94; RMSEA=0.06, 90% CI=0.05-0.07). For simplicity, all error terms have been omitted and variable names have been abbreviated (CVD=cardiovascular disease; BRCA=breast cancer; LUCA=lung cancer). All values are standardized path coefficients. *= p ≤ 0.05; **= p ≤ 0.01; ***= p ≤ 0.001.
In this final model, no variables were associated with core beliefs about risk. Social influences were weakly associated with specific disease risk perceptions (standardized coefficients ranging from 0.12-0.16). Social exposure to CVD was positively associated with women’s global perceptions of CVD risk, and was uniquely associated with perceptions of personal risk for CVD. Women who believed that others stigmatized those afflicted with CVD, breast cancer, and lung cancer had greater perceptions of risk for each disease. Several objective risk factors were, in general, more strongly related to risk perceptions (standardized coefficients ranging from 0.10-0.42). Women with a family history of CVD, breast cancer, and lung cancer had greater perceptions of global and personal risk for each disease than did women without a family history. Ever smoking was associated with increased perceptions of personal risk for CVD and lung cancer.
Correlates of Perceptions of Disease Risk among Middle-Aged Women
We incorporated measures of age and education into the hypothesized model for middle-aged women (Figure 1). The hypothesized model was a poor fit to the data (χ2(540)=1001.76, p<0.001; CFI=0.71; RMSEA=0.07, 90% CI=0.07-0.08). Social exposure to breast cancer and education were significantly associated with core beliefs about risk. By using these variables as a starting point for the model-generating approach, we achieved a model that was a good fit to the data in the normally-distributed subsample of 167 middle-aged women (χ2(175)=269.79, p<0.001; CFI=0.93; RMSEA=0.06, 90% CI=0.04-0.07; Figure 3).
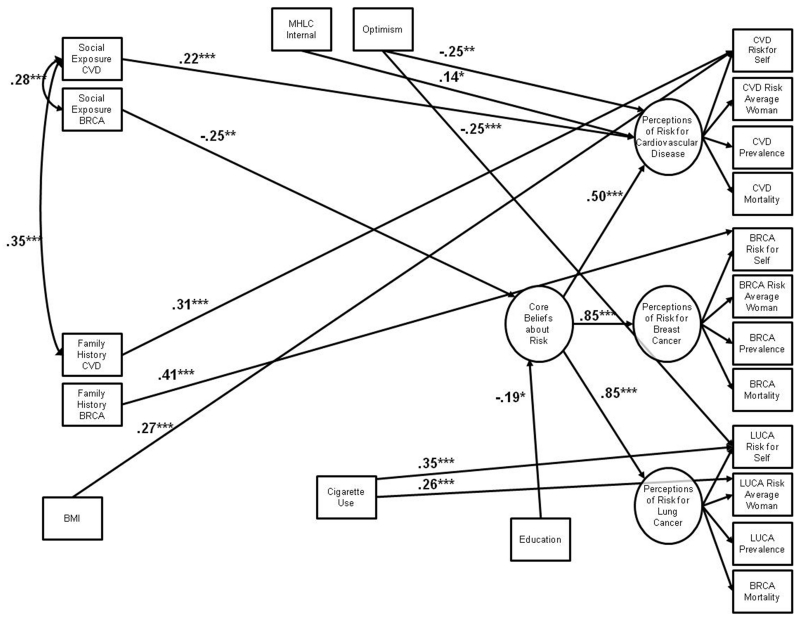
Figure 3.
Final structural equation model of psychosocial correlates of middle-aged women’s risk perceptions for chronic disease. The model was a good fit to the data in the sample of 167 middle-aged women (χ2(175)=269.79, p<0.001; CFI=0.93; RMSEA=0.06, 90% CI=0.04-0.07). For simplicity, all error terms have been omitted and variable names have been abbreviated (MHLC=Multidimensional Health Locus of Control; CVD=cardiovascular disease; BRCA=breast cancer; BMI=body mass index; LUCA=lung cancer). All values are standardized path coefficients. *= p ≤ 0.05; **= p ≤ 0.01; ***= p ≤ 0.001.
Social exposure to breast cancer (standardized coefficient= −0.25) and education (standardized coefficient= −0.19) were inversely associated with core beliefs about risk. Thus, women with more social exposure to breast cancer and more education perceived lower risks for chronic diseases, compared to those with less social exposure and education. Women’s individual traits and social influences were also moderately associated with perceptions of specific disease risks (standardized coefficients ranging from −0.25-0.22). Women with greater beliefs in the ability to control their health perceived greater CVD risk. Women who were more optimistic perceived lower CVD risk and lower personal lung cancer risk. Women with greater social exposure to CVD perceived greater CVD risk. Several objective risk factors were strongly positively associated with specific disease risk perceptions (standardized coefficients ranging from 0.26-0.41). Women with a family history of CVD and breast cancer perceived greater personal risk for each disease than did women without a family history. Women with a greater BMI perceived greater personal CVD risk than did women with a lower BMI. Finally, ever smoking cigarettes was associated with increased perceptions of personal lung cancer risk and perceptions of lung cancer risk for the average woman.
Discussion
This study identified notable differences between younger and middle-aged women in the number, types, and strength of associations between psychosocial factors and their perceptions of chronic disease risk. Among younger women, none of the hypothesized variables was associated with the underlying factor representing core beliefs about risk. However, social influence factors were associated with younger women’s disease risk perceptions. Consistent with the availability heuristic (Tversky & Kahneman, 1973, 1974), those who knew more women afflicted with CVD perceived greater risks for CVD than did women with less social exposure. We also hypothesized that women would be motivated to believe they were less susceptible to diseases that they perceived as highly stigmatized. However, younger women’s perceptions of stigma were positively associated with their perceptions of disease risk. Perhaps such beliefs about stigma were simply indicative of a greater awareness of chronic disease, such that younger women who perceived greater stigma were more attuned to the threat of a disease.
A few objective risk factors were also associated with younger women’s perceptions of disease risk. Younger women with a positive family history perceived greater personal risk for the three diseases, and to a lesser extent, perceived greater global perceptions of disease risk, than did women without a family history. Such findings are consistent with the well-established relationship between family history and elevated disease risk perceptions (e.g., Katapodi et al., 2004). Furthermore, cigarette smoking was strongly associated with younger women’s perceptions of personal risk for lung cancer, and to a lesser extent, with perceptions of personal risk for CVD. Adverse health effects of cigarette smoking are often topics in public health messages aimed at this age group (e.g., Apollonio & Malone, 2009), and this finding suggests that younger women had some awareness of the consequences of this behavior.
Among middle-aged women, individual traits, including optimism, education, and health locus of control, contributed to their risk perceptions. Middle-aged women who were more optimistic perceived lower global risk for CVD, and lower personal risk for lung cancer. Similarly, higher levels of education were associated with lower core beliefs about risk. Education may confer greater confidence and knowledge about how to cope with health threats, leading to lower perceptions of overall risk. Education has also been associated with the adoption of protective health behaviors (Gorin & Heck, 2005; Huisman et al., 2005), which in turn lowers risk for chronic disease. Contrary to expectations, middle-aged women with greater beliefs in their own ability to control their health perceived greater risk for CVD. The reason for this finding is unknown; perhaps this finding reflects women’s understanding of the role of largely controllable behavioral risk factors, such as diet, exercise, and smoking, in the development of CVD. It seems likely that this explanation would also result in a similar association of this trait with perceptions of risk for lung cancer – yet no such association was observed.
Social exposure to disease was inconsistently associated with middle-aged women’s risk beliefs. Greater social exposure to CVD was associated with greater perceived disease risk; yet, greater social exposure to breast cancer was inversely associated with core beliefs about risk. Breast cancer treatments and survival rates have improved (American Cancer Society, 2013), and perhaps observing positive cancer-related outcomes in close others contributed to a reduction in women’s general beliefs about disease vulnerability. Alternatively, greater social exposure to a disease may lead to feelings of vulnerability that are countered by defensive cognitive representations of risk (e.g., cognitive dissonance theory; Festinger & Carlsmith, 1959). Indeed, across the diseases under investigation, middle-aged women reported the greatest social exposure to breast cancer. These possibilities warrant future investigation. Associations between objective risk factors and middle-aged women’s risk beliefs were more consistent with our predictions. Family history, BMI, and cigarette smoking were fairly strongly positively associated with middle-aged women’s disease risk perceptions.
A greater number and variety of factors were associated with middle-aged women’s perceptions of risk compared to younger women in this study. Furthermore, associations between psychosocial factors and perceptions of risk were, on average, stronger among middle-aged women than among younger women. Although both groups’ risk beliefs were related to social influences and some objective risk factors, only middle-aged women’s risk perceptions were associated with their underlying individual traits. Such traits, particularly optimism, may help middle-aged women manage their heightened risk for chronic diseases of aging and any associated distress. To cope with this threat, middle-aged women may think about their disease risk differently than younger women. For instance, by optimistically evaluating their future outcomes, middle-aged women may protect themselves from uncomfortable feelings due to their age-related risk. Similarly, such optimistic beliefs may help justify their adoption of controllable but unhealthy risk behaviors. It is notable that greater optimism was associated with lower perceptions of personal risk for lung cancer. Whether such optimistic beliefs may interact with risky behaviors such as cigarette smoking is unknown (such possibilities could not be explored here given the small number of middle-aged smokers, n=31). Furthermore, it is unclear whether this process is effortful and motivated as opposed to nonconscious and automatic.
Findings regarding objective risk factors are notable. Among both younger and middle-aged women, family history and smoking were associated with greater perceptions of risk. Interestingly , in both groups, a family history of breast cancer was the strongest correlate of women’s risk beliefs. All chronic diseases have a genetic component; yet awareness may be particularly high for breast cancer given recent advances with hereditary breast cancer syndromes and direct-to-consumer marketing emphasizing the contribution of family history to cancer and other conditions (e.g., Gray & Olopade, 2003; Hamilton et al., 2009). Another objective risk factor, BMI, was only associated with perceptions of CVD risk among middle-aged women. This is troubling given the adverse health effects of early-life obesity (Reilly & Kelly, 2011; Saydah et al., 2013). Well-established obesity prevention behaviors were also unrelated to women’s perceptions of risk in spite of frequent public health messages about their benefits. Although a few objective risk factors are relevant to women’s risk beliefs, multiple psychosocial variables including optimism, health locus of control, social exposure to disease, and perceptions of stigma by others are also powerfully associated with women’s perceptions of disease risk. It appears that, to some extent, women base their risk estimates on factors beyond those considered important by healthcare providers.
Study Limitations
This was a correlational cross-sectional study; thus reverse causality or bidirectionality was possible in the analyzed relationships. Although it is unlikely that many of the observed relationships, such as the associations of optimism, social exposure to disease, and family history with risk perceptions, would operate in the opposite direction, the cross-sectional nature of this study likely limited our ability to detect the dynamic associations known to exist between risk perceptions and health behaviors (e.g., Brewer et al., 2004). We assessed most study variables with validated, frequently used instruments; nonetheless, the measure of health locus of control demonstrated modest reliability in this study, and findings related to this measure should be interpreted cautiously. We also designed novel items based on a review of relevant literature, and the reliability and validity of such items are unknown. Furthermore, the non-random sampling approach used to identify younger and middle-aged participants may have introduced selection bias into the observed estimates so that the results may not be generalizable. Although the subsample of younger women was racially/ethnically and socioeconomically diverse, the subsample of middle-aged women was less diverse, and both subsamples reported median incomes above the U.S. median household income (Bishaw & Semega, 2008). These factors, as well as the possibility of social desirability and participation bias, may also limit the generalizability of these findings. Similarly, it is noteworthy that a theory- and data-driven model-generating approach produced the final SEM models (Joreskog, 1993). Thus, efforts should be made to replicate these results to determine their robustness. Finally, it is not clear whether these findings would also be valid for men. Conducting a similar study in men using diseases that affect both sexes (e.g., CVD), and sex-specific diseases (e.g., prostate cancer), would clarify whether women and men differ in the factors associated with their risk beliefs.
Future Directions and Conclusions
These results demonstrated that younger and middle-aged women in our sample differed in the factors associated with their disease risk perceptions; yet, why these differences emerged is unknown. Middle-aged women’s more varied and complex conceptualization of disease risk may result from increased understanding and experience. As people advance from young adulthood through later life, they may acquire and integrate knowledge about diseases of aging through formal (e.g., education) and social (e.g., family) sources, and may have a greater understanding of behavioral contributors to disease risk. Younger women, who have had fewer of these informative experiences, may therefore have a less sophisticated understanding of disease risk. Alternatively, middle-aged and younger women may vary in their awareness of disease risk due to differences in their exposure to public health campaigns or media coverage of disease-affected public figures. Middle-aged women in this study may have a more complex set of factors associated with their understanding of health threats simply because they have lived through different public health events than younger women. Longitudinal studies examining changes in women’s risk perceptions could inform our understanding of such age-related differences.
Future longitudinal investigations should also assess the mechanistic relationships between psychosocial factors, risk perceptions, and health behaviors with both broad and disease-specific benefits. Such studies would clarify the theorized association between disease risk perceptions and precautionary behavior. If heightened risk perceptions are reliably associated with behavioral intentions and subsequent behavioral change as some have demonstrated (e.g., Dillard et al., 2010; Katapodi et al., 2004), it may be possible to design and test interventions involving psychosocial correlates of perceived risk. For example, interventions could encourage women to actively think about disease-affected women in their social networks or their family history as a means of reducing feelings of invulnerability, and possibly increasing motivation for adopting healthy behaviors. However, such interventions should carefully monitor participants’ distress, and ethical challenges may be associated with increasing risk perceptions.
Researchers and clinicians need to understand that psychosocial factors can shape women’s risk beliefs, and these associations may differ based on women’s ages and experiences. In this study, although some objective risk factors emerged as correlates of women’s risk perceptions, their individual traits and social influences were also relevant. Individual and social factors are rarely considered in formal health communication efforts about disease risk; yet, such factors may be novel targets for increasing awareness of disease risk and ultimately motivating behavior change.
Acknowledgments
The authors would like to acknowledge the research support provided by an Individual Development Award from United University Professors and funding from the Department of Psychology at Stony Brook University.
Footnotes
The questionnaire also included several additional measures (i.e., preferences for medical treatments, beliefs about disease etiology, traditional gender roles, coping) that were not germane to this study.
References
- Aiken LS, Gerend MA, Jackson KM, Ranby KW. Subjective risk and health-protective behavior: Prevention and early detection. In: Baum A, Revenson TA, Singer J, editors. Handbook of Health Psychology. 2nd ed. Psychology Press; New York, NY: 2012. [Google Scholar]
- American Cancer Society . Cancer Facts & Figures 2013. ACS; Atlanta: 2013. [Google Scholar]
- Apollonio DE, Malone RE. Turning negative into positive: Public health mass media campaigns and negative advertising. Health Educ Res. 2009;24:483–95. doi: 10.1093/her/cyn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle JL. AMOS 18.0.0. Amos Development Corporation; Crawfordville, FL: 2009. [Google Scholar]
- Barth KR, Cook RL, Downs JS, Switzer GE, Fischhoff B. Social stigma and negative consequences: Factors that influence college students’ decisions to seek testing for sexually transmitted infections. J Am Coll Health. 2002;50:153–9. doi: 10.1080/07448480209596021. [DOI] [PubMed] [Google Scholar]
- Bishaw A, Semega J. Income, earnings, and poverty data from the 2007 American Community Survey. U.S. Census Bureau, American Community Survey Reports, ACS-09. U.S. Government Printing Office; Washington, DC: 2008. [Google Scholar]
- Brewer NT, Weinstein ND, Cuite CL, Herrington JE. Risk perceptions and their relation to risk behavior. Ann Behav Med. 2004;27:125–30. doi: 10.1207/s15324796abm2702_7. [DOI] [PubMed] [Google Scholar]
- Byrne BM. Structural Equation Modeling with AMOS. Lawrence Erlbaum Associates; Mahwah, NJ: 2001. [Google Scholar]
- Carver CS, Pozo C, Harris SD, Noriega V, Scheier MF, Robinson DS, et al. How coping mediates the effect of optimism on distress: A study of women with early-stage breast-cancer. J Pers Soc Psychol. 1993;65:375–90. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction. 2003;98:1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- DeLuca RS, Lobel M. Conception, commitment, and health behavior practices in medically high-risk pregnant women. Womens Health. 1995;1:257–71. [PubMed] [Google Scholar]
- Dillard AJ, Couper MP, Zikmund-Fisher BJ. Perceived risk of cancer and patient reports of participation in decisions about screening: The DECISIONS study. Med Decis Making. 2010;30:96S–105S. doi: 10.1177/0272989X10377660. [DOI] [PubMed] [Google Scholar]
- DiLorenzo TA, Schnur J, Montgomery GH, Erblich J, Winkel G, Bovbjerg DH. A model of disease-specific worry in heritable disease: The influence of family history, perceived risk and worry about other illnesses. J Behav Med. 2006;29:37–49. doi: 10.1007/s10865-005-9039-y. [DOI] [PubMed] [Google Scholar]
- Eibner F, Barth J, Bengel J. Predicting perceived vulnerability for breast cancer among women with an average breast cancer risk. Br J Health Psychol. 2006;11:607–21. doi: 10.1348/135910705X71425. [DOI] [PubMed] [Google Scholar]
- Erblich J, Bovbjerg DH, Norman C, Valdimarsdottir HB, Montgomery GH. It won’t happen to me: Lower perception of heart disease risk among women with family histories of breast cancer. Prev Med. 2000;31:714–21. doi: 10.1006/pmed.2000.0765. [DOI] [PubMed] [Google Scholar]
- Festinger L, Carlsmith JM. Cognitive consequences of forced compliance. J Abnorm Soc Psychol. 1959;58:203–10. doi: 10.1037/h0041593. [DOI] [PubMed] [Google Scholar]
- Gerend MA, Aiken LS, West SG. Personality factors in older women’s perceived susceptibility to diseases of aging. J Pers. 2004;72:243–70. doi: 10.1111/j.0022-3506.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- Gerend MA, Aiken LS, West SG, Erchull MJ. Beyond medical risk: Investigating the psychological factors underlying women’s perceptions of susceptibility to breast cancer, heart disease, and osteoporosis. Health Psychol. 2004;23:247–58. doi: 10.1037/0278-6133.23.3.247. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin SS, Heck JE. Cancer screening among Latino subgroups in the United States. Prev Med. 2005;40:515–26. doi: 10.1016/j.ypmed.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Gray S, Olopade OI. Direct-to-consumer marketing of genetic tests for cancer: Buyer beware. J Clin Oncol. 2003;21:3191–3. doi: 10.1200/JCO.2003.12.069. [DOI] [PubMed] [Google Scholar]
- Hamilton JG, Lobel M. Types, patterns, and predictors of coping with stress during pregnancy: Examination of the Revised Prenatal Coping Inventory in a diverse sample. J Psychosom Obstet Gynaecol. 2008;29:97–104. doi: 10.1080/01674820701690624. [DOI] [PubMed] [Google Scholar]
- Hamilton JG, Lobel M. Passing years, changing fears? Conceptualizing and measuring risk perceptions for chronic disease in younger and middle-aged women. J Behav Med. 2012;35:124–38. doi: 10.1007/s10865-011-9342-8. [DOI] [PubMed] [Google Scholar]
- Hamilton JG, Lobel M, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: A meta-analytic review. Health Psychol. 2009;28:510–8. doi: 10.1037/a0014778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwell TS, Blades LL, Oser CS, Dietrich DW, Okon NJ, Rodriguez DV, et al. Perceived risk for developing stroke among older adults. Prev Med. 2005;41:791–4. doi: 10.1016/j.ypmed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Horiuchi S, Finch CE, Mesle F, Vallin J. Differential patterns of age-related mortality increase in middle age and old age. J Gerontol A Biol Sci Med Sci. 2003;58:495–507. doi: 10.1093/gerona/58.6.b495. [DOI] [PubMed] [Google Scholar]
- Huisman M, Kunst AE, Mackenbach JP. Inequalities in the prevalence of smoking in the European Union: Comparing education and income. Prev Med. 2005;40:756–64. doi: 10.1016/j.ypmed.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The Health Belief Model: A decade later. Health Educ Quart. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Joreskog KG. Testing structural equation models. In: Bollen KA, Lang JS, editors. Testing structural equation models. Sage; Newbury Park, CA: 1993. [Google Scholar]
- Kahn JA, Slap GB, Bernstein DI, Tissot AM, Kollar LM, Hillard PA, et al. Personal meaning of human papillomavirus and pap test results in adolescent and young adult women. Health Psychol. 2007;26:192–200. doi: 10.1037/0278-6133.26.2.192. [DOI] [PubMed] [Google Scholar]
- Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: A meta-analytic review. Prev Med. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. 3rd ed. Guilford Press; New York: 2011. [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent CJ, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604–15. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- Lobel M, DeVincent CJ, Kaminer A, Meyer BA. The impact of prenatal maternal stress and optimistic disposition on birth outcomes in medically high-risk women. Health Psychol. 2000;19:544–53. doi: 10.1037//0278-6133.19.6.544. [DOI] [PubMed] [Google Scholar]
- McQueen A, Vernon SW, Meissner HI, Rakowski W. Risk perceptions and worry about cancer: Does gender make a difference? J Health Commun. 2008;13:56–79. doi: 10.1080/10810730701807076. [DOI] [PubMed] [Google Scholar]
- Meischke H, Sellers DE, Robbins ML, Goff DC, Daya MR, Meshack A, et al. Factors that influence personal perceptions of the risk of an acute myocardial infarction. Behav Med. 2000;26:4–13. doi: 10.1080/08964280009595748. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Erblich J, DiLorenzo T, Bovbjerg DH. Family and friends with disease: Their impact on perceived risk. Prev Med. 2003;37:242–9. doi: 10.1016/s0091-7435(03)00120-8. [DOI] [PubMed] [Google Scholar]
- Moran S, Glazier G, Armstrong K. Women smokers’ perceptions of smoking-related health risks. J Womens Health. 2003;12:363–71. doi: 10.1089/154099903765448871. [DOI] [PubMed] [Google Scholar]
- Mosca L, Mochari H, Christian A, Berra K, Taubert K, Mills T, et al. National study of women’s awareness, preventive action, and barriers to cardiovascular health. Circulation. 2006;113:525–34. doi: 10.1161/CIRCULATIONAHA.105.588103. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute [Accessed August 26];Calculate your body mass index. 2008 http://www.nhlbi.nih.gov/guidelines/obesity/BMI/bmicalc.htm.
- Nelson DE, Kreps GL, Hesse BW, Croyle RT, Willis G, Arora NK, et al. The Health Information National Trends Survey (HINTS): Development, design, and dissemination. J Health Commun. 2004;9:443–60. doi: 10.1080/10810730490504233. [DOI] [PubMed] [Google Scholar]
- Norman P, Brain K. Does dispositional optimism predict psychological responses to counseling for familial breast cancer? J Psychosom Res. 2007;63:247–54. doi: 10.1016/j.jpsychores.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int J Obesity. 2011;35:891–8. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- Renner B, Knoll N, Schwarzer R. Age and body make a difference in optimistic health beliefs and nutrition behaviors. Int J Behav Med. 2000;7:143–59. [Google Scholar]
- Robb KA, Miles A, Wardle J. Demographic and psychosocial factors associated with perceived risk for colorectal cancer. Cancer Epidem Biomar. 2004;13:366–72. [PubMed] [Google Scholar]
- Rogers RW. A protection motivation theory of fear appeals and attitude change. J Psychol. 1975;91:93–114. doi: 10.1080/00223980.1975.9915803. [DOI] [PubMed] [Google Scholar]
- Rowe JL, Montgomery GH, Duberstein PR, Bovbjerg DH. Health locus of control and perceived risk for breast cancer in healthy women. Behav Med. 2005;31:33–40. doi: 10.3200/BMED.31.1.33-42. [DOI] [PubMed] [Google Scholar]
- Satia-Abouta J, Patterson RE, King IB, Stratton KL, Shattuck AL, Kristal AR, et al. Reliability and validity of self-report of vitamin and mineral supplement use in the Vitamins and Lifestyle Study. Am J Epidemiol. 2003;157:944–54. doi: 10.1093/aje/kwg039. [DOI] [PubMed] [Google Scholar]
- Saydah S, Bullard KM, Imperatore G, Geiss L, Gregg EW. Cardiometabolic risk factors among US adolescents and young adults and risk of early mortality. Pediatrics. 2013;131:e679–86. doi: 10.1542/peds.2012-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–47. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Matthews KA, Owens JF, Schulz R, Bridges MW, Magovern GJ, et al. Optimism and rehospitalization after coronary artery bypass graft surgery. Arch Intern Med. 1999;159:829–34. doi: 10.1001/archinte.159.8.829. [DOI] [PubMed] [Google Scholar]
- Slovic P. Perceptions of risk. Science. 1987;236:280–5. doi: 10.1126/science.3563507. [DOI] [PubMed] [Google Scholar]
- Slovic P, Peters E, Finucane ML, MacGregor DG. Affect, risk, and decision making. Health Psychol. 2005;24:S35–S40. doi: 10.1037/0278-6133.24.4.S35. [DOI] [PubMed] [Google Scholar]
- Timperio A, Salmon J, Crawford D. Validity and reliability of a physical activity recall instrument among overweight and non-overweight men and women. J Sci Med Sport. 2003;6:477–91. doi: 10.1016/s1440-2440(03)80273-6. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Availability: A heuristic for judging frequency and probability. Cognitive Psychol. 1973;5:207–32. [Google Scholar]
- Tversky A, Kahneman D. Judgment under uncertainty: Heuristics and biases. Science. 1974;185:1124–31. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- Vassy JL, Meigs JB. Despite underestimated familial risk by self-report, family history correlates with perceived risk and worry about chronic diseases such as coronary heart disease and diabetes. Curr Cardiovasc Risk Rep. 2011;5:7–9. doi: 10.1007/s12170-010-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon SW, Vogel VG, Halabi S, Bondy ML. Factors associated with perceived risk of breast cancer among women attending a screening program. Breast Cancer Res Treat. 1993;28:137–44. doi: 10.1007/BF00666426. [DOI] [PubMed] [Google Scholar]
- Wallston KA. Multidimensional Health Locus of Control Scale. In: Christensen AJ, Martin R, Smyth J, editors. Encyclopedia of Health Psychology. Kluwer Academic/Plenum; New York: 2004. [Google Scholar]
- Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) scales. Health Educ Monogr. 1978;6:160–70. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- Weiner B, Perry RP, Magnusson J. An attributional analysis of reactions to stigmas. J Pers Soc Psychol. 1988;55:738–48. doi: 10.1037//0022-3514.55.5.738. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Unrealistic optimism about susceptibility to health problems: Conclusions from a community-wide sample. J Behav Med. 1987;10:481–500. doi: 10.1007/BF00846146. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. The precaution adoption process. Health Psychol. 1988;7:355–86. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- Weinstein ND, Klein WM. Resistance of personal risk perceptions to debiasing interventions. Health Psychol. 1995;14:132–40. doi: 10.1037//0278-6133.14.2.132. [DOI] [PubMed] [Google Scholar]
- Yung YF, Bentler PM. Bootstrap-corrected ADF test statistics in covariance structure analysis. Brit J Math Stat Psy. 1994;47:63–84. doi: 10.1111/j.2044-8317.1994.tb01025.x. [DOI] [PubMed] [Google Scholar]