Abstract
One of the most challenging problems in critical care medicine is the management of patients with the acute respiratory distress syndrome. Increasing evidence from experimental and clinical studies suggests that mechanical ventilation, which is necessary for life support in patients with acute respiratory distress syndrome, can cause lung fibrosis, which may significantly contribute to morbidity and mortality. The role of mechanical stress as an inciting factor for lung fibrosis versus its role in lung homeostasis and the restoration of normal pulmonary parenchymal architecture is poorly understood. In this review, the authors explore recent advances in the field of pulmonary fibrosis in the context of acute respiratory distress syndrome, concentrating on its relevance to the practice of mechanical ventilation, as commonly applied by anesthetists and intensivists. The authors focus the discussion on the thesis that mechanical ventilation—or more specifically, that ventilator-induced lung injury—may be a major contributor to lung fibrosis. The authors critically appraise possible mechanisms underlying the mechanical stress–induced lung fibrosis and highlight potential therapeutic strategies to mitigate this fibrosis.
The acute respiratory distress syndrome (ARDS) is a major cause of mortality.1 ARDS is characterized by its acute onset, bilateral pulmonary infiltrates, severe hypoxemia, and pulmonary edema of noncardiac origin.2–5 Pronounced morphological changes occur in the lung parenchyma and are associated with impaired lung function, which is partly reversible.5 Mechanical ventilation is the most important supportive therapy for patients with ARDS, but it can induce or aggravate lung injury—an entity referred to as ventilator-induced lung injury (VILI).6,7 ARDS is also characterized pathologically by an early exudative, inflammatory phase, followed in many patients by a fibrotic phase. The inflammatory phase is the focus of more studies—a PubMed search for ARDS AND inflammation yielded 561 articles, whereas a search for ARDS AND fibrosis yielded 260 articles.
In this review, we explore recent advances in the field of pulmonary fibrosis in the context of ARDS, concentrating on its relevance to the practice of mechanical ventilation, as commonly applied by anesthetists and intensivists. We focus our discussion on the thesis that mechanical ventilation—or more specifically, that VILI—may be a major contributor to lung fibrosis. We critically appraise possible mechanisms underlying the mechanical stress–induced lung fibrosis and highlight potential therapeutic strategies to mitigate this fibrosis.
Clinical Evidence of Lung Fibrosis in ARDS
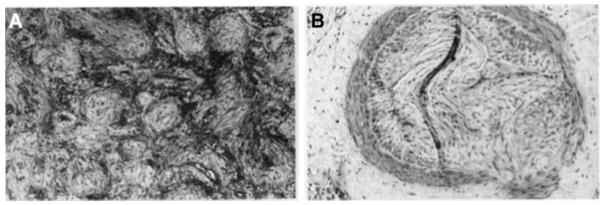
Many patients with ARDS survive the acute phase, but subsequently go on to die, often with evidence of significant pulmonary fibrosis.8 Severe fibrosis was demonstrated to be a frequent complication in ARDS as early as the 1990s.9 Lung histologic studies of patients with late ARDS suggested ongoing inflammatory injury together with progressive fibrosis. 10–12 Areas of exudation are found adjacent to advanced fibrosis, and epithelial and endothelial injury is pronounced in the late phase of ARDS10–12 (fig. 1).
Figure 1.
Histologic findings of hematoxylin-eosin staining at open-lung biopsy in a patient with acute respiratory distress syndrome. The photomicrograph shows myxoid fibrosis, fibroblastic and inflammatory cell infiltration of the interstitium, and scattered collapsed alveoli (A) and subintimal deposition of loose myxoid collagen in an arteriole (B). Reproduced, with permission, from the American College of Chest Physicians and adapted from Meduri GU et al. Chest 1994; 105:1516–27
Patients with severe ARDS frequently require prolonged mechanical ventilation, with a mean duration of approximately 12 days reported in this subgroup.13,14 A recent study15 showed that patients with ARDS with greater fibrotic changes required more prolonged mechanical ventilation, and this in turn was associated with an increased severity of systemic organ failure.
On the basis of the open-lung biopsies, Papazian et al.16 found evidence of pulmonary fibrosis in 53% of ventilated patients who had ARDS for 5 days or more. In a prospective cohort study of 25 consecutive patients with ARDS who were receiving mechanical ventilation, Martin et al.13 reported that the mortality rate was 57% (8 of 14 patients) in those who developed lung fibrosis with zero mortality in patients without evidence of fibrosis. Madtes et al.17 studied 74 consecutive patients during the first 2 weeks after the onset of ARDS. Transforming growth factor-β (TGF-β) was detected in the lung lavage fluid of 90% of patients with ARDS but was not detectable in 13 normal volunteers. The mortality rate was four times higher in patients with both increased concentrations of TGF-β and procollagen type III (PCIII) in lung lavage fluids at day 7 compared with that in patients who had low TGF-β and PCIII levels. Marshall et al.18 demonstrated that the PCIII concentrations were significantly increased in nonsurvivors of ARDS as compared with survivors. The patients with ARDS who survived at least 14 days had less active fibroproliferation as reflected by lower baseline levels of PCIII in the lung.19 These human studies suggest that, in ARDS survivors, a key event is the transition to a normal repair process, such as that evidenced by reduced collagen content in lung lavage fluids, and this is consistent with the resolution phase of ARDS.17
The development of fibrosis seems to be an important determinant of mortality attributable to mechanical ventilation regardless of the cause of ARDS.13 Taken together, the clinical data support the concept that pulmonary fibrosis represents a pathologic response in patients with ARDS.
There seems to be a “fibrosis paradox,” in that fibrosis leads to prolongation of ARDS and critical illness, and worsens outcome. Patients who die of ARDS show clear evidence of pulmonary fibrosis, even when they die in relatively early stages of ARDS. Yet, it is hard to find much trace of fibrosis in ARDS survivors. In fact, most long-term survivors of ARDS have relatively little evidence of fibrosis as measured by PCIII levels in the lung lavage although they may have mildly reduced vital capacity and diffusion capacity.20 This apparent “paradox” has two important implications. First, mechanical ventilation may be a key driver of the fibroproliferative response. Consequently, the removal of mechanical ventilation as early as possible may be a key—and previously under recognized—factor in enabling normal lung repair. Second, strategies aimed directly at attenuating the fibroproliferative response may enhance survival in patients with ARDS.
Pathophysiology of VILI
The time course of lung structural damage in ARDS has been classically thought to occur in three phases: (1) an inflammatory exudative phase characterized by diffuse alveolar injury with necrosis of alveolar type I cells, increase in vascular permeability, and influx of inflammatory cells; (2) a proliferative phase, starting approximately 72 h after the initial insult and lasting approximately 7 days, associated with alveolar epithelial type II cell repair; mesenchymal cells, including interstitial fibroblasts and myofibroblasts, migrate, proliferate, and produce extracellular matrix (ECM) proteins such as collagen18,21–24; and (3) a fibrotic phase with up- regulation of collagen synthesis.25 The proinflammatory and profibrotic responses may become persistent or uncontrolled during mechanical ventilation, and can lead to pulmonary fibrosis, with subsequent decline in lung function. However, the classic time course of these events is not as distinct as portrayed above. For example, there is evidence of fibrotic change in the earliest stages of ARDS.15,18,26 Several studies have demonstrated that PC III concentrations in serum and lung lavage fluids are increased in the ARDS group compared with control patients at 24 h18 with collagen synthesis commencing within 24 h of the development of ARDS.26 In one study, 47% of patients had computed tomography evidence of fibrosis on the first day of ARDS.15 Given the robust correlation between early collagen synthesis and mortality, mechanical ventilation may have altered collagen synthesis early in the course of ARDS.
Biophysical Insults
The forces generated during mechanical ventilation can impact this time course by causing VILI27 and can impact clinical outcomes. For example, a ventilation strategy aimed at decreasing lung stretch significantly decreased mortality.28 Conversely, ventilation with high transpulmonary pressures can lead to injury due to “barotrauma,” that is, air leaks caused by overdistension. Alveolar overdistension can also lead to “volutrauma,” characterized by increased alveolar- capillary leak and pulmonary edema.29,30 Moreover, repeated opening and collapsing of alveolar space can also contribute to injury via a mechanism called “atelectrauma.” 31 There is emerging evidence demonstrating that barotrauma, volutrauma, or atelectrauma may influence the course of lung remodeling.32,33
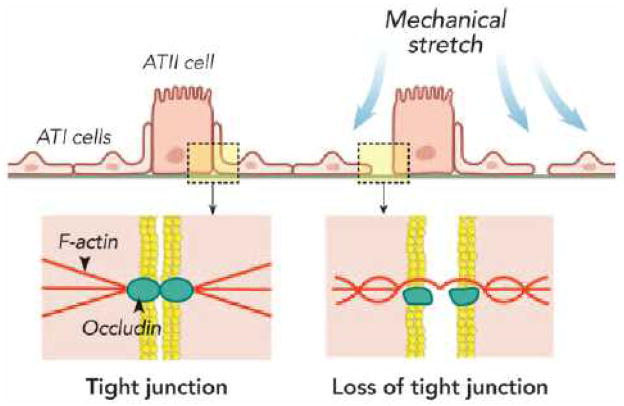
At the cellular level, mechanical stretch of alveolar epithelial cells can result in loss of tight junction structure and cell–cell attachment associated with a decrease in the intensity of the peripheral occludin band and actin perturbations.34 Disruption of the epithelial layer and failed repair mechanisms after mechanical stretch can result in epithelial–mesenchymal transition (EMT),35 a mechanism discussed in greater detail later in the article. A recent study using a three-dimensional cell culture system observed F-actin clumps during mechanical stretch, suggesting that actin cytoskeleton remodeling plays an important role in fibrosis formation36 (fig. 2).
Figure 2.
Mechanical stretch impairs alveolar epithelial integrity. The alveolar epithelial tight junction is consists of several constituents of connected proteins. Occludin is a transmembrane protein known to be associated with F-actin, either directly or indirectly modulating the tight junction structure. Mechanical stretch of alveolar epithelial cells can result in loss of tight junction structure and cell–cell attachment associated with decrease in the expression or increase in degradation of occluding and actin perturbations. The actin cytoskeleton remodeling plays an important role in fibrosis formation in the lung. ATI = alveolar type I; ATII = alveolar type II.
Biomechanical interactions between cells and the ECM proteins can lead to the reorganization and remodeling of the ECM. Collagen is the most important stress-bearing constituent of the parenchymal tissue and plays a critical role in determining the cellular responses to injury and mechanotransduction in lung repair and fibrosis development.37 PCIII is a byproduct of type III collagen synthesis and a potential marker of collagen secretion and has been considered to be a marker of early and active stages of fibrosis.38–42 In the isolated rat lung or lung parenchymal strips, mechanical stretch resulted in enhanced PCIII gene expression.38,41 Frequent applications of recruitment maneuvers associated with atelectasis have been shown to increase PCIII gene expression in animal models of ARDS.42–44 In open-chest rabbits, mechanical ventilation with a high-positive endexpiratory pressures led to a greater gene expression of PCIII and procollagen IV, fibronectin (a fibroblast growth factor), and TGF-β1, a classical growth factor for fibrosis formation.45 In contrast, ventilation with a low-positive end-expiratory pressure did not impact expression of these genes.40 Taken together, these studies40,42–45 suggest that atelectasis and alveolar overdistension are harmful and can lead to development of fibrosis.
Biochemical Insults
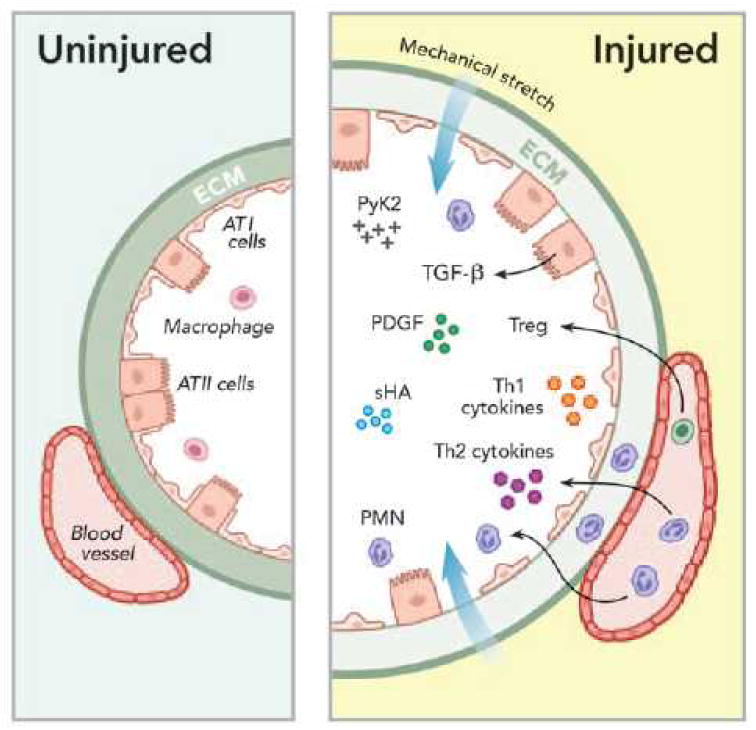
It has become clear that mechanical ventilation causes injury not only by structural disruption of the lung but also by induction of inflammatory responses associated with mediator release that can worsen lung injury and potentially cause systemic organ dysfunction.46 This is termed “biotrauma.” The physical forces generated during mechanical ventilation can induce the release of cytokines, chemokines, and growth factors47 in lungs with preexisting injury or in previously healthy lungs (fig. 3).
Figure 3.
Mechanical stretch causes inflammatory responses associated with release of mediators that can worsen lung injury leading to “biotrauma.” Mechanical stretch of alveoli results in increased expression of small fragment hyaluronan (sHA) and activation of cytoplasmic proline-rich tyrosine kinase- 2 (PyK2); polymorphonuclear leukocyte (PMN) infiltration that release soluble mediators such as cytokines and platelet-derived growth factor (PDGF); increased production of extracellular matrix (ECM) proteins including transforming growth factor- β1 (TGF-β1), collagen, elastin, fibronectin laminin, lumican, proteoglycan, and glycosaminoglycans.
During the exudative phase of acute respiratory distress syndrome, the influx of T regulatory cells (Treg) may play a critical role in the crosstalk between innate and adaptive immune systems that normally would modulate the transition from injury to repair in resolving lung injury. ATI = alveolar type I; ATII = alveolar type II.
The type-1 and type-2 helper T-cell (Th1 and Th2) cytokines and chemokines released in response to lung stretch or strain, together with inflammatory cell recruitment, may play a role in the progression from injury to fibroproliferation. The Th1 response, with increased expression of interferon-γ, interleukin (IL)-2, IL-12, and IL-18, may play a role helping tissue repair, whereas the Th2 cytokines, including IL-4, IL-5, IL-10, and IL-13, tend to promote fibroproliferation.48 Certain chemokine receptors expressed on lung epithelial cells, such as CXCR349–51 for Th1 responses, and CCR4,52 CCR8,53 and CXCR454–56 for Th2 responses, can modulate the transition from lung restoration and repair to progressive lung fibrosis.
In a model of endotoxin-induced lung injury in mice, investigators demonstrated that a subset of CD4+ lymphocytes named T regulatory cells, expressing the surface marker CD25 (IL-2 receptor α) and the transcription factor Forkhead box protein 3, played an important role in resolving lung injury.57 This finding suggests that the influx of inflammatory cells such as T regulatory cells during the exudative phase may play a critical role in the crosstalk between innate and adaptive immune systems that normally would modulate the transition from injury to repair in the lung.
Activation of TGF-β has been reported in response to in vitro mechanical stretch in lung epithelial cells.58 We recently demonstrated that TGF-β activation is also an important mechanism involved in lung remodeling after mechanical ventilation in a murine model of acute lung injury induced by acid aspiration.59 The degree and reversibility of lung fibrosis were dependent on the severity of VILI in this two- hit model.59 Increased expression of TGF-β and activation of collagen synthesis combined with inhibition of collagenase production60–62 seem to be the key events contributing to the lung-remodeling process after VILI, leading to the development of pulmonary fibrosis.
Cellular Mechanisms of Mechanical Ventilation–associated Lung Fibrosis
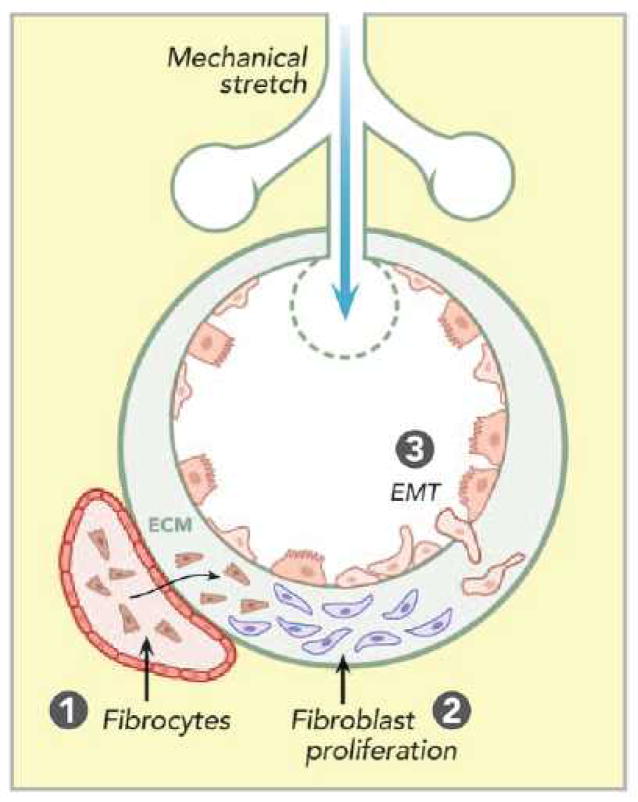
The pathophysiology of mechanical ventilation–associated lung fibrosis may involve a number of distinct cellular mechanisms including activation of stretch-sensitive ion channels in pulmonary epithelial and endothelial cells, disruption of cell plasma membranes, and direct conformational changes in membrane-associated molecules and their cell–cell or cell–ECM interactions.63,64 We will focus the following discussion on possible cell sources and activation of a few signaling pathways in the context of mechanical ventilation–associated lung fibrosis (fig. 4).
Figure 4.
Proposed cell sources of mechanical ventilation– associated lung fibrosis in acute respiratory distress syndrome. Mechanical stretch of alveoli results in (1) increased circulating fibrocytes recruitment into the lung by chemokines, contributing to local fibrosis formation; (2) accelerated fibroproliferation so that resident fibroblasts can proliferate and participate in the lung repair process; and (3) epithelial– mesenchymal transition (EMT) whereby epithelial cells undergo transition to a mesenchymal phenotype in the process of epithelial repair following injury. ECM = extracellular matrix
Cell Sources
Epithelial–Mesenchymal Transition
Epithelial–mesenchymal transition is a biological process whereby epithelial cells undergo transition to a mesenchymal phenotype, that is, fibroblasts and myofibroblasts. EMT plays an important role in the process of epithelial repair after injury.65–67 EMT can be characterized by loss of lung epithelial markers including surfactant proteins (surfactant protein B, C, and D, junctional and cell–cell adhesion proteins (i.e., E-cadherin and claudin), and cytoskeletal organization proteins (i.e., cytokeratin- 8) associated with gain of mesenchymal markers including α-smooth muscle actin, N-cadherin, vimentin, and fibronectin.65,68–70 The failure of lung epithelial repair in patients with ARDS may potentially lead to activation of EMT-signaling pathways.
In a recent study, the application of cyclic mechanical stretch induced EMT in vitro in primary murine alveolar type II epithelial cells.35 Using an in vivo mouse model of acid aspiration–induced ARDS followed by a ventilator strategy causing overdistension, we demonstrated impaired lung mechanics associated with increased lung hydroxyproline content and higher expression of TGF-β, β-catenin, and mesenchymal markers including α-smooth muscle actin and vimentin at both gene and protein levels.59 In contrast, the expression of epithelial markers including cytokeratin-8, E-cadherin, and prosurfactant protein B was decreased.59 The specific EMT pattern in response to mechanical stretch was reproduced in an in vitro system using cultured human lung epithelial cells.59 These results suggest that mechanical stretch alone can induce EMT and hence may play an important role in mediating the VILI-associated lung fibrosis.59
Fibroproliferation
Resident fibroblasts can proliferate and participate in the lung repair process. Pulmonary fibroblasts can be activated in response to Th2 cytokines and growth factors, thereby increasing fibroproliferation.71 Mechanical ventilation using high tidal volumes (30 ml/kg) has been demonstrated to induce pulmonary fibroproliferation in a mouse model of ARDS.72 Similar results were obtained in rats ventilated with more clinically relevant (7 ml/kg) tidal volumes.73 In fetal human lung fibroblasts, exposure to cyclic stretch for 48 h resulted in an increased expression of the cell cycle–regulated gene calcyclin. Calcyclin gene expression was also up-regulated in isolated-perfused rat lungs exposed to high stretch for 4 h.74 Whether the calcyclin gene is a biomarker of the fibroblast cell cycle or plays a direct role in the mechanotransduction signaling pathways leading to fibroproliferation remains to be elucidated.
In a prospective observational cohort clinical study, Ichikado et al.15 performed high-resolution computed tomography in 85 patients on the day of diagnosis of ARDS. They demonstrated that lung fibroproliferation as assessed by computed tomography was a prognostic indicator for ventilator dependency, increased mortality, and increased susceptibility to multiple organ dysfunction.
Circulating Fibrocytes
Fibrocytes are a distinct subpopulation of bone marrow–derived fibroblast-like cells which can be found in tissue75 or as circulating cells in peripheral blood.76 They are defined as cells that dually express leukocyte (CD45) and mesenchymal (collagen I) markers.77,78 Fibrocytes can express α-smooth muscle actin, indicative of myofibroblast differentiation.79 Studies have shown that circulating fibrocytes can travel to the lung and serve as progenitors for interstitial fibroblasts.78,80,81 In a clinical setting, Quesnel et al.82 reported that fibrocytes were detected in bronchoalveolar lavage fluid of 90 of 92 patients (98%) with ARDS treated with mechanical ventilation. They suggested that a fibrocyte percentage greater than 6% in lung lavage fluid was an independent predictor for mortality in mechanically ventilated patients with ARDS.82 Larger studies are needed to confirm these findings.
Immune Cells
Neutrophil infiltration into the alveolar space is a hallmark of ARDS.83 Although immune cells— other than fibrocytes—are not directly transformed into fibroblasts, the persistence of neutrophils in the lung likely plays a role in modulating the fibrotic process.84,85 Activated neutrophils release matrix proteins and chemotactic factors that not only induce further neutrophil lung infiltration86–89 but also facilitate epithelial cell remodeling.90 A correlation between the numbers of alveolar macrophages and pulmonary fibrosis was reported in a recent study.91 The investigators demonstrated that ventilated patients with ARDS who had greater numbers of fibroblasts in lung lavage fluid had a higher percentage of alveolar macrophages although whether the alveolar macrophages contributed to the fibrotic development was not addressed in this clinical study.91 It is noteworthy that activated macrophages are categorized into M1 and M2 phenotypes depending on the patterns of their inflammatory responses. Stimulation of normal human alveolar macrophages with the Th2 cytokines IL-4 and/or IL-10 resulted in an alveolar macrophage phenotype shift to M2 that has been implicated in the pathogenesis of pulmonary fibrosis.92 Taken together, the literature suggests that alveolar macrophages may not simply be bystanders but may play an important role contributing to the development of pulmonary remodeling in ARDS.
Signaling Pathways Involved in Mechanical Ventilation–associated Lung Fibrosis
Extracellular matrix plays an important role in the biomechanical behavior of the lung parenchyma, and mechanical ventilation has been shown to activate ECM elements such as collagen, elastin, fibronectin, laminin, lumican, and proteoglycan. 41,93–97 The expression of different components of the pulmonary ECM varies during the course of lung fibrosis. PCIII fibers are predominate early in the course of lung injury, whereas PCI is more prevalent in the late phase.94 The ECM interacts with TGF-β and mesenchymal tissue growth factors modulating the lung-remodeling processes.98–101
Mechanical stretch induced accumulation of the short fragment hyaluronan (sHA) of ECM glycosaminoglycan associated with an increase in IL-8 production in pulmonary fibroblasts and lung epithelial cells.102 The increased inflammatory responses, lung injury, neutrophil infiltration, cytokine production, and lung edema observed in wild-type mice ventilated with high tidal volume were attenuated in hyaluronan synthase-3 gene knockout mice.102,103 Moreover, a recent study demonstrated that cyclic mechanical stretch of primary murine alveolar type II epithelial cells for 4 h resulted in an increased expression of the sHA in the absence of fibroblasts. 35 Furthermore, mechanical stretch–induced sHA production has been shown to up-regulate the Wnt-inducible signaling protein 1. In turn, the Wnt/β-catenin signaling pathway is known to mediate EMT,35 which has been reported to be activated by mechanical ventilation at high tidal volumes in animals without preexisting lung injury.10. These data suggest that sHA produced and released during lung injury induces EMT in alveolar type II epithelial cells, and sHA may be a novel therapeutic target in ventilator- associated lung fibrosis.
It is noteworthy that, in the kidney, mechanical stretch and subsequent renal tubular epithelial cell distension induced up-regulation of reactive oxygen species that in turn activated the cytoplasmic proline-rich tyrosine kinase-2 (Pyk2).105 Although this pathway has not been examined in the mechanically ventilated lung or in pulmonary epithelial cells subjected to in vitro mechanical stretch, human lung epithelial cells do express Pyk2.106 In mice deficient in the Pyk2 gene, the renal expression of TGF-β1 and connective tissue growth factor induced by mechanical stretch was significantly reduced.105 Thus, Pyk2 may be important in initiating stretch-induced fibrosis in the lung although this hypothesis has not been directly examined.
Therapeutic Strategies
There are no pharmacological therapies that have been proven to be effective in large-scale trials for ventilator- induced lung fibrosis. Meduri et al.107 reported that all patients with unresolving ARDS had a progressive increase in PCI and PCIII in plasma and in bronchoalveolar lavage fluids, and that administration of methylprednisolone decreased these molecules and improved patient outcome. In the ARDS Network study, patients with low baseline lung lavage levels of PCIII had a 60-day mortality of 35% in the methylprednisolone treated arm versus 9% in the placebo arm. In contrast, there was a trend to lower mortality in the methylprednisolone group (4%) compared with that in the placebo group (19%) in patients with high baseline lavage levels of PCIII.19 This study suggests that the corticosteroids may be beneficial in the subset of patients most at risk of developing pulmonary fibrosis.
The mechanisms of action of methylprednisolone may be related to its antiinflammatory and/or antifibrotic properties. 107 Corticosteroids have numerous actions that mitigate inflammation, such as inhibition of the adhesion, migration of leukocytes across the capillary wall, and blockade of nuclear factor-κB nuclear translocation.108 A recent review article suggested several therapeutic targets to limit ARDS- associated lung fibrosis by pharmacological interventions, including tyrosine kinase inhibitors, Src kinase inhibitors, histone deacetylase inhibitors, monoclonal antibodies, and blocking peptides that directly bind to growth factors or block receptor ligation binding to inhibit synthesis of matrix proteins.109 The potential for these approaches to attenuate mechanical stretch–induced fibrosis is unknown.
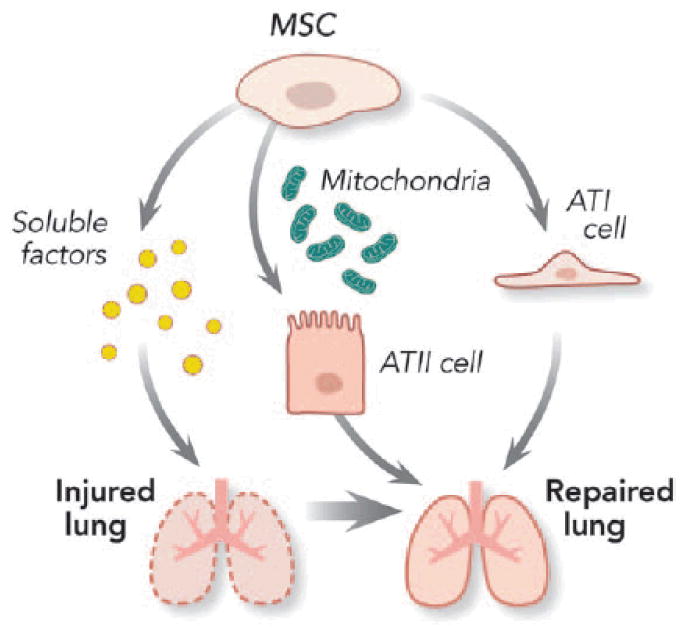
Because both ARDS and VILI are associated with inflammatory injury to the alveolar epithelial cells,110 mesenchymal stromal cells (MSCs) are interesting candidates to facilitate lung regeneration and repair (fig. 5). The demonstration that human MSCs exert benefit in a variety of in vitro and in vivo preclinical lung injury models is particularly exciting.111–119 MSCs are reported to secrete multiple paracrine factors that can protect epithelial cell membranes from damage, decrease inflammation, and inhibit bacterial growth.
Figure 5.
Potential mechanisms of mesenchymal stromal cells (MSCs) in the lung repair process in acute respiratory distress syndrome. MSCs exert a number of properties to enhance repair and restoration of physiologic function after ventilator- induced lung injury. The effects seem to be paracrine mediated and dependent in part on keratinocyte growth factor produced by the stromal cells. The bone marrow–derived MSC could transfer their mitochondria into lung epithelial cells resulting in increased alveolar adenosine triphosphate concentrations and enhanced cellular bioenergetics and improved lung function. The MSC may also be able to differentiate into alveolar type I (ATI) and type II (ATII) epithelial cells.
Mesenchymal stromal cells have been demonstrated to enhance repair and restoration of physiologic function after VILI.120 The effects seem to be paracrine mediated and dependent in part on keratinocyte growth factor produced by the stromal cells. Both intratracheal and intravenous MSC delivery seem to enhance lung repair after VILI.121 A recent study in a mouse model of endotoxin-induced lung injury demonstrated that bone marrow–derived MSCs could transfer their mitochondria into lung epithelial cells resulting in increased alveolar adenosine triphosphate concentrations.122 The investigators speculated that the mitochondrial transfer might have enhanced cellular bioenergetics and improved lung function. However, one has to be careful in potential application to humans because MSCs derived from mouse bone marrow and human umbilical cord blood have been shown to produce soluble factors that mediate lung fibroblast growth.123 At present, although MSCs demonstrate considerable promise, additional studies are needed to address significant deficits in our knowledge regarding mechanism(s) of action and the efficacy and the safety of MSCs in the treatment of patients with ARDS.
Conclusions
Recent evidence demonstrates that mechanical ventilation, particularly where significant overstretch occurs, may drive the pathogenesis of fibrosis in patients with ARDS. The application of mechanical ventilation in animal models of acute lung injury or the application of mechanical stress in vitro in lung epithelial cells can induce the development of lung fibrosis through fibroproliferation and EMT. Future studies are required to improve our understanding of these mechanisms so that we can develop novel approaches— pharmacologic or other—to prevent or treat the pulmonary fibrosis associated with mechanical ventilation in patients with ARDS.
Acknowledgments
This study was supported in part by Instituto de Salud Carlos III, Madrid, Spain (grant nos. PI10/0393, CB06/06/1088), and by Canadian Institutes of Health Research, Ottawa, Ontario, Canada. Dr. Laffey holds a merit award from the Department of Anesthesia, University of Toronto, Toronto, Ontario, Canada.
Footnotes
Competing Interests
Dr. Slutsky consults for Gambro Inc. (Grobenzell, Germany), Maquet Medical (Solna, Sweden), Novalung GmbH (Heilbronn, Germany), GSK (Mississauga, Ontario, Canada), and Apeiron Biologics AG (Vienna, Austria). The other authors declare no competing interests.
References
- 1.Villar J. Ventilator or physician-induced lung injury? Minerva Anestesiol. 2005;71:255–8. [PubMed] [Google Scholar]
- 2.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med. 2004;141:460–70. doi: 10.7326/0003-4819-141-6-200409210-00012. [DOI] [PubMed] [Google Scholar]
- 5.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 6.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 7.Dreyfuss D, Saumon G. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 8.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1β and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–73. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 9.Entzian P, Hückstädt A, Kreipe H, Barth J. Determination of serum concentrations of type III procollagen peptide in mechanically ventilated patients. Pronounced augmented concentrations in the adult respiratory distress syndrome. Am Rev Respir Dis. 1990;142:1079–82. doi: 10.1164/ajrccm/142.5.1079. [DOI] [PubMed] [Google Scholar]
- 10.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977;116:589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- 11.Tomashefski JF, Jr, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112:112–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Meduri GU, Chinn AJ, Leeper KV, Wunderink RG, Tolley E, Winer-Muram HT, Khare V, Eltorky M. Corticosteroid rescue treatment of progressive fibroproliferation in late ARDS. Patterns of response and predictors of outcome. Chest. 1994;105:1516–27. doi: 10.1378/chest.105.5.1516. [DOI] [PubMed] [Google Scholar]
- 13.Martin C, Papazian L, Payan MJ, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995;107:196–200. doi: 10.1378/chest.107.1.196. [DOI] [PubMed] [Google Scholar]
- 14.Meduri GU, Rocco PR, Annane D, Sinclair SE. Prolonged glucocorticoid treatment and secondary prevention in acute respiratory distress syndrome. Expert Rev Respir Med. 2010;4:201–10. doi: 10.1586/ers.10.2. [DOI] [PubMed] [Google Scholar]
- 15.Ichikado K, Muranaka H, Gushima Y, Kotani T, Nader HM, Fujimoto K, Johkoh T, Iwamoto N, Kawamura K, Nagano J, Fukuda K, Hirata N, Yoshinaga T, Ichiyasu H, Tsumura S, Kohrogi H, Kawaguchi A, Yoshioka M, Sakuma T, Suga M. Fibroproliferative changes on high-resolution CT in the acute respiratory distress syndrome predict mortality and ventilator dependency: A prospective observational cohort study. BMJ Open. 2012;2:e000545. doi: 10.1136/bmjopen-2011-000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papazian L, Doddoli C, Chetaille B, Gernez Y, Thirion X, Roch A, Donati Y, Bonnety M, Zandotti C, Thomas P. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med. 2007;35:755–62. doi: 10.1097/01.CCM.0000257325.88144.30. [DOI] [PubMed] [Google Scholar]
- 17.Madtes DK, Rubenfeld G, Klima LD, Milberg JA, Steinberg KP, Martin TR, Raghu G, Hudson LD, Clark JG. Elevated transforming growth factor-α levels in bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158:424–30. doi: 10.1164/ajrccm.158.2.9711112. [DOI] [PubMed] [Google Scholar]
- 18.Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162:1783–8. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network: Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–84. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 20.Meduri GU, Marik PE, Chrousos GP, Pastores SM, Arlt W, Beishuizen A, Bokhari F, Zaloga G, Annane D. Steroid treatment in ARDS: A critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008;34:61–9. doi: 10.1007/s00134-007-0933-3. [DOI] [PubMed] [Google Scholar]
- 21.Pastor CM, Matthay MA, Frossard JL. Pancreatitis-associated acute lung injury: New insights. Chest. 2003;124:2341–51. doi: 10.1378/chest.124.6.2341. [DOI] [PubMed] [Google Scholar]
- 22.Rocco PR, Negri EM, Kurtz PM, Vasconcellos FP, Silva GH, Capelozzi VL, Romero PV, Zin WA. Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am J Respir Crit Care Med. 2001;164:1067–71. doi: 10.1164/ajrccm.164.6.2007062. [DOI] [PubMed] [Google Scholar]
- 23.Zapol WM, Trelstad RL, Coffey JW, Tsai I, Salvador RA. Pulmonary fibrosis in severe acute respiratory failure. Am Rev Respir Dis. 1979;119:547–54. doi: 10.1164/arrd.1979.119.4.547. [DOI] [PubMed] [Google Scholar]
- 24.Raghu G, Striker LJ, Hudson LD, Striker GE. Extracellular matrix in normal and fibrotic human lungs. Am Rev Respir Dis. 1985;131:281–9. doi: 10.1164/arrd.1985.131.2.281. [DOI] [PubMed] [Google Scholar]
- 25.Curley GF, Contreras M, Higgins B, O’Kane C, McAuley DF, O’Toole D, Laffey JG. Evolution of the inflammatory and fibroproliferative responses during resolution and repair after ventilator-induced lung injury in the rat. Anesthesiology. 2011;115:1022–32. doi: 10.1097/ALN.0b013e31823422c9. [DOI] [PubMed] [Google Scholar]
- 26.Chesnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am J Respir Crit Care Med. 1997;156(3 Pt 1):840–5. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 27.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A. Active Bacterial Core Surveillance of the Emerging Infections Program Network: Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl JMed. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 28.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 29.Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Curr Opin Crit Care. 2002;8:12–20. doi: 10.1097/00075198-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Pecchiari M, Monaco A, Koutsoukou A, D’Angelo E. Plasma membrane disruptions with different modes of injurious mechanical ventilation in normal rat lungs*. Crit Care Med. 2012;40:869–75. doi: 10.1097/CCM.0b013e318232da2b. [DOI] [PubMed] [Google Scholar]
- 31.Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116(1 suppl):9S–15. doi: 10.1378/chest.116.suppl_1.9s-a. [DOI] [PubMed] [Google Scholar]
- 32.Copland IB, Reynaud D, Pace-Asciak C, Post M. Mechanotransduction of stretch-induced prostanoid release by fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L487–95. doi: 10.1152/ajplung.00510.2005. [DOI] [PubMed] [Google Scholar]
- 33.Torday JS, Torres E, Rehan VK. The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation, and repair in vitro. Pediatr Pathol Mol Med. 2003;22:189–207. doi: 10.1080/pdp.22.3.189.207. [DOI] [PubMed] [Google Scholar]
- 34.Cavanaugh KJ, Jr, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;25:584–91. doi: 10.1165/ajrcmb.25.5.4486. [DOI] [PubMed] [Google Scholar]
- 35.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial- mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011;286:17435–44. doi: 10.1074/jbc.M110.137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SL, Nekouzadeh A, Butler B, Pryse KM, McConnaughey WB, Nathan AC, Legant WR, Schaefer PM, Pless RB, Elson EL, Genin GM. Physically-induced cytoskeleton remodeling of cells in three-dimensional culture. PLoS One. 2012;7:e45512. doi: 10.1371/journal.pone.0045512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: Critical roles of collagen and mechanical forces. J Appl Physiol (1985) 2005;98:1892–9. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- 38.Garcia CS, Rocco PR, Facchinetti LD, Lassance RM, Caruso P, Deheinzelin D, Morales MM, Romero PV, Faffe DS, Zin WA. What increases type III procollagen mRNA levels in lung tissue: Stress induced by changes in force or amplitude? Respir Physiol Neurobiol. 2004;144:59–70. doi: 10.1016/j.resp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Farias LL, Faffe DS, Xisto DG, Santana MC, Lassance R, Prota LF, Amato MB, Morales MM, Zin WA, Rocco PR. Positive end-expiratory pressure prevents lung mechanical stress caused by recruitment/derecruitment. J Appl Physiol (1985) 2005;98:53–61. doi: 10.1152/japplphysiol.00118.2004. [DOI] [PubMed] [Google Scholar]
- 40.Berg JT, Fu Z, Breen EC, Tran HC, Mathieu-Costello O, West JB. High lung inflation increases mRNA levels of ECM components and growth factors in lung parenchyma. J Appl Physiol (1985) 1997;83:120–8. doi: 10.1152/jappl.1997.83.1.120. [DOI] [PubMed] [Google Scholar]
- 41.Parker JC, Breen EC, West JB. High vascular and airway pressures increase interstitial protein mRNA expression in isolated rat lungs. J Appl Physiol (1985) 1997;83:1697–705. doi: 10.1152/jappl.1997.83.5.1697. [DOI] [PubMed] [Google Scholar]
- 42.Riva DR, Oliveira MB, Rzezinski AF, Rangel G, Capelozzi VL, Zin WA, Morales MM, Pelosi P, Rocco PR. Recruitment maneuver in pulmonary and extrapulmonary experimental acute lung injury. Crit Care Med. 2008;36:1900–8. doi: 10.1097/CCM.0b013e3181760e5d. [DOI] [PubMed] [Google Scholar]
- 43.Steimback PW, Oliveira GP, Rzezinski AF, Silva PL, Garcia CS, Rangel G, Morales MM, Lapa E, Silva JR, Capelozzi VL, Pelosi P, Rocco PR. Effects of frequency and inspiratory plateau pressure during recruitment manoeuvres on lung and distal organs in acute lung injury. Intensive Care Med. 2009;35:1120–8. doi: 10.1007/s00134-009-1439-y. [DOI] [PubMed] [Google Scholar]
- 44.Rocco PR, Dos Santos C, Pelosi P. Lung parenchyma remodeling in acute respiratory distress syndrome. Minerva Anestesiol. 2009;75:730–40. [PubMed] [Google Scholar]
- 45.Tatler AL, Jenkins G. TGF-β activation and lung fibrosis. Proc Am Thorac Soc. 2012;9:130–6. doi: 10.1513/pats.201201-003AW. [DOI] [PubMed] [Google Scholar]
- 46.Tremblay LN, Slutsky AS. Pathogenesis of ventilator-induced lung injury: Trials and tribulations. Am J Physiol Lung Cell Mol Physiol. 2005;288:L596–8. doi: 10.1152/ajplung.00438.2004. [DOI] [PubMed] [Google Scholar]
- 47.Tremblay LN, Slutsky AS. Ventilator-induced injury: From barotrauma to biotrauma. Proc Assoc Am Physicians. 1998;110:482–8. [PubMed] [Google Scholar]
- 48.Keane MP. The role of chemokines and cytokines in lung fibrosis. Eur Respir Rev. 2008;17:151–6. [Google Scholar]
- 49.Ji R, Lee CM, Gonzales LW, Yang Y, Aksoy MO, Wang P, Brailoiu E, Dun N, Hurford MT, Kelsen SG. Human type II pneumocyte chemotactic responses to CXCR3 activation are mediated by splice variant A. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1187–96. doi: 10.1152/ajplung.00388.2007. [DOI] [PubMed] [Google Scholar]
- 50.Tudhope SJ, Catley MC, Fenwick PS, Russell RE, Rumsey WL, Newton R, Barnes PJ, Donnelly LE. The role of IkappaB kinase 2, but not activation of NF-kappaB, in the release of CXCR3 ligands from IFN-gamma-stimulated human bronchial epithelial cells. J Immunol. 2007;179:6237–45. doi: 10.4049/jimmunol.179.9.6237. [DOI] [PubMed] [Google Scholar]
- 51.Shahabuddin S, Ji R, Wang P, Brailoiu E, Dun N, Yang Y, Aksoy MO, Kelsen SG. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: Role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol. 2006;291:C34–9. doi: 10.1152/ajpcell.00441.2005. [DOI] [PubMed] [Google Scholar]
- 52.Bonner K, Pease JE, Corrigan CJ, Clark P, Kay AB. CCL17/thymus and activation-regulated chemokine induces calcitonin gene-related peptide in human airway epithelial cells through CCR4. J Allergy Clin Immunol. 2013;132:942–50. e1–3. doi: 10.1016/j.jaci.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, Arrigoni G, Fabbri LM, Sinigaglia F. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–64. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.May LA, Kicic A, Rigby P, Heel K, Pullen TL, Crook M, Charles A, Banerjee B, Ravine D, Saxena A, Musk M, Stick SM, Chambers DC. Cells of epithelial lineage are present in blood, engraft the bronchial epithelium, and are increased in human lung transplantation. J Heart Lung Transplant. 2009;28:550–7. doi: 10.1016/j.healun.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 55.Eddleston J, Christiansen SC, Zuraw BL. Functional expression of the C-X-C chemokine receptor CXCR4 by human bronchial epithelial cells: Regulation by proinflammatory mediators. J Immunol. 2002;169:6445–51. doi: 10.4049/jimmunol.169.11.6445. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh MC, Makena PS, Gorantla V, Sinclair SE, Waters CM. CXCR4 regulates migration of lung alveolar epithelial cells through activation of Rac1 and matrix metalloproteinase-2. Am J Physiol Lung Cell Mol Physiol. 2012;302:L846–56. doi: 10.1152/ajplung.00321.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Maciejewski BS, Soto-Reyes D, Lee HS, Warburton D, Sanchez-Esteban J. Mechanical stretch promotes fetal type II epithelial cell differentiation via shedding of HB-EGF and TGF-α. J Physiol. 2009;587(Pt 8):1739–53. doi: 10.1113/jphysiol.2008.163899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cabrera-Benítez NE, Parotto M, Post M, Han B, Spieth PM, Cheng WE, Valladares F, Villar J, Liu M, Sato M, Zhang H, Slutsky AS. Mechanical stress induces lung fibrosis by epithelial- mesenchymal transition. Crit Care Med. 2012;40:510–7. doi: 10.1097/CCM.0b013e31822f09d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF-β 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 61.Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-β: A mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med. 2003;31(4 suppl):S258–64. doi: 10.1097/01.CCM.0000057901.92381.75. [DOI] [PubMed] [Google Scholar]
- 62.Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci U S A. 2000;97:1778–83. doi: 10.1073/pnas.97.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rocco PR, Dos Santos C, Pelosi P. Pathophysiology of ventilator- associated lung injury. Curr Opin Anaesthesiol. 2012;25:123–30. doi: 10.1097/ACO.0b013e32834f8c7f. [DOI] [PubMed] [Google Scholar]
- 64.Frank JA, Matthay MA. Science review: Mechanisms of ventilator- induced injury. Crit Care. 2003;7:233–41. doi: 10.1186/cc1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 67.Willis BC, Borok Z. TGF-β-induced EMT: Mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 68.Zavadil J, Böttinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 69.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med (Berl) 2004;82:175–81. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- 70.De Wever O, Westbroek W, Verloes A, Bloemen N, Bracke M, Gespach C, Bruyneel E, Mareel M. Critical role of N-cadherin in myofibroblast invasion and migration in vitro stimulated by colon-cancer-cell-derived TGF-β or wounding. J Cell Sci. 2004;117(Pt 20):4691–703. doi: 10.1242/jcs.01322. [DOI] [PubMed] [Google Scholar]
- 71.Phan SH. Fibroblast phenotypes in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(3 suppl):S87–92. [PubMed] [Google Scholar]
- 72.Li LF, Liao SK, Huang CC, Hung MJ, Quinn DA. Serine/threonine kinase-protein kinase B and extracellular signal- regulated kinase regulate ventilator-induced pulmonary fibrosis after bleomycin-induced acute lung injury: A prospective, controlled animal experiment. Crit Care. 2008;12:R103. doi: 10.1186/cc6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foda HD, Rollo EE, Drews M, Conner C, Appelt K, Shalinsky DR, Zucker S. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: Attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340) Am J Respir Cell Mol Biol. 2001;25:717–24. doi: 10.1165/ajrcmb.25.6.4558f. [DOI] [PubMed] [Google Scholar]
- 74.Breen EC, Fu Z, Normand H. Calcyclin gene expression is increased by mechanical strain in fibroblasts and lung. Am J Respir Cell Mol Biol. 1999;21:746–52. doi: 10.1165/ajrcmb.21.6.3312. [DOI] [PubMed] [Google Scholar]
- 75.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1819–28. doi: 10.1164/ajrccm.154.6.8970376. [DOI] [PubMed] [Google Scholar]
- 76.Labat ML, Bringuier AF, Arys-Philippart C, Arys A, Wellens F. Monocytic origin of fibrosis. In vitro transformation of HLA-DR monocytes into neo-fibroblasts: Inhibitory effect of all-trans retinoic acid on this process. Biomed Pharmacother. 1994;48:103–11. doi: 10.1016/0753-3322(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 77.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 78.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–81. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 80.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow- derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–52. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quesnel C, Piednoir P, Gelly J, Nardelli L, Garnier M, Leçon V, Lasocki S, Bouadma L, Philip I, Elbim C, Mentré F, Soler P, Crestani B, Dehoux M. Alveolar fibrocyte percentage is an independent predictor of poor outcome in patients with acute lung injury. Crit Care Med. 2012;40:21–8. doi: 10.1097/CCM.0b013e31822d718b. [DOI] [PubMed] [Google Scholar]
- 83.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malech HL, Gallin JI. Current concepts: Immunology. Neutrophils in human diseases. N Engl J Med. 1987;317:687–94. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 85.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 86.Davidson JM. Biochemistry and turnover of lung interstitium. Eur Respir J. 1990;3:1048–63. [PubMed] [Google Scholar]
- 87.O’Connor CM, FitzGerald MX. Matrix metalloproteases and lung disease. Thorax. 1994;49:602–9. doi: 10.1136/thx.49.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shapiro SD, Campbell EJ, Welgus HG, Senior RM. Elastin degradation by mononuclear phagocytes. Ann N Y Acad Sci. 1991;624:69–80. doi: 10.1111/j.1749-6632.1991.tb17007.x. [DOI] [PubMed] [Google Scholar]
- 89.Vartio T, Seppä H, Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981;256:471–7. [PubMed] [Google Scholar]
- 90.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 91.Quesnel C, Nardelli L, Piednoir P, Leçon V, Marchal-Somme J, Lasocki S, Bouadma L, Philip I, Soler P, Crestani B, Dehoux M. Alveolar fibroblasts in acute lung injury: Biological behavior and clinical relevance. Eur Respir J. 2010;35:1312–21. doi: 10.1183/09031936.00074709. [DOI] [PubMed] [Google Scholar]
- 92.Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, Luttmann W, Friedrich K, Müller-Quernheim J, Zissel G. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 93.Al-Jamal R, Ludwig MS. Changes in proteoglycans and lung tissue mechanics during excessive mechanical ventilation in rats. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1078–87. doi: 10.1152/ajplung.2001.281.5.L1078. [DOI] [PubMed] [Google Scholar]
- 94.Pelosi P, Rocco PR. Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med. 2008;34:631–9. doi: 10.1007/s00134-007-0964-9. [DOI] [PubMed] [Google Scholar]
- 95.Ying S, Shiraishi A, Kao CW, Converse RL, Funderburgh JL, Swiergiel J, Roth MR, Conrad GW, Kao WW. Characterization and expression of the mouse lumican gene. J Biol Chem. 1997;272:30306–13. doi: 10.1074/jbc.272.48.30306. [DOI] [PubMed] [Google Scholar]
- 96.Dolhnikoff M, Morin J, Roughley PJ, Ludwig MS. Expression of lumican in human lungs. Am J Respir Cell Mol Biol. 1998;19:582–7. doi: 10.1165/ajrcmb.19.4.2979. [DOI] [PubMed] [Google Scholar]
- 97.Li LF, Chen BX, Tsai YH, Kao WW, Yang CT, Chu PH. Lumican expression in diaphragm induced by mechanical ventilation. PLoS One. 2011;6:e24692. doi: 10.1371/journal.pone.0024692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK, Haley KJ, Lilly CM, So PT, Lauffenburger DA, Kamm RD, Drazen JM. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83–6. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schild C, Trueb B. Mechanical stress is required for high-level expression of connective tissue growth factor. Exp Cell Res. 2002;274:83–91. doi: 10.1006/excr.2001.5458. [DOI] [PubMed] [Google Scholar]
- 100.Sheppard D. Transforming growth factor-β: A central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3:413–7. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gutierrez JA, Perr HA. Mechanical stretch modulates TGF- β1 and -α1(I) collagen expression in fetal human intestinal smooth muscle cells. Am J Physiol. 1999;277(5 Pt 1):G1074–80. doi: 10.1152/ajpgi.1999.277.5.G1074. [DOI] [PubMed] [Google Scholar]
- 102.Bai KJ, Spicer AP, Mascarenhas MM, Yu L, Ochoa CD, Garg HG, Quinn DA. The role of hyaluronan synthase 3 in ventilator- induced lung injury. Am J Respir Crit Care Med. 2005;172:92–8. doi: 10.1164/rccm.200405-652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mascarenhas MM, Day RM, Ochoa CD, Choi WI, Yu L, Ouyang B, Garg HG, Hales CA, Quinn DA. Low molecular weight hyaluronan from stretched lung enhances interleukin-8 expression. Am J Respir Cell Mol Biol. 2004;30:51–60. doi: 10.1165/rcmb.2002-0167OC. [DOI] [PubMed] [Google Scholar]
- 104.Villar J, Cabrera NE, Casula M, Valladares F, Flores C, López-Aguilar J, Blanch L, Zhang H, Kacmarek RM, Slutsky AS. WNT/β-catenin signaling is modulated by mechanical ventilation in an experimental model of acute lung injury. Intensive Care Med. 2011;37:1201–9. doi: 10.1007/s00134-011-2234-0. [DOI] [PubMed] [Google Scholar]
- 105.Sonomura K, Okigaki M, Kimura T, Matsuoka E, Shiotsu Y, Adachi T, Kado H, Ishida R, Kusaba T, Matsubara H, Mori Y. The kinase Pyk2 is involved in renal fibrosis by means of mechanical stretch-induced growth factor expression in renal tubules. Kidney Int. 2012;81:449–57. doi: 10.1038/ki.2011.403. [DOI] [PubMed] [Google Scholar]
- 106.Liang L, Woodward OM, Chen Z, Cotter R, Guggino WB. A novel role of protein tyrosine kinase2 in mediating chloride secretion in human airway epithelial cells. PLoS One. 2011;6:e21991. doi: 10.1371/journal.pone.0021991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1432–41. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 108.Suter PM. Lung Inflammation in ARDS—Friend or foe? N Engl J Med. 2006;354:1739–42. doi: 10.1056/NEJMe068033. [DOI] [PubMed] [Google Scholar]
- 109.Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: Mechanisms and clinical significance. Eur Respir J. 2014;43:276–85. doi: 10.1183/09031936.00196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davey A, McAuley DF, O’Kane CM. Matrix metalloproteinases in acute lung injury: Mediators of injury and drivers of repair. Eur Respir J. 2011;38:959–70. doi: 10.1183/09031936.00032111. [DOI] [PubMed] [Google Scholar]
- 111.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211–22. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin- induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 114.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide- induced lung injury. J Immunol. 2004;172:1266–72. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- 116.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–52. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–41. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 118.Zhen G, Liu H, Gu N, Zhang H, Xu Y, Zhang Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008;13:3415–22. doi: 10.2741/2936. [DOI] [PubMed] [Google Scholar]
- 119.Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472–81. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 120.Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, O’Brien T, O’Toole D, Laffey JG. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 121.Curley GF, Ansari B, Hayes M, Devaney J, Masterson C, Ryan A, Barry F, O’Brien T, Toole DO, Laffey JG. Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology. 2013;118:924–32. doi: 10.1097/ALN.0b013e318287ba08. [DOI] [PubMed] [Google Scholar]
- 122.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–65. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Salazar KD, Lankford SM, Brody AR. Mesenchymal stem cells produce Wnt isoforms and TGF-β1 that mediate proliferation and procollagen expression by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1002–11. doi: 10.1152/ajplung.90347.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]