Abstract
Imaging plays an increasingly important role in the staging and management of colorectal cancer. In recent years, magnetic resonance imaging (MRI) has supplanted transrectal ultrasound as the preferred modality for the locoregional staging of rectal cancer. Furthermore, the advent of both diffusion-weighted imaging and hepatobiliary contrast agents has significantly enhanced the ability of MRI to detect colorectal liver metastases. In clinical practice, MRI routinely provides prognostic information, helps to guide surgical strategy, and determines the need for neoadjuvant therapies related to both the primary tumor and metastatic disease. Expanding on these roles for MRI, positron emission tomography (PET)/MRI is the newest clinical hybrid imaging modality and combines the metabolic information of PET with the high soft tissue contrast of MRI. The addition of PET/MRI to the clinical staging armamentarium has the potential to provide comprehensive state-of-the-art colorectal cancer staging in a single examination.
Keywords: colorectal cancer staging, transrectal ultrasound, MRI, circumferential margin
Colorectal cancer (CRC) is the third most common malignancy and second most common cause of cancer mortality in the United States, with over 132,000 new diagnoses and 49,000 deaths each year.1 As these numbers indicate, CRC is curable in a substantial percentage of cases. Accurate staging is critical to the selection of a suitable treatment strategy and, consequently, to the achievement of optimal clinical outcomes. To this point, radiologic examinations are playing an increasingly important role in both the initial staging of CRC and the evaluation of response to neoadjuvant therapy. Moreover, imaging facilitates preoperative planning by delineating relationships between tumors and the adjacent anatomy, thereby defining the best surgical approach and minimizing risk of injury to surrounding structures.
The imaging and treatment of CRC can be generally divided into two main components: (1) locoregional staging and management of primary tumors; and (2) the identification and management of distant metastases, which most commonly occur in the liver. The primary goal of locoregional treatment strategies is to achieve a margin-negative surgical resection, either primarily (for colon and rectal cancer) or after neoadjuvant chemotherapy and/or radiation (for rectal cancer only). Transrectal ultrasound (TRUS), computed tomography (CT), and magnetic resonance imaging (MRI) are the main imaging modalities for locoregional staging (Table 1) and are focused on defining the local extent of the primary tumor (T-staging) as well as the status of local and regional lymph nodes (N-staging).
Table 1. Imaging modalities for locoregional staging of rectal cancer.
| Modality | Protocol notes | Advantages | Disadvantages |
|---|---|---|---|
| TRUS | Often performed in office by clinician | • No radiation exposure • Real-time evaluation • Good visualization of discrete rectal wall layers |
• Operator dependence • Limited field of view |
| CT | Performed with IV contrast; portal venous phase only | • Wide availability • Short acquisition • Large field of view |
• Radiation exposure • Poor soft tissue contrast |
| MRI | Perform without and with ECCA, using multiphase dynamic protocol | • No radiation exposure • Excellent soft tissue contrast • Large field of view • Validated prognostic features |
• Multiple contraindications (e.g., claustrophobia) • Long acquisition |
Abbreviations: CT, computed tomography; ECCA, extracellular contrast agent (gadolinium-based); IV, intravenous; MRI, magnetic resonance imaging; TRUS, transrectal ultrasound.
While locoregional tumor management is important for preventing or minimizing invasion of adjacent structures and avoiding the associated morbidity, mortality in CRC is primarily determined by the hepatic disease burden.2 Thus, the current treatment paradigm for liver metastases from both colon cancer and rectal cancer is surgical resection, which maximizes the chances for long-term survival.3 4 For liver metastases deemed unresectable, neoadjuvant chemotherapy and percutaneous interventions can be employed to achieve hepatic tumor control and may eventually make resection feasible by reducing metastatic disease burden.5 6 7 The imaging modalities most commonly utilized for identifying hepatic and other distant metastases (M-staging) are MRI, CT, and positron emission tomography (PET)/CT (Table 2).
Table 2. Imaging modalities for distant metastatic staging of colorectal cancer.
| Modality | Protocol notes | Advantages | Disadvantages |
|---|---|---|---|
| CT | Perform with IV contrast; arterial, portal venous, and delayed phases | • High sensitivity for lung metastases • Fast acquisition • Wide availability |
• Radiation exposure • Reduced sensitivity for liver metastases in hepatic steatosis |
| PET/CT | PET images acquired 60–70 min after FDG injection; CT obtained without or with IV contrast | • High sensitivity for most distant metastatic disease • Quantification of tumor metabolism with SUV (can be followed to assess response) |
• Radiation exposure • Normal liver FDG uptake can obscure metastases • Nonspecific FDG uptake by infection or inflammation leads to false positives |
| MRI | Perform without and with HBCA, using multiphase dynamic protocol | • No radiation exposure • High sensitivity for liver metastases • Excellent soft tissue contrast |
• Multiple contraindications (e.g., claustrophobia) • Variable quality from institution to institution |
Abbreviations: CT, computed tomography; FDG, 2-deoxy-2-[18F]fluoro-D-glucose; HBCA, hepatobiliary contrast agent (gadolinium-based); IV, intravenous; PET; positron emission tomography; SUV, standardized uptake value.
This review article provides an update on emerging imaging strategies for both locoregional and hepatic disease. The role of imaging (in particular MRI) in initial staging, preoperative planning, and evaluation of response to neoadjuvant therapies is discussed.
Locoregional Staging and Prognostication
For colon cancer, locoregional treatment typically involves a radical hemicolectomy with removal of the associated mesentery and lymph nodes. The utility of preoperative imaging for locoregional staging is limited given the lack of impact on surgical strategy (aside from cases of perforation, obstruction, or locally advanced disease). Furthermore, neoadjuvant therapies for colon cancer have not yet been shown to improve the clinical outcomes achieved with surgery alone, making imaging as a means of selecting patients for presurgical treatments unnecessary; however, some ongoing studies are reexamining this question.8 Consequently, our discussion of locoregional staging will focus on rectal cancer.
Local surgical approaches to rectal cancer depend on the proximity of the primary tumor and associated mesorectal lymph nodes to the mesorectal fascia and the peritoneal reflection. This distance is known as the circumferential resection margin (CRM). Beyond the circumferential boundaries, a critical determinant in achieving negative margins is the relationship of the tumor to the anal sphincter complex and pelvic floor musculature. Tumor extension into the mesorectal fascia or invasion through this layer into adjacent structures necessitates a more extensive and complex surgical approach and confers a worse clinical prognosis.9 Recently, high-risk rectal cancers, as defined by preoperative imaging, have been shown to benefit from preoperative chemoradiation, which reduces local recurrence and improves overall survival.10 11 Thus, the identification of locally advanced and high-risk rectal cancers is the goal of imaging performed for initial locoregional staging. After neoadjuvant therapy, the preoperative assessment of treatment response becomes the primary indication for imaging.
Transrectal Ultrasound
TRUS has long been the gold standard for the locoregional staging (i.e., T/N-staging) of rectal cancer. By clearly depicting the individual layers of the rectal wall, TRUS can achieve T-staging accuracies of 80 to 94%.12 13 14 15 16 However, TRUS is less reliable for the differentiation of minimal T3 from advanced T3 tumors and of minimal T3 from T2 tumors.17 18 TRUS also tends to understage T4 tumors.19 Such inaccuracies can have significant implications regarding the need for neoadjuvant treatments and the selection of surgical approaches. Furthermore, TRUS has limited sensitivity and accuracy in determining nodal stage, in part because its small field of view restricts evaluation to the mesorectal and lateral pelvic nodes.20
Computed Tomography
Contrast-enhanced CT similarly has a T-staging accuracy of 64 to 74%.21 22 23 In light of its inability to resolve the individual layers of the rectal wall, CT performs poorly for low T-stage tumors but somewhat better for advanced T3 and T4 tumors.22 Like TRUS, CT is an unreliable indicator of N-stage given its use of size as the primary criterion for nodal involvement.24 25 Nevertheless, CT is still a valuable tool for presurgical planning due to its utility in detecting complications associated with rectal cancer, such as perforation or obstruction.
Magnetic Resonance Imaging
In recent years, MRI has emerged as a powerful modality for the preoperative locoregional staging of rectal cancer. Like TRUS, MRI can clearly depict the mural layers in high-resolution (Fig. 1), especially when imaging is performed with 3 Tesla (3T) magnets and endorectal coils.26 While some studies advocate the use of endorectal coils, imaging with modern MRI scanners without endorectal coils is sufficient in clinical practice, as long as protocols are optimized. Agreement between TRUS and MRI with respect to T-staging is quite high (kappa = 0.93) for T3 and T4 tumors.27 However, other studies highlight the substantial operator dependence of TRUS relative to MRI and the potential impacts on accuracy and reproducibility, especially with respect to determining nodal involvement.28 29 Much of the evidence supporting the use of MRI in the preoperative assessment of rectal cancer was generated by the MERCURY Study Group, which found a high correlation between preoperative MRI and histopathology with respect to the extramural depth (EMD) of tumor spread.30 Similarly, other authors have reported the high accuracy of MRI for assessing both EMD and the involvement of the mesorectal fascia.31 32 33 34 Overall, the information provided by initial staging MRI is essential in selecting an optimal surgical strategy and assessing the need for neoadjuvant therapy.
Fig. 1.
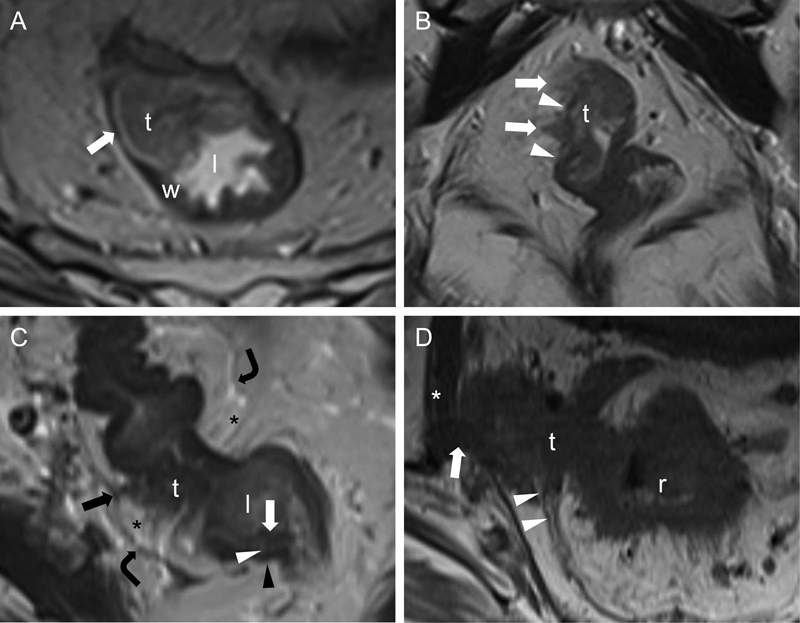
Various rectal cancer T-stages and rectal mural anatomy on MRI. Transaxial (A, C, and D) and coronal (B) T2WIs (A–D) through the rectum show T2 (A), T3 (B and C), and T4 (D) rectal tumors. In (A), a rectal tumor (t) arising from the mucosa extends into the lumen and rectal wall but does not breach the outer surface (arrow), consistent with T2 disease. In (B), a rectal tumor (t) with hypointense spiculations (arrows) extends beyond the wall (arrowheads) into the perirectal fat but does not involve the mesorectal fascia, consistent with T3 disease. In (C), similar hypointense spiculations from a rectal tumor (t) extend into the perirectal fat (asterisks) to tether (black arrow) the mesorectal fascia (curved arrows) on the right, consistent with advanced T3 disease. Also, this image nicely displays the mural anatomy of the rectum, including the hypointense mucosa (white arrow) lining the lumen (l), the hyperintense submucosa (white arrowhead), and the hypointense muscularis propria (black arrowhead). In (D), an exophytic tumor (t) arising from the rectum (r) invades through the mesorectal fascia (arrowheads) to involve (arrow) the right obturator internus (asterisk) muscle, consistent with T4 disease. MRI, magnetic resonance imaging; T2WI, T2-weighted images.
Beyond accurately determining rectal cancer T-stage (Fig. 1), preoperative MRI has also been shown to provide valuable prognostic information (Table 3). When assessed before any neoadjuvant therapy, MRI-based CRMs of < 1 mm predict significantly higher local recurrence rates and lower 5-year survival rates, compared with MRI-based CRMs of ≥ 1 mm.35 Similarly, MRI accurately predicts whether surgical margins will be clear of tumor, which in turn correlates with the risk of local tumor recurrence.36 MRI findings of EMD > 5 mm and lymph node involvement indicate a lower probability of responding favorably to standard neoadjuvant therapy; such criteria can thus be used to select patients for more intensive presurgical treatment regimens.37 Additionally, high-risk tumor features including EMD > 5 mm, T4 status, extramural vascular invasion, and CRM < 1 mm, when identified on initial staging MRI (Fig. 2), predict a higher likelihood of distant metastatic disease.38 These findings suggest that patients with such primary tumor features would benefit from imaging studies such as liver MRI or PET/CT to ensure the accurate TNM-staging.
Table 3. Established MRI-based prognostic features of rectal tumors on locoregional staging.
| Higher risk of distant metastatic disease38: • EMD > 5 mm • T4 status • Extramural vascular invasion • CRM < 1 mm |
| Higher risk of local recurrence and lower overall survival at 5 y35: • CRM < 1 mm |
| Lower probability of responding favorably to neoadjuvant therapy37: • EMD > 5 mm • Evidence of nodal involvement |
| Low risk of local recurrence after surgery without neoadjuvant or adjuvant therapies42: • CRM > 1 mm • EMD < 5 mm |
| Low recurrence rates and high DFS and OS rates without neoadjuvant therapy41: • CRM > 2 mm • No evidence of nodal involvement |
Abbreviations: CRM, circumferential resection margin; DFS, disease-free survival; EMD, extramural depth; OS, overall survival.
Fig. 2.
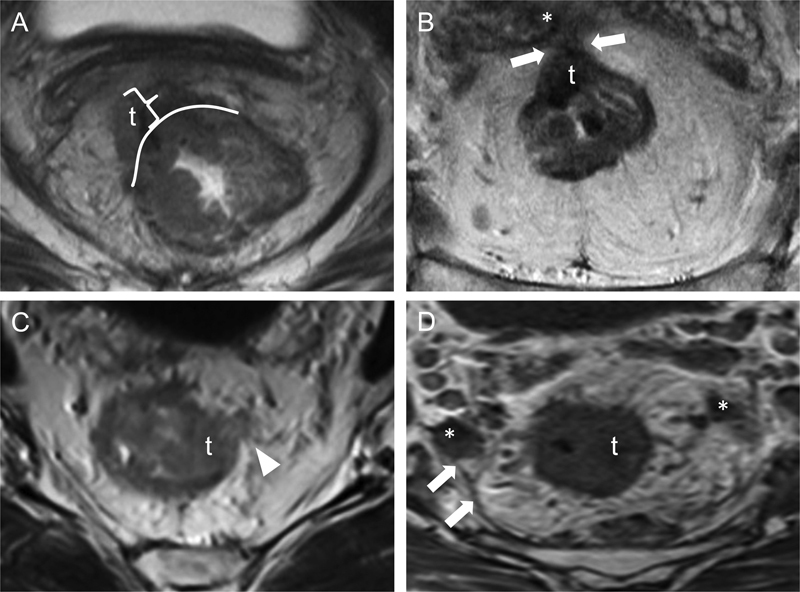
High-risk rectal cancer features on MRI. Transaxial T2WIs from four different patients show various high-risk primary tumor features. In (A), a right anterolateral rectal tumor (t) extends beyond the external aspect of the rectal wall (white curved line) into the mesorectal fat, with an extramural depth of greater than 5 mm (bracket). In (B), an anterior rectal tumor (t) extends through the mesorectal fat to involve the mesorectal fascia (arrows) and the right seminal vesicle (asterisk); these findings are compatible with T4 disease. In (C), a left lateral rectal tumor (t) exhibits extramural vascular invasion (arrowhead); the serpiginous hypointense structure represents tumor within the vessel lumen. In (D), a left lateral rectal tumor (t) with spiculations extending into the perirectal fat is associated with several enlarged lymph nodes (asterisks) that abut the mesorectal fascia (arrows), resulting in a circumferential resection margin of < 1 mm. MRI, magnetic resonance imaging; T2WI, T2-weighted images.
In the United States, neoadjuvant therapy for rectal cancer typically includes fractionated external beam pelvic radiation and systemic chemotherapy with 5-fluorouracil (5-FU) or capecitabine.39 As these treatments can result in adverse effects, such as anorectal dysfunction,40 determining which rectal cancers are sufficiently low-risk to warrant omission of neoadjuvant therapy can reduce morbidity. Several studies have already evaluated whether high-risk local tumor features, as identified by initial staging MRI, can be employed to determine which patients should receive neoadjuvant therapy versus primary surgical resection. One group found that patients with CRMs > 2 mm and negative lymph nodes by MRI could be spared neoadjuvant therapy and still achieve low recurrence and high disease-free and overall survival rates after surgical resection.41 Similarly, the MERCURY Study Group determined that rectal cancers with MRI-based CRMs > 1 mm and EMD < 5 mm (regardless of nodal status) had postoperative local recurrence rates of just 3%, without either neoadjuvant or adjuvant therapies.42 In patients deemed to have rectal cancers with high-risk features, this same group of investigators found the assessment of tumor response to neoadjuvant therapies by MRI (Fig. 3) to be predictive of clinical outcomes, as poor tumor regression correlated with lower 5-year progression-free and overall survival.43 44 Hence, MRI can provide prognostic information on initial staging examinations, as well as on follow-up evaluations after neoadjuvant therapy. The role of MRI in adaptive prospective trials remains an area of active research.
Fig. 3.
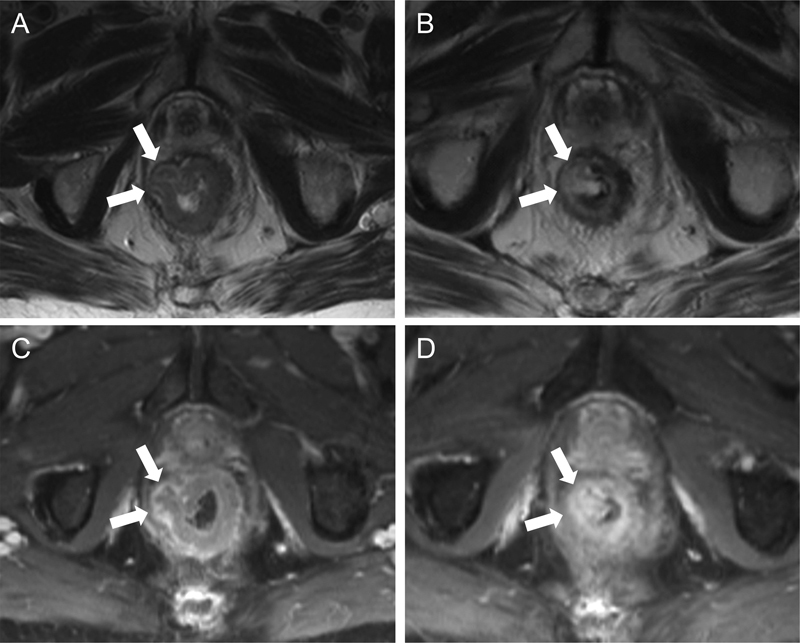
Rectal tumor response to neoadjuvant therapy on MRI. Transaxial T2WIs (A and B) and transaxial T1WIs with gadolinium-based contrast (C and D) were obtained before (A and C) and after (B and D) neoadjuvant therapy. A nodular rectal tumor arising from the right rectal wall and protruding into the adjacent fat was T2-intermediate (arrows in [A]) and peripherally enhancing (arrows in [C]) on initial imaging. After neoadjuvant therapy, the tumor became T2-hyperintense (arrows in [B]) relative to the normal rectal submucosa, suggestive of necrosis/degeneration; a discrete extramural enhancing nodule was no longer evident on the postcontrast images (arrows in [D]). Histologic specimens from subsequent resection showed a complete pathologic response. MRI, magnetic resonance imaging; T1WI, T1-weighted images; T2WI, T2-weighted images.
As with TRUS and CT, the determination of nodal status in rectal cancer remains challenging for MRI, in marked contrast to its high accuracy in T-staging. MRI is sensitive for the detection of enlarged pelvic lymph nodes and is capable of assessing stations outside of the mesorectal space, in contradistinction to the relatively limited field of view of TRUS. However, many lymph nodes not meeting standard size criteria (i.e., > 1 cm) for lymphadenopathy contain metastatic deposits when evaluated by histopathology.24 25 Using a short-axis diameter of ≤ 5 mm as an indicator of the benignity by MRI results in 94% sensitivity and a negative predictive value of 86% but a specificity of 13% and overall accuracy of only 34%.45 Though accuracy of N-staging may be improved by MRI performed with nodal compounds such as superparamagnetic iron oxide, these contrast agents are not yet available for use in the United States.46 Interestingly, the MERCURY Study Group found that pelvic sidewall nodes (i.e., nodes lateral to the mesorectal fascia) deemed suspicious on initial MRI independently conferred a worse 5-year disease-free survival in patients undergoing surgery only but not in patients treated with neoadjuvant therapy.47 While this finding suggests that MRI may provide utility in identifying patients with possible nodal metastasis to undergo presurgical chemoradiation, further research is needed to determine the optimal combination of MRI features for accurately predicting nodal disease status. As will be discussed in subsequent sections, the combination of PET and MRI may provide additional value in determining accurate nodal stage.
Distant Metastases: Diagnosing and Managing Hepatic Disease Burden
Despite the differences between colon cancer and rectal cancer with respect to locoregional staging and treatment strategies, their diagnostic and therapeutic algorithms for distant metastases are the same. The liver is the most common location of distant metastatic disease in CRC, with roughly 30% of patients presenting with synchronous liver metastases and 70% ultimately developing hepatic disease.2 As with locoregional management of rectal cancer, complete surgical resection is the treatment paradigm for colorectal liver metastases.
Preoperative imaging is critical for determining the size and number of metastatic lesions and their relationship to surrounding anatomic landmarks. Surgical resection is considered suitable if the metastases can be completely resected, if at least two adjacent liver segments can be spared, and if the future liver remnant (FLR) is adequate in size—usually ≥ 20% of total liver volume.3 Additionally, patients with metastatic burdens not suitable for immediate resection generally receive neoadjuvant chemotherapy, typically consisting of 5-FU plus irinotecan or oxaliplatin, with or without bevacizumab.5 Minimally invasive procedures, such as cryoablation, radiofrequency ablation, microwave ablation, chemoembolization, or radioembolization, may provide an alternate means of converting hepatic metastases to a resectable state.48 Following neoadjuvant chemotherapy or percutaneous interventions, imaging plays a key role in assessing the response to treatment and determining the technical feasibility of resection, the ultimate determinant of survival.6
Computed Tomography
When contrast-enhanced protocols are employed, CT can identify liver metastases with 97% specificity, 90% negative predictive value, 73% sensitivity, and 85% overall accuracy.21 23 49 Multiphase acquisitions by multidetector CT with appropriate bolus timing and optimized imaging parameters have even been shown to outperform MRI,50 though such results were obtained before the advent of the hepatobiliary contrast agents (as discussed below). Importantly, CT has impaired sensitivity in the setting of hepatic steatosis, which can obscure hypodense focal liver lesions. This limitation is especially relevant after neoadjuvant therapy, as several of the chemotherapeutic agents commonly used in this setting (5-FU and irinotecan) can induce lipid accumulation in the liver,51 thereby reducing the conspicuity of liver metastases.
Nuclear Medicine
The primary role of PET/CT in CRC is the assessment for nodal and distant metastatic disease. Typically performed with the radiolabeled glucose analogue 2-deoxy-2-[18F]fluoro-D-glucose (FDG), PET/CT has high sensitivity (89%) for metastatic disease.52 A major advantage of PET/CT over CT and MRI is its high sensitivity for nodal and distant metastasis in patients for whom high-morbidity, intent-to-cure surgeries are planned. However, because the relatively high uptake of FDG by the normal hepatic parenchyma can obscure focal hypermetabolic lesions, PET/CT has substantially lower sensitivity for liver metastases (55%) than either contrast-enhanced CT or MRI.52 53 The sensitivity of PET/CT for residual hepatic disease after neoadjuvant therapy is similarly impaired. Consequently, PET/CT should not be used as the sole modality for surgical planning in this setting.54 However, PET/CT may have utility in monitoring the response of liver metastases to neoadjuvant therapy via standardized uptake values (SUVs), which allow for quantification of metabolic activity.55
Magnetic Resonance Imaging
By virtue of its high soft tissue contrast, MRI has become the examination of choice in many institutions for the evaluation of liver lesions, including metastases from CRC. A meta-analysis comparing CT, MRI, and FDG-PET (without CT) found per-lesion sensitivities for untreated colorectal liver metastases of 74, 80, and 81%, respectively; MRI performed especially well compared with CT for lesions < 10 mm in diameter.56 Importantly, these numbers were derived from studies performed before the introduction of hepatobiliary phase MRI, which further improves the sensitivity of MRI for liver metastases relative to CT. In contrast to the conventional gadolinium-based extracellular contrast agents, the hepatobiliary contrast agents (HBCAs) undergo dual elimination, with a portion of contrast excreted by glomerular filtration and another portion excreted by the biliary system following uptake by hepatocytes.57 For the HBCA gadoxetic acid (Eovist/Primovist, Bayer HealthCare LLC., Whippany, NJ), peak hepatic enhancement occurs around 20 minutes after administration. Lesions containing a relative paucity of functional hepatocytes, such as metastases, will appear hypointense on T1-weighted images (T1WIs) relative to the surrounding liver parenchyma. With HBCAs, MRI has been shown to have a 17% higher sensitivity than CT for liver metastases, with even greater differences for lesions < 10 mm.58 59 This sensitivity advantage of MRI over CT is especially pronounced in the setting of hepatic steatosis (Fig. 4), when typically hypodense colorectal liver metastases become less apparent on CT.60
Fig. 4.
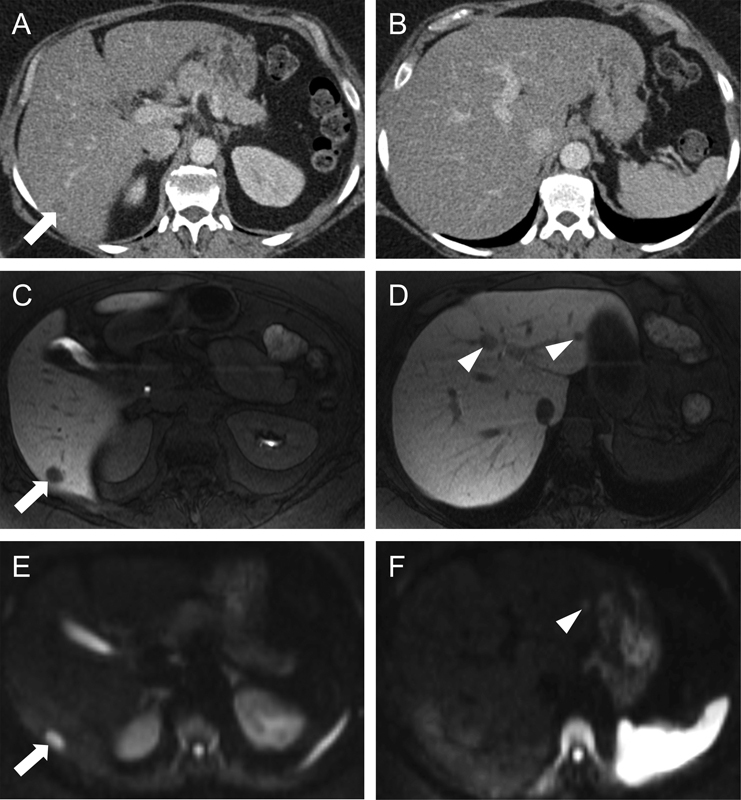
Colorectal liver metastasis conspicuity on CT versus MRI. Transaxial contrast-enhanced CT images (A and B) reveal diffuse hepatic steatosis with a subtle hypodense mass in segment 6 (arrow in [A]) but no definite disease more superiorly (B). The background hepatic steatosis, as manifested by diffuse liver hypoattenuation, made the detection of liver metastases challenging. On an MRI obtained around the same time, transaxial T1WIs acquired with a HBCA display the same segment 6 metastasis much more conspicuously as a focal hypointense lesion (arrow in [C]). Additional metastases were evident more superiorly within segments 2 and 4A (arrowheads in [D]), at a level where no disease was appreciated on CT (B). Such differences in perceived hepatic disease burden can have significant implications for resectability. Transaxial diffusion-weighted images (E and F) likewise demonstrated the segment 6 lesion (arrow in [E]) and the segment 2 lesion (arrowhead in [F]). The segment 4A lesion seen on hepatobiliary phase images (D) was visible on a more superior slice of the diffusion-weighted images (not shown). CT, computed tomography; HBCA, hepatobiliary contrast agents; MRI, magnetic resonance imaging; T2WI, T2-weighted images.
An important consideration in using a highly sensitive modality such as HBCA MRI is the false-positive rate. Any focal liver lesion (benign or malignant) containing a paucity of functional hepatocytes relative to normal liver will have a T1-hypointense appearance on hepatobiliary phase images. Consequently, there has been concern that the use of HBCAs might result in more false-positive diagnoses of hepatic metastasis or might overestimate the total hepatic disease burden. A prospective study using intraoperative ultrasound and histopathology found a false-positive rate of 3.9% (10 of 257 findings; 95% confidence interval: 1.9%, 7.1%) for contrast-enhanced CT.61 The false-positive rate HBCA MRI for liver metastases is less definitively established. Using histopathology as the reference standard, one study in which three readers independently evaluated the hepatic metastatic burden for 32 patients with 96 proven metastases found a false-positive rate for HBCA MRI of 8.4% (8 of 95 findings) to 11.1% (11 of 99 findings).58 For example, it is relatively common in clinical practice to struggle with differentiating certain benign liver lesions from small mucinous colorectal metastases. Further research is needed to determine the false-positive rate of HBCA MRI in prospective studies. In our practice, patients often initially undergo MRI with conventional extracellular contrast agents or contrast-enhanced CT, followed by HBCA MRI for monitoring treatment response and for surgical planning.
In addition to HBCA MRI, several authors have investigated the use of diffusion-weighted imaging (DWI) for both lesion characterization and detection. DWI is a type of MRI acquisition in which water molecules located in environments restricting their movement produce more signal and appear brighter than water molecules located in environments permitting relatively unhindered motion. The high cellular density of malignant tumors results in restricted diffusion of cytoplasmic water molecules, causing lesions such as liver metastases to be bright on DWI (Fig. 4). The addition of DWI to standard protocols can improve the sensitivity and specificity of MRI for colorectal liver metastases.62 63 As mentioned above, colorectal liver metastases with mucinous histology are often T2-hyperintense and nonenhancing and can appear nearly identical to hepatic cysts or hemangiomas on MRI. However, mucinous metastases, unlike these benign lesions, may exhibit restricted diffusion, potentially allowing DWI to distinguish among these entities. As expected from its ability to differentiate hypercellular tumor from benign hepatic masses, DWI has been found to increase the overall accuracy of MRI for focal liver lesions.64 Despite these improvements in MRI from both DWI and HBCAs (Table 4), the impact of false-positive lesions is not entirely understood, and MRI quality may vary from institution to institution. Some institutions remain loyal to CT for the staging of distant CRC metastases and have published excellent outcomes.65 66
Table 4. Benefits of hepatobiliary contrast agents and diffusion-weighted imaging.
| MRI with hepatobiliary contrast agents • Higher sensitivity than CT for liver metastases, especially for lesions < 10 mm58 59 • Superior to CT for detecting liver metastases in setting of hepatic steatosis60 |
| MRI with diffusion-weighted imaging • Improved sensitivity and specificity for liver metastases, relative to standard MRI62 63 • Increased accuracy for diagnosis of focal liver lesions, relative to standard MRI64 |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
A final important concept to address when discussing liver MRI for operative planning after neoadjuvant treatment of hepatic metastases is that of the so-called “disappearing” lesions. In patients undergoing chemotherapy to reduce hepatic disease burden before surgical resection, some initially identified lesions not only respond to treatment but also cease to be visible on subsequent imaging (Fig. 5). Even in the case of complete imaging response, residual disease is still found at surgery and on pathology in a significant percentage of patients.67 To this point, one study of 242 patients with liver metastases from CRC found that patients who underwent HBCA MRI both before and after neoadjuvant chemotherapy had significantly lower rates of intrahepatic recurrence (48 vs. 65%, p = 0.04) and fewer repeat hepatectomies (13 vs. 25%, p = 0.03), compared with patients who underwent hepatobiliary phase MRI only after neoadjuvant therapy.68 As the authors explain, when hepatobiliary phase MRI was obtained only after neoadjuvant therapy, the surgical resection may have inappropriately excluded radiographically occult but still viable lesions that had “disappeared” during the course of preoperative chemotherapy, leading to higher rates of recurrence. Consequently, the goal of surgical resection should be to include all lesions identified at the time of initial imaging, even if such lesions are no longer apparent on repeat imaging after neoadjuvant therapy.
Fig. 5.
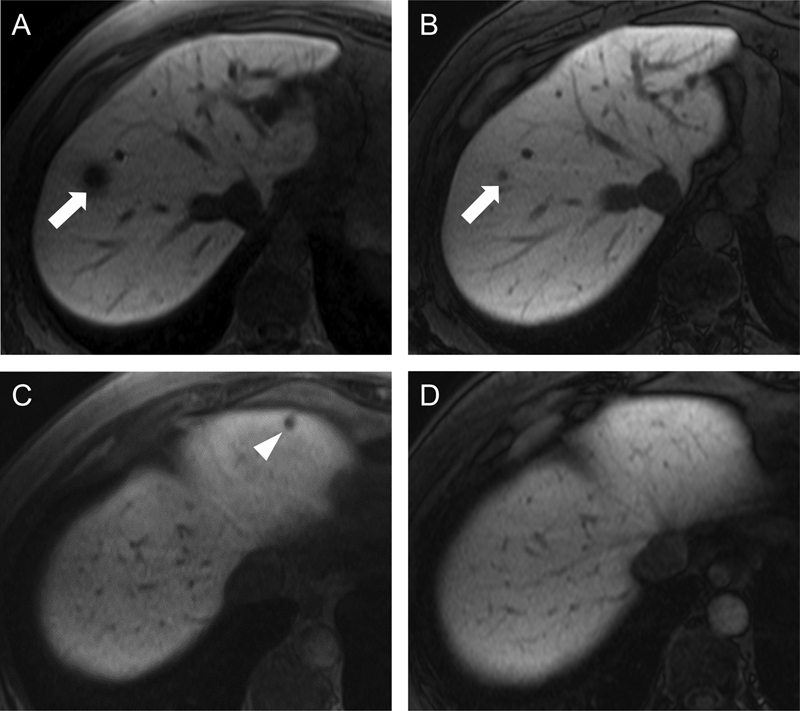
Disappearing hepatic metastases on MRI. Transaxial T1WIs were obtained 20 minutes after gadoxetic acid administration (i.e., in the hepatobiliary phase), both before (A and C) and after (B and D) neoadjuvant therapy, in a patient initially deemed to have unresectable liver disease. A metastasis in segment 8 (arrow in [A]) had significantly decreased in size but was still identifiable on the posttreatment images (arrow in [B]). In contrast, a segment 2 metastasis (arrowhead in [C]) was no longer visible on the posttreatment images (D). Importantly, the disappearance of lesions on imaging does not imply complete pathologic response, as microscopic disease may persist. MRI, magnetic resonance imaging; T1WI, T1-weighted images.
New Imaging Modalities in Colorectal Cancer: PET/MRI
As discussed previously, PET/CT has revolutionized oncologic imaging by exploiting metabolic differences between malignant tumors and normal background tissues and, consequently, has become the standard of care for the initial staging and subsequent restaging of many oncologic conditions. However, PET/CT has several important limitations, especially with respect to local tumor staging and the diagnosis of malignant spread to organs with high background FDG uptake, such as the liver, brain, and heart. In such scenarios, MRI is commonly acquired to supplement the information obtained from PET/CT and thereby ensure optimal staging accuracy. As the newest clinical hybrid imaging modality, PET/MRI combines the metabolic information of PET (conferring sensitivity for nodal and distant metastasis) with the excellent soft-tissue contrast of MRI (conferring high T-staging accuracy) and thus has the potential to serve as a single comprehensive imaging examination for CRC (Fig. 6).
Fig. 6.
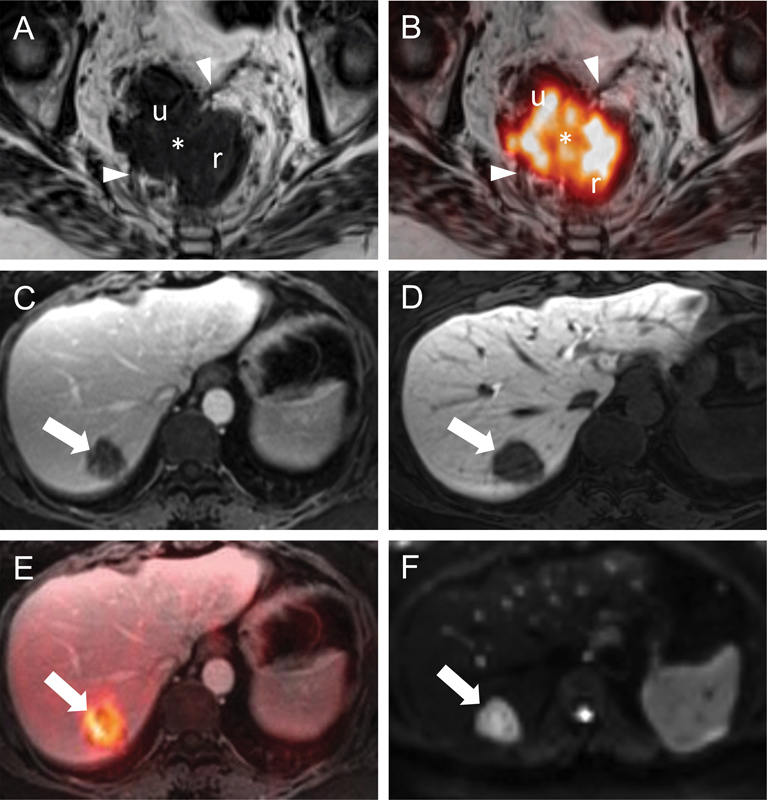
Locoregional and hepatic staging of rectal cancer by PET/MRI. Transaxial T2WIs (A) through the rectum (r) show a right anterolateral rectal tumor (asterisk) that invades the mesorectal fascia (arrowheads). The relationship of the tumor (i.e., abutment vs. invasion) to the uterus (u) is unclear on this image alone. Transaxial T2WIs with FDG-PET fusion (B) at the same level better delineate the borders of this FDG-avid mass (asterisk) with respect to the mesorectal fascia (arrowheads) and confirm invasion of the left posterolateral aspect of the uterus (u). A normal-sized right internal iliac lymph node (not shown) was also FDG-avid and suspicious for regional nodal disease. Transaxial T1WIs (C and D) obtained with gadoxetic acid (Eovist, Bayer HealthCare LLC., Whippany, NJ) in the portal venous (C) and hepatobiliary (D) phases show a well-defined hypointense mass (arrows). This mass is FDG-avid on the fused FDG-PET images (arrow in [E]) and diffusion-restricting on DWI (arrow in [F]). These findings are consistent with a colorectal liver metastasis. DWI, diffusion-weighted imaging; FDG, 2-deoxy-2-[18F]fluoro-D-glucose; PET; positron emission tomography; MRI, magnetic resonance imaging; T2WI, T2-weighted images.
While much research is still needed to generate the data necessary to support the routine clinical use of PET/MRI, several small studies have examined the utility of PET/MRI in the evaluation of CRC and liver metastases. For example, a pilot study of 12 patients with CRC found PET/MRI to provide higher accuracy for T-staging than PET/CT; of course, such results require validation in larger studies.69 Another study of 15 patients with CRC found PET/MRI with DWI to have higher accuracy than PET/CT for the diagnosis of liver metastases, with comparable accuracy for peritoneal lesions and lymph node metastases.70 More generally, PET/MRI has been shown to increase radiologist confidence for the diagnosis of liver metastasis (from CRC and other primaries), compared with PET/CT.71 This study also found PET/MRI to be more sensitive (p = 0.002) than PET/CT for liver metastases. As with MRI alone, PET/MRI can be also used to monitor anatomic responses to neoadjuvant therapy in locally advanced rectal cancer. In this setting, tumor regression indices, as assessed by MRI after neoadjuvant therapy but before surgical resection, are predictive of clinical outcomes.43 44 Furthermore, rectal cancer SUV reductions on FDG-PET following neoadjuvant therapy have been shown to increase the likelihood of a complete pathologic response at surgery.72 Thus, PET/MRI has the ability to serve as a comprehensive indicator of response to neoadjuvant therapy by simultaneously interrogating both anatomic and metabolic tumor alterations. Such information could potentially be used to select patients with favorable responses for less invasive surgical approaches, such as transanal resection. Overall, PET/MRI is a promising modality for the staging of CRC, as well as for surgical planning, assessing response to neoadjuvant therapies, and monitoring for recurrent disease.
Conclusions
MRI is critical for identifying rectal cancer patients with high-risk primary tumor characteristics, such as T4 status, extramural vascular invasion, circumferential resection margin less than 1 mm, or extramural depth of greater than 5 mm. Such patients benefit from neoadjuvant chemoradiation for local tumor control, followed by preoperative MRI for assessing tumor response and for surgical planning. Staging for distant metastases, in particular liver metastases, should be considered in this high-risk rectal cancer population. For diagnosing and managing liver metastases, both CT and MRI provide high accuracy, with several studies suggesting superiority of MRI with HBCAs and DWI over CT. Patients with complex or bilobar hepatic disease should receive neoadjuvant chemotherapy, with the ultimate goal of making an intent-to-cure resection feasible. Furthermore, HBCA MRI has proven useful for monitoring the response of liver metastases to neoadjuvant treatments and for subsequent surgical planning. Finally, PET/MRI is a promising new hybrid modality that promises high accuracy of locoregional tumor staging, as well as high sensitivity for liver metastases (MRI), nodal metastases (PET), and distant extrahepatic disease (PET). PET data reflect tumor metabolism may be useful in quantifying treatment response.
References
- 1.Siegel R L, Miller K D, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier A-M. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberts S R, Poston G J. Treatment advances in liver-limited metastatic colorectal cancer. Clin Colorectal Cancer. 2011;10(4):258–265. doi: 10.1016/j.clcc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Berri R N, Abdalla E K. Curable metastatic colorectal cancer: recommended paradigms. Curr Oncol Rep. 2009;11(3):200–208. doi: 10.1007/s11912-009-0029-z. [DOI] [PubMed] [Google Scholar]
- 5.Wagman L D. Importance of response to neoadjuvant therapy in patients with liver-limited mCRC when the intent is resection and/or ablation. Clin Colorectal Cancer. 2013;12(4):223–232. doi: 10.1016/j.clcc.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Folprecht G, Grothey A, Alberts S, Raab H-R, Köhne C-H. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16(8):1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 7.Abdalla E K Vauthey J-N Ellis L M et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases Ann Surg 20042396818–825., discussion 825–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foxtrot Collaborative Group . Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13(11):1152–1160. doi: 10.1016/S1470-2045(12)70348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glynne-Jones R, Tan D, Goh V. Pelvic MRI for guiding treatment decisions in rectal cancer. Oncology (Williston Park) 2014;28(8):667–677. [PubMed] [Google Scholar]
- 10.Nogué M, Salud A, Vicente P. et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16(5):614–620. doi: 10.1634/theoncologist.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velenik V, Ocvirk J, Music M. et al. Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: results of an open-label phase II study. Radiat Oncol. 2011;6:105. doi: 10.1186/1748-717X-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog U, von Flüe M, Tondelli P, Schuppisser J P. How accurate is endorectal ultrasound in the preoperative staging of rectal cancer? Dis Colon Rectum. 1993;36(2):127–134. doi: 10.1007/BF02051167. [DOI] [PubMed] [Google Scholar]
- 13.Jochem R J, Reading C C, Dozois R R, Carpenter H A, Wolff B G, Charboneau J W. Endorectal ultrasonographic staging of rectal carcinoma. Mayo Clin Proc. 1990;65(12):1571–1577. doi: 10.1016/s0025-6196(12)62192-2. [DOI] [PubMed] [Google Scholar]
- 14.Snady H, Merrick M A. Improving the treatment of colorectal cancer: the role of EUS. Cancer Invest. 1998;16(8):572–581. doi: 10.3109/07357909809032887. [DOI] [PubMed] [Google Scholar]
- 15.Waizer A, Zitron S, Ben-Baruch D, Baniel J, Wolloch Y, Dintsman M. Comparative study for preoperative staging of rectal cancer. Dis Colon Rectum. 1989;32(1):53–56. doi: 10.1007/BF02554726. [DOI] [PubMed] [Google Scholar]
- 16.Yimei J, Ren Z, Lu X, Huan Z. A comparison between the reference values of MRI and EUS and their usefulness to surgeons in rectal cancer. Eur Rev Med Pharmacol Sci. 2012;16(15):2069–2077. [PubMed] [Google Scholar]
- 17.Jürgensen C, Teubner A, Habeck J-O, Diener F, Scherübl H, Stölzel U. Staging of rectal cancer by EUS: depth of infiltration in T3 cancers is important. Gastrointest Endosc. 2011;73(2):325–328. doi: 10.1016/j.gie.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Badger S A, Devlin P B, Neilly P JD, Gilliland R. Preoperative staging of rectal carcinoma by endorectal ultrasound: is there a learning curve? Int J Colorectal Dis. 2007;22(10):1261–1268. doi: 10.1007/s00384-007-0273-3. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Esparrach G, Ayuso-Colella J R, Sendino O. et al. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointest Endosc. 2011;74(2):347–354. doi: 10.1016/j.gie.2011.03.1257. [DOI] [PubMed] [Google Scholar]
- 20.Landmann R G, Wong W D, Hoepfl J. et al. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum. 2007;50(10):1520–1525. doi: 10.1007/s10350-007-9019-0. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharjya S, Bhattacharjya T, Baber S, Tibballs J M, Watkinson A F, Davidson B R. Prospective study of contrast-enhanced computed tomography, computed tomography during arterioportography, and magnetic resonance imaging for staging colorectal liver metastases for liver resection. Br J Surg. 2004;91(10):1361–1369. doi: 10.1002/bjs.4699. [DOI] [PubMed] [Google Scholar]
- 22.Balthazar E J, Megibow A J, Hulnick D, Naidich D P. Carcinoma of the colon: detection and preoperative staging by CT. AJR Am J Roentgenol. 1988;150(2):301–306. doi: 10.2214/ajr.150.2.301. [DOI] [PubMed] [Google Scholar]
- 23.Zerhouni E A, Rutter C, Hamilton S R. et al. CT and MR imaging in the staging of colorectal carcinoma: report of the Radiology Diagnostic Oncology Group II. Radiology. 1996;200(2):443–451. doi: 10.1148/radiology.200.2.8685340. [DOI] [PubMed] [Google Scholar]
- 24.Ju H, Xu D, Li D, Chen G, Shao G. Comparison between endoluminal ultrasonography and spiral computerized tomography for the preoperative local staging of rectal carcinoma. Biosci Trends. 2009;3(2):73–76. [PubMed] [Google Scholar]
- 25.Perez R O, Pereira D D, Proscurshim I. et al. Lymph node size in rectal cancer following neoadjuvant chemoradiation—can we rely on radiologic nodal staging after chemoradiation? Dis Colon Rectum. 2009;52(7):1278–1284. doi: 10.1007/DCR.0b013e3181a0af4b. [DOI] [PubMed] [Google Scholar]
- 26.Kim S H, Lee J M, Lee M W, Kim G H, Han J K, Choi B I. Diagnostic accuracy of 3.0-Tesla rectal magnetic resonance imaging in preoperative local staging of primary rectal cancer. Invest Radiol. 2008;43(8):587–593. doi: 10.1097/RLI.0b013e31817e9083. [DOI] [PubMed] [Google Scholar]
- 27.Rafaelsen S R, Vagn-Hansen C, Sørensen T, Pløen J, Jakobsen A. Transrectal ultrasound and magnetic resonance imaging measurement of extramural tumor spread in rectal cancer. World J Gastroenterol. 2012;18(36):5021–5026. doi: 10.3748/wjg.v18.i36.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phang P T, Gollub M J, Loh B D. et al. Accuracy of endorectal ultrasound for measurement of the closest predicted radial mesorectal margin for rectal cancer. Dis Colon Rectum. 2012;55(1):59–64. doi: 10.1097/DCR.0b013e318235b885. [DOI] [PubMed] [Google Scholar]
- 29.Li J CM, Liu S YW, Lo A WI. et al. The learning curve for endorectal ultrasonography in rectal cancer staging. Surg Endosc. 2010;24(12):3054–3059. doi: 10.1007/s00464-010-1085-z. [DOI] [PubMed] [Google Scholar]
- 30.MERCURY Study Group . Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243(1):132–139. doi: 10.1148/radiol.2431051825. [DOI] [PubMed] [Google Scholar]
- 31.Del Vescovo R, Trodella L E, Sansoni I. et al. MR imaging of rectal cancer before and after chemoradiation therapy. Radiol Med (Torino) 2012;117(7):1125–1138. doi: 10.1007/s11547-012-0804-2. [DOI] [PubMed] [Google Scholar]
- 32.Kim S H, Lee J M, Park H S, Eun H W, Han J K, Choi B I. Accuracy of MRI for predicting the circumferential resection margin, mesorectal fascia invasion, and tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging. 2009;29(5):1093–1101. doi: 10.1002/jmri.21742. [DOI] [PubMed] [Google Scholar]
- 33.Purkayastha S, Tekkis P P, Athanasiou T, Tilney H S, Darzi A W, Heriot A G. Diagnostic precision of magnetic resonance imaging for preoperative prediction of the circumferential margin involvement in patients with rectal cancer. Colorectal Dis. 2007;9(5):402–411. doi: 10.1111/j.1463-1318.2006.01104.x. [DOI] [PubMed] [Google Scholar]
- 34.Videhult P, Smedh K, Lundin P, Kraaz W. Magnetic resonance imaging for preoperative staging of rectal cancer in clinical practice: high accuracy in predicting circumferential margin with clinical benefit. Colorectal Dis. 2007;9(5):412–419. doi: 10.1111/j.1463-1318.2006.01167.x. [DOI] [PubMed] [Google Scholar]
- 35.Wieder H A, Rosenberg R, Lordick F. et al. Rectal cancer: MR imaging before neoadjuvant chemotherapy and radiation therapy for prediction of tumor-free circumferential resection margins and long-term survival. Radiology. 2007;243(3):744–751. doi: 10.1148/radiol.2433060421. [DOI] [PubMed] [Google Scholar]
- 36.MERCURY Study Group . Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang G J, You Y N, Park I J. et al. Pretreatment high-resolution rectal MRI and treatment response to neoadjuvant chemoradiation. Dis Colon Rectum. 2012;55(4):371–377. doi: 10.1097/DCR.0b013e31824678e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter C J, Garant A, Vuong T. et al. Adverse features on rectal MRI identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann Surg Oncol. 2012;19(4):1199–1205. doi: 10.1245/s10434-011-2036-1. [DOI] [PubMed] [Google Scholar]
- 39.Hofheinz R-D, Wenz F, Post S. et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–588. doi: 10.1016/S1470-2045(12)70116-X. [DOI] [PubMed] [Google Scholar]
- 40.Loos M, Quentmeier P, Schuster T. et al. Effect of preoperative radio(chemo)therapy on long-term functional outcome in rectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20(6):1816–1828. doi: 10.1245/s10434-012-2827-z. [DOI] [PubMed] [Google Scholar]
- 41.Engelen S ME, Maas M, Lahaye M J. et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer. 2013;49(10):2311–2320. doi: 10.1016/j.ejca.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Taylor F GM, Quirke P, Heald R J. et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253(4):711–719. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- 43.Patel U B, Taylor F, Blomqvist L. et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29(28):3753–3760. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- 44.Shihab O C, Taylor F, Salerno G. et al. MRI predictive factors for long-term outcomes of low rectal tumours. Ann Surg Oncol. 2011;18(12):3278–3284. doi: 10.1245/s10434-011-1776-2. [DOI] [PubMed] [Google Scholar]
- 45.Doyon F, Attenberger U I, Dinter D J, Schoenberg S O, Post S, Kienle P. Clinical relevance of morphologic MRI criteria for the assessment of lymph nodes in patients with rectal cancer. Int J Colorectal Dis. 2015;30(11):1541–1546. doi: 10.1007/s00384-015-2339-y. [DOI] [PubMed] [Google Scholar]
- 46.Koh D-M, George C, Temple L. et al. Diagnostic accuracy of nodal enhancement pattern of rectal cancer at MRI enhanced with ultrasmall superparamagnetic iron oxide: findings in pathologically matched mesorectal lymph nodes. AJR Am J Roentgenol. 2010;194(6):W505-13. doi: 10.2214/AJR.08.1819. [DOI] [PubMed] [Google Scholar]
- 47.Shihab O C, Taylor F, Bees N. et al. Relevance of magnetic resonance imaging-detected pelvic sidewall lymph node involvement in rectal cancer. Br J Surg. 2011;98(12):1798–1804. doi: 10.1002/bjs.7662. [DOI] [PubMed] [Google Scholar]
- 48.Mahnken A H, Pereira P L, de Baère T. Interventional oncologic approaches to liver metastases. Radiology. 2013;266(2):407–430. doi: 10.1148/radiol.12112544. [DOI] [PubMed] [Google Scholar]
- 49.Cance W G, Cohen A M, Enker W E, Sigurdson E R. Predictive value of a negative computed tomographic scan in 100 patients with rectal carcinoma. Dis Colon Rectum. 1991;34(9):748–751. doi: 10.1007/BF02051063. [DOI] [PubMed] [Google Scholar]
- 50.Numminen K, Isoniemi H, Halavaara J. et al. Preoperative assessment of focal liver lesions: multidetector computed tomography challenges magnetic resonance imaging. Acta Radiol. 2005;46(1):9–15. doi: 10.1080/02841850510016108. [DOI] [PubMed] [Google Scholar]
- 51.Zorzi D, Laurent A, Pawlik T M, Lauwers G Y, Vauthey J-N, Abdalla E K. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94(3):274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 52.Ramos E, Valls C, Martinez L. et al. Preoperative staging of patients with liver metastases of colorectal carcinoma. Does PET/CT really add something to multidetector CT? Ann Surg Oncol. 2011;18(9):2654–2661. doi: 10.1245/s10434-011-1670-y. [DOI] [PubMed] [Google Scholar]
- 53.Shin S S, Jeong Y Y, Min J J, Kim H R, Chung T W, Kang H K. Preoperative staging of colorectal cancer: CT vs. integrated FDG PET/CT. Abdom Imaging. 2008;33(3):270–277. doi: 10.1007/s00261-007-9262-9. [DOI] [PubMed] [Google Scholar]
- 54.Spatz J, Holl G, Sciuk J, Anthuber M, Arnholdt H M, Märkl B. Neoadjuvant chemotherapy affects staging of colorectal liver metastasis—a comparison of PET, CT and intraoperative ultrasound. Int J Colorectal Dis. 2011;26(2):165–171. doi: 10.1007/s00384-010-1065-8. [DOI] [PubMed] [Google Scholar]
- 55.Capirci C, Rubello D, Pasini F. et al. The role of dual-time combined 18-fluorodeoxyglucose positron emission tomography and computed tomography in the staging and restaging workup of locally advanced rectal cancer, treated with preoperative chemoradiation therapy and radical surgery. Int J Radiat Oncol Biol Phys. 2009;74(5):1461–1469. doi: 10.1016/j.ijrobp.2008.10.064. [DOI] [PubMed] [Google Scholar]
- 56.Niekel M C, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257(3):674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 57.Zech C J, Herrmann K A, Reiser M F, Schoenberg S O. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci. 2007;6(1):43–52. doi: 10.2463/mrms.6.43. [DOI] [PubMed] [Google Scholar]
- 58.Scharitzer M, Ba-Ssalamah A, Ringl H. et al. Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur Radiol. 2013;23(8):2187–2196. doi: 10.1007/s00330-013-2824-z. [DOI] [PubMed] [Google Scholar]
- 59.Muhi A, Ichikawa T, Motosugi U. et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2011;34(2):326–335. doi: 10.1002/jmri.22613. [DOI] [PubMed] [Google Scholar]
- 60.Kulemann V, Schima W, Tamandl D. et al. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol. 2011;79(2):e1–e6. doi: 10.1016/j.ejrad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Valls C, Andía E, Sánchez A. et al. Hepatic metastases from colorectal cancer: preoperative detection and assessment of resectability with helical CT. Radiology. 2001;218(1):55–60. doi: 10.1148/radiology.218.1.r01dc1155. [DOI] [PubMed] [Google Scholar]
- 62.Bruegel M, Gaa J, Waldt S. et al. Diagnosis of hepatic metastasis: comparison of respiration-triggered diffusion-weighted echo-planar MRI and five t2-weighted turbo spin-echo sequences. AJR Am J Roentgenol. 2008;191(5):1421–1429. doi: 10.2214/AJR.07.3279. [DOI] [PubMed] [Google Scholar]
- 63.Parikh T, Drew S J, Lee V S. et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246(3):812–822. doi: 10.1148/radiol.2463070432. [DOI] [PubMed] [Google Scholar]
- 64.Holzapfel K, Eiber M J, Fingerle A A, Bruegel M, Rummeny E J, Gaa J. Detection, classification, and characterization of focal liver lesions: Value of diffusion-weighted MR imaging, gadoxetic acid-enhanced MR imaging and the combination of both methods. Abdom Imaging. 2012;37(1):74–82. doi: 10.1007/s00261-011-9758-1. [DOI] [PubMed] [Google Scholar]
- 65.Brouquet A, Abdalla E K, Kopetz S. et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29(8):1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shindoh J, Loyer E M, Kopetz S. et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30(36):4566–4572. doi: 10.1200/JCO.2012.45.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bischof D A, Clary B M, Maithel S K, Pawlik T M. Surgical management of disappearing colorectal liver metastases. Br J Surg. 2013;100(11):1414–1420. doi: 10.1002/bjs.9213. [DOI] [PubMed] [Google Scholar]
- 68.Knowles B, Welsh F KS, Chandrakumaran K, John T G, Rees M. Detailed liver-specific imaging prior to pre-operative chemotherapy for colorectal liver metastases reduces intra-hepatic recurrence and the need for a repeat hepatectomy. HPB (Oxford) 2012;14(5):298–309. doi: 10.1111/j.1477-2574.2012.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paspulati R M, Partovi S, Herrmann K A, Krishnamurthi S, Delaney C P, Nguyen N C. Comparison of hybrid FDG PET/MRI compared with PET/CT in colorectal cancer staging and restaging: a pilot study. Abdom Imaging. 2015;40(6):1415–1425. doi: 10.1007/s00261-015-0474-0. [DOI] [PubMed] [Google Scholar]
- 70.Brendle C, Schwenzer N F, Rempp H. et al. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur J Nucl Med Mol Imaging. 2016;43(1):123–132. doi: 10.1007/s00259-015-3137-z. [DOI] [PubMed] [Google Scholar]
- 71.Donati O F, Hany T F, Reiner C S. et al. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J Nucl Med. 2010;51(5):692–699. doi: 10.2967/jnumed.109.068510. [DOI] [PubMed] [Google Scholar]
- 72.Cascini G L, Avallone A, Delrio P. et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47(8):1241–1248. [PubMed] [Google Scholar]


