Abstract
Drug addiction is a major public health concern in the United States costing taxpayers billions in health care costs, lost productivity and law enforcement. However, the availability of effective treatment options remains limited. The development of novel therapeutics will not be possible without a better understanding of the addicted brain. Studies in both clinical and preclinical models indicate that chronic drug use leads to alterations in the body and brain’s response to stress. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis may shed light on the ability of stress to increase vulnerability to relapse. Further, within both the HPA axis and limbic brain regions, corticotropin-releasing factor (CRF) is critically involved in the brain’s response to stress. Alterations in both central and peripheral CRF activity seen following chronic drug use provide a mechanism by which substance use can alter stress reactivity, thus mediating addictive phenotypes. While many reviews have focused on how stress alters drug-mediated changes in physiology and behavior, the goal of this review is to focus on how substance use alters responses to stress.
Keywords: Substance Use, Addiction, Stress, CRF, HPA axis
Recreational drug use has existed in nearly every society throughout history. However, this recreational use can spiral into addiction for a subset of vulnerable individuals. One of the factors mitigating this vulnerability is stress. Clinical research demonstrates that chronic stress is a risk factor in the development of addiction [1, 2], and as many as 70% of addicts have experienced trauma within their lifetime [3]. Furthermore, life stress is a critical factor mediating treatment outcomes and relapse rates [4–6]. In light of this, treatments aimed at reducing stress could increase addiction treatment success [7].
To promote the identification of therapies to reduce stress in addicts, we must first determine the neurobiological mechanisms underlying the interactions between drugs of abuse and stress. Many studies have examined the ability of stress exposure to potentiate addictive phenotypes [8–11]. However, it is just as important to examine how addicts respond to stress and how drugs of abuse can alter stress responsivity. This review will focus on how chronic drug administration in both preclinical and clinical models can lead to alterations in behavioral and physiological responses to stress. Furthermore, we will discuss the neurobiological alterations underlying the ability of chronic drug use to affect responses to stress and the implications of these alterations in designing new treatment options.
Stress-related Psychiatric Disorders and Substance Abuse Comorbidity
Preclinical research has clearly demonstrated that drugs and stressful stimuli exhibit cross-sensitization, whereby experience with drugs leads to an enhanced response to stress and vice versa [12–15]. This cross-sensitization between drugs and stress is likely mediated by the overlap in neural circuitry. Both preclinical and clinical studies have demonstrated that acute stress and drug exposure lead to the activation of similar brain regions [5, 16, 17].
As there is such a clear relationship between the neurobiological circuits underlying stress and addiction, it is not surprising that there is a high comorbidity between substance use disorders (SUD) and post-traumatic stress disorder (PTSD). While overall estimates of SUD prevalence are 3–7% nationally [18], within individuals with PTSD, the lifetime prevalence increases to 19–35% for SUD and 36–52% for alcohol use disorder (AUD) [19–21]. Likewise, PTSD is more common in individuals with SUD, with an estimated lifetime PTSD prevalence of 26–52% (compared to 8% in the total population) [3, 22, 23].
Individuals with both PTSD and SUD have more severe symptomology and exhibit poorer treatment outcomes than those with PTSD or SUD alone [24, 25], suggesting a magnifying effect of substance use on stress responsivity. Poorer treatment outcomes for these disorders, are compounded by additional physical and psychiatric health problems, including higher incidence of depression and anxiety disorders [22, 25–27], as well as cardiovascular and neurological problems [28]. Furthermore, these individuals are more likely to be unemployed and are more prone to violence [24, 29, 30]. Taken together, this suggests that gaining a better understanding of the neurobiological mechanisms underlying this relationship could help reveal unique treatment options for this population.
While much research has focused on patients with PTSD using alcohol and drugs to “self-medicate”, there is evidence that SUD can predate PTSD. Individuals with SUD have a heightened likelihood of trauma exposure, which in turn, leads to a heightened risk of PTSD [31, 32]. Furthermore, SUD, as well as nicotine dependence, can increase PTSD vulnerability after trauma exposure [26, 33, 34]. Regardless of the temporal order of SUD and PTSD, it is clear that SUD can sustain, prolong, or worsen PTSD symptoms [35].
For example, within a population of PTSD patients, those with lifetime substance use, specifically cocaine and marijuana use, exhibited significantly higher PTSD symptomology than those without drug use [36]. Furthermore, PTSD patients that smoke exhibit more severe withdrawal from nicotine and this withdrawal leads to exacerbated PTSD symptomology [37, 38]. Additionally, PTSD patients that successfully quit smoking exhibit improved negative affect compared to those who are unsuccessful [39]. Moreover, alcohol withdrawal and craving can increase response to trauma cues in alcoholics with comorbid PTSD [40]. Consistent with this, a study of individuals with comorbid SUD and PTSD reported intensified trauma symptoms following relapse [41].
Although the effects of drugs of abuse on stress reactivity may be most prominently seen in individuals with PTSD, they are not the only individuals affected. Drug abuse is often comorbid with other stress-related psychiatric disorders. For example, approximately 20% of individuals with mood disorders and 15% with anxiety disorders report at least one concurrent SUD. Major depression is strongly associated with SUD as individuals with major depression are 7 times more likely to exhibit drug abuse or dependence [42]. When examining lifetime SUD prevalence, 50% of individuals with generalized anxiety disorder (GAD) report problems with substance abuse. The rates of SUD increase individuals with concurrent anxiety disorders, as 46% of individuals with concurrent panic disorder, social phobia, and generalized anxiety disorders report comorbid SUD [43]. As with PTSD, substance use can exacerbate symptoms of anxiety and initiate additional anxiety disorders [44].
Chronic drug use leads to an increased response to stress, even in individuals who do not exhibit comorbid psychiatric conditions. Unfortunately, clinical studies are limited by extreme environmental and genetic variability, which can obscure the data and misinform scientific interpretations. Therefore, this review will focus on the way individuals with SUD respond to stress by further examining how preclinical studies have informed us on potential neurobiological mechanisms underlying the interaction between chronic drug use and stress responsivity.
Corticotropin-Releasing Factor: HPA Axis & Beyond
The high rates of comorbidity between substance abuse and stress-related psychiatric disorders may be mediated by the ability of drugs and stress to activate similar neural circuits. Consistent with this, animal models have demonstrated that both stress and drug exposure lead to an increase in mesolimbic dopamine transmission [45–48]. Though an increase in dopamine transmission may explain how stress can augment the effects of abused substances, it does not explain how drug use affects stress reactivity.
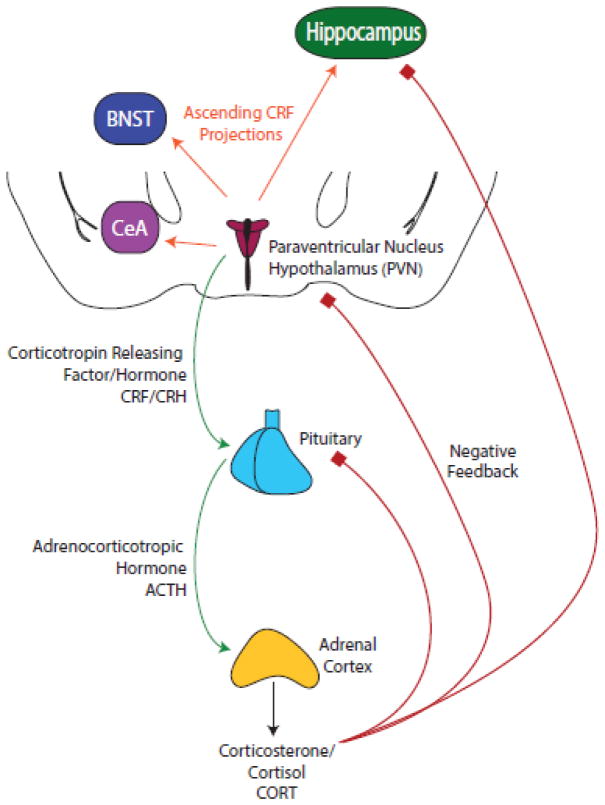
A key system mediating the body’s response to stressful stimuli is the hypothalamic-pituitary-adrenal (HPA) axis (see Fig. 1). In response to stress, there is an increased production of corticotropin releasing factor (CRF) in the hypothalamic paraventricular nucleus (PVN). CRF is a 41 amino acid polypeptide that controls behavioral, hormonal, and sympathetic responses to stress [49, 50] and its release onto the anterior pituitary gland induces the release of adrenocorticotropic hormone (ACTH) and subsequently the release of glucocorticoids from the adrenal cortex [51].
Fig. 1.
CRF serves as the primary mediator in the HPA response to stress in addition to its independent roles in brain stress systems [52–55]. It binds to two types of G-protein coupled receptors, CRF receptor type 1 (CRFR1) and CRF receptor type 2 (CRFR2), with high and moderate potency respectively [56, 57]. CRF activates the HPA axis stress response via its actions at CRFR1 in the anterior pituitary but CRF receptors are widely distributed throughout the brain with particularly high concentrations in the cell bodies of the paraventricular nucleus of the hypothalamus, the basal forebrain and the brainstem [58]. While CRFR1 is widely expressed throughout the brain, distribution of CRFR2 is more restricted, with the highest density in the olfactory bulb, lateral septum, bed nucleus of the stria terminalis (BNST), ventral hippocampus, and the amygdala [59].
One clear mechanism by which chronic drug use can alter stress response is via actions on CRF signaling. Acute administration of cocaine, morphine, nicotine, cannabinoids and alcohol lead to increases in CRF release, mRNA, and/or immunoreactivity [60–65]. While transient increases are seen following acute drug administration, chronic drug administration leads to sustained increases in CRF release [66, 67] and CRF immunoreactivity [68]. Furthermore, withdrawal from drugs of abuse leads to persistent activation of CRF release and immunoreactivity [68–73]. Further, CRF antagonists block many withdrawal-mediated anxiety-like and addictive behaviors [74]. As CRF administration enhances stress related behaviors such as acoustic startle, conditioned fear and stress-induced freezing behavior [75, 76], the increase in activation of stress systems following chronic drug use provides a mechanism by which addiction leads to a dysregulation of stress responding in addicts.
This dysregulation of CRF activity impacts a number of downstream targets. For example, chronic cocaine leads to an increase in excitatory signaling within the bed nucleus of the stria terminalis and this increase is dependent upon CRF activation [77]. Another downstream target of CRF that has been implicated in addiction is dynorphin and the kappa opioid system [78]. The kappa opioid system has been demonstrated to be critically involved in the ability of stress to initiate drug relapse as well as withdrawal-induced increases in stress responsivity [79–83].
Clinical Studies of Substance Use and Stress Responsivity
The high rates of comorbidity in SUD and PTSD diagnoses clearly suggest that there is a relationship between substance use and stress. As we have discussed, much of the work examining this relationship has focused on the influence of chronic stress and trauma exposure on the likelihood of an individual becoming an addict [35, 84]. However, the relationship between stress and addiction does not end there. Along with increasing the vulnerability to developing SUD, stress exposure also triggers relapse in abstaining addicts [85]. Furthermore, abstinence from drugs of abuse can lead to increased anxiety [86], making addicts even more vulnerable to the effects of stress. Therefore, understanding how addicts respond to stress, and how this differs from non-addicts, could provide us with valuable information for the development of therapeutics for SUD as well as comorbid PTSD.
The link between stress and relapse may be due, in part, to the ability of stress to activate the HPA axis. Baseline activity of the HPA axis, specifically levels of plasma adrenocorticotropic hormone (ACTH) and cortisol, is increased in individuals with SUD compared to healthy controls [87–89]. When presented with stressful stimuli, heroin addicts exhibit higher levels of cortisol (both saliva and serum levels) as well as ACTH [90]. These increases in stress-induced cortisol seem to persist after longer periods of abstinence [91]. Furthermore, acute administration of the stress hormone, cortisol, leads to increased cocaine craving in addicted individuals compared to a placebo injection [92].
Activation of the HPA axis is often accompanied by noradrenergic activation of the sympathetic nervous system, including increases in heart rate and blood pressure. Heroin and cocaine addicts both exhibit an increase in plasma levels of norepinephrine that parallels their duration of use [93]. A similar increase in plasma norepinephrine is seen in recently abstinent alcoholics compared to healthy controls in response to a yohimbine challenge [94] (which increases norepinephrine release by blocking alpha-2 adrenergic receptors [95]). As expected, these changes in noradrenergic signaling are paralleled by changes in cardiovascular measures of stress reactivity. When exposed to stress, male cocaine addicts exhibit greater increase in heart rate and blood pressure compared to controls [96]. Even further, increases in noradrenergic signaling, induced by a yohimbine challenge lead to increased craving in alcoholics [97].
Along with these physiological differences in the response to stress, there appear to also be behavioral differences in the stress reactivity of individuals with SUD. For example, when presented with fearful faces, heroin addicts report higher levels of anxiety compared to healthy controls [90]. Additionally, when given a yohimbine challenge, heroin addicts reported higher levels of subjective anxiety than healthy controls [98]. The severity of drug use may contribute to an addict’s response to stress. When high frequency cocaine abusers are presented with stressful stimuli they exhibit higher levels of craving and anxiety than low frequency abusers. Additionally, the high frequency abusers exhibit higher increases in heart rate and blood pressure in response to these stressful stimuli [99].
In contrast to what is seen with other abusive substances, smokers exhibit a blunted cardiovascular and decreased salivary cortisol levels in response to stress [100]. However, this decrease in stress reactivity may reverse during withdrawal, with former smokers exhibiting higher responses to stress than healthy controls [101, 102].
This brings up the important consideration of withdrawal state when examining the clinical literature. During acute withdrawal, addicts exhibit an increase in anxiety [103, 104]. Accompanying this behavioral response is an increase in CRF and this dysregulation can persist after longer periods of abstinence [105–108]. For example, during acute withdrawal, heroin addicts exhibit an increased stress-potentiated acoustic startle response, accompanied by an increase in circulating cortisol [109]. Similar increases in acoustic startle have been seen in alcoholics during acute withdrawal [110]. However, after more prolonged periods of abstinence, cocaine addicts exhibit decreased acoustic startle compared with healthy controls, suggesting a dampened stress response [111]. Additionally, stimulant dependent individuals exhibit decreased cortisol response to a stressful situation, as do alcoholics after longer periods of abstinence [94, 112, 113]. However, when marijuana dependence is added to the equation, the results are quite different. In a sample of treatment seeking individuals dependent upon cocaine and alcohol, those who were also marijuana dependent showed an increase in basal anxiety as well as stress-induced cortisol and ACTH compared to those who were not marijuana dependent as well as healthy controls [114]. Despite these decreases in peripheral markers of stress reactivity seen in alcoholics, post mortem microarray analysis of their brains reveal an upregulation of gene expression of genes that have been associated with stress signaling, suggesting that the dampened physiological response to stress may not reflect a decrease in the activity of brain stress systems [115].
As these studies were done in postmortem tissue, it is not possible to examine how substance use affects the ability of acute stress to activate stress-related genes in humans. Therefore, we must look to preclinical studies to determine how chronic drug administration affects the brain’s response to stress. A focus of many of these animal studies has been the role of CRF in addiction and the response to stress after drug administration.
Preclinical studies of drug administration and stress responsivity
Preclinical studies in animal models of drug administration and addiction allow us to more directly examine how drugs of abuse modulate stress response, both behaviorally and physiologically. Furthermore, clinical studies do not allow for the examination of causality for a variety of reasons. As we know, chronic early life stress can predispose individuals towards addiction and chronic stress can alter future responses to stress, making it difficult to determine how drug administration affects stress response in human addicts. Additionally, many drug addicts are also alcoholics or polydrug users, making it difficult to determine which drugs of abuse might be causing the differences in stress responsivity. Therefore, focusing on the HPA axis and brain CRF systems, we will discuss how individual drugs of abuse alter stress responses in animal models.
Stimulants
Effect on HPA axis
Acute stimulant administration activates the HPA axis, leading to increases in corticosterone (CORT) and ACTH [116, 117]. Furthermore, chronic stimulant exposure can lead to increased basal HPA activity as seen with the increased basal CORT and ACTH levels following binge cocaine administration [117, 118]. Stimulant administration also leads to an increase in the ability of stress to elicit HPA activation. For example, repeated experimenter-administered amphetamine administration led to augmented CORT and ACTH release following a restraint stress [119]. Similar increases in restraint-induced CORT are seen following cocaine self-administration [120]. Binge cocaine exposure during adolescence leads to exaggerated CORT responses to the elevated plus maze and forced swim stress [121, 122]. Although stimulants do not cause a typical withdrawal syndrome, there are changes in the HPA axis that occur during stimulant withdrawal. For example, animals exhibit decreased ACTH and CORT responses to amphetamine challenge when withdrawn 28 days from chronic amphetamine [123]. However, there appears to be an enhanced ability for stress to activate the system during withdrawal, as evidenced by increases in restraint-induced CORT following acute withdrawal from chronic cocaine administration [124].
Behavioral alterations
These alterations in HPA response to stress seen following stimulant administration are accompanied by alterations in behavioral stress reactivity. For example, acute cocaine administration leads to an increase in acoustic startle response in rhesus monkeys [125]. It is difficult to examine stress responsivity following stimulant administration because many behavioral paradigms are confounded by stimulant-induced increases in locomotor activity [126]. However, there is evidence from a runway model that acute cocaine administration induces anxiety-like behavior [127]. The majority of work examining anxiety-like behavior and stress responses following stimulant exposure has focused on the withdrawal period. Withdrawal from chronic cocaine leads to enhanced responsivity to forced swim stress and increased anxiety-like behavior within the elevated plus maze and defensive burying test [128–130]. This enhanced responsivity to stress may last well beyond the initial withdrawal period, as rats exhibit an increase in immobility in the forced swim test after 12 days of withdrawal from repeated cocaine administration [131].
Neurobiological underpinnings
The increases in anxiety seen during stimulant administration and withdrawal from stimulants are likely influenced by alterations in CRF signaling. Acute cocaine stimulates hypothalamic CRF release [132]. Furthermore, the ability of acute cocaine to increase anxiety-like behavior is attenuated by administration of CRF antagonists in the ventral tegmental area [133]. However, this increase in release undergoes habituation with chronic cocaine administration. During a prolonged cocaine self-administration session, CRF release decreases by ~25% in central amygdala [70]. However, this decrease is reversed during withdrawal, when there is an increase in both CRF release and immunoreactivity [70, 134]. Furthermore, levels of CRFR1 are increased in the anterior pituitary following two weeks of withdrawal from binge cocaine administration [117].
Additionally, there is evidence that this augmentation of CRF signaling following chronic stimulant use has functional consequences. For example, chronic cocaine administration leads to an increase in the ability of CRF to potentiate glutamate release and excitatory transmission within ventral tegmental area dopamine neurons [135, 136], thus demonstrating a mechanism by which stress may promote drug seeking. Additionally, chronic cocaine leads to a CRF-mediated increase in synaptic strength in the amygdala [137, 138], providing a mechanism by which cocaine could alter anxiety and stress reactivity. In fact, mice with a CRF receptor type 2 (CRFR2) deficiency do not exhibit stress-induced memory deficits seen during cocaine withdrawal, suggesting that downregulating the CRF system could decrease cocaine-induced increases in stress responsivity [139].
Opiates
Effect on HPA axis
Heroin and other opiate agonists have a complex interaction with the HPA axis, as acute exposure in naïve animals results in elevated levels of CORT and ACTH, while animals that have received chronic administration and withdrawal show decreased CORT and ACTH in response to a heroin challenge [140, 141]. This may be due, in part, to the increases in basal ACTH and CORT seen following chronic opiate administration and acute withdrawal [140, 142]. These increases in CORT have been seen as early as two hours after cessation of chronic opiate administration, but seem to disappear after long periods of abstinence [143]. Along with these alterations in basal and drug-induced HPA responses, opiates also affect the HPA response to stress. Chronic morphine increases the ACTH and CORT response to a mild novelty stress in juveniles [144]. During acute withdrawal (12hr), animals exhibit a heightened CORT and ACTH response to restraint stress [142].
Behavioral alterations
These alterations in the physiological responses to stress are paralleled by behavioral alterations following opiate administration. Despite the ability of acute opiates to activate the HPA axis, acute opiate administration can lead to decreased anxiety-like behavior in the elevated plus maze following restraint stress as well as decreased fear potentiated startle responses [145–147]. In contrast, chronic morphine leads to increased response to forced swim stress and tail suspension stress during both short (24hr) and long (1 week) withdrawal periods [143, 148]. These increases in stress responsivity seem to persist through even more prolonged periods of withdrawal as animals show augmented responses to tail suspension and forced swim stress after 4 weeks of withdrawal [143, 149].
Neurobiological underpinnings
These behavioral and physiological responses to opiates are accompanied by regionally specific alterations in brain stress systems. Within the hypothalamus, acute morphine triggers the release of CRF [141, 150, 151]. However, this release is attenuated in animals that have received chronic morphine [141]. Within the BNST, chronic morphine leads to a decrease in transport of CRF to the cell membrane, suggesting decreased CRF release [152]. However, this potential decrease in synaptic CRF may be countered by an engagement of more individual neurons, as chronic morphine increases activation of CRF+ cells with the BNST [153]. Within the central nucleus of the amygdala, chronic morphine upregulates CRF mRNA and increases the ability of morphine to activate CRF+ cells [153, 154]. Within the dorsal raphe the morphine-induced alterations in the CRF system occur at the receptor level. After chronic morphine exposure there is a decrease in CRFR1 mRNA within the dorsal raphe during both acute (3hr) and prolonged (7d) withdrawal [155]. In contrast, increases in CRFR2 mRNA were seen in the raphe during prolonged withdrawal [155]. Taken together, it is clear that opiates alter the CRF system in a profound way but these regional alterations may mediate different aspects of stress responsivity and withdrawal.
Alcohol
Effect on HPA axis
Acute alcohol administration has been clearly shown to activate the HPA axis in both humans and laboratory animals [65, 156]. However, work in laboratory animals has allowed us to determine that this increase in ACTH secretion is mediated through activation of CRF, as inhibiting CRF blocks this effect [65]. Furthermore, chronic alcohol administration has been shown to lead to alterations in the HPA axis. For example, binge-like alcohol administration leads to an increase in basal plasma CORT levels [157]. However, alcohol dependence seen after self-administration leads to a relative dampening in alcohol-evoked CORT and ACTH, suggesting a habituation in these animals [158].
Behavioral alterations
Despite this attenuated HPA response to alcohol following chronic alcohol use, behavioral and physiological responses to stress are sensitized. For example, chronic alcohol exposure leads to a sensitization of the stress response to a forced swim stress [159, 160]. Furthermore, there is a correlation between animals that exhibit higher behavioral reactivity to stress and those that self-administer more alcohol [161]. However, alcohol administration may dampen the response to stress in alcohol dependent animals, as seen in fear potentiated startle paradigms [162]. The anxiogenic effects of alcohol withdrawal may in part, mediate this effect. During withdrawal from ethanol, animals exhibit an increased responsiveness to external stimuli, as shown by an increased acoustic startle response [163]. Animals exhibit other signs of anxiety-like behavior during withdrawal as well, such as decreased open arm exploration in the elevated plus maze and decreased social interaction [164–166].
Neurobiological underpinnings
These increases in anxiety-like behavior may be due to alterations in the CRF system. Alcohol withdrawal leads to an increase in extracellular CRF release within the central amygdala [167–169] and the BNST [170]. This increase in CRF activity within the BNST is normalized by alcohol administration [170], paralleling the behavior on fear potentiated startle [162]. Along with these baseline alterations in signaling seen following alcohol administration, there is also an effect of chronic alcohol on neuronal responses to stress. When exposed to a forced swim stress, animals with a history of chronic alcohol intake exhibit increased neuronal activation, as indicated by increased c-Fos immunoreactivity, within the central nucleus of the amygdala [159]. Double-labeling revealed that this increase in stress-induced activation following chronic alcohol is mediated by CRF-positive cells [159], suggesting that stress engages the CRF system more following chronic alcohol. This is consistent with data demonstrating an increase in CRFR1 mRNA following chronic alcohol use and may be due, in part, to a decrease in basal CRF release [158, 171]. Taken together this would suggest an increased signal to noise in the CRF system that could be the cause of the increased stress reactivity seen in alcoholics. In support of this, neuropeptide Y (NPY) activity is decreased following binge alcohol drinking and NPY activation within the extended amygdala leads to an inhibition of CRF neurons [172].
Nicotine
Effect on HPA axis
Acute nicotine leads to an activation of the HPA axis, as evidenced by a dose-dependent increase in ACTH and CORT [173–175]. Furthermore, nicotine can enhance the ability of stress to activate the HPA axis, leading to a further increase in stress-induced CORT and epinephrine [176]. Chronic nicotine also has the ability to augment stress-induced HPA activation. Following nicotine self-administration, rats exhibit a greater increase in ACTH and CORT following a mild stressor [177, 178]. Additionally, nicotine self-administration augments stress-induced activation of the paraventricular nucleus, a central player in the HPA axis [179].
Behavioral alterations
These nicotine-induced changes in HPA activity are accompanied by changes in behavior. Acute nicotine leads to enhanced anxiety-like behavior in the elevated zero maze, particularly in adult females and adolescent males [73]. However, there is also evidence that nicotine can decrease stress responsivity in the forced swim test and marble-burying test [180, 181]. Similarly, chronic nicotine can decrease the behavioral response to a forced swim stress as well as stress response to novelty [180, 182, 183]. In contrast, withdrawal from nicotine can also lead to increased anxiety-like behavior as well as enhanced stress responsivity. For example, after 1 day of withdrawal from chronic nicotine, mice exhibit higher levels of anxiety-like behavior in the novelty-induced hypophasia test [184]. Similar effects are also seen in the elevated plus maze and forced swim test, with animals exhibiting augmented responses to these stressors [185–188]. These withdrawal induced increases in anxiety-like behavior and stress responsivity are persistent; animals withdrawn from nicotine exhibit an augmented response to swim stress after 30 days of withdrawal [189].
Neurobiological underpinnings
As seen with other drugs of abuse, the behavioral effects of nicotine on stress may be mediated by changes within the brain CRF system. Chronic nicotine self-administration leads to a basal decrease in the number of CRF positive cells as well as a decrease in CRF mRNA within the paraventricular nucleus [179]. However, withdrawal from nicotine leads to an increase in CRF immunoreactivity and CRF mRNA within the amygdala [72, 74]. This increase in CRF mRNA is also seen within the nucleus accumbens during nicotine withdrawal [73]. Furthermore, CRFR1 antagonists blunt nicotine withdrawal-induced anxiety-like behavior and dysphoria [74, 190]. This suggests that while chronic nicotine may lead to a decrease in HPA activity, brain CRF systems remain sensitized. This sensitization of the CRF system is likely responsible for the changes in stress reactivity and normalizing these differences could provide therapeutic relief [191].
Marijuana
Effect on HPA axis
Systemic injections of delta(9)-tetrahydrocannabinol (THC, the main psychoactive component of marijuana) do not alter HPA activity on its own and subchronic THC seems to decrease HPA activity [192]. This may be due, in part, to peripheral effects, as centrally administered THC (intracerebroventricular injections) leads to a marked increase in plasma ACTH and CORT at multiple doses [193]. Along with these changes in basal HPA activity, it seems clear that higher doses of THC can potentiate the ability of stressors to activate the HPA axis. For example, THC and/or other CB1 agonists can potentiate CORT release in response to forced swim stress [194], restraint stress [195] and footshock stress [196]. Chronic THC has also been shown to potentiate the CORT response to restraint stress [197].
Behavioral alterations
Similar to the dose dependent effects of cannabinoids on HPA activity, the behavioral effects of THC can be both anxiolytic and anxiogenic. There is evidence that low doses of THC and other CB1 receptor agonists can reduce anxiety-like behavior and stress responsivity [198–200], while higher doses of the drug have the opposite effect [201–204]. High doses of a CB1 agonist lead to increased anxiety-like behavior in the elevated plus maze [205]. Additionally, acute administration of a highly potent cannabinoid agonist led to an increased stress response in a defensive burying task [64]. Similarly, systemic injections or direct infusion of THC into the amygdala increases anxiety in the elevated plus maze [203, 206]. Further, acute THC potentiates the behavioral response to forced swim stress and systemic administration of a CB1 agonist also increases the ability of chronic stress exposure to potentiate anxiety-like behavioral responses [194, 202]. Although there is much less work examining the effects of chronic THC exposure, there is evidence that it too can lead to increased anxiety. For example, chronic cannabinoid administration leads to greater anxiety-like behavior in the open field test and the light-dark test [197, 207].
Neurobiological underpinnings
Just as with other drugs of abuse, both acute and chronic THC leads to alterations in brain CRF systems that may help explain the behavioral effects. Acute administration of the endogenous cannabinoid agonist anandamide leads to increased hypothalamic CRF release [208]. Chronic THC treatment leads to a decrease in CRF mRNA in the central amygdala [205], while increasing CRF mRNA in the hypothalamus [209]. However, during withdrawal from chronic cannabinoid administration, an increase in CRF mRNA and CRF release is seen within the amygdala [71, 205]. These withdrawal-induced changes in the CRF system may explain the increased reactivity to stressors seen after chronic THC use. In support of this, increases in behavioral response to stress seen following cannabinoid agonist administration are blocked by a CRF receptor antagonist [64].
Development of Novel Drug Therapies
The large body of literature suggesting the involvement of the CRF system in addiction has led researchers to examine the possibility of targeting this dysregulated stress system in the development of therapeutics. In support of this, small molecule CRFR1 antagonists can reduce alcohol withdrawal induced increases in anxiety without altering anxiety in non-dependent controls [169, 210–213]. Similarly, anxiogenic withdrawal responses from cocaine, nicotine, cannabinoids, and opiates have been reversed by CRFR1 antagonists [71, 74, 214–218]. This has led to the examination of small molecular CRF antagonists for the treatment of addiction and alcoholism. However, to date, the studies examining these compounds have not yielded promising results. A recent examination of pexacerfont, an orally available and brain-penetrant CRFR1 antagonist, for the treatment of alcoholics found no evidence for therapeutic efficacy. The treatment group failed to exhibit a decrease in craving or subjective distress responses [219]. However, CRFR1 antagonists with different receptor kinetics have yielded promising results for the treatment of depression [220], so it is possible that more drug development is needed to find a compound that will be effective. However, these compounds have been developed to affect brain CRF activity without affecting the HPA axis to aid in their therapeutic safety. Therefore, the sympathetic response to stress remains increased in these alcoholics. It is possible that for a therapy to be effective it must dampen both brain and sympathetic responses to stress.
Interestingly, recent studies suggest that manipulating the HPA axis could be a more effective treatment option. Within a small sample of addicts, Walter et al. [221] found that cortisol administration led to a decrease in craving within low dose heroin addicts while not affecting heavier users. Additionally, clonidine, which decreases noradrenergic activity through presynaptic activation of alpha-2 receptors [222], is currently used in the treatment of opiate withdrawal [223], further supporting an idea that peripheral stress responsivity may be critically involved in addictive behaviors. The use of another antihypertensive drug, prazosin, which decreases noradrenergic activity via inhibition of postsynaptic alpha-1 receptors, may be effective in reducing stress reactivity in alcoholics. Whereas four weeks of placebo treatment caused an increase in stress and cue-elicited alcohol craving and anxiety, this was not seen in individuals treated with prazosin [224]. Further, the blunted cortisol response to stress seen in the placebo group was not seen in the prazosin group suggesting this drug was able to normalize HPA axis activity. As such, a clinical trial is currently underway to test the efficacy of prazosin in patients with comorbid PTSD and SUD (NCT007440055; clinical trials.gov).
It is also possible that the failure of CRFR1 antagonists in clinical trials is because other stress-related neurotransmitters play a more critical role in addictive phenotypes. One such neurotransmitter is substance P, which binds preferentially to the neurokinin 1 receptor (NK1R). A small trial in recently detoxified alcoholics demonstrated that an NK1R antagonist blunted spontaneous craving as well as stress and cue-elicited craving [225]. These decreases in craving were accompanied by a decrease in cortisol, further supporting the idea that therapeutics that also act on the peripheral HPA axis may be more effective.
Conclusion
These studies clearly demonstrate that drugs of abuse and alcohol alter the stress response, behaviorally, neurochemically, and physiologically. These changes may be linked to the CRF system, however clinical trials of CRF antagonists have not been effective. Although preclinical data suggest that dampening the CRF system should decrease craving and relapse, it is clear that there are differences in basal tone that have to be considered as well as stimulus-evoked responses of the system. Furthermore, there seem to be differences between how drugs of abuse affect the central and peripheral CRF systems and any current therapeutics are hitting both of these systems equally. Perhaps most importantly, it is clear that although there are similarities in how different drugs of abuse affect the CRF system, the alterations are not identical. The majority of drug addicts use more than one drug, including alcohol and nicotine and therefore more work into how different drug combinations might affect the system are needed. More research into the interplay between these systems and the development of more targeted therapies is necessary to best treat the alterations in the stress system that occur following drug addiction.
Highlights.
Drugs of abuse and alcohol alter behavioral & physiological responses to stress
Drug use leads to altered CRF response to stress
CRF antagonists have not been effective in treating SUD
Therapies targeting both central and peripheral stress responses may be more effective
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R00DA033372 to LAB). We thank Alexandra Ellis, Rob Juza, and Jeffrey Lenz for editorial assistance.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ouimette P, Coolhart D, Funderburk JS, Wade M, Brown PJ. Precipitants of first substance use in recently abstinent substance use disorder patients with PTSD. Addict Behav. 2007;32:1719–27. doi: 10.1016/j.addbeh.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 2.McFall ME, Mackay PW, Donovan DM. Combat-related posttraumatic stress disorder and severity of substance abuse in Vietnam veterans. J Stud Alcohol. 1992;53:357–63. doi: 10.15288/jsa.1992.53.357. [DOI] [PubMed] [Google Scholar]
- 3.Dragan M, Lis-Turlejska M. Lifetime exposure to potentially traumatic events in a sample of alcohol-dependent patients in Poland. Journal of traumatic stress. 2007;20:1041–51. doi: 10.1002/jts.20259. [DOI] [PubMed] [Google Scholar]
- 4.Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–45. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and alcohol review. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 6.Dewart T, Frank B, Schmeidler J. The impact of 9/11 on patients in New York City’s substance abuse treatment programs. The American journal of drug and alcohol abuse. 2006;32:665–72. doi: 10.1080/00952990600919435. [DOI] [PubMed] [Google Scholar]
- 7.Fox H, Sinha R. The role of guanfacine as a therapeutic agent to address stress-related pathophysiology in cocaine-dependent individuals. Advances in pharmacology. 2014;69:217–65. doi: 10.1016/B978-0-12-420118-7.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briand LA, Blendy JA. Not all stress is equal: CREB is not necessary for restraint stress reinstatement of cocaine-conditioned reward. Behav Brain Res. 2013;246:63–8. doi: 10.1016/j.bbr.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, Blendy JA. Stress-induced potentiation of cocaine reward: a role for CRF R1 and CREB. Neuropsychopharmacology. 2009;34:2609–17. doi: 10.1038/npp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128:408–12. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- 11.Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119:334–41. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- 12.Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 1996;128:256–64. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- 13.Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–12. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- 14.Yap JJ, Chartoff EH, Holly EN, Potter DN, Carlezon WA, Jr, Miczek KA. Social defeat stress-induced sensitization and escalated cocaine self-administration: the role of ERK signaling in the rat ventral tegmental area. Psychopharmacology (Berl) 2015;232:1555–69. doi: 10.1007/s00213-014-3796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holly EN, Shimamoto A, Debold JF, Miczek KA. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology (Berl) 2012;224:179–88. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J Neurosci. 2010;30:16149–59. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28:904–14. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 18.Administration., S. A. a. M. H. S; Studies OoA, editor. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, MD: 2010. [Google Scholar]
- 19.Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry. 1992;149:671–5. doi: 10.1176/ajp.149.5.671. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 21.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of anxiety disorders. 2011;25:456–65. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills KL, Teesson M, Ross J, Peters L. Trauma, PTSD, and substance use disorders: Findings from the Australian National Survey of Mental Health and Well-Being. The American journal of psychiatry. 2006:163. doi: 10.1176/ajp.2006.163.4.652. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds M, Hinchliffe K, Asamoah V, Kouimtsidis C. Trauma and post-traumatic stress disorder in a drug treatment community service. The Psychiatrist. 2011:35. [Google Scholar]
- 24.Drapkin ML, Yusko D, Yasinski C, Oslin D, Hembree EA, Foa EB. Baseline functioning among individuals with posttraumatic stress disorder and alcohol dependence. Journal of substance abuse treatment. 2011;41:186–92. doi: 10.1016/j.jsat.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read JP, Brown PJ, Kahler CW. Substance use and posttraumatic stress disorders: symptom interplay and effects on outcome. Addict Behav. 2004;29:1665–72. doi: 10.1016/j.addbeh.2004.02.061. [DOI] [PubMed] [Google Scholar]
- 26.Schafer I, Najavits LM. Clinical challenges in the treatment of patients with posttraumatic stress disorder and substance abuse. Current opinion in psychiatry. 2007;20:614–8. doi: 10.1097/YCO.0b013e3282f0ffd9. [DOI] [PubMed] [Google Scholar]
- 27.Najavits LM, Gastfriend DR, Barber JP, Reif S, Muenz LR, Blaine J, et al. Cocaine dependence with and without PTSD among subjects in the National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Am J Psychiatry. 1998;155:214–9. doi: 10.1176/ajp.155.2.214. [DOI] [PubMed] [Google Scholar]
- 28.Ouimette P, Goodwin E, Brown PJ. Health and well being of substance use disorder patients with and without posttraumatic stress disorder. Addict Behav. 2006;31:1415–23. doi: 10.1016/j.addbeh.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Klaw EL, Demers AL, Da Silva N. Predicting Risk Factors for Intimate Partner Violence Among Post-9/11 College Student Veterans. Journal of interpersonal violence. 2014 doi: 10.1177/0886260514556102. [DOI] [PubMed] [Google Scholar]
- 30.Barrett EL, Teesson M, Mills KL. Associations between substance use, post-traumatic stress disorder and the perpetration of violence: A longitudinal investigation. Addict Behav. 2014;39:1075–80. doi: 10.1016/j.addbeh.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Howard DE, Qi Wang M. Risk procedures of adolescent girls who were victims of dating violence. Adolescence. 2003:38. [PubMed] [Google Scholar]
- 32.Kingston S, Raghavan C. The relationship of sexual abuse, early initiation of substance use, and adolescent trauma to PTSD. Journal of traumatic stress. 2009;22:65–8. doi: 10.1002/jts.20381. [DOI] [PubMed] [Google Scholar]
- 33.Kilpatrick DG, Acierno R, Resnick HS, Saunders BE, Best CL. A 2-year longitudinal analysis of the relationships between violent assault and substance use in women. Journal of consulting and clinical psychology. 1997;65:834–47. doi: 10.1037//0022-006x.65.5.834. [DOI] [PubMed] [Google Scholar]
- 34.Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Archives of general psychiatry. 2005;62:1258–65. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- 35.Stewart SH, Conrod PJ. Psychosocial models of functional associations between posttraumatic stress disorder and substance use disorder. In: Brown POPJ, editor. Trauma and substance abuse: Causes, consequences, and treatment of comorbid disorders. Washington, DC, US: American Psychological Association; 2003. pp. 29–55. [Google Scholar]
- 36.Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ. Substance use, childhood traumatic experience, and posttraumatic stress disorder in an urban civilian population. Depress Anxiety. 2010:27. doi: 10.1002/da.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beckham JC, Lytle BL, Vrana SR, Hertzberg MA, Feldman ME, Shipley RH. Smoking withdrawal symptoms in response to a trauma-related stressor among Vietnam combat veterans with posttraumatic stress disorder. Addict Behav. 1996;21:93–101. doi: 10.1016/0306-4603(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 38.Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, et al. Impact of Posttraumatic Stress Disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine Tob Res. 2008;10:1415–27. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]
- 39.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 40.Coffey SF, Schumacher JA, Stasiewicz PR, Henslee AM, Baillie LE, Landy N. Craving and physiological reactivity to trauma and alcohol cues in posttraumatic stress disorder and alcohol dependence. Experimental and clinical psychopharmacology. 2010;18:340–9. doi: 10.1037/a0019790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown PJ, Stout RL, Gannon-Rowley J. Substance use disorder-PTSD comobidity: Patients’ perceptions of symptom interplay and treatment issues. Sep–Oct 1998. Journal of substance abuse treatment. 1998:15. doi: 10.1016/s0740-5472(97)00286-9. [DOI] [PubMed] [Google Scholar]
- 42.Grant BF. Comorbidity between DSM-IV drug use disorders and major depression: results of a national survey of adults. Journal of substance abuse. 1995;7:481–97. doi: 10.1016/0899-3289(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 43.McEvoy PM, Shand F. The effect of comorbid substance use disorders on treatment outcome for anxiety disorders. Journal of anxiety disorders. 2008;22:1087–98. doi: 10.1016/j.janxdis.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Alegria AA, Hasin DS, Nunes EV, Liu SM, Davies C, Grant BF, et al. Comorbidity of generalized anxiety disorder and substance use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2010;71:1187–95. doi: 10.4088/JCP.09m05328gry. quiz 252–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–8. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 46.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry. 1989;25:913–28. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- 48.Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–4. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- 49.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–4. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 50.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual review of pharmacology and toxicology. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 51.Mitsukawa K, Mombereau C, Lotscher E, Uzunov DP, van der Putten H, Flor PJ, et al. Metabotropic glutamate receptor subtype 7 ablation causes dysregulation of the HPA axis and increases hippocampal BDNF protein levels: implications for stress-related psychiatric disorders. Neuropsychopharmacology. 2006;31:1112–22. doi: 10.1038/sj.npp.1300926. [DOI] [PubMed] [Google Scholar]
- 52.Berridge CW, Dunn AJ. Corticotropin-releasing factor elicits naloxone sensitive stress-like alterations in exploratory behavior in mice. Regulatory peptides. 1986;16:83–93. doi: 10.1016/0167-0115(86)90196-5. [DOI] [PubMed] [Google Scholar]
- 53.Dunn AJ, Berridge CW. Corticotropin-releasing factor administration elicits a stress-like activation of cerebral catecholaminergic systems. Pharmacol Biochem Behav. 1987;27:685–91. doi: 10.1016/0091-3057(87)90195-x. [DOI] [PubMed] [Google Scholar]
- 54.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 55.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 56.Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–7. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J Neurochem. 2001;76:509–19. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- 58.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 59.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 60.Gardi J, Biro E, Sarnyai Z, Vecsernyes M, Julesz J, Telegdy G. Time-dependent alterations in corticotropin-releasing factor-like immunoreactivity in different brain regions after acute cocaine administration to rats. Neuropeptides. 1997;31:15–8. doi: 10.1016/s0143-4179(97)90013-5. [DOI] [PubMed] [Google Scholar]
- 61.Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Alterations of corticotropin-releasing factor-like immunoreactivity in different brain regions after acute cocaine administration in rats. Brain Res. 1993;616:315–9. doi: 10.1016/0006-8993(93)90224-b. [DOI] [PubMed] [Google Scholar]
- 62.Maj M, Turchan J, Smialowska M, Przewlocka B. Morphine and cocaine influence on CRF biosynthesis in the rat central nucleus of amygdala. Neuropeptides. 2003;37:105–10. doi: 10.1016/s0143-4179(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 63.Puder M, Weidenfeld J, Chowers I, Nir I, Conforti N, Siegel RA. Corticotrophin and corticosterone secretion following delta 1-Tetrahydrocannabinol, in intact and in hypothalamic deafferentated male rats. Experimental brain research. Experimentelle Hirnforschung. 1982;46:85–8. doi: 10.1007/BF00238101. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, et al. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- 65.Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–31. [PubMed] [Google Scholar]
- 66.Richter RM, Pich EM, Koob GF, Weiss F. Sensitization of cocaine-stimulated increase in extracellular levels of corticotropin-releasing factor from the rat amygdala after repeated administration as determined by intracranial microdialysis. Neurosci Lett. 1995;187:169–72. doi: 10.1016/0304-3940(95)11365-4. [DOI] [PubMed] [Google Scholar]
- 67.el Daly ES. Influence of acute and chronic morphine or stadol on the secretion of adrenocorticotrophin and its hypothalamic releasing hormone in the rat. Life Sci. 1996;59:1881–90. doi: 10.1016/s0024-3205(96)00535-8. [DOI] [PubMed] [Google Scholar]
- 68.Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, et al. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–13. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–47. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–61. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–4. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 72.Aydin C, Oztan O, Isgor C. Vulnerability to nicotine abstinence-related social anxiety-like behavior: molecular correlates in neuropeptide Y, Y2 receptor and corticotropin releasing factor. Neurosci Lett. 2011;490:220–5. doi: 10.1016/j.neulet.2010.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE. Behavioral, Biochemical, and Molecular Indices of Stress are Enhanced in Female Versus Male Rats Experiencing Nicotine Withdrawal. Frontiers in psychiatry. 2013;4:38. doi: 10.3389/fpsyt.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cole BJ, Koob GF. Propranolol antagonizes the enhanced conditioned fear produced by corticotropin releasing factor. J Pharmacol Exp Ther. 1988;247:902–10. [PubMed] [Google Scholar]
- 76.Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berl) 1986;88:147–52. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- 77.Nobis WP, Kash TL, Silberman Y, Winder DG. beta-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biol Psychiatry. 2011;69:1083–90. doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–54. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polter AM, Bishop RA, Briand LA, Graziane NM, Pierce RC, Kauer JA. Poststress block of kappa opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking. Biol Psychiatry. 2014;76:785–93. doi: 10.1016/j.biopsych.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–76. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–73. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinha R. The role of stress in addiction relapse. Current psychiatry reports. 2007;9:388–95. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- 86.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Archives of general psychiatry. 1986;43:107–13. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 87.Gerra G, Leonardi C, Cortese E, Zaimovic A, Dell’Agnello G, Manfredini M, et al. Adrenocorticotropic hormone and cortisol plasma levels directly correlate with childhood neglect and depression measures in addicted patients. Addict Biol. 2008;13:95–104. doi: 10.1111/j.1369-1600.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 88.Parrott AC, Sands HR, Jones L, Clow A, Evans P, Downey LA, et al. Increased cortisol levels in hair of recent Ecstasy/MDMA users. Eur Neuropsychopharmacol. 2014;24:369–74. doi: 10.1016/j.euroneuro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Xie P, Wang TJ, Yin G, Yan Y, Xiao LH, Li Q, et al. Metabonomic Study of Biochemical Changes in Human Hair of Heroin Abusers by Liquid Chromatography Coupled with Ion Trap-Time of Flight Mass Spectrometry. Journal of molecular neuroscience : MN. 2015 doi: 10.1007/s12031-015-0655-x. [DOI] [PubMed] [Google Scholar]
- 90.Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rossler A, et al. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol Psychiatry. 2014;76:289–96. doi: 10.1016/j.biopsych.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 91.McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, et al. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Archives of general psychiatry. 1994;51:713–9. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- 92.Elman I, Lukas SE, Karlsgodt KH, Gasic GP, Breiter HC. Acute cortisol administration triggers craving in individuals with cocaine dependence. Psychopharmacology bulletin. 2003;37:84–9. [PubMed] [Google Scholar]
- 93.Macedo T, Ribeiro CA, Cotrim D, Tavares P, Morgadinho MT, Caramona M, et al. Catecholamine and MHPG plasma levels, platelet MAO activity, and 3H-imipramine binding in heroin and cocaine addicts. Molecular neurobiology. 1995;11:21–9. doi: 10.1007/BF02740681. [DOI] [PubMed] [Google Scholar]
- 94.Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, et al. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153:83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- 95.Aghajanian GK. Feedback regulation of central monoaminergic neurons: evidence from single cell recording studies. Essays Neurochem Neuropharmacol. 1978;3:1–32. [PubMed] [Google Scholar]
- 96.Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, et al. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;36:1178–86. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–51. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- 99.Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–91. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 100.al’Absi M, Nakajima M, Grabowski J. Stress response dysregulation and stress-induced analgesia in nicotine dependent men and women. Biological psychology. 2013;93:1–8. doi: 10.1016/j.biopsycho.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ginty AT, Jones A, Carroll D, Roseboom TJ, Phillips AC, Painter R, et al. Neuroendocrine and cardiovascular reactions to acute psychological stress are attenuated in smokers. Psychoneuroendocrinology. 2014;48:87–97. doi: 10.1016/j.psyneuen.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 102.McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AF, Picciotto MR, et al. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J Psychopharmacol. 2015;29:300–11. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller NS, Dackis CA, Gold MS. The relationship of addiction, tolerance, and dependence to alcohol and drugs: a neurochemical approach. Journal of substance abuse treatment. 1987;4:197–207. doi: 10.1016/s0740-5472(87)80014-4. [DOI] [PubMed] [Google Scholar]
- 104.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 105.Kreek MJ. Tolerance and dependence: implications for the pharmacological treatment of addiction. NIDA research monograph. 1987;76:53–62. [PubMed] [Google Scholar]
- 106.Kreek MJ, Ragunath J, Plevy S, Hamer D, Schneider B, Hartman N. ACTH, cortisol and beta-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides. 1984;5:277–8. doi: 10.1016/0143-4179(84)90081-7. [DOI] [PubMed] [Google Scholar]
- 107.Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, et al. Nalmefene causes greater hypothalamic-pituitary-adrenal axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcoholism, clinical and experimental research. 1998;22:1430–6. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- 108.Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of general psychiatry. 2011;68:942–52. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stine SM, Grillon CG, Morgan CA, 3rd, Kosten TR, Charney DS, Krystal JH. Methadone patients exhibit increased startle and cortisol response after intravenous yohimbine. Psychopharmacology (Berl) 2001;154:274–81. doi: 10.1007/s002130000644. [DOI] [PubMed] [Google Scholar]
- 110.Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, 3rd, et al. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP) Psychopharmacology (Berl) 1997;131:207–15. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- 111.Corcoran S, Norrholm SD, Cuthbert B, Sternberg M, Hollis J, Duncan E. Acoustic startle reduction in cocaine dependence persists for 1 year of abstinence. Psychopharmacology (Berl) 2011;215:93–103. doi: 10.1007/s00213-010-2114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kemper A, Koalick F, Thiele H, Retzow A, Rathsack R, Nickel B. Cortisol and beta-endorphin response in alcoholics and alcohol abusers following a high naloxone dosage. Drug Alcohol Depend. 1990;25:319–26. doi: 10.1016/0376-8716(90)90158-b. [DOI] [PubMed] [Google Scholar]
- 113.Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcoholism, clinical and experimental research. 2000;24:651–8. [PubMed] [Google Scholar]
- 114.Fox HC, Tuit KL, Sinha R. Stress system changes associated with marijuana dependence may increase craving for alcohol and cocaine. Human psychopharmacology. 2013;28:40–53. doi: 10.1002/hup.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McClintick JN, Xuei X, Tischfield JA, Goate A, Foroud T, Wetherill L, et al. Stress-response pathways are altered in the hippocampus of chronic alcoholics. Alcohol (Fayetteville, NY) 2013;47:505–15. doi: 10.1016/j.alcohol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28:836–62. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 117.Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res. 2003;964:187–99. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]
- 118.Sarnyai Z, Dhabhar FS, McEwen BS, Kreek MJ. Neuroendocrine-related effects of long-term, ‘binge’ cocaine administration: diminished individual differences in stress-induced corticosterone response. Neuroendocrinology. 1998;68:334–44. doi: 10.1159/000054382. [DOI] [PubMed] [Google Scholar]
- 119.Barr AM, Hofmann CE, Weinberg J, Phillips AG. Exposure to repeated, intermittent d-amphetamine induces sensitization of HPA axis to a subsequent stressor. Neuropsychopharmacology. 2002;26:286–94. doi: 10.1016/S0893-133X(01)00308-6. [DOI] [PubMed] [Google Scholar]
- 120.Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, et al. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–11. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alves CJ, Magalhaes A, Melo P, de Sousa L, Tavares MA, Monteiro PR, et al. Long-term effects of chronic cocaine exposure throughout adolescence on anxiety and stress responsivity in a Wistar rat model. Neuroscience. 2014;277:343–55. doi: 10.1016/j.neuroscience.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 122.Alves CJ, Magalhaes A, Summavielle T, Melo P, De Sousa L, Tavares MA, et al. Hormonal, neurochemical, and behavioral response to a forced swim test in adolescent rats throughout cocaine withdrawal. Ann N Y Acad Sci. 2008;1139:366–73. doi: 10.1196/annals.1432.047. [DOI] [PubMed] [Google Scholar]
- 123.Russig H, Pryce CR, Feldon J. Amphetamine withdrawal leads to behavioral sensitization and reduced HPA axis response following amphetamine challenge. Brain Res. 2006;1084:185–95. doi: 10.1016/j.brainres.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 124.Mantsch JR, Taves S, Khan T, Katz ES, Sajan T, Tang LC, et al. Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neurosci Lett. 2007;415:269–73. doi: 10.1016/j.neulet.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kirkland Henry P, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 2009;205:237–47. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morisot N, Millan MJ, Contarino A. CRF1 receptor-deficiency induces anxiety-like vulnerability to cocaine. Psychopharmacology (Berl) 2014;231:3965–72. doi: 10.1007/s00213-014-3534-1. [DOI] [PubMed] [Google Scholar]
- 127.Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology (Berl) 1991;103:455–61. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- 128.Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–64. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Valzachi MC, Teodorov E, Marcourakis T, Bailey A, Camarini R. Enhancement of behavioral sensitization, anxiety-like behavior, and hippocampal and frontal cortical CREB levels following cocaine abstinence in mice exposed to cocaine during adolescence. PLoS One. 2013;8:e78317. doi: 10.1371/journal.pone.0078317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.El Hage C, Rappeneau V, Etievant A, Morel AL, Scarna H, Zimmer L, et al. Enhanced anxiety observed in cocaine withdrawn rats is associated with altered reactivity of the dorsomedial prefrontal cortex. PLoS One. 2012;7:e43535. doi: 10.1371/journal.pone.0043535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zilkha N, Feigin E, Barnea-Ygael N, Zangen A. Induction of depressive-like effects by subchronic exposure to cocaine or heroin in laboratory rats. J Neurochem. 2014;130:575–82. doi: 10.1111/jnc.12753. [DOI] [PubMed] [Google Scholar]
- 132.Calogero AE, Gallucci WT, Kling MA, Chrousos GP, Gold PW. Cocaine stimulates rat hypothalamic corticotropin-releasing hormone secretion in vitro. Brain Res. 1989;505:7–11. doi: 10.1016/0006-8993(89)90109-1. [DOI] [PubMed] [Google Scholar]
- 133.Ettenberg A, Cotten SW, Brito MA, Klein AK, Ohana TA, Margolin B, et al. CRF antagonism within the ventral tegmental area but not the extended amygdala attenuates the anxiogenic effects of cocaine in rats. Pharmacol Biochem Behav. 2015;138:148–55. doi: 10.1016/j.pbb.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–81. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- 135.Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009;29:6535–44. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fu Y, Pollandt S, Liu J, Krishnan B, Genzer K, Orozco-Cabal L, et al. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors. J Neurophysiol. 2007;97:937–41. doi: 10.1152/jn.00349.2006. [DOI] [PubMed] [Google Scholar]
- 138.Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, et al. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–43. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- 139.Morisot N, Le Moine C, Millan MJ, Contarino A. CRF(2) receptor-deficiency reduces recognition memory deficits and vulnerability to stress induced by cocaine withdrawal. Int J Neuropsychopharmacol. 2014;17:1969–79. doi: 10.1017/S1461145714000625. [DOI] [PubMed] [Google Scholar]
- 140.Zhou Y, Leri F, Ho A, Kreek MJ. Suppression of hypothalamic-pituitary-adrenal axis by acute heroin challenge in rats during acute and chronic withdrawal from chronic heroin administration. Neurochemical research. 2013;38:1850–60. doi: 10.1007/s11064-013-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Buckingham JC, Cooper TA. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology. 1984;38:411–7. doi: 10.1159/000123927. [DOI] [PubMed] [Google Scholar]
- 142.Houshyar H, Galigniana MD, Pratt WB, Woods JH. Differential responsivity of the hypothalamic-pituitary-adrenal axis to glucocorticoid negative-feedback and corticotropin releasing hormone in rats undergoing morphine withdrawal: possible mechanisms involved in facilitated and attenuated stress responses. Journal of neuroendocrinology. 2001;13:875–86. doi: 10.1046/j.1365-2826.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- 143.Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, et al. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–44. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nock B, Cicero TJ, Wich M. Chronic morphine increases the pituitary-adrenocortical response of juvenile rats to mild stress. Pharmacol Biochem Behav. 2005;80:77–85. doi: 10.1016/j.pbb.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 145.Anand R, Gulati K, Ray A. Pharmacological evidence for the role of nitric oxide in the modulation of stress-induced anxiety by morphine in rats. Eur J Pharmacol. 2012;676:71–4. doi: 10.1016/j.ejphar.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 146.Glover EM, Davis M. Anxiolytic-like effects of morphine and buprenorphine in the rat model of fear-potentiated startle: tolerance, cross-tolerance, and blockade by naloxone. Psychopharmacology (Berl) 2008;198:167–80. doi: 10.1007/s00213-008-1112-0. [DOI] [PubMed] [Google Scholar]
- 147.Joshi JC, Ray A, Gulati K. Effects of morphine on stress induced anxiety in rats: role of nitric oxide and Hsp70. Physiol Behav. 2015;139:393–6. doi: 10.1016/j.physbeh.2014.11.056. [DOI] [PubMed] [Google Scholar]
- 148.Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology (Berl) 2001;157:217–20. doi: 10.1007/s002130100793. [DOI] [PubMed] [Google Scholar]
- 149.Lutz PE, Reiss D, Ouagazzal AM, Kieffer BL. A history of chronic morphine exposure during adolescence increases despair-like behaviour and strain-dependently promotes sociability in abstinent adult mice. Behav Brain Res. 2013;243:44–52. doi: 10.1016/j.bbr.2012.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Buckingham JC, Cooper TA. Pharmacological characterization of opioid receptors influencing the secretion of corticotrophin releasing factor in the rat. Neuroendocrinology. 1986;44:36–40. doi: 10.1159/000124618. [DOI] [PubMed] [Google Scholar]
- 151.Buckingham JC, Cooper TA. Interrelationships of opioidergic and adrenergic mechanisms controlling the secretion of corticotrophin releasing factor in the rat. Neuroendocrinology. 1987;46:199–206. doi: 10.1159/000124820. [DOI] [PubMed] [Google Scholar]
- 152.Jaferi A, Lane DA, Pickel VM. Subcellular plasticity of the corticotropin-releasing factor receptor in dendrites of the mouse bed nucleus of the stria terminalis following chronic opiate exposure. Neuroscience. 2009;163:143–54. doi: 10.1016/j.neuroscience.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garcia-Carmona JA, Milanes MV, Laorden ML. Brain stress system response after morphine-conditioned place preference. Int J Neuropsychopharmacol. 2013;16:1999–2011. doi: 10.1017/S1461145713000588. [DOI] [PubMed] [Google Scholar]
- 154.McNally GP, Akil H. Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience. 2002;112:605–17. doi: 10.1016/s0306-4522(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 155.Lunden JW, Kirby LG. Opiate exposure and withdrawal dynamically regulate mRNA expression in the serotonergic dorsal raphe nucleus. Neuroscience. 2013;254:160–72. doi: 10.1016/j.neuroscience.2013.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rivier C, Vale W. Interaction between ethanol and stress on ACTH and beta-endorphin secretion. Alcoholism, clinical and experimental research. 1988;12:206–10. doi: 10.1111/j.1530-0277.1988.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 157.Cippitelli A, Damadzic R, Hamelink C, Brunnquell M, Thorsell A, Heilig M, et al. Binge-like ethanol consumption increases corticosterone levels and neurodegneration whereas occupancy of type II glucocorticoid receptors with mifepristone is neuroprotective. Addict Biol. 2014;19:27–36. doi: 10.1111/j.1369-1600.2012.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–53. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Retson TA, Hoek JB, Sterling RC, Van Bockstaele EJ. Amygdalar neuronal plasticity and the interactions of alcohol, sex, and stress. Brain structure & function. 2014 doi: 10.1007/s00429-014-0851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gomez JL, Luine VN. Female rats exposed to stress and alcohol show impaired memory and increased depressive-like behaviors. Physiol Behav. 2014;123:47–54. doi: 10.1016/j.physbeh.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bertholomey ML, West CH, Jensen ML, Li TK, Stewart RB, Weiss JM, et al. Genetic propensities to increase ethanol intake in response to stress: studies with selectively bred swim test susceptible (SUS), alcohol-preferring (P), and non-preferring (NP) lines of rats. Psychopharmacology (Berl) 2011;218:157–67. doi: 10.1007/s00213-011-2381-6. [DOI] [PubMed] [Google Scholar]
- 162.Barrenha GD, Coon LE, Chester JA. Effects of alcohol on the acquisition and expression of fear-potentiated startle in mouse lines selectively bred for high and low alcohol preference. Psychopharmacology (Berl) 2011;218:191–201. doi: 10.1007/s00213-011-2285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology (Berl) 1992;106:351–8. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- 164.File SE, Zharkovsky A, Hitchcott PK. Effects of nitrendipine, chlordiazepoxide, flumazenil and baclofen on the increased anxiety resulting from alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:87–93. doi: 10.1016/0278-5846(92)90011-3. [DOI] [PubMed] [Google Scholar]
- 165.File SE, Zharkovsky A, Gulati K. Effects of baclofen and nitrendipine on ethanol withdrawal responses in the rat. Neuropharmacology. 1991;30:183–90. doi: 10.1016/0028-3908(91)90202-m. [DOI] [PubMed] [Google Scholar]
- 166.File SE, Baldwin HA, Hitchcott PK. Flumazenil but not nitrendipine reverses the increased anxiety during ethanol withdrawal in the rat. Psychopharmacology (Berl) 1989;98:262–4. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- 167.Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–32. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–9. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–45. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 170.Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–20. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 171.Lee S, Rivier C. Alcohol increases the expression of type 1, but not type 2 alpha corticotropin-releasing factor (CRF) receptor messenger ribonucleic acid in the rat hypothalamus. Brain research. Molecular brain research. 1997;52:78–89. doi: 10.1016/s0169-328x(97)00226-x. [DOI] [PubMed] [Google Scholar]
- 172.Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18:545–52. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Skwara AJ, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Influence of environmental enrichment on hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine by osmotic mini-pumps, and nicotine withdrawal by mecamylamine in male and female rats. Behav Brain Res. 2012;234:1–10. doi: 10.1016/j.bbr.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Faraday MM, Blakeman KH, Grunberg NE. Strain and sex alter effects of stress and nicotine on feeding, body weight, and HPA axis hormones. Pharmacol Biochem Behav. 2005;80:577–89. doi: 10.1016/j.pbb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 175.Matta SG, Beyer HS, McAllen KM, Sharp BM. Nicotine elevates rat plasma ACTH by a central mechanism. J Pharmacol Exp Ther. 1987;243:217–26. [PubMed] [Google Scholar]
- 176.Morse DE. Neuroendocrine responses to nicotine and stress: enhancement of peripheral stress responses by the administration of nicotine. Psychopharmacology (Berl) 1989;98:539–43. doi: 10.1007/BF00441956. [DOI] [PubMed] [Google Scholar]
- 177.Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropsychopharmacology. 2008;33:721–30. doi: 10.1038/sj.npp.1301466. [DOI] [PubMed] [Google Scholar]
- 178.Yu G, Sharp BM. Nicotine self-administration diminishes stress-induced norepinephrine secretion but augments adrenergic-responsiveness in the hypothalamic paraventricular nucleus and enhances adrenocorticotropic hormone and corticosterone release. J Neurochem. 2010;112:1327–37. doi: 10.1111/j.1471-4159.2009.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yu G, Chen H, Zhao W, Matta SG, Sharp BM. Nicotine self-administration differentially regulates hypothalamic corticotropin-releasing factor and arginine vasopressin mRNAs and facilitates stress-induced neuronal activation. J Neurosci. 2008;28:2773–82. doi: 10.1523/JNEUROSCI.3837-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. J Pharmacol Exp Ther. 2010;334:665–72. doi: 10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vazquez-Palacios G, Bonilla-Jaime H, Velazquez-Moctezuma J. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with fluoxetine [correction of flouxetine] Pharmacol Biochem Behav. 2004;78:165–9. doi: 10.1016/j.pbb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 182.Djuric VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M. Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav. 1999;67:533–7. doi: 10.1016/s0031-9384(99)00091-8. [DOI] [PubMed] [Google Scholar]
- 183.Mannucci C, Tedesco M, Bellomo M, Caputi AP, Calapai G. Long-term effects of nicotine on the forced swimming test in mice: an experimental model for the study of depression caused by smoke. Neurochemistry international. 2006;49:481–6. doi: 10.1016/j.neuint.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 184.Yohn NL, Turner JR, Blendy JA. Activation of alpha4beta2*/alpha6beta2* nicotinic receptors alleviates anxiety during nicotine withdrawal without upregulating nicotinic receptors. J Pharmacol Exp Ther. 2014;349:348–54. doi: 10.1124/jpet.113.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–34. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- 186.Isola R, Vogelsberg V, Wemlinger TA, Neff NH, Hadjiconstantinou M. Nicotine abstinence in the mouse. Brain Res. 1999;850:189–96. doi: 10.1016/s0006-8993(99)02131-9. [DOI] [PubMed] [Google Scholar]
- 187.Kotagale NR, Chopde CT, Umekar MJ, Taksande BG. Chronic agmatine treatment prevents behavioral manifestations of nicotine withdrawal in mice. Eur J Pharmacol. 2015;754:190–8. doi: 10.1016/j.ejphar.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 188.Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–84. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- 189.Thanos P, Delis F, Rosko L, Volkow ND. Passive Response to Stress in Adolescent Female and Adult Male Mice after Intermittent Nicotine Exposure in Adolescence. Journal of addiction research & therapy. 2013;(Suppl 6):007. doi: 10.4172/2155-6105.S6-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bruijnzeel AW, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M, et al. Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacol Biochem Behav. 2012;101:62–8. doi: 10.1016/j.pbb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Qi X, Shan Z, Ji Y, Guerra V, Alexander JC, Ormerod BK, et al. Sustained AAV-mediated overexpression of CRF in the central amygdala diminishes the depressive-like state associated with nicotine withdrawal. Translational psychiatry. 2014;4:e385. doi: 10.1038/tp.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Patel V, Borysenko M, Kumar MS, Millard WJ. Effects of acute and subchronic delta 9-tetrahydrocannabinol administration on the plasma catecholamine, beta-endorphin, and corticosterone levels and splenic natural killer cell activity in rats. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1985;180:400–4. doi: 10.3181/00379727-180-42195. [DOI] [PubMed] [Google Scholar]
- 193.Manzanares J, Corchero J, Fuentes JA. Opioid and cannabinoid receptor-mediated regulation of the increase in adrenocorticotropin hormone and corticosterone plasma concentrations induced by central administration of delta(9)-tetrahydrocannabinol in rats. Brain Res. 1999;839:173–9. doi: 10.1016/s0006-8993(99)01756-4. [DOI] [PubMed] [Google Scholar]
- 194.Sano K, Koushi E, Irie K, Higuchi S, Tsuchihashi R, Kinjo J, et al. Delta(9)-tetrahydrocannabinol enhances an increase of plasma corticosterone levels induced by forced swim-stress. Biological & pharmaceutical bulletin. 2009;32:2065–7. doi: 10.1248/bpb.32.2065. [DOI] [PubMed] [Google Scholar]
- 195.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–8. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- 196.Jacobs JA, Dellarco AJ, Manfredi RA, Harclerode J. The effects of delta 9-tetrahydrocannabinol, cannabidiol, and shock on plasma corticosterone concentrations in rats. The Journal of pharmacy and pharmacology. 1979;31:341–2. doi: 10.1111/j.2042-7158.1979.tb13516.x. [DOI] [PubMed] [Google Scholar]
- 197.Hill MN, Gorzalka BB. Increased sensitivity to restraint stress and novelty-induced emotionality following long-term, high dose cannabinoid exposure. Psychoneuroendocrinology. 2006;31:526–36. doi: 10.1016/j.psyneuen.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 198.Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163:111–7. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- 199.Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- 200.Haring M, Grieb M, Monory K, Lutz B, Moreira FA. Cannabinoid CB(1) receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations. Neuropharmacology. 2013;65:83–9. doi: 10.1016/j.neuropharm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 201.Genn RF, Tucci S, Marco EM, Viveros MP, File SE. Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharmacol Biochem Behav. 2004;77:567–73. doi: 10.1016/j.pbb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 202.Hill MN, Gorzalka BB. Enhancement of anxiety-like responsiveness to the cannabinoid CB(1) receptor agonist HU-210 following chronic stress. Eur J Pharmacol. 2004;499:291–5. doi: 10.1016/j.ejphar.2004.06.069. [DOI] [PubMed] [Google Scholar]
- 203.Onaivi ES, Chakrabarti A, Gwebu ET, Chaudhuri G. Neurobehavioral effects of delta 9-THC and cannabinoid (CB1) receptor gene expression in mice. Behav Brain Res. 1995;72:115–25. doi: 10.1016/0166-4328(96)00139-8. [DOI] [PubMed] [Google Scholar]
- 204.Marco EM, Perez-Alvarez L, Borcel E, Rubio M, Guaza C, Ambrosio E, et al. Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behav Pharmacol. 2004;15:21–7. doi: 10.1097/00008877-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 205.Caberlotto L, Rimondini R, Hansson A, Eriksson S, Heilig M. Corticotropin-releasing hormone (CRH) mRNA expression in rat central amygdala in cannabinoid tolerance and withdrawal: evidence for an allostatic shift? Neuropsychopharmacology. 2004;29:15–22. doi: 10.1038/sj.npp.1300296. [DOI] [PubMed] [Google Scholar]
- 206.Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191:867–77. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- 207.Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol. 2010;13:861–76. doi: 10.1017/S1461145709990605. [DOI] [PubMed] [Google Scholar]
- 208.Weidenfeld J, Feldman S, Mechoulam R. Effect of the brain constituent anandamide, a cannabinoid receptor agonist, on the hypothalamo-pituitary-adrenal axis in the rat. Neuroendocrinology. 1994;59:110–2. doi: 10.1159/000126646. [DOI] [PubMed] [Google Scholar]
- 209.Corchero J, Manzanares J, Fuentes JA. Role of gonadal steroids in the corticotropin-releasing hormone and proopiomelanocortin gene expression response to Delta(9)-tetrahydrocannabinol in the hypothalamus of the rat. Neuroendocrinology. 2001;74:185–92. doi: 10.1159/000054685. [DOI] [PubMed] [Google Scholar]
- 210.Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–82. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–26. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084; flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol (Fayetteville, NY) 2004;32:101–11. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–13. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- 215.Park PE, Vendruscolo LF, Schlosburg JE, Edwards S, Schulteis G, Koob GF. Corticotropin-releasing factor (CRF) and alpha 2 adrenergic receptors mediate heroin withdrawal-potentiated startle in rats. Int J Neuropsychopharmacol. 2013;16:1867–75. doi: 10.1017/S1461145713000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- 217.Skelton KH, Oren D, Gutman DA, Easterling K, Holtzman SG, Nemeroff CB, et al. The CRF1 receptor antagonist, R121919, attenuates the severity of precipitated morphine withdrawal. Eur J Pharmacol. 2007;571:17–24. doi: 10.1016/j.ejphar.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 218.Tucci S, Cheeta S, Seth P, File SE. Corticotropin releasing factor antagonist, alpha-helical CRF(9-41), reverses nicotine-induced conditioned, but not unconditioned, anxiety. Psychopharmacology (Berl) 2003;167:251–6. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- 219.Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–63. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–81. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 221.Walter M, Bentz D, Schicktanz N, Milnik A, Aerni A, Gerhards C, et al. Effects of cortisol administration on craving in heroin addicts. Translational psychiatry. 2015;5:e610. doi: 10.1038/tp.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Silverstone PH, Done C, Sharp T. Clonidine but not nifedipine prevents the release of noradrenaline during naloxone-precipitated opiate withdrawal: an in vivo microdialysis study in the rat. Psychopharmacology (Berl) 1992;109:235–8. doi: 10.1007/BF02245506. [DOI] [PubMed] [Google Scholar]
- 223.Gold MS, Pottash AC, Sweeney DR, Kleber HD. Opiate withdrawal using clonidine. A safe, effective, and rapid nonopiate treatment. JAMA. 1980;243:343–6. [PubMed] [Google Scholar]
- 224.Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, et al. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcoholism, clinical and experimental research. 2012;36:351–60. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–9. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]