Abstract
The aim of this article is to evaluate geographic variation in the incidence of diverticulitis and examine behavioral and environmental factors associated with high rates of diverticulitis across the United States. We used state hospital discharge data from 20 states to determine rates of inpatient diverticulitis from January 2002 to December 2004 at patient's county of residence. Next, we merged the county level data with behavioral and environmental survey data from the Behavioral Risk Factor Surveillance System (BRFSS). Finally, we determined the association between behavioral and environmental factors (i.e., teeth removal, dental cleaning, air quality, smoking, alcohol, vaccine, vitamins, and mental health) and high rates of diverticulitis. From January 1, 2002, to December 31, 2004, a total of 345,216 hospitalizations for acute diverticulitis were recorded for 1,055 counties. We identified rates of diverticulitis that ranged from 35.4 to 332.7 per 100,000 population. On univariate analysis, high diverticulitis burden was associated with regions of the country with substantial tooth loss from dental disease (45.8% for high diverticulitis counties vs. 37.5% for low diverticulitis counties; p = 0.0001). There is considerable variability in diverticulitis cases by county of residence across the nation. Potential triggers of diverticulitis may be associated with tooth removal and sun exposure.
Keywords: diverticulitis, population, BRFSS, CDC, epidemiology, seasonal variation
Diverticular disease is one of the most common conditions affecting men and women in developed countries1; yet potential causes of acute or chronic disease are unknown. There has been a substantial increase in rates of diverticulitis over the recent past with considerably more patients younger than 50 years experiencing diverticular disease.2 3 Much of the increased prevalence of diverticulosis is thought to be due to changes in diet, specifically more processed food and a deficiency of insoluble fiber.4 This hypothesis is further reinforced by the high rate of diverticulosis in Japanese immigrants after assimilating a Western diet and lifestyle despite the low rate of diverticulosis in Japan.5 6
Acute diverticulitis is the most frequent complication of diverticulosis.7 Despite some understanding of the association between diet and the development of diverticulosis, no actual cause for an acute episode of diverticulitis has been identified. Previously, it was assumed that the consumption of nuts, seeds, and corn led to acute diverticular disease through luminal trauma8 9 10; however, more recent data have revealed no such association.11 In fact, the consumption of some of these food items is now proposed to be protective for diverticulitis.11
Recently, seasonal variation in diverticulitis cases was described, with excess cases during summer months.12 A potential trigger that is more prevalent in the summer may thus account for the increased burden of disease in the summer months. Given our prior work on the seasonality of diverticulitis, it is possible that some summer time behavioral or other environmental factor may trigger diverticulitis. To answer this hypothesis, we first evaluated diverticulitis cases across 1,055 counties, 20 states, and 4 census regions. Next, we compared rates of age-adjusted diverticulitis cases at the county level to determine populations with relatively high burden of diverticular disease. Finally, we studied associations between those counties with a high burden of diverticulitis cases and behavioral, environmental, and other health-related factors.
Materials and Methods
Data Source— Agency for Healthcare Research and Quality
We obtained state hospital discharge data from the Agency for Healthcare Research and Quality (AHRQ) and the Office of Statewide Health Planning and Development in California. We included data for 20 states with county-level place of residence information in Arizona, California, Colorado, Florida, Iowa, Kentucky, Maryland, Michigan, North Carolina, Nebraska, New Jersey, New York, Oregon, Rhode Island, South Carolina, Utah, Vermont, Washington, Wisconsin, and West Virginia.13 Our office has a data-use agreement with AHRQ and the California Office of Statewide Health Planning and Development. In addition, our study protocol was considered exempt by our local Institutional Review Board.
Data Source—Behavioral Risk Factor Surveillance System
The Centers for Disease Control and Prevention maintains the world's longest, continuously conducted health survey, the Behavioral Risk Factor Surveillance System (BRFSS). This survey reports 400,000 health-related telephone interviews in all 50 states, the District of Columbia, and three U.S. territories through an annual core questionnaire and optional state models to assess health-related risk behaviors, conditions, and use of preventative services among civilian, noninstitutionalized adults (≥ 18 years). The Selected Metropolitan/Micropolitan Area Risk Trends of BRFSS (SMART BRFSS) uses BRFSS data at the city and county level to provide prevalence rates for selected conditions and behaviors.14
Data Source—U.S. Census
We obtained population data from the 2000 U.S. Census for each county sampled from AHRQ. Census information was used with discharge data to determine population age categories and calculate age-adjusted rates of diverticulitis for each county sampled during the three years of the study period. In addition, U.S. Census categories were used to define boundaries of four geographic regions: Northeast, Midwest, South, and West.15
Study Population
We used International Classification of Diseases-9 (ICD-9) diagnostic codes to identify all patients discharged with a primary or secondary diagnosis of diverticulitis (ICD-9 code: 562.11 or 562.13) from January 1, 2002, through December 31, 2004. We did not include patients with only diverticulosis or those with small bowel diverticulitis.
We used standard formulas to adjust county of residence diverticulitis rates to the 2000 U.S. standard population. Age adjustment was performed for each county from January 2002 through December 2004 and presented as the incidence of diverticulitis hospitalizations per 100,000 population. We then determined the mean rate for each county, analyzing all three years and averaging the rates for one estimate. We encountered incomplete data in 2002 for the state of Utah and thus determined the mean rate by the average of the age-adjusted rates for 2003 and 2004 for each Utah counties. We identified 103 counties for which we had both AHRQ and BRFSS data and grouped diverticulitis burden into tertiles based on mean diverticulitis rates.
Behavioral Risk Factor Surveillance System—Variables
We used survey data from the 2004 SMART BRFSS to develop an understanding of local population behavioral and environmental factors. We selected nine variables for all sampled counties: (1) teeth removal, (2) dental cleaning, (3) air quality, (4) smoking, (5) alcohol, (6) vaccine, (7) sunburn, (8) vitamins, and (9) mental health. Each of these variables corresponded to a specific question within the BRFSS survey, as follows:
Teeth removal: “How many of your permanent teeth have been removed because of tooth decay or gum disease?”
Dental cleaning: “How long has it been since you had your teeth cleaned by a dentist or dental hygienist?”
Air quality: “In the past 12 months have you had an illness or symptoms that you think was caused by something in the air inside a home, office, or other building?”
Smoking: “Have you smoked at least 100 cigarettes in your life?”
Alcohol: “Adults who report having had at least one drink of alcohol in the past 30 days.”
Vaccine: “Have you ever had a pneumonia shot?”
Sunburn: “Have you had a sunburn within the past 12 months?”
Vitamins: “Do you currently take any vitamin pills or supplements?”
Mental health: “Now thinking about your mental health, which includes stress, depression, and problems with emotions, for how many days during the past 30 days was your mental health not good?”16
Data were standardized according to the recommended county-level weighting procedure17 to adjust for age, sex, race, and geographic location and extrapolated to estimate prevalence within each of the 103 counties sampled. We then calculated the answers based on each respective BRFSS survey question for the variables selected. For questions containing multiple answer choices, we aggregated data into binary categories that could be simplified into simple yes/no answers. For dental cleaning, we aggregated responses into two groups: those whose last dental cleaning was within the past 5 years and those whose last dental cleaning was 5 or more years ago. For mental health, we aggregated all nonzero responses (ranging from 1 to 30 days of poor mental health in the past month). Table 1 lists behavioral and environmental variables for counties with adequate sample size and available data.
Table 1. Behavioral and environmental data for counties with low, medium, and high rates of diverticulitis.
| Low diverticulitis counties (n = 34) | Moderate diverticulitis counties (n = 35) | High diverticulitis counties (n = 34) | p | |
|---|---|---|---|---|
| Population (%) | 20,649,778 (31.2) | 22,409,651 (33.9) | 23,113,522 (34.9) | |
| 3-y diverticulitis count (%) | 26,651 (16.7) | 49,616 (31.0) | 83,715 (52.3) | |
| Range of diverticulitis rate (per 100,000) | 9.1–69.6 | 70.1–95.5 | 96.3–149.0 | |
| Median diverticulitis rate (per 100,000) | 60.3 | 75.3 | 108.3 | |
| Characteristics | ||||
| Teeth removal | 37.5 ± 8.0% | 41.8 ± 9.3% | 45.8 ± 5.7% | 0.0001 |
| Dental cleaning | 86.2 ± 4.8% | 85.8 ± 4.3% | 86.1 ± 5.1% | 0.9 |
| Air quality | 20.4 ± 3.0% | 10.8 ± 3.8% | 20.6 ± 3.4% | 0.9 |
| Smoking | 18.2 ± 5.3% | 20.5 ± 3.8% | 20.1 ± 3.6% | 0.06 |
| Alcohol | 55.0 ± 13.1% | 54.9 ± 9.5% | 57.6 ± 10.6% | 0.5 |
| Vaccine | 21.2 ± 3.3% | 20.2 ± 3.3% | 21.3 ± 3.8% | 0.3 |
| Sunburn | 39.9 ± 9.8% | 32.4 ± 9.8% | 30.8 ± 7.0% | <0.0001 |
| Vitamins | 17.7 ± 27.9% | 25.6 ± 30.1% | 18.6 ± 27.5% | 0.4 |
| Mental health | 35.3 ± 4.2% | 33.0 ± 4.7% | 33.0 ± 4.4% | 0.05 |
Notes: Behavioral and environmental data from the 2004 SMART BRFSS. The 3-y diverticulitis count for the state of Utah was projected based on data from 2003 and 2004.
Data Merge
Of 1,100 counties in the 20 states for which we had state discharge data, 45 were excluded for incomplete data. After aggregating county data into 3-year, age-adjusted rates of diverticulitis incidence, we then identified those with a population of at least 20,000 and for which we had BRFSS survey data. This resulted in a total of 103 counties with a total population of 66,172,951, of which approximately 52.9 million were older than 18 years. Based on the mean 3-year diverticulitis rates, we divided these counties into tertiles, corresponding to “low,” “moderate,” and “high” diverticulitis counties.
The data merge was performed at the level of patients' county of residence. The master file recorded county-level information for (1) diverticulitis rate from the State Inpatient Discharge Data by county, (2) population data from the U.S. Census, and (3) responses to the SMART BRFSS questionnaire.
Statistical Analysis
We divided counties into tertiles based on mean diverticulitis rates. We then used ANOVA for continuous data and chi-square analysis for proportions to compare variables for all three groups. The answers to the BRFSS questionnaire were included as categorical variables to determine county-level determinants. For the dental cleaning and mental health variables, survey questions included multiple responses, and thus data were dichotomized into non-overlapping categories (yes/no) to simplify analyses. Prevalence of each variable type was determined and tertiles were compared. Given the multiple statistical testing performed, we used a Bonferroni adjustment to reduce the potential for type I error. We performed all statistical analyses using SAS 9.3 (SAS Institute, Cary, NC) and considered a value of 0.006 as significant.
Results
All Counties Data
Data were obtained from 20 states representing a total of 1,055 counties with a total population of 141,920,780 and 51,315,128 total inpatient discharges. A total of 345,216 (0.7%) discharges were coded with a primary or secondary diagnosis of diverticulitis during the years 2002 to 2004. The yearly total diverticulitis case count was as follows: 108,790 (rate of 76.7 per 100,000) in 2002; 114,913 (rate of 81.0 per 100,000) in 2003; and 121,513 (rate of 86.6 per 100,000) in 2004. The average rate over the study period was 81.1 cases per year per 100,000 population.
At the state level, the rates of age-adjusted diverticulitis per 100,000 population ranged from a low of 35.4 cases in California to a high of 134.2 cases in Florida. At the county level, average annual rates per 100,000 population ranged from a low of 7.5 cases to 332.7 cases in Okeechobee, FL, with a mean of 91.5 ± 42.8 across all counties. Rates of diverticulitis for each county are plotted in Fig. 1.
Fig. 1.
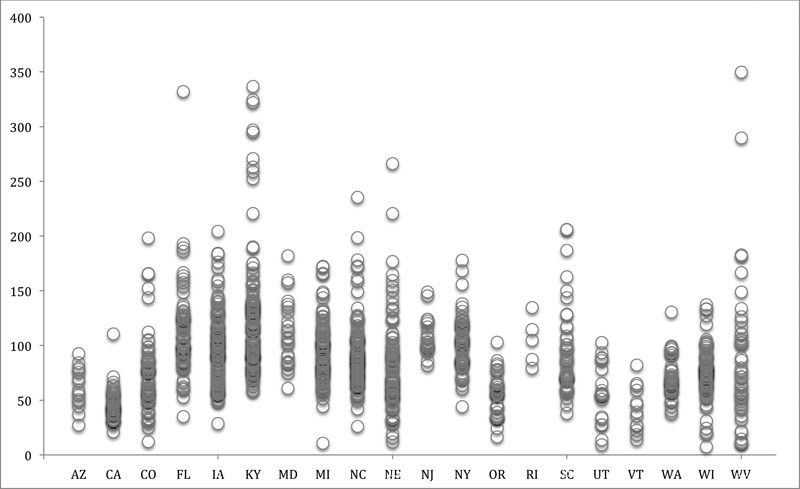
Mean annual rate of diverticulitis per 100,000 population in 20 states by county. Each circle represents the mean rate of diverticulitis from 2002 to 2004 in each county for which we have state hospital discharge data from the Agency for Healthcare Research and Quality and the Office of Statewide Health Planning and Development in California. Note that we only included data for 2003 and 2004 for the state of Utah.
In the master file, 103 counties met our inclusion criteria for sample size and data availability and were divided into tertiles based on average annual diverticulitis rate per 100,000 population: “low” (9.1–69.6, median: 60.3), “moderate” (70.1–95.4, median: 75.3), and “high” (96.3–149.0, median: 108.3). In addition, at the regional level, we also observed geographic variability across the four U.S. Census regions. Ranges for diverticulitis rates per 100,000 population for each region are as follows: Northeast (18.8–148.9, median: 98.2), Midwest (55.2–131.6, median: 73.4), South (45.7–123.5, median: 85.2), and West (9.1–93.6, median: 65.2). We noted that 17 (50%) of the high diverticulitis counties in our study sample were found in the Northeastern region of the country, while 20 (58.8%) of the low diverticulitis counties were found in the West (Table 2). These 103 counties were included in subsequent analysis using BRFSS variables (Table 1).
Table 2. Regional distribution for counties with low, moderate, and high rates of diverticulitis.
| Northeast | Midwest | South | West | |
|---|---|---|---|---|
| Total counties (%) | 31 (30.1) | 9 (8.7) | 33 (32.0) | 30 (29.1) |
| Population (%) | 20,119,483 (30.4) | 6,233,434 (9.4) | 15,672,640 (23.7) | 24,147,395 (36.5) |
| 3-y diverticulitis count (%) | 58,202 (36.4) | 18,082 (11.3) | 47,833 (30.0) | 35,864 (22.4) |
| Range of diverticulitis rates (per 100,000) | 18.8–148.9 | 55.2–131.6 | 45.7–123.5 | 9.1–93.6 |
| Median diverticulitis rate (per 100,000) | 98.2 | 73.4 | 85.2 | 65.2 |
| Low diverticulitis counties (n = 34) | 5 (16.1%) | 3 (33.3%) | 6 (18.2%) | 20 (66.7%) |
| Moderate diverticulitis counties (n = 35) | 9 (29.0%) | 3 (33.3%) | 13 (39.4%) | 10 (33.3%) |
| High diverticulitis counties (n = 34) | 17 (54.8%) | 3 (33.3%) | 14 (42.4%) | 0 (0%) |
Notes: Defined by the U.S. Census Bureau. The 3-y diverticulitis count for the state of Utah was projected based on data from 2003 and 2004.
Behavioral and Environmental Analysis
These data reveal no association between county age-adjusted diverticulitis rates and the BRFSS variables of dental cleaning, air quality, smoking, alcohol, vaccine, vitamins, or mental health. However, we did find a statistically significant association between the highest tertile of diverticulitis cases and teeth removal (p = 0.0001). In addition, counties with high rates of diverticulitis also had decreased rates of sunburn history (p < 0.0001).
Discussion
We found significant geographic variation in the incidence rates of diverticulitis at county, state, and regional levels. The majority (54.8%) of counties sampled in the Northeast were identified in the highest tertile of diverticulitis cases, while the counties in the West were never classified in the highest tertile of diverticulitis cases. In fact, the majority (66.7%) of counties in the Western part of the country belonged to the lowest tertile of diverticulitis cases.
In our study, we identified substantial variability in population rates of inpatient diverticulitis cases across the country. These findings are consistent with other studies demonstrating regional variation in diverticulitis attacks,18 as well as in other conditions such as inflammatory bowel disease,19 and esophageal cancer.20 Although we expected to find some degree of variability, we were surprised to find rates of diverticulitis that differed by an order of magnitude and greater.
We also identified particularly low rates of diverticulitis in the Western part of the country. Although this may be explained by increased fiber consumption21 or lower rates of obesity22 in this region, this explanation does not explain the high rates in the Northeast. Our findings do not clearly indicate a north–south gradient, as some of the highest rates of diverticulitis were found in Florida, while lower rates were noted in Vermont and Oregon.
Our study is meant to be hypothesis generating. In our analysis of potential environmental or behavioral factors, we found that high diverticulitis counties were more likely to report high rates of tooth loss, specified as being due to disease (including infection), not injury or orthodontics. Periodontal disease has been associated with other conditions such as coronary heart disease23 24 and rheumatoid arthritis.25 26 In addition, tooth loss and edentulism (missing all teeth) have been associated with gastrointestinal cancer,26 heart disease,26 stroke,26 27 28 and diabetes.29 Our population data suggest that diverticulitis may be yet another disease associated with tooth loss. This is further reinforced, given the association of diverticulitis with increased age. However, these findings are just associations, as poor dental health practices may also be an indicator of poor general health status or socioeconomic factors connected to health-care access. Interestingly, we found no significant association between frequency of dental cleaning and diverticulitis cases; thus, patterns of tooth removal do not directly correspond to behaviors of preventive oral health. Direct mechanisms of disease etiology have been suggested that may relate to oral bacteria and inflammation in the link between dental disease, particularly tooth loss, and stroke.27 28 As inflammation is directly involved in diverticulitis pathophysiology, the ecological association between diverticulitis and dental health is worth further clinical investigation.
The inverse association between diverticulitis and sunburn is difficult to understand. Given the seasonal variation in diverticulitis incidence, with more cases occurring in the summer months,12 we expected to find more sunburns in the counties with high diverticulitis burden. Our findings are further perplexing given that sunburn is essentially an inflammatory response to UVB radiation. In mouse models, sunburn has been found to activate caspase-1, a protein directly involved in inflammation30 that has also been implicated in colitis.31 However, it is possible that those individuals prone to sunburn derived some anti-inflammatory effect from increased vitamin D. Others have similarly shown reduced inflammation with increased vitamin D levels. Sunburn may be interpreted as a proxy measure for sun exposure, which has been found to be inversely associated with multiple sclerosis32 33 and Crohn disease.34 In addition, sunlight is the principle source for vitamin D production in the skin. Vitamin D levels have been observed to fluctuate seasonally,35 although the use of sun-blocking agents and oral supplementation may complicate issues.36 Vitamin D deficiency has been noted in many diseases of the digestive tract, including inflammatory bowel disease and colon cancer.37 38 Furthermore, a recent study of a Boston cohort of diverticulitis patients revealed a significant relationship between prediagnostic serum vitamin D levels and diverticular disease severity.39
It should be noted that the geographic variation in diverticulitis rates does not easily correspond to latitude, as described earlier, and thus there may be some behavioral factor at play. For example, obesity may be associated with increased complications of diverticulitis,40 and adiposity reduces serum levels of vitamin D due to sequestration.36 Sunscreen blocks most ultraviolet radiation and thus may be an important confounder. Further studies among patients living at various latitudes with data including sun exposure, sunscreen use, and use of vitamin D supplementation are thus warranted.
The present investigation is limited by study design. The ecological methodology is subject to ecological fallacy and overinterpretation. We had no access to patient records and actual behaviors of diverticulitis patients. In addition, state discharge data do not capture number of episodes of disease, complications, severity, diagnostic methods, or treatment decisions. Patients treated in the outpatient setting were also excluded. In our population analysis, we had a small number of counties due to the limitations of survey data. BRFSS survey data are self-reported and so inherently subject to bias; however, BRFSS data have been shown to be reliable and valid compared with similar surveys.41 Despite these limitations, the strength of this study lies in its sheer size, geographic scope, and large number of variables tested. Our findings do not provide an obvious cause or trigger for diverticulitis but lead to hypothesis generation and suggest further avenues for research. This has great clinical utility, given the frequency of diverticulitis incidence and increasing rates of disease.
In conclusion, our ecologic study of diverticulitis and population behavioral risk factors reinforced the understanding of geographic variability of disease incidence. We identified potential associations of diverticulitis hospitalization with tooth removal and sun exposure. We were unable to find significant associations between diverticulitis cases and dental cleaning, air pollution, smoking, alcohol use, pneumonia vaccination, vitamin consumption, or mental health status. Our study should be used to propel further work into behavioral factors associated or triggering diverticulitis.
Acknowledgment
The datasets used in this project were purchased with grant funding from the American Society of Colon and Rectal Surgeons.
Footnotes
Disclosures None. Contributions All authors were responsible for drafting of the manuscript, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. R.R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The study has been approved by all authors in the present format.
References
- 1.Parks T G. Natural history of diverticular disease of the colon. Clin Gastroenterol. 1975;4(1):53–69. [PubMed] [Google Scholar]
- 2.Ricciardi R, Baxter N N, Read T E, Marcello P W, Hall J, Roberts P L. Is the decline in the surgical treatment for diverticulitis associated with an increase in complicated diverticulitis? Dis Colon Rectum. 2009;52(9):1558–1563. doi: 10.1007/DCR.0b013e3181a90a5b. [DOI] [PubMed] [Google Scholar]
- 3.Etzioni D A, Mack T M, Beart R W Jr, Kaiser A M. Diverticulitis in the United States: 1998-2005: changing patterns of disease and treatment. Ann Surg. 2009;249(2):210–217. doi: 10.1097/SLA.0b013e3181952888. [DOI] [PubMed] [Google Scholar]
- 4.Aldoori W H, Giovannucci E L, Rockett H R, Sampson L, Rimm E B, Willett W C. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128(4):714–719. doi: 10.1093/jn/128.4.714. [DOI] [PubMed] [Google Scholar]
- 5.Miura S, Kodaira S, Shatari T, Nishioka M, Hosoda Y, Hisa T K. Recent trends in diverticulosis of the right colon in Japan: retrospective review in a regional hospital. Dis Colon Rectum. 2000;43(10):1383–1389. doi: 10.1007/BF02236634. [DOI] [PubMed] [Google Scholar]
- 6.Ohi G, Minowa K, Oyama T. et al. Changes in dietary fiber intake among Japanese in the 20th century: a relationship to the prevalence of diverticular disease. Am J Clin Nutr. 1983;38(1):115–121. doi: 10.1093/ajcn/38.1.115. [DOI] [PubMed] [Google Scholar]
- 7.Hughes L E. Postmortem survey of diverticular disease of the colon. I. Diverticulosis and diverticulitis. Gut. 1969;10(5):336–344. doi: 10.1136/gut.10.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman L G, Burdick D, Heitzman E R, Prior J T. A critical reappraisal of sigmoid peridiverticulitis. Surg Gynecol Obstet. 1968;127(3):481–491. [PubMed] [Google Scholar]
- 9.Fleischner F G, Ming S C. Revised concepts on diverticular disease of the colon II, so-called diverticulitis; diverticular sigmoiditis and perisigmoidosis, diverticular abscess, fistula, and frank peritonitis. Radiology. 1965;84:599–609. doi: 10.1148/84.4.599. [DOI] [PubMed] [Google Scholar]
- 10.Morson B C. The muscle abnormality in diverticular disease of the colon. Proc R Soc Med. 1963;56:798–800. doi: 10.1177/003591576305600916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strate L L, Liu Y L, Syngal S, Aldoori W H, Giovannucci E L. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300(8):907–914. doi: 10.1001/jama.300.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricciardi R, Roberts P L, Read T E. et al. Cyclical increase in diverticulitis during the summer months. Arch Surg. 2011;146(3):319–323. doi: 10.1001/archsurg.2011.27. [DOI] [PubMed] [Google Scholar]
- 13.Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality, Rockville, MD Available at: www.hcup-us.ahrq.gov/sidoverview.jsp. Accessed March 2009 [PubMed]
- 14.Centers for Disease Control and Prevention (CDC) . Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. Behavioral Risk Factor Surveillance System Survey Data. [Google Scholar]
- 15.U.S. Census Bureau Census regions and divisions of the United States Available at: https://www.census.gov/geo/maps-data/maps/pdfs/reference/us_regdiv.pdf Accessed July 9, 2014
- 16.Centers for Disease Control and Prevention (CDC) . Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. Behavioral Risk Factor Surveillance System Survey Codebook Report. [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) 2004 SMART BRFSS County Methodology Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. Available at: http://www.cdc.gov/brfss/smart/2004/2004_SMART_BRFSS_County_Methodology.rtf. Accessed February 17, 2014 [Google Scholar]
- 18.Nguyen G C, Sam J, Anand N. Epidemiological trends and geographic variation in hospital admissions for diverticulitis in the United States. World J Gastroenterol. 2011;17(12):1600–1605. doi: 10.3748/wjg.v17.i12.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kappelman M D, Rifas-Shiman S L, Kleinman K. et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Drahos J, Wu M, Anderson W F. et al. Regional variations in esophageal cancer rates by census region in the United States, 1999-2008. PLoS ONE. 2013;8(7):e67913. doi: 10.1371/journal.pone.0067913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajjar I, Kotchen T. Regional variations of blood pressure in the United States are associated with regional variations in dietary intakes: the NHANES-III data. J Nutr. 2003;133(1):211–214. doi: 10.1093/jn/133.1.211. [DOI] [PubMed] [Google Scholar]
- 22.Singh G K, Kogan M D, van Dyck P C. A multilevel analysis of state and regional disparities in childhood and adolescent obesity in the United States. J Community Health. 2008;33(2):90–102. doi: 10.1007/s10900-007-9071-7. [DOI] [PubMed] [Google Scholar]
- 23.DeStefano F, Anda R F, Kahn H S, Williamson D F, Russell C M. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306(6879):688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desvarieux M, Demmer R T, Rundek T. et al. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Stroke. 2003;34(9):2120–2125. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Pablo P, Dietrich T, McAlindon T E. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35(1):70–76. [PubMed] [Google Scholar]
- 26.Abnet C C, Qiao Y-L, Dawsey S M, Dong Z-W, Taylor P R, Mark S D. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol. 2005;34(2):467–474. doi: 10.1093/ije/dyh375. [DOI] [PubMed] [Google Scholar]
- 27.Lopes-Virella M F, Virella G. Immunological and microbiological factors in the pathogenesis of atherosclerosis. Clin Immunol Immunopathol. 1985;37(3):377–386. doi: 10.1016/0090-1229(85)90107-2. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida M, Akagawa Y. The relationship between tooth loss and cerebral stroke. Jpn Dent Sci Rev. 2011;47:157–160. [Google Scholar]
- 29.Patel M H, Kumar J V, Moss M E. Diabetes and tooth loss: an analysis of data from the National Health and Nutrition Examination Survey, 2003-2004. J Am Dent Assoc. 2013;144(5):478–485. doi: 10.14219/jada.archive.2013.0149. [DOI] [PubMed] [Google Scholar]
- 30.Faustin B, Reed J C. Sunburned skin activates inflammasomes. Trends Cell Biol. 2008;18(1):4–8. doi: 10.1016/j.tcb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Siegmund B. Interleukin-1β converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol. 2002;64(1):1–8. doi: 10.1016/s0006-2952(02)01064-x. [DOI] [PubMed] [Google Scholar]
- 32.Munger K L, Levin L I, Hollis B W, Howard N S, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 33.Lucas R M, Ponsonby A L. Considering the potential benefits as well as adverse effects of sun exposure: can all the potential benefits be provided by oral vitamin D supplementation? Prog Biophys Mol Biol. 2006;92(1):140–149. doi: 10.1016/j.pbiomolbio.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Nerich V, Jantchou P, Boutron-Ruault M-C. et al. Low exposure to sunlight is a risk factor for Crohn's disease. Aliment Pharmacol Ther. 2011;33(8):940–945. doi: 10.1111/j.1365-2036.2011.04601.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosecrans R, Dohnal J C. Seasonal vitamin D changes and the impact on health risk assessment. Clin Biochem. 2014;47(7–8):670–672. doi: 10.1016/j.clinbiochem.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Holick M F. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 37.Ulitsky A, Ananthakrishnan A N, Naik A. et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35(3):308–316. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 38.Jørgensen S P, Hvas C L, Agnholt J, Christensen L A, Heickendorff L, Dahlerup J F. Active Crohn's disease is associated with low vitamin D levels. J Crohn's Colitis. 2013;7(10):e407–e413. doi: 10.1016/j.crohns.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Maguire L H, Song M, Strate L E, Giovannucci E L, Chan A T. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol. 2013;11(12):1631–1635. doi: 10.1016/j.cgh.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strate L L, Liu Y L, Aldoori W H, Syngal S, Giovannucci E L. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology. 2009;136(1):115–1220. doi: 10.1053/j.gastro.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierannunzi C, Hu S S, Balluz L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004-2011. BMC Med Res Methodol. 2013;13:49. doi: 10.1186/1471-2288-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]


