Summary
Background
The burden of severe bleeding in adults and children with immune thrombocytopenia (ITP) has not been established.
Objectives
To describe the frequency and severity of bleeding events in patients with ITP, and the methods used to measure bleeding in ITP studies.
Patients/Methods
We performed a systematic review of all prospective ITP studies that enrolled 20 or more patients. Two reviewers searched Medline, Embase, CINAHL and the Cochrane registry up to May 2014. Overall weighted proportions were estimated using a random effects model. Measurement properties of bleeding assessment tools were evaluated.
Results
We identified 118 studies that reported bleeding (n = 10 908 patients). Weighted proportions for intracerebral hemorrhage (ICH) were 1.4% for adults (95% confidence interval [CI], 0.9–2.1%) and 0.4% for children (95% CI, 0.2–0.7%; P < 0.01), most of whom had chronic ITP. The weighted proportion for severe (non-ICH) bleeding was 9.6% for adults (95% CI, 4.1–17.1%) and 20.2% for children (95% CI, 10.0–32.9%; P < 0.01) with newly-diagnosed or chronic ITP. Methods of reporting and definitions of severe bleeding were highly variable in primary studies. Two bleeding assessment tools (Buchanan 2002 for children; Page 2007 for adults) demonstrated adequate interrater reliability and validity in independent assessments.
Conclusions
ICH was more common in adults and tended to occur during chronic ITP; other severe bleeds were more common in children and occurred at all stages of disease. Reporting of non-ICH bleeding was variable across studies. Further attention to ITP-specific bleeding measurement in clinical trials is needed to improve standardization of this important outcome for patients.
Keywords: bleeding, intracranial hemorrhages, outcome assessment health care, platelets, purpura, thrombocytopenic
Introduction
Immune thrombocytopenia (ITP) is a hematological disorder characterized by a reduced number of circulating platelets and an increased risk of bleeding. The platelet count is most often used to assess disease status and response to therapy; however, bleeding is the most clinically important outcome [1] because it has a direct impact on morbidity, mortality, quality of life and treatment decisions [2–4].
The frequency of bleeding events across a broad range of adults and children with ITP has not been established. Furthermore, the measurement of bleeding poses significant challenges: First, patients commonly experience bruising, purpura and petechiae, which are difficult to quantify; second, the criteria used to define ‘severe’ bleeding have not been standardized; and third, life threatening bleeding such as intracerebral hemorrhage (ICH) is relatively uncommon [5]. Current estimates of bleeding derive largely from ITP registries; however, registry data generally cannot capture day-to-day bleeding events prospectively [6].
Standard measurement tools for the assessment of bleeding have been developed for specific patient groups, including chemotherapy-induced thrombocytopenia [7], surgical patients receiving anti-thrombotic therapy [8] and critically ill patients [9]. A widely-accepted and validated bleeding measurement tool that is specific for ITP is lacking. Several previous tools have been used and a new tool has recently been proposed [10].
We performed a systematic review of all prospective ITP studies with two objectives. The primary objective was to determine the frequency of overall bleeding, severe bleeding and ICH in adults and children. The secondary objective was to evaluate the methods used to measure bleeding to help standardize bleeding assessments and reporting.
Methods
Article search and selection
To identify eligible studies for this systematic review, two reviewers (CN and DA) independently searched Medline, Embase, CINAHL and the Cochrane central registry of controlled trials using the subject headings ‘Purpura, Thrombocytopenic, Idiopathic’, and the keywords ‘idiopathic thrombocytopenic purpura’, ‘immune thrombocytopenic purpura’, ‘ITP’ and ‘immune thrombocytopenia’ up to May 2014. Results were combined with a search of prospective studies, randomized controlled trials and prospective cohort studies. Articles published before 1970 were excluded to ensure consistency in methods of counting platelets. We hand-searched bibliographies of relevant review articles and canvassed experts for additional studies.
Included studies enrolled at least 20 patients of any age with primary ITP. We excluded: laboratory-based and biomarker studies; assessments of different surgical techniques for splenectomy; retrospective studies; studies of secondary ITP in the context of drugs, pregnancy, infection or malignancy; duplicate and redundant publications; review articles; and non-English-language and abstract-only publications. Registry studies with periodic clinical assessments were excluded from this analysis because bleeding evaluations in those studies relied on retrospective information. Three reviewers (CN, NN and DA) screened article titles for relevance and assessed articles for eligibility based on a review of the abstract and full text. Agreement on article selection was determined by Cohen’s kappa [11]. Disagreements were resolved by consensus in all cases.
Data abstraction
Data abstraction was carried out in duplicate and independently (by CN, NN and DA in pairs). We collected patient demographics, study design and methods of reporting bleeding events for each study. Discrepancies in data abstraction were verified at source and adjudicated by both reviewers.
Measurement properties of bleeding tools
We assessed the quality of reporting of bleeding in primary studies based on the characteristics of the bleeding assessment tool used. We defined high-quality reporting to be bleeding assessments that were specifically developed for ITP patients. Moderate quality of reporting was defined as bleeding assessments by severity grades with clear definitions. Otherwise, reporting of bleeding was considered low quality. In addition, we evaluated whether reliability and validity of bleeding measurement tools were assessed, and if so, whether reliability and validity were good. These assessments were based on criteria established in the consensus-based standards for the selection of health measurement instruments (COSMIN) criteria [12] and standard definitions with the aid of an expert psychometrician (GN) experienced in health measurement scales.
Statistical analysis
Weighted proportions, reported as percentages with 95% confidence intervals (CIs), for ICH and severe bleeding were estimated by meta-analysis using a random effects model (using Stats Direct). Differences between subgroups of adults vs. children and chronic vs. newly-diagnosed ITP were evaluated by logistic regression (SAS 9.3; SAS Institution Inc, Cary, NC, USA). We explored the correlation between platelet count and bleeding where possible.
Results
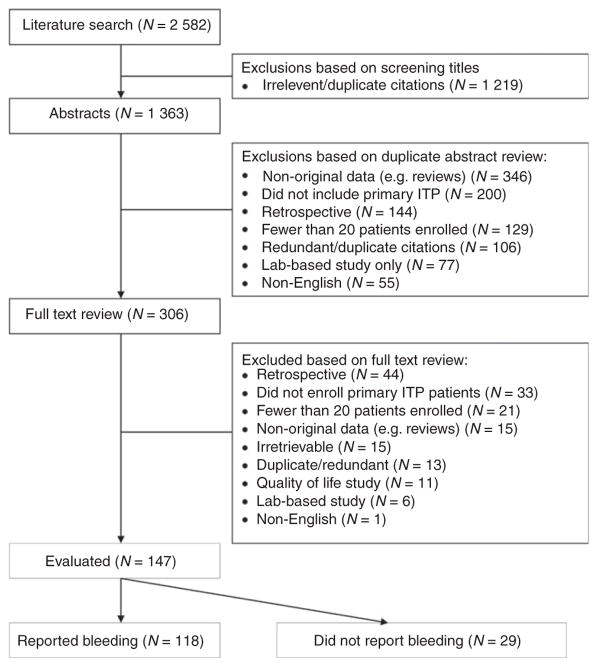
We identified 2582 citations in our initial literature search. After exclusions, we reviewed 1363 abstracts and full text articles in duplicate for eligibility. In the end, 147 clinical studies that prospectively enrolled 20 or more adults or children with primary ITP were included in this systematic review (Fig. 1). Agreement between reviewers on study selection was very good (kappa = 0.76).
Fig. 1.
Article search and selection.
All 147 ITP studies reported platelet count levels. Of those, 118 (80.3%) enrolling 10 908 patients reported bleeding events (Table 1). The other 29 studies (19.7%) did not report bleeding. Bleeding was reported as an efficacy outcome in 67 of 118 studies (56.8%; n = 4810), and in 30 of 51 randomized trials (58.8%; n = 2082). Otherwise, bleeding was reported as a safety outcome (n = 12 studies) or as a baseline variable or inclusion criterion only (n = 39 studies).
Table 1.
Studies that reported bleeding in prospective studies of children and adults with primary ITP
| Number of studies (%) (N = 118; n = 10 908) | |
|---|---|
| Population (n) | |
| Adults | 5336 |
| Children | 5572 |
| How bleeding was reported | |
| As an efficacy outcome | 67 (56.8) |
| As a safety outcome | 12 (10.2) |
| Other* | 39 (33.1) |
| How bleeding was assessed | |
| Presence or absence of bleeding only | 24 (20.3) |
| By anatomical site only | 37 (31.4) |
| By severity only | 7 (5.9) |
| By anatomical site and severity | 49 (41.5) |
| Not reported | 1 (0.8) |
| How bleeding information was obtained | |
| From history | 1 (0.8) |
| From history and physical examination | 14 (11.9) |
| Not specified | 103 (87.3) |
| Bleeding measurement tools | |
| ITP-specific tool | 29 (24.6) |
| WHO score | 5 (4.2) |
| Adverse events tool (e.g. CTCAE) | 3 (2.5) |
| Thrombosis bleeding assessment tool | 1 (0.8) |
| No pre-existing tool used | 80 (67.8) |
As a baseline variable or eligibility criterion.
CTCAE, Common Terminology Criteria for Adverse Events.
Intracerebral hemorrhage
The estimated frequency of ICH was obtained from 51 studies (n = 4782 patients) that specifically reported the presence or absence of ICH (Table 2). The weighted proportion of patients with ICH was 1.0% overall (95% CI, 0.7–1.3). Weighted proportions of adults and children with ICH were 1.4% (95% CI, 0.9–2.1) and 0.4% (95% CI, 0.2–0.7), respectively (P < 0.01). ICH occurred more often in patients who had chronic ITP (1.6%; 95% CI, 1.0–2.2) at the time of study enrollment compared with patients who were newly diagnosed (0.4%; 95% CI, 0.1–0.8) (P < 0.01).
Table 2.
Intracerebral hemorrhage in adults and children with immune thrombocytopenia (weighted proportions, reported as percentage with 95% confidence intervals)
| Newly-diagnosed, % | Chronic, % | All disease stages, % | |
|---|---|---|---|
| Children only (n = 1965) | 0.4 (0.1–0.9) | 1.3 (0.4–2.7) | 0.4 (0.2–0.7) |
| Adults only (n = 1896) | 0.6 (0–1.8) | 1.8 (0.9–2.8) | 1.4 (0.9–2.1) |
| Either children or adults* (n = 921) | 0.2 (0.2–1.6) | 1.6 (0.5–3.1) | 1.2 (0.4–2.4) |
| Overall (n = 4782) | 0.4 (0.1–0.8) | 1.6 (1.0–2.2) | 1.0 (0.7–1.3) |
Data for children and adults were not reported separately in these studies.
Severe bleeding
Information on the frequency of severe (non-ICH) bleeding was restricted to 29 studies (N = 2225 patients) that used a predefined bleeding measurement tool and reported bleeding as a categorical variable (e.g. grade 1, 2, 3) rather than an overall score (Table S1). The weighted proportion of severe bleeding was 15.0% overall (95% CI, 9.3–21.8). We analyzed rates of severe bleeding for adults and children from studies that reported these populations separately: Severe bleeding occurred in 9.6% (95% CI, 4.1–17.1) of adults and 20.2% (95% CI, 10.0–32.9) of children (P < 0.01). Heterogeneity among studies precluded a statistical comparison of severe bleeding for newly diagnosed and chronic ITP patients. The definition of non-ICH severe bleeding was not consistent across studies due to inherent differences in the bleeding measurement tools used; however, extensive mucosal bleeding was the minimum criterion in most studies. Anatomical sites of severe bleeding events were seldom reported; rather, bleeding events (including multiple sites of bleeding) were typically reported in aggregate by severity grade.
Predictors of severe bleeding
Predictors of severe bleeding as reported in individual studies were: severe thrombocytopenia, defined as a platelet count either less than 10 × 109 L−1 [13] or 20 × 109 L−1 [14] (Table S2); newly-diagnosed ITP [13–15]; and previous minor bleeding [15,16]. In several randomized clinical trials, such as those examining the effect of thrombopoietin (TPO) receptor agonists, response to therapy reduced the frequency of significant bleeding [17–21]; however, this effect did not appear to be treatment specific [22].
Correlation between bleeding and platelet count
The correlation between low platelet counts and bleeding was examined in five studies (Table S2). Khellaf et al. reported a moderate correlation between a platelet count < 20 × 109 L−1 and bleeding (r = −0.40; P < 0.01) [23]. Page et al. did not observe a correlation between thrombocytopenia (< 30 × 109 L−1) and bleeding [24]. Pansy et al. observed an association between platelets and bleeding score at diagnosis among treated patients (r = −0.61, P = 0.022) and at days 5–8 among untreated patients (r = −0.65, P = 0.015); however, there was not a consistent correlation across all study visits or patient groups [25]. In a large follow-up study of 292 patients treated with romiplostim, 51% of all bleeding events (mostly mild) occurred at platelet count levels below 50 × 109 L−1 [26]. In that study, 61% of severe bleeding events occurred at platelet counts < 20 × 109 L−1 and none were considered life threatening. Overall, most bleeding events occurred with platelets < 30 × 109 L−1, with frequent exceptions, including one patient with ICH and a platelet count of 120 × 109 L−1 [27].
Quality of reporting of bleeding outcomes
The quality of reporting of bleeding was low in the majority of ITP studies because they reported bleeding by its presence or absence only, by anatomical site only, or without clear definitions of severity grades (n = 77 studies, 65.3%). Quality of reporting of bleeding was moderate in those studies that used a generic assessment tool such as the World Health Organization (WHO) bleeding score [28] or the Common Terminology Criteria for Adverse Events (CTCAE) [29] (n = 8 studies, 6.8%), in studies that used non-descriptive categorical criteria (e.g. severe, life-threatening or fatal) [19,30] and in studies that used a tool that lacked specific severity criteria [31,32] (n = 4, 3.4%). One study used a bleeding tool designed for anticoagulation studies [33]. Quality of reporting of bleeding was high in 28 studies that used one of 10 ITP-specific bleeding measurement tools [10,13,23,24,34–39] (Table 3). Two of those tools had undergone an independent validation study [13,24] and both demonstrated good inter-rater reliability and validity compared with platelet count criteria (Table S3). The measurement properties of the WHO bleeding scale for ITP patients were also evaluated in a separate validation study [40], but the findings did not conclusively give information on reliability or validity.
Table 3.
ITP-specific bleeding measurement tools used in prospective studies (n = 10)
| Bleeding tool | Experience (Studies (patients, n)) | Population | Description | Measurement properties* |
|---|---|---|---|---|
| Buchanan [13] | 11 (480) | Pediatric | Grades (none, minor, mild, moderate, severe) for three anatomical sites, and overall | Good reliability and validity |
| Mazzucconi [37] | 4 (201) | Adult | Severity grades 0–4, blood loss with or without sequelae | Not reported |
| Page [24] | 4 (187) | Pediatric and adult | Ordinal scale from 0 (no bleeding) to 2 (more marked bleeding) at 11 anatomical sites, no overall score | Good reliability and validity |
| Buchanan [35] | 2 (143) | Pediatric | Ordinal bleeding score 0 (definitely no new bleeding) to 4 (bleeding with a drop in hemoglobin> 1 g dL−1) | Not reported |
| Godeau [36] | 1 (122) | Adult | Severity scores at seven anatomical sites plus age and overall | Not reported |
| Khellaf [23] | 2 (120) | Adult | Severity scores for six anatomical sites and age, and overall | Not reported |
| Zhou [38] | 1 (86) | Pediatric and adult | Ordinal scale from 1 to 4 | Not reported |
| Dutch national pediatric ITP protocol [39] | 1 (60) | Pediatric | Ordinal bleeding score 0 (none) to 4 (life threatening bleeding) | Not reported |
| Blanchette [34] | 1 (53) | Pediatric | Bleeding grade: moderate or severe | Not reported |
| SMOG score [10] | 1 (50) | Adult | Each of three anatomical sites (skin, mucosa, body organ) are graded from 0 to 4 based on explicit descriptions | Not reported |
Good reliability and validity were determined based on published criteria [12] by an expert in evaluation. For further details, see Table S3.
Discussion
Bleeding is the most clinically important outcome in ITP studies. It is what motivates physicians to institute treatment [41,42]; it provokes physician, patient and parental anxiety; and it is an important cause of morbidity and mortality [43]. Our findings show that the rates of ICH were higher in adults than children (1.4% vs. 0.4%) and rates of (non-ICH) severe bleeding were higher in children than adults (20.2% vs. 9.6%).
Our estimates of ICH are supported by previous data [4,44–46], including the Intercontinental Cooperative ITP Study (ICIS) Registry II, in which the frequency of ICH at diagnosis among 863 children was 0.15% [4]. The frequency of ICH may be higher in adults due to frequent comorbidities, anti-hemostatic medications or longer periods of observation. Other severe bleeds may be more common in children because children are more prone to trauma and less likely to have thrombocytopenia detected incidentally (as part of other investigations). The rate of severe bleeding in children reported in the ICIS II Registry (n = 1106) was 3% [5]; however, severe bleeding was defined as requiring hospitalization, requiring a blood transfusion or interfering seriously with quality of life [47]. The Nordic registry also reported a 3% rate of severe bleeding among children (n = 501) at diagnosis, defined as requiring a blood transfusion [14]. Our estimate of severe bleeding in children may be higher because of the more liberal definitions applied in primary studies, more frequent assessments in prospective studies and the inclusion of both incident and prevalent bleeds. Conversely, severe bleeding in adults may have been underestimated in our review because of the exclusion of some patients from primary studies with severe bleeding before enrollment.
Predictors of severe bleeding were not readily identified in primary studies, but platelet counts below 10–20 × 109 L−1 and previous minor bleeding were frequent associations. Other retrospective studies have identified older age as a risk factor for severe bleeding [44,48]. A formal regression analysis of predictors was not possible in our study due to the lack of consistent reporting of incident bleeding and variability in bleeding definitions.
Bleeding was often omitted as an efficacy outcome even in randomized trials. Efficacy outcomes mandate more rigorous assessments than safety outcomes and improve the fidelity of reporting [49]. Our systematic review uncovered other limitations in the measurement and reporting of bleeding in ITP studies, including the lack of consistency in defining severity grades, the need for analyses that include time to bleeding events, and the low quality of reporting. While the data were insufficient to endorse any specific bleeding measurement tool, the Buchanan tool (for children) and the Page score (for adults) have been extensively used and have demonstrated adequate measurement properties in independent validation studies [13,24].
Recently, a group of experts designed the ‘SMOG’ bleeding score to quantify bleeding severity from different sites: skin (s), visible mucosa (m) and internal organs (o) [10]. A severity grade from 0 (none) to 5 (fatal) is assigned for each site and a cumulative score is calculated. A pilot study of 50 patients from five centers showed that the time needed to complete the SMOG questionnaire ranged from 5 to 20 min. The performance of this tool has not yet been evaluated in clinical studies.
Our study has limitations. For one, we found extreme heterogeneity in the definitions and methods of measuring bleeding, especially severe (non-ICH) bleeding. Thus, we reported rates of severe bleeding from each individual study in addition to the overall estimate with its broad confidence limits (see Table S1). Second, case mix may explain the variability in the frequency of severe bleeding across studies. For example, studies of rapid-acting treatments (e.g. intravenous immune globulin) may have included patients with more severe bleeding, whereas trials of therapies with a slower onset of action (e.g. rituximab or TPO receptor agonists) tended to exclude patients with significant bleeding manifestations at the time of enrollment. Third, confounding by treatment may have led to an underestimate of bleeding risk overall. We excluded ITP bleeding scales that were applied retrospectively, including that of Bolton-Maggs and Moon [47], which has been widely used in pediatric studies. Our review did not capture global health assessment tools, which may have included bleeding, including the tool used in ICIS studies [50].
Our study represents the most comprehensive summary of bleeding frequency and severity from the ITP literature. These data improve our understanding of the natural history of ITP and help inform patients and physicians of the risks of withholding treatment during periods of observation, which is often indicated in managing ITP patients. Additional studies are needed to better delineate clinical predictors of bleeding and to further validate existing bleeding assessment tools for ITP so that we can standardize the assessment of this important outcome for patients.
Acknowledgments
We thank V. Blanchette for his ideas at the conception stage of this project and G. Wang for statistical support. This study was funded by the McMaster Division of Hematology & Thromboembolism and by the Canadian Institutes for Health Research (grant #102446).
Footnotes
Disclosure of Conflict of Interests
D. M. Arnold reports grants from Amgen and Glaxo-SmithKline and honoraria from Amgen and Bristol Meyers Squibb outside of the submitted work. The other authors state that they have no conflict of interests.
Addendum
C. Neunert performed the research, analyzed the data and wrote the paper; N. Noroozi performed the research, secured funding and wrote the paper; G. Norman performed the analysis and wrote the paper; G. R. Buchanan performed the research, analyzed the results and edited the paper; J. Goy performed the research and analyzed the results; I. Nazi and J. G. Kelton analyzed the results and edited the paper; D. M. Arnold performed the research, analyzed the results, secured funding and wrote the paper.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Severe bleeding events: definition and frequency in clinical studies of adults and children with immune thrombocytopenia that reported bleeding as a categorical variable (n = 29).
Table S2. Reported associations between platelet count and bleeding in prospective clinical studies of adults or children with immune thrombocytopenia.
Table S3. Evaluation of the measurement properties of bleeding assessment tools used in clinical studies of immune thrombocytopenia.
References
- 1.Arnold DM. Immune thrombocytopenia: getting back to basics. Am J Hematol. 2012;87:841–2. doi: 10.1002/ajh.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuter DJ, Mathias SD, Rummel M, Mandanas R, Giagounidis AA, Wang X, Deuson RR. Health-related quality of life in non-splenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol. 2012;87:558–61. doi: 10.1002/ajh.23163. [DOI] [PubMed] [Google Scholar]
- 3.McMillan R, Bussel JB, George JN, Lalla D, Nichol JL. Self-reported health-related quality of life in adults with chronic immune thrombocytopenic purpura. Am J Hematol. 2008;83:150–4. doi: 10.1002/ajh.20992. [DOI] [PubMed] [Google Scholar]
- 4.Neunert CE, Buchanan GR, Blanchette V, Barnard D, Young NL, Curtis C, Klaassen RJ. Relationships among bleeding severity, health-related quality of life, and platelet count in children with immune thrombocytopenic purpura. Pediatr Blood Cancer. 2009;53:652–4. doi: 10.1002/pbc.21978. [DOI] [PubMed] [Google Scholar]
- 5.Neunert CE, Buchanan GR, Imbach P, Bolton-Maggs PH, Bennett CM, Neufeld E, Vesely SK, Adix L, Blanchette VS, Kuhne T. Bleeding manifestations and management of children with persistent and chronic immune thrombocytopenia: data from the Intercontinental Cooperative ITP Study Group (ICIS) Blood. 2013;121:4457–62. doi: 10.1182/blood-2012-12-466375. [DOI] [PubMed] [Google Scholar]
- 6.Heddle NM, Cook RJ, Webert KE, Sigouin C, Rebulla P. Methodologic issues in the use of bleeding as an outcome in transfusion medicine studies. Transfusion. 2003;43:742–52. doi: 10.1046/j.1537-2995.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 7.Webert KE, Arnold DM, Lui Y, Carruthers J, Arnold E, Heddle NM. A new tool to assess bleeding severity in patients with chemotherapy-induced thrombocytopenia. Transfusion. 2012;52:2466–74. doi: 10.1111/j.1537-2995.2012.03634.x. [DOI] [PubMed] [Google Scholar]
- 8.Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–4. doi: 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 9.Arnold DM, Donahoe L, Clarke FJ, Tkaczyk AJ, Heels-Ansdell D, Zytaruk N, Cook R, Webert KE, McDonald E, Cook DJ. Bleeding during critical illness: a prospective cohort study using a new measurement tool. Clin Invest Med. 2007;30:E93–102. doi: 10.25011/cim.v30i2.985. [DOI] [PubMed] [Google Scholar]
- 10.Rodeghiero F, Michel M, Gernsheimer T, Ruggeri M, Blanchette V, Bussel JB, Cines DB, Cooper N, Godeau B, Greinacher A, Imbach P, Khellaf M, Klaassen RJ, Kuhne T, Liebman H, Mazzucconi MG, Newland A, Pabinger I, Tosetto A, Stasi R. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121:2596–606. doi: 10.1182/blood-2012-07-442392. [DOI] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 12.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HC. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19:539–49. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchanan GR, Adix L. Grading of hemorrhage in children with idiopathic thrombocytopenic purpura. J Pediatr. 2002;141:683–8. doi: 10.1067/mpd.2002.128547. [DOI] [PubMed] [Google Scholar]
- 14.Rosthoj S, Rajantie J, Treutiger I, Zeller B, Tedgard U, Henter JI. Duration and morbidity of chronic immune thrombocytopenic purpura in children: five-year follow-up of a Nordic cohort. Acta Paediatr. 2012;101:761–6. doi: 10.1111/j.1651-2227.2012.02671.x. [DOI] [PubMed] [Google Scholar]
- 15.Robak T, Mainau C, Pyringer B, Chojnowski K, Warzocha K, Dmoszynska A, Straub J, Imbach P. Efficacy and safety of a new intravenous immunoglobulin 10% formulation (octagam(R) 10%) in patients with immune thrombocytopenia. Hematology. 2010;15:351–9. doi: 10.1179/102453310X12719010991867. [DOI] [PubMed] [Google Scholar]
- 16.Praituan W, Rojnuckarin P. Faster platelet recovery by high-dose dexamethasone compared with standard-dose prednisolone in adult immune thrombocytopenia: a prospective randomized trial. J Thromb Haemost. 2009;7:1036–8. doi: 10.1111/j.1538-7836.2009.03359.x. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CM, Rogers ZR, Kinnamon DD, Bussel JB, Mahoney DH, Abshire TC, Sawaf H, Moore TB, Loh ML, Glader BE, McCarthy MC, Mueller BU, Olson TA, Lorenzana AN, Mentzer WC, Buchanan GR, Feldman HA, Neufeld EJ. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107:2639–42. doi: 10.1182/blood-2005-08-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, Salama A, Jenkins JM, Roychowdhury D, Mayer B, Stone N, Arning M. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:641–8. doi: 10.1016/S0140-6736(09)60402-5. [DOI] [PubMed] [Google Scholar]
- 19.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161–71. doi: 10.1182/blood-2008-04-150078. [DOI] [PubMed] [Google Scholar]
- 20.Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, Arning M, Stone NL, Bussel JB. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377:393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 21.Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, Rodeghiero F, Chong BH, Wang X, Berger DP. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889–99. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 22.Zeng Y, Duan X, Xu J, Ni X. TPO receptor agonist for chronic idiopathic thrombocytopenic purpura. Cochrane Database Syst Rev. 2011:CD008235. doi: 10.1002/14651858.CD008235.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khellaf M, Michel M, Schaeffer A, Bierling P, Godeau B. Assessment of a therapeutic strategy for adults with severe autoimmune thrombocytopenic purpura based on a bleeding score rather than platelet count. Haematologica. 2005;90:829–32. [PubMed] [Google Scholar]
- 24.Page LK, Psaila B, Provan D, Michael HJ, Jenkins JM, Elish AS, Lesser ML, Bussel JB. The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. Br J Haematol. 2007;138:245–8. doi: 10.1111/j.1365-2141.2007.06635.x. [DOI] [PubMed] [Google Scholar]
- 25.Pansy J, Minkov M, Dengg R, Quehenberger F, Lackner H, Nebl A, Sovinz P, Schwinger W, Urban C, Benesch M. Evaluating bleeding severity in children with newly diagnosed immune thrombocytopenia: a pilot study. Klin Padiatr. 2010;222:374–7. doi: 10.1055/s-0030-1267150. [DOI] [PubMed] [Google Scholar]
- 26.Kuter DJ, Bussel JB, Newland A, Baker RI, Lyons RM, Wasser J, Viallard JF, Macik G, Rummel M, Nie K, Jun S. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. BrJ Haematol. 2013;161:411–23. doi: 10.1111/bjh.12260. [DOI] [PubMed] [Google Scholar]
- 27.Shirasugi Y, Ando K, Miyazaki K, Tomiyama Y, Okamoto S, Kurokawa M, Kirito K, Yonemura Y, Mori S, Usuki K, Iwato K, Hashino S, Wei H, Lizambri R. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized Phase III clinical trial. Int J Hematol. 2011;94:71–80. doi: 10.1007/s12185-011-0886-8. [DOI] [PubMed] [Google Scholar]
- 28.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 30.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM, Sanz MA, Liebman HA, Slovick FT, de Wolf JT, Bourgeois E, Guthrie TH, Jr, Newland A, Wasser JS, Hamburg SI, Grande C, Lefrere F, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 31.Robak T, Salama A, Kovaleva L, Vyhovska Y, Davies SV, Mazzucconi MG, Zenker O, Kiessling P. Efficacy and safety of Privigen, a novel liquid intravenous immunoglobulin formulation, in adolescent and adult patients with chronic immune thrombocytopenic purpura. Hematology. 2009;14:227–36. doi: 10.1179/102453309X439773. [DOI] [PubMed] [Google Scholar]
- 32.Strullu M, Rakotonjanahary J, Tarral E, Savagner C, Thomas C, Mechinaud F, Reguerre Y, Poignant S, Boutet A, Bassil J, Medinger D, Quemener E, Young NL, Rachieru P, Klaassen RJ, Pellier I. Evaluation of health related quality of life in children with immune thrombocytopenia with the PedsQL 4.0 Generic Core Scales: a study on behalf of the pays de Loire pediatric hematology network. Health Qual Life Outcomes. 2013;11:193. doi: 10.1186/1477-7525-11-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George JN, Raskob GE, Vesely SK, Moore D, Jr, Lyons RM, Cobos E, Towell BL, Klug P, Guthrie TH. Initial management of immune thrombocytopenic purpura in adults: a randomized controlled trial comparing intermittent anti-D with routine care. Am J Hematol. 2003;74:161–9. doi: 10.1002/ajh.10424. [DOI] [PubMed] [Google Scholar]
- 34.Blanchette VS, Luke B, Andrew M, Sommerville-Nielsen S, Barnard D, de Veber B, Gent M. A prospective, randomized trial of high-dose intravenous immune globulin G therapy, oral prednisone therapy, and no therapy in childhood acute immune thrombocytopenic purpura. J Pediatr. 1993;123:989–95. doi: 10.1016/s0022-3476(05)80400-7. [DOI] [PubMed] [Google Scholar]
- 35.Buchanan GR, Holtkamp CA. Prednisone therapy for children with newly diagnosed idiopathic thrombocytopenic purpura. A randomized clinical trial. Am J Pediatr Hematol Oncol. 1984;6:355–61. doi: 10.1097/00043426-198424000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Godeau B, Chevret S, Varet B, Lefrere F, Zini JM, Bassompierre F, Cheze S, Legouffe E, Hulin C, Grange MJ, Fain O, Bierling P. Intravenous immunoglobulin or high-dose methylprednisolone, with or without oral prednisone, for adults with untreated severe autoimmune thrombocytopenic purpura: a randomised, multicentre trial. Lancet. 2002;359:23–9. doi: 10.1016/S0140-6736(02)07275-6. [DOI] [PubMed] [Google Scholar]
- 37.Mazzucconi MG, Fazi P, Bernasconi S, De Rossi G, Leone G, Gugliotta L, Vianelli N, Avvisati G, Rodeghiero F, Amendola A, Baronci C, Carbone C, Quattrin S, Fioritoni G, D’Alfonso G, Mandelli F. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood. 2007;109:1401–7. doi: 10.1182/blood-2005-12-015222. [DOI] [PubMed] [Google Scholar]
- 38.Zhou YM, Huang ZQ, Hu MH, Zhou SH, Huang T, Xu Y, Lu JH, Gan XF, Zhu WW. Clinical study on the effect of Shengxueling on idiopathic thrombocytopenic purpura. Chin J Integr Med. 2005;11:60–4. doi: 10.1007/BF02835753. [DOI] [PubMed] [Google Scholar]
- 39.Bruin M, Bierings M, Uiterwaal C, Revesz T, Bode L, Wiesman ME, Kuijpers T, Tamminga R, de Haas M. Platelet count, previous infection and FCGR2B genotype predict development of chronic disease in newly diagnosed idiopathic thrombocytopenia in childhood: results of a prospective study. BrJ Haematol. 2004;127:561–7. doi: 10.1111/j.1365-2141.2004.05235.x. [DOI] [PubMed] [Google Scholar]
- 40.Fogarty PF, Tarantino MD, Brainsky A, Signorovitch J, Grotzinger KM. Selective validation of the WHO Bleeding Scale in patients with chronic immune thrombocytopenia. Curr Med Res Opin. 2012;28:79–87. doi: 10.1185/03007995.2011.644849. [DOI] [PubMed] [Google Scholar]
- 41.Tarantino MD, Fogarty P, Mayer B, Vasey SY, Brainsky A. Efficacy of eltrombopag in management of bleeding symptoms associated with chronic immune thrombocytopenia. Blood Coagul Fibrinolysis. 2013;24:284–96. doi: 10.1097/MBC.0b013e32835fac99. [DOI] [PubMed] [Google Scholar]
- 42.Guidry JA, George JN, Vesely SK, Kennison SM, Terrell DR. Corticosteroid side-effects and risk for bleeding in immune thrombocytopenic purpura: patient and hematologist perspectives. Eur J Haematol. 2009;83:175–82. doi: 10.1111/j.1600-0609.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 43.Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97:2549–54. doi: 10.1182/blood.v97.9.2549. [DOI] [PubMed] [Google Scholar]
- 44.Cohen YC, Djulbegovic B, Shamai-Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160:1630–8. doi: 10.1001/archinte.160.11.1630. [DOI] [PubMed] [Google Scholar]
- 45.Kuhne T, Berchtold W, Michaels LA, Wu R, Donato H, Espina B, Tamary H, Rodeghiero F, Chitlur M, Rischewski J, Imbach P. Newly diagnosed immune thrombocytopenia in children and adults: a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica. 2011;96:1831–7. doi: 10.3324/haematol.2011.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kime C, Klima J, Rose MJ, O’Brien SH. Patterns of inpatient care for newly diagnosed immune thrombocytopenia in US children’s hospitals. Pediatrics. 2013;131:880–5. doi: 10.1542/peds.2012-2021. [DOI] [PubMed] [Google Scholar]
- 47.Bolton-Maggs PH, Moon I. Assessment of UK practice for management of acute childhood idiopathic thrombocytopenic purpura against published guidelines. Lancet. 1997;350:620–3. doi: 10.1016/s0140-6736(97)04143-3. [DOI] [PubMed] [Google Scholar]
- 48.Cortelazzo S, Finazzi G, Buelli M, Molteni A, Viero P, Barbui T. High risk of severe bleeding in aged patients with chronic idiopathic thrombocytopenic purpura. Blood. 1991;77:31–3. [PubMed] [Google Scholar]
- 49.Cook D, Lauzier F, Rocha MG, Sayles MJ, Finfer S. Serious adverse events in academic critical care research. CMAJ. 2008;178:1181–4. doi: 10.1503/cmaj.071366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imbach P, Kuhne T, Zimmerman S. New developments in idiopathic thrombocytopenic purpura (ITP): cooperative, prospective studies by the Intercontinental Childhood ITP Study Group. J Pediatr Hematol Oncol. 2003;25(Suppl 1):S74–6. doi: 10.1097/00043426-200312001-00017. [DOI] [PubMed] [Google Scholar]