Highlights
-
•
Colon is a potential site for solitary metastasis from renal cell carcinoma (RCC).
-
•
Recurrent disease after curative nephrectomy usually occurs within three years.
-
•
Metastatic RCC should be considered in RCC patients with bowel obstruction.
Abbreviations: AUA, American Urological Association; NCCN, National Comprehensive Cancer Network; OS, overall survival; RCC, renal cell carcinoma
Keywords: Carcinoma, Renal cell, Neoplasm metastasis
Abstract
Introduction
Renal cell carcinoma (RCC) is the most common renal malignancy in adults. Metastatic disease is relatively common at presentation and frequently involves the lung, bone, brain, liver and adrenal glands. After curative resection, there is a 30–40% risk of recurrence, and a 10% risk of developing metastatic disease after 5 years. The gastrointestinal tract, particularly the colon, represents a very uncommon site of late metastatic disease.
Presentation of Case
We present a case of a 67 year-old-male who underwent a left radical nephrectomy for RCC 9 years before presenting with a metastatic large bowel obstruction. He was later found to have a near-completely obstructing mass in the rectosigmoid colon and underwent a sigmoidectomy with anterior resection of the upper rectum. Histopathology confirmed metastatic RCC confined to the colonic wall with negative microscopic margins.
Discussion
The tendency of RCC to metastasize to unusual sites such as the pancreas or thyroid gland has been widely reported. However, cases of colon metastasis from RCC are extremely rare. Despite the absence of randomized prospective data, widespread consensus supports the surgical treatment of solitary and oligometastatic disease in light of the poor patient outcomes in non-surgically treated disease (Milovic et al., 2013) [3]. Multiple groups have reported favorable outcomes for surgically resected solitary metastatic disease with long disease-free intervals and good performance status.
Conclusion
The colon is a potential, though uncommon, site for solitary metastasis from RCC. The clinical presentation is frequently several years after initial curative resection. Oncologic resection with negative margins may result in long-term survival in patients with isolated metastatic disease.
1. Introduction
Renal cell carcinoma (RCC) accounts for the majority of adult renal malignancies. Surgical resection remains the primary curative treatment in patients with localized disease. Even with appropriate oncologic resection, nearly 40% of patients will develop metastases after nephrectomy, and about 10% will be diagnosed with late metastatic disease after 5 years [1]. The most common sites of metastases are the lung, liver, bone, and brain [2]. Metastasis to the gastrointestinal tract, specifically the colon, is extremely rare. Less than 10 cases of solitary colon metastases have been reported previously in the worldwide in the literature to date (Table 1) [3], [4], [5], [6], [7], [8]. We report a case of a patient who presented with a late recurrence of RCC in the rectosigmoid colon and was treated successfully with colorectal resection.
Table 1.
Literature review of cases of colonic metastases from renal cell carcinoma after nephrectomy.
| Title | Author | Journal | Year Published | Case Details |
|---|---|---|---|---|
| Rare locations of metastatic renal cell carcinoma: a presentation of three cases | Milovic N et al. [3] | Vojnosanit Pregl | 2013 | Patient 1: Patient with a history of RCC developed metastatic disease in the rectosigmoid colon after undergoing previous treatment with left nephrectomy. He underwent a subsequent lymphadenectomy and left hemicolectomy. Patient 2: Patient with history of RCC treated by left radical nephrectomy later developed metastatic disease at multiple sites and underwent partial right nephrectomy, lung metastasectomy, small bowel and cecum resection and right orchiectomy. The patient subsequently died from brain and bone metastases. Patient 3: Patient with RCC treated by right nephrectomy and developed metastatic disease in the cecum four years later. |
| Renal cell carcinoma with colon metastases: an infrequent site for metastases | Valdespino-Castillo VE et al. [4] | Cir Cir | 2008 | Patient 4: Patient with history of RCC who underwent radical nephrectomy and interferon. He presented 8 years later with hematochezia and found to have a splenic flexure tumor and underwent left hemicolectomy. Pathology revealed metastatic RCC. |
| Localization of monoclonal antibody CC49 in colonic metastasis from renal cell carcinoma | Avital S et al. [5] | Eur J Surg Oncol | 1998 | Patient 5: Patient with history of RCC presented with primary colonic tumor 5 years after nephrectomy. A monoclonal antibody (CC49) was used in radio-immuno-guided surgery to localize the tumor, |
| Solitary colonic metastasis of renal cell carcinoma seven years after nephrectomy: a case report | Tokonabe S et al. [6] | Int J Urol | 1996 | Patient 6: Patient with history of right radical nephrectomy 7 years previously for RCC, presented with melena and an abdominal mass. Barium enema, colonoscopy, computed tomography, and arteriography were completed and consistent with a hypervascular mass in the transverse colon. A colectomy was performed and pathologic review revealed metastatic clear cell carcinoma. |
| Solitary colonic metastasis from renal cell carcinoma 17 years after nephrectomy: report of a case | Thomason PA et al. [7] | Dis Colon Rectum | 1991 | Patient 7: Patient with RCC who developed late recurrence 17 years after nephrectomy with metastasis to the colon. |
| Renal cell carcinoma: late recurrence in 2 cases | Ruiz JL et al. [8] | Eur Urol | 1991 | Patient 8: Patient with RCC who developed late recurrence 11 years after nephrectomy with solitary metastatic disease to the colon. Other case in this series developed metastatic disease the externo vastus muscle. |
RCC = renal cell carcinoma.
2. Presentation of case
The patient is a 67-year-old male who initially presented in 2005 with localized clear cell carcinoma of the left kidney. He underwent a left radical nephrectomy through a retroperitoneal approach. Pathology showed T3a (stage III) disease and microscopically negative margins. Five years after initial presentation, the patient was found to have a biopsy-proven left retroperitoneal recurrence in the psoas muscle and underwent laparoscopic excision with negative margins. The patient remained disease-free for five years thereafter.
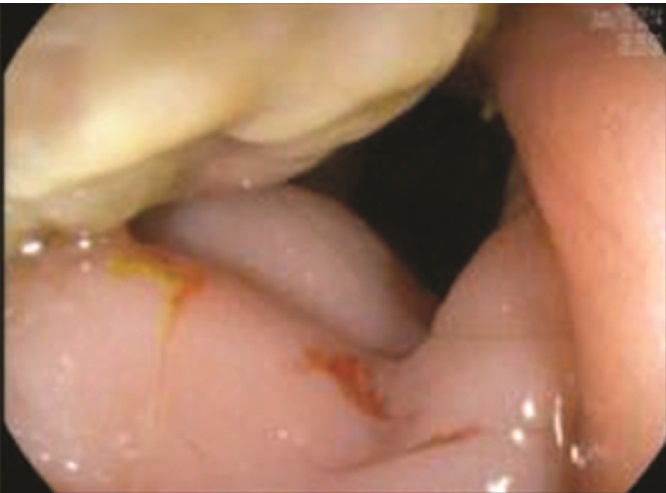
He subsequently presented to his primary care physician complaining of two weeks of nausea, vomiting, abdominal distention, hematochezia, and a decrease in stool caliber. Examination revealed a soft, non-tender, but distended abdomen, and a computed tomography scan showed an infiltrative mass in the distal sigmoid colon (Fig. 1). The patient was admitted and underwent colonoscopy, which revealed a near obstructing, circumferential, ulcerated mass in the lumen of the sigmoid colon, approximately 20 cm from the anal verge (Fig. 2, Fig. 3). The biopsy was non-specific and showed fragments of a fibrinopurulent exudate and normal colonic mucosa without evidence of malignancy.
Fig. 1.
Computed tomography image showing an infiltrative mass in the sigmoid colon.
Fig. 2.
Colonoscopy image showing the near obstructing, circumferential mass in the sigmoid colon.
Fig. 3.
Colonoscopy image showing the ulcerated mass in the sigmoid colon.
The patient developed progressively worsening obstructive symptoms and was taken urgently to the operating room for an exploratory laparotomy. He was found to have a complete obstruction with a competent ileocecal valve and underwent sigmoidectomy with anterior resection of the upper rectum, primary anastomosis and diverting ileostomy. Intraoperative pathology assessment revealed adenocarcinoma, with widely negative circumferential, proximal and distal margins. Final pathology showed a 2.8 cm sigmoid colon mass consistent with metastatic clear cell carcinoma with negative margins as well as diverticulitis with associated abscess formation.
The patient recovered uneventfully and was discharged home on postoperative day 5. He underwent an uneventful ileostomy reversal 12 weeks after the initial resection. During surveillance examination 6 months later, he was found to have liver metastases and is currently undergoing systemic chemotherapy.
3. Discussion
The tendency of RCC to metastasize to unusual sites such as the pancreas or thyroid gland has been widely reported. However, cases of colon metastasis from RCC are extremely rare. A comprehensive review of the English literature yielded less than 10 such cases. The majority of colon metastases were found during work up for symptoms such as lower gastrointestinal bleeding. Solitary metastases, regardless of the site, have been noted in less than 10% of patients with RCC. In particular, colonic metastasis is usually associated with disseminated disease [2]. Saitoh et al. studied 1173 autopsy reports of patients with RCC and reported intestinal involvement in 9% of cases, but none were solitary metastases [2].
The majority of recurrent disease following curative nephrectomy occurs within three years. Late recurrence after five years occurs in less than 10% of patients with recurrent disease, but is typical of colon metastases [1], [9]. The pathologic features of late recurrences tend to be somewhat unique in that they are more often associated with primary tumors of T2 stage or greater, and lower histologic grade [1]. With respect to outcome, patients with late recurrence generally have better prognosis than patients with early recurrence [1].
Despite the absence of randomized prospective data, widespread consensus supports the surgical treatment of solitary and oligometastatic disease in light of the poor patient outcomes in non-surgically treated disease [9]. Multiple groups have reported favorable outcomes for surgically resected solitary metastatic disease with long disease-free intervals and good performance status [1], [9]. In a series of 141 patients undergoing surgical treatment of metastatic disease, those who underwent curative metastectomy with negative margins had a significantly better overall survival than those who underwent either non-curative surgery or non-surgical treatment (OS 44% vs. 14 and 11%, respectively). Factors associated with favorable outcome after surgery included disease-free interval of greater than 12 months, solitary site at first recurrence, performance of curative metastectomy, and age under 60 years [9]. Patients who developed subsequent metastases after initially curative metastectomy and underwent curative resection of their second and third recurrence had a 5 year overall survival rate of 46% and 44%, respectively [9].
Given the high rate of recurrent disease, surveillance is an important part of the management of RCC. Both the National Comprehensive Cancer Network (NCCN) and the American Urological Association (AUA) recommend routine surveillance during the first 5 years following nephrectomy, but provide no clear guidelines for surveillance beyond 5 years. However, a long term longitudinal study of 3651 patients after surgery for M0 RCC showed that up to a third of RCC recurrences would have been missed if NCCN and AUA guidelines were strictly followed [10]. Given the established potential for late RCC recurrence, it may be necessary to extend postoperative surveillance beyond 15 years. Additionally, colonoscopy may serve as an important diagnostic adjunct in patients who develop gastrointestinal symptoms during extended RCC surveillance.
4. Conclusion
The colon is a potential, though uncommon, site for solitary metastasis from RCC. The clinical presentation is frequently several years after initial curative resection. Metastatic RCC should be within the differential diagnosis in patients presenting with large bowel obstruction who have a previous history of RCC resection. Colonoscopy with tissue examination should be performed when clinically feasible. Oncologic resection with negative margins may result in long-term survival in patients with isolated metastatic disease.
This work is reported in line with the CARE criteria [11].
Conflicts of interest
None of the contributing authors have any conflict of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript. This work was not supported by extramural funding.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This study was waived from requiring ethical approval.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Elaine Vo, M.D: conception and design, acquisition of data, drafting of the manuscript.
Carlos H. Palacio, M.D.: conception and design, drafting of the manuscript.
Ronald Omino, M.D.: conception and design, drafting of the manuscript.
Richard E. Link, M.D., Ph.D.: critical review of the manuscript for intellectual content.
Yvonne Sada, M.D., M.P.H.: critical review of the manuscript for intellectual content.
Avo Artinyan, M.D., M.S., FACS: conception and design, drafting of the manuscript, critical review of the manuscript for intellectual content, supervision.
Guarantor
Dr. Avo Artinyan.
Acknowledgements
We would like to thank two members of the Baylor College of Medicine Surgical Research Core Team, who helped us with the preparation of this manuscript: Ana María Rodríguez, PhD for her editorial assistance and Scott Holmes, CMI for his preparation of the figures.
Footnotes
This work originated from the Baylor College of Medicine at 1 Baylor Plaza, Houston, TX 77030, United States.
References
- 1.Fujii Y., Ikeda M., Kurosawa K. Different clinicopathological features between patients who developed early and late recurrence following surgery for renal cell carcinoma. Int. J. Clin. Oncol. 2015;20:802–807. doi: 10.1007/s10147-014-0775-2. [DOI] [PubMed] [Google Scholar]
- 2.Saitoh H. Distant metastasis of renal adenocarcinoma. Cancer. 1981;48:1487–1491. doi: 10.1002/1097-0142(19810915)48:6<1487::aid-cncr2820480635>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Milovic N., Lazic M., Aleksic P. Rare locations of metastatic renal cell carcinoma: a presentation of three cases. Vojnosanit. Pregl. 2013;70(9):881–886. doi: 10.2298/vsp120515014m. [DOI] [PubMed] [Google Scholar]
- 4.Valdespino-Castillo V.E., Ruiz-Jaime A. Renal cell carcinoma with colon metastases: an infrequent site for metastases. Cir. Cir. 2008;76(4):339–342. [PubMed] [Google Scholar]
- 5.Avital S., Hitchcock C.L., Baratz M., Haddad R., Skornick Y., Schneebaum S. Localization of monoclonal antibody CC49 in colonic metastasis from renal cell carcinoma. Eur. J. Surg. Oncol. 1998;24(2):149–151. doi: 10.1016/s0748-7983(98)91719-x. [DOI] [PubMed] [Google Scholar]
- 6.Tokonabe S., Sugimoto M., Komine Y., Horii H., Matsukuma S. Solitary colonic metastasis of renal cell carcinoma seven years after nephrectomy: a case report. Int. J. Urol. 1996;3(6):501–503. doi: 10.1111/j.1442-2042.1996.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 7.Thomason P.A., Peterson L.S., Staniunas R.J. Solitary colonic metastasis from renal-cell carcinoma 17 years after nephrectomy. Report of a case. Dis. Colon Rectum. 1991;34(8):709–712. doi: 10.1007/BF02050356. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz J.L., Vera C., Server G., Osca J.M., Boronat F., Jimenez Cruz J.F. Renal cell carcinoma: late recurrence in 2 cases. Eur. Urol. 1991;20(2):167–169. doi: 10.1159/000471690. [DOI] [PubMed] [Google Scholar]
- 9.Kavolius J.P., Mastorakos D.P., Pavlovich C., Russo P., Burt M.E., Brady M.S. Resection of metastatic renal cell carcinoma. J. Clin. Oncol. 1998;16:2261–2266. doi: 10.1200/JCO.1998.16.6.2261. [DOI] [PubMed] [Google Scholar]
- 10.Stewart S.B., Thompson R.H., Psutka S.P. Evaluation of the national comprehensive cancer network and american urological association renal cell carcinoma surveillance guidelines. J. Clin. Oncol. 2014;32:4059–4065. doi: 10.1200/JCO.2014.56.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnier J.J., Kienle G., Altman D.G. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013 doi: 10.1186/1752-1947-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]