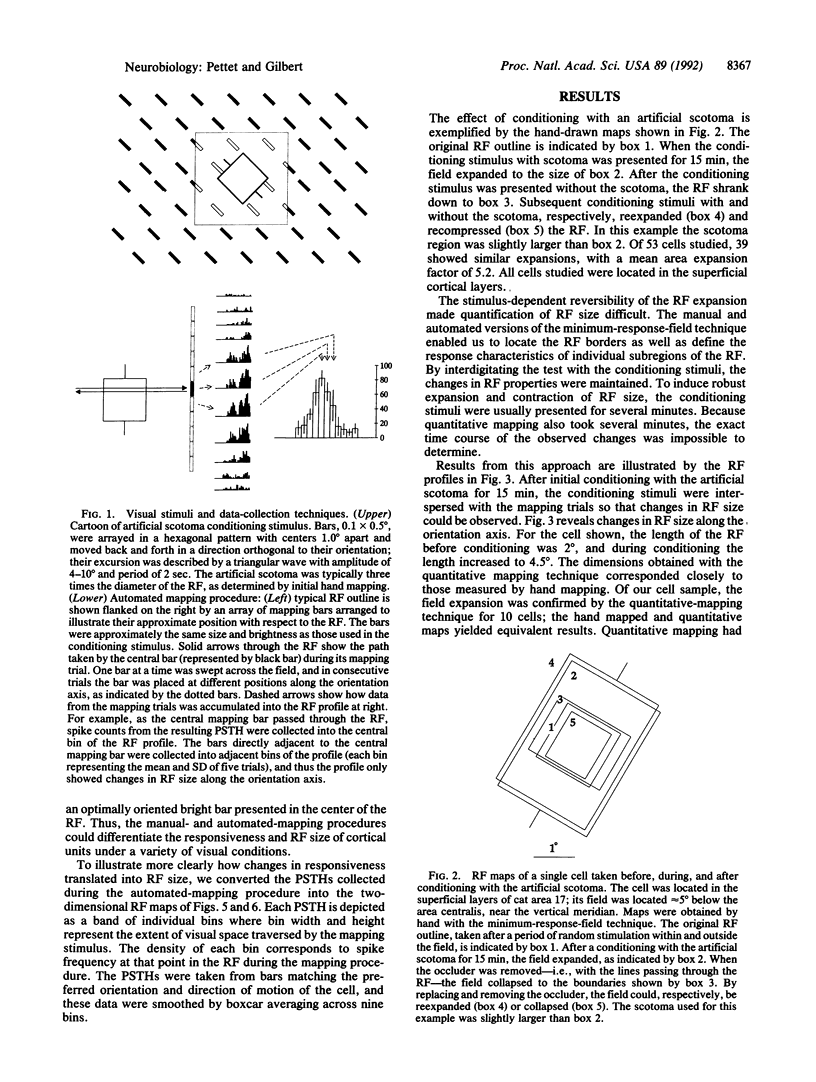
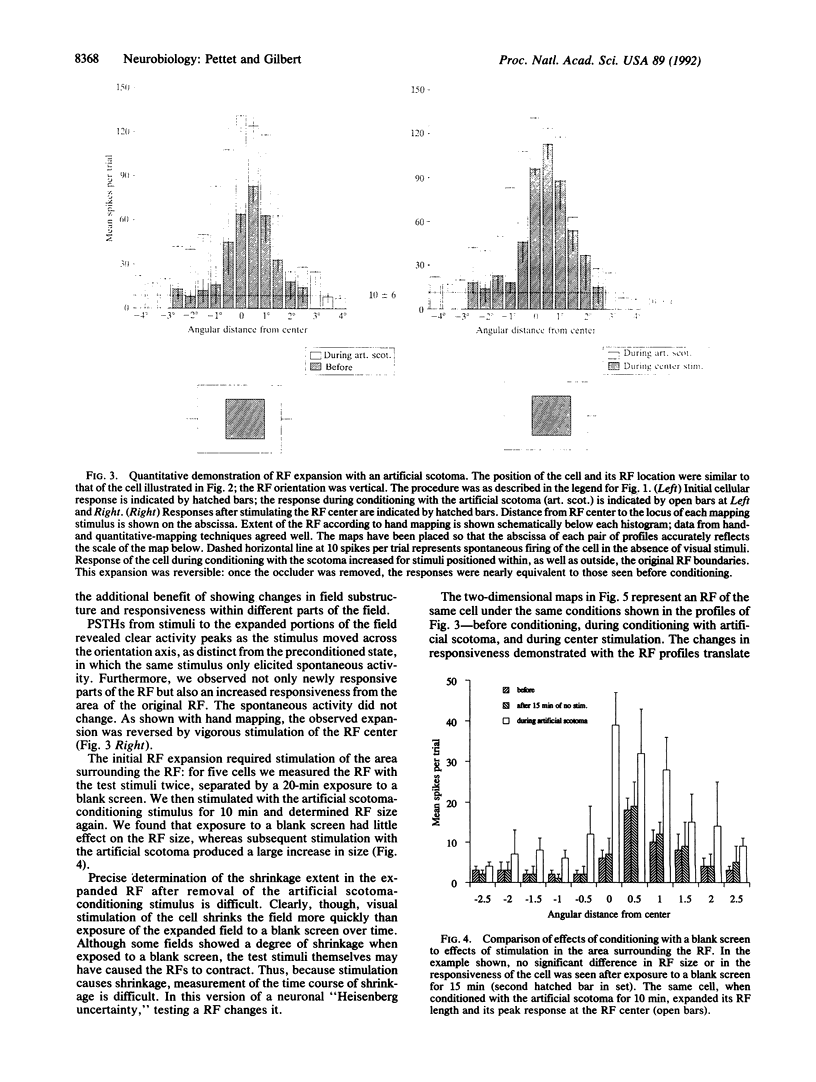
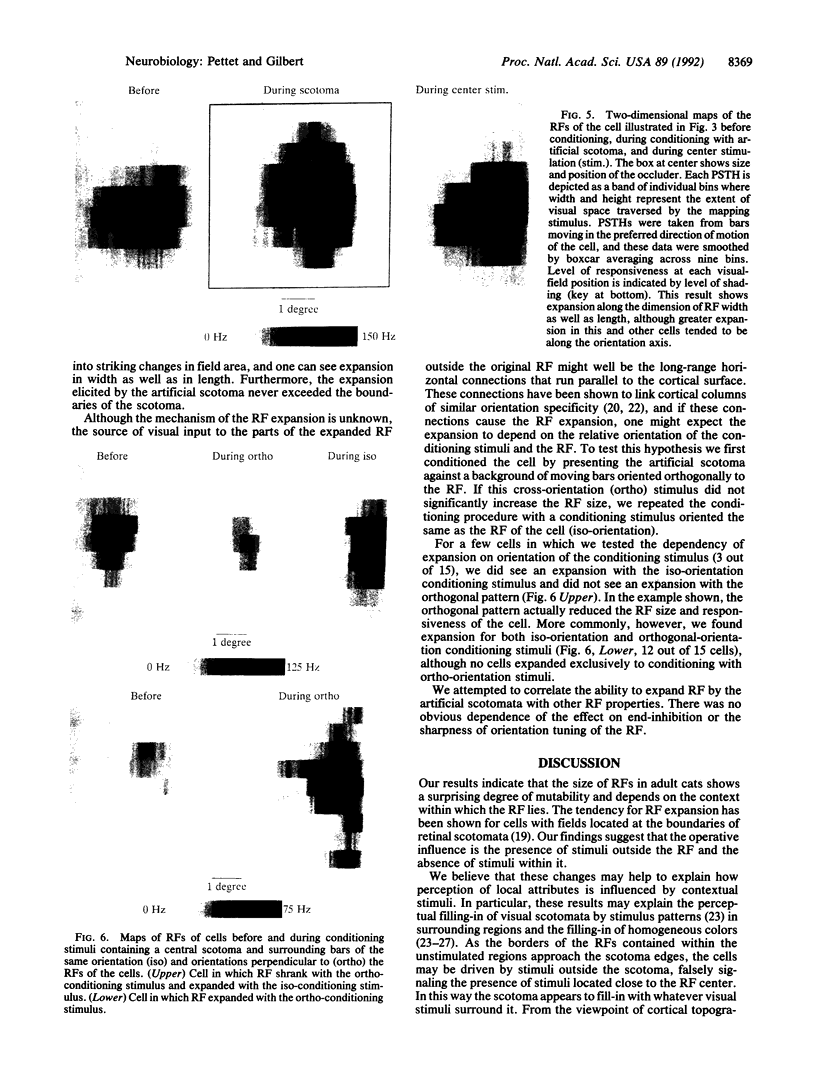
Abstract
Immediately after focal retinal lesions, receptive fields (RFs) in primary visual cortex expand considerably, even when the retinal damage is limited to the photoreceptor layer. The time course of these changes suggests that mere lack of stimulation in the vicinity of the RF accompanied by stimulation in the surrounding region causes the RF expansion. While recording from single cells in cat area 17, we simulated this pattern of stimulation with a pattern of moving lines in the visual field, masking out an area covering the RF of the recorded cell, thereby producing an "artificial scotoma." Over approximately 10 min this masking resulted in a 5-fold average expansion in RF area. Stimulating the RF center caused the field to collapse in size, returning to near its original extent; reconditioning with the masked stimulus led to RF reexpansion. Stimulation in the surrounding region was required for the RF expansion to occur--little expansion was seen during exposure to a blank screen. We propose that the expansion may account for visual illusions, such as perceptual fill-in of stabilized images and illusory contours and may constitute the prodrome of altered cortical topography after retinal lesions. These findings support the idea that even in adult animals RFs are dynamic, capable of being altered by the sensory context.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allman J., Miezin F., McGuinness E. Direction- and velocity-specific responses from beyond the classical receptive field in the middle temporal visual area (MT). Perception. 1985;14(2):105–126. doi: 10.1068/p140105. [DOI] [PubMed] [Google Scholar]
- Bonds A. B. Temporal dynamics of contrast gain in single cells of the cat striate cortex. Vis Neurosci. 1991 Mar;6(3):239–255. doi: 10.1017/s0952523800006258. [DOI] [PubMed] [Google Scholar]
- Crane H. D., Piantanida T. P. On seeing reddish green and yellowish blue. Science. 1983 Sep 9;221(4615):1078–1080. doi: 10.1126/science.221.4615.1078. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Hirsch J. A., Wiesel T. N. Lateral interactions in visual cortex. Cold Spring Harb Symp Quant Biol. 1990;55:663–677. doi: 10.1101/sqb.1990.055.01.063. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983 May;3(5):1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Receptive field dynamics in adult primary visual cortex. Nature. 1992 Mar 12;356(6365):150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. The influence of contextual stimuli on the orientation selectivity of cells in primary visual cortex of the cat. Vision Res. 1990;30(11):1689–1701. doi: 10.1016/0042-6989(90)90153-c. [DOI] [PubMed] [Google Scholar]
- Gulyás B., Orban G. A., Duysens J., Maes H. The suppressive influence of moving textured backgrounds on responses of cat striate neurons to moving bars. J Neurophysiol. 1987 Jun;57(6):1767–1791. doi: 10.1152/jn.1987.57.6.1767. [DOI] [PubMed] [Google Scholar]
- Heinen S. J., Skavenski A. A. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp Brain Res. 1991;83(3):670–674. doi: 10.1007/BF00229845. [DOI] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- KRAUSKOPF J. Effect of retinal image stabilization on the appearance of heterochromatic targets. J Opt Soc Am. 1963 Jun;53:741–744. doi: 10.1364/josa.53.000741. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Krubitzer L. A., Chino Y. M., Langston A. L., Polley E. H., Blair N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990 Apr 13;248(4952):229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The unresponsive regions of visual cortical receptive fields. Vision Res. 1976;16(10):1131–1139. doi: 10.1016/0042-6989(76)90253-4. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol. 1984 Aug;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. I., Frost B. J. Intracortical facilitation among co-oriented, co-axially aligned simple cells in cat striate cortex. Exp Brain Res. 1985;61(1):54–61. doi: 10.1007/BF00235620. [DOI] [PubMed] [Google Scholar]
- Nelson J. I., Frost B. J. Orientation-selective inhibition from beyond the classic visual receptive field. Brain Res. 1978 Jan 13;139(2):359–365. doi: 10.1016/0006-8993(78)90937-x. [DOI] [PubMed] [Google Scholar]
- Ohzawa I., Sclar G., Freeman R. D. Contrast gain control in the cat's visual system. J Neurophysiol. 1985 Sep;54(3):651–667. doi: 10.1152/jn.1985.54.3.651. [DOI] [PubMed] [Google Scholar]
- Orban G. A., Gulyás B., Vogels R. Influence of a moving textured background on direction selectivity of cat striate neurons. J Neurophysiol. 1987 Jun;57(6):1792–1812. doi: 10.1152/jn.1987.57.6.1792. [DOI] [PubMed] [Google Scholar]
- Paradiso M. A., Nakayama K. Brightness perception and filling-in. Vision Res. 1991;31(7-8):1221–1236. doi: 10.1016/0042-6989(91)90047-9. [DOI] [PubMed] [Google Scholar]
- Peterhans E., von der Heydt R. Mechanisms of contour perception in monkey visual cortex. II. Contours bridging gaps. J Neurosci. 1989 May;9(5):1749–1763. doi: 10.1523/JNEUROSCI.09-05-01749.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V. S., Gregory R. L. Perceptual filling in of artificially induced scotomas in human vision. Nature. 1991 Apr 25;350(6320):699–702. doi: 10.1038/350699a0. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Lund J. S. Intrinsic laminar lattice connections in primate visual cortex. J Comp Neurol. 1983 May 20;216(3):303–318. doi: 10.1002/cne.902160307. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Hikosaka K., Saito H., Yukie M., Fukada Y., Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci. 1986 Jan;6(1):134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts'o D. Y., Gilbert C. D., Wiesel T. N. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci. 1986 Apr;6(4):1160–1170. doi: 10.1523/JNEUROSCI.06-04-01160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G., Shimamura K., McKee S. P. Interference with line-orientation sensitivity. J Opt Soc Am. 1976 Apr;66(4):332–338. doi: 10.1364/josa.66.000332. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Simultaneous orientation contrast for lines in the human fovea. Vision Res. 1990;30(11):1913–1921. doi: 10.1016/0042-6989(90)90167-j. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in the domain of disparity signals in human stereoscopic vision. J Physiol. 1986 Jan;370:619–629. doi: 10.1113/jphysiol.1986.sp015954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Heydt R., Peterhans E. Mechanisms of contour perception in monkey visual cortex. I. Lines of pattern discontinuity. J Neurosci. 1989 May;9(5):1731–1748. doi: 10.1523/JNEUROSCI.09-05-01731.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]