Abstract
The urea cycle is the main pathway for the disposal of excess nitrogen. Carbamoylphosphate synthetase 1 (CPS1), the first and rate-limiting enzyme of urea cycle, is activated by N-acetylglutamate (NAG), and thus N-acetylglutamate synthase (NAGS) is an essential part of the urea cycle. Although NAGS deficiency is the rarest urea cycle disorder, it is the only one that can be specifically and effectively treated by a drug, N-carbamylglutamate, a stable structural analogous of NAG that activates CPS1. Here we report an infant with NAGS deficiency who presented with neonatal hyperammonemia. She was found to have a novel homozygous splice-site mutation, c.1097-2A>T, in the NAGS gene. We describe the clinical course of this infant, who had rapid response to N-carbamylglutamate treatment. In addition, we reviewed the clinical and molecular spectra of previously reported individuals with NAGS deficiency, which presents in most cases with neonatal hyperammonemia, and in some cases the presentation is later, with a broad spectrum of ages and manifestations. With this broad later-onset phenotypic spectrum, maintaining a high index of suspicion is needed for the early diagnosis of this treatable disease.
Keywords: NAGS, Urea cycle disorders, Hyperammonemia, N-carbamylglutamate
1. Introduction
The urea cycle is the main pathway for the disposal of excess nitrogen generated from the breakdown of protein and other nitrogen-containing molecules. Urea cycle disorders, which are characterized by hyperammonemia and distorted amino acid metabolism, can have variable clinical presentations. Severe deficiencies are typically associated with neonatal hyperammonemia, presenting with poor feeding, vomiting, lethargy, tachypnea, convulsions, and coma. In milder deficiencies, ammonia accumulation may be triggered by illness or stress and the disease can present at almost any age. In infancy, partial deficiencies can present with poor developmental progress, behavioral disturbances, hepatomegaly, and gastrointestinal symptoms. Children and adults with partial deficiencies can develop migraine headaches, behavioral changes, confusion, and cyclic vomiting [26].
Carbamoylphosphate synthetase 1 (CPS1) is the first and rate-limiting enzyme of urea cycle. CPS1 is activated by N-acetylglutamate (NAG), which is necessary for CPS1 activity [20]. Therefore, N-acetylglutamate synthase (NAGS), the only enzyme that makes NAG, is an essential part of the urea cycle [3]. In this report we describe an infant with NAGS deficiency who presented in neonatal period with hyperammonemia and was found to have a novel mutation in the NAGS gene. In addition, we discuss the clinical and molecular spectra for previously reported individuals with NAGS deficiency.
2. Case report
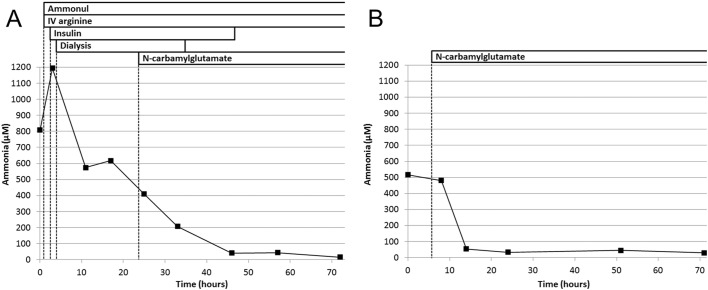
A girl was born at term after an uncomplicated antenatal course. Her physical examination was normal and she was discharged home at 2 days old. Hours later, she developed poor sucking and decreased activity, and her parents brought her to the emergency room (ER) at the age of 3 days. In ER she was lethargic, tachypnic, and developed convulsions in the form of abnormal movements of her right upper and lower extremities. Septic and metabolic tests were performed, and antibiotics and antiepileptic medications were initiated. Initial investigation showed high ammonia (387 μM) and respiratory alkalosis; therefore, a urea cycle defect was highly suspected and she was transferred to a tertiary medical center. Upon arrival, she was lethargic and hypotonic, and her ammonia was 809 μM. The routine management of hyperammonemia was immediately started with Ammonul® (sodium phenylacetate and sodium benzoate), intravenous (IV) arginine, IV high dextrose, and continuous insulin infusion. Hemodialysis was not available, therefore, a peritoneal catheter was inserted and peritoneal dialysis was started after 4 h. At that time ammonia was 1194 μM. After that ammonia level decreased rapidly with the dialysis and medical management. In addition to elevated glutamine and glycine, plasma amino acids showed undetectable citrulline level suggesting a proximal urea cycle defect, which includes NAGS deficiency. Therefore, N-carbamylglutamate was subsequently initiated after about 24 h. After < 48 h, ammonia normalized and dialysis and insulin infusion were discontinued, while Ammonul, IV arginine, and N-carbamylglutamate were continued (Fig. 1A). Protein intake was initiated using infant formula based on essential amino acids. She regained normal consciousness and her activity improved gradually. Ammonia remained within normal levels while the IV medications were changed to oral medications and protein intake was gradually increased. As N-carbamylglutmate was started after the initiation of dialysis and other medications, it was difficult to judge whether the neonate responded to the N-carbamylglutamate or the reduced ammonia was just due to the dialysis and other medications. In addition, the rarity of NAGS deficiency, the limited availability of N-carbamylglutamate in the tertiary center, and the desire to have a simplified treatment regimen were also considered and a decision was made at that time to discontinue the N-carbamylglutamate while maintaining oral citrulline and sodium benzoate, and protein-restricted diet. Her ammonia levels remained normal while she was on this treatment regimen. The infant was discharged home after two weeks on oral citrulline (300 mg/kg/day) and sodium benzoate (300 mg/kg/day) and protein restricted (2 g/kg/day) formula composed of a mixture of essential amino acid formula and regular infant formula. A genetic panel for urea cycle disorders including the CPS1, NAGS, OTC, ASS1, ASL, and ARG1 genes was sent during the hospitalization. Parents were cousins and this was their first child.
Fig. 1.
Ammonia levels and treatment during the first (A) and second (B) hospitalizations.
Three weeks after discharge, the results of the genetic panel became available, demonstrating a novel homozygous mutation, c.1097-2A>T, in the NAGS gene consistent with a diagnosis of NAGS deficiency. When a call was made to discuss the results and arrange for a clinic visit to initiate N-carbamylglutamate, the family mentioned that the child was not feeding well and had been less active for the past day. Therefore, the child was immediately assessed in ER and found to be lethargic with ammonia of 571 μM. Knowing that the child has NAGS deficiency N-carbamylglutamate was initiated (200 mg/kg/day) and ammonia normalized within 8 h (Fig. 1B). Within 24 h, the child regained her normal activity and feeding was initiated using regular infant formula. The ammonia remained normal and the infant was discharged home after 5 days with N-carbamylglutamate monotherapy and regular infant formula.
3. Discussion
The overall incidence of urea cycle disorders is 1:35,000. NAGS deficiency is the rarest and accounts for only 0.5–1% of urea cycle disorders; therefore, its estimated incidence is 1:3,500,000–7,000,000 [23]. In 1981, this disease was first described in a neonate with hyperammonemia who had undetectable NAGS enzyme activity in liver tissue [2]. Subsequently, very few reports described NAGS deficiency, reflecting not only the rarity of the disease but also the difficulty in confirming the diagnosis which initially required enzyme assessment in liver tissue [8]. In 2002, the NAGS gene was identified and mutations found in affected individuals [4], [10]. Since then, the diagnosis of NAGS deficiency has become more common. With the infant described here, molecularly confirmed NAGS deficiency has been reported in 59 individuals from 45 families (Table 1).
Table 1.
Individuals with molecularly confirmed NAGS deficiency.
| Family | Cases | Onset and clinical presentation | NAGS gene mutations | References |
|---|---|---|---|---|
| 1 | 1 | Neonatal hyperammonemia | Hom c.779C > T (p.P260L) | [21] |
| 2 | 1 | Neonatal hyperammonemia | Hom c.791C > T (p.T264M) | [21] |
| 3 | 2 | Neonatal hyperammonemia | Hom c.1228T > C (p.S410P) | [22] |
| 4 | 1 | Neonatal hyperammonemia | Hom c.1228T > C (p.S410P) | [21] |
| 5 | 2 | Neonatal hyperammonemia | Hom c.1241G > C (p.R414P) | [19] |
| 6 | 1 | Neonatal hyperammonemia | Hom c.1289T > C (p.L430P) | [14], [22] |
| 7 | 1 | Neonatal hyperammonemia | Hom c.1289T > C (p.L430P) | [21] |
| 8 | 1 | Neonatal hyperammonemia | Hom c.1299G > C (p.E433D) | [14] |
| 9 | 1 | Neonatal hyperammonemia | Hom c.1370G > A (p.G457D) | [21] |
| 10 | 3 | Neonatal hyperammonemia | Hom c.1450T > C (p.W484R) | [14], [22] |
| 11 | 1 | Neonatal hyperammonemia | Hom c.1450T > C (p.W484R) | [21] |
| 12 | 1 | Neonatal hyperammonemia | Hom c.1450T > C (p.W484R) | [21] |
| 13 | 1 | Neonatal hyperammonemia | Hom c.1450T > C (p.W484R) | [21] |
| 14 | 1 | Neonatal hyperammonemia | Hom c.1450T > C (p.W484R) | [21] |
| 15 | 1 | Neonatal hyperammonemia | Hom c.1450T > C (p.W484R) | [25] |
| 16 | 1 | Neonatal hyperammonemia | Hom c.1552G > A (p.A518T) | [22] |
| 17 | 1 | Neonatal hyperammonemia | Hom c.971G > A (p.W324⁎) | [5] |
| 18 | 2 | Neonatal hyperammonemia | Hom c.971G > A (p.W324⁎) | [11], [14] |
| 19 | 2 | Neonatal hyperammonemia | Hom c.991C > T (p.Q324⁎) | [21] |
| 20 | 1 | Neonatal hyperammonemia | Hom c.1264G > T (p.E422⁎) | [21] |
| 21 | 1 | Neonatal hyperammonemia | Hom c.545delC (p.A182Vfs⁎23) | [13] |
| 22 | 1 | Neonatal hyperammonemia | Hom c.545delC (p.A182Vfs⁎23) | [21] |
| 23 | 2 | Neonatal hyperammonemia | Hom c.1025delG (p.R342Pfs⁎50) | [5] |
| 24 | 2 | Neonatal hyperammonemia | Hom c.1036dupC (p.H346Pfs⁎10) | [10] |
| 25 | 1 | Neonatal hyperammonemia | Hom c.1313dupG (p.T439Hfs⁎52) | [21] |
| 26 | 2 | Neonatal hyperammonemia | Hom c.1313delG (p.G438Afs⁎10) | [21] |
| 27 | 1 | Neonatal hyperammonemia | Hom c.1097-2A > T | This report |
| 28 | 1 | Neonatal hyperammonemia | Het c.1172T > G (p.L391R) Het c.1450T > C (p.W484R) |
[21] |
| 29 | 1 | Neonatal hyperammonemia | Het c.916-2A > T Het c.1307dupC (p.T439Hfs⁎52) |
[14], [15] |
| 30 | 1 | Neonatal hyperammonemia | Het c.499A > G (p.M167V) Het c.278delC (p.93Qfs⁎18) |
[21] |
| 31 | 1 | Neonatal hyperammonemia | Het c.929T > C (p.V310A) Het c.1464_1465del (p.H488Qfs⁎2) |
[17] |
| 32 | 1 | Neonatal hyperammonemia | Het c.1494G>A (p.W498⁎) (second mutation was not found) |
[21] |
| 33 | 2 | Infantile (2 months): hypotonia, hepatomegaly, failure-to-thrive | Hom c.598T > C (p.C200R) | [22] |
| 34 | 1 | Infantile (3 months): hypotonia, developmental delay | Hom c.598T > C (p.C200R) | [21] |
| 35 | 1 | Childhood (13 months): protein aversion and episodes of vomiting, lethargy, confusion, aggressiveness, restlessness, disorientation, and hyperreflexia | Hom c.835G > A (p.A279T) | [14] |
| 36 | 4 | Late-onset and mild course | Hom c.872T > A (p.I291N) | [21] |
| 37 | 1 | Childhood (4 years): seizures and microcephaly | Hom c.1552G > A (p.A518T) | [21] |
| 38 | 2 | Childhood (1 month and 9 years): episodes of vomiting, lethargy, anorexia, irritability, and seizures | Het c.1526G > A (p.R509Q) Het c.1097-1G > C |
[7] |
| 39 | 1 | Childhood (15 year): somnolence and decreased consciousness with a urinary tract infection | Het c.1192A > T (p.S398C) (second mutation was not found) |
[21] |
| 40 | 1 | Childhood (16 year): Reye-like syndrome | Het c.1535A > G (p.Y512C) (second mutation was not found) |
[21] |
| 41 | 1 | Adult-onset (46 years): confusion and coma after pelvic fracture | Hom c.603G > C (p.K201N) | [18] |
| 42 | 1 | Adult-onset (33 years): combativeness, confusion, coma, seizure brain edema, and death after caesarean section. | Het c.518T > A (p.V173E) Het c.1292C > T (p.T431I) |
[7] |
| 43 | 1 | Adult-onset (27 years): seizures and coma during pregnancy | Het c.935T > C (p.L312P) Het c.1292C > T (p.T431I) |
[8] |
| 44 | 1 | Adult-onset (40 years): migraine headaches and episodes of staring spells, nausea, vomiting, lethargy, and ataxia followed by coma. | Het c.1048G > A (p.V350I) Het c.1326C > G (p.L442V) |
[8], [24] |
| 45 | 1 | Adult-onset (20 years): episodes of nausea, vomiting, confusion, and behavioral changes | Het c.1298A > G (p.E433G) Het c.1451+5G>A |
[9] |
NAGS deficiency typically presents with neonatal hyperammonemia. However, late-onset presentations have been reported in infancy, childhood, and adulthood. Neonatal hyperammonemia was the presentation in 41 (~ 70%) reported individuals with NAGS deficiency (Table 1). Neonates with this presentation can have poor feeding, vomiting, lethargy, tachypnea, respiratory distress, respiratory alkalosis, hypothermia, hypotonia, irritability, seizures, brain edema, coma, and death. Other neonatal manifestations may include failure-to-thrive, hepatomegaly, oliguria, diarrhea, metabolic acidosis, trembling, jitteriness, and hypertonia [21]. The infant in this report had a typical neonatal presentation. Infantile and childhood presentation occurred in 13 (~ 22%). These children had more variable phenotypes with common manifestations being hypotonia, developmental delay, seizures, behavioral problems, and lethargy. Less common manifestations included protein aversion, Reye-like syndrome, failure-to-thrive, and hepatomegaly (Table 1). Finally, adult-onset was found in 5 (~ 8%). The manifestations, which were precipitated by major stressors in 3 of the 5, included headaches, seizures, behavioral problems, confusion, lethargy, and coma (Table 1). Therefore, NAGS deficiency presents with neonatal hyperammonemia in most cases, whereas a broad spectrum of manifestations occurring at any age beyond the neonatal period occurs in a minority of cases. Maintaining a high index of suspicion and lower threshold of doing ammonia level are needed for early diagnosis particularly for late-onset diseases.
Besides the hyperammonemia, the biochemical profile in NAGS deficiency is similar to CPS1 deficiency which is characterized by low plasma citrulline and the absence of orotic aciduria [26]. Measuring enzyme activity requires liver biopsy which is difficult and not completely reliable [8]. Therefore, molecular tests sequencing NAGS gene have become the practical standard for diagnosing NAGS deficiency [16]. The infant described here had the classic biochemical profile and a novel homozygous mutation, c.1097-2A>T, in the NAGS gene. This mutation has not been previously reported; however, it is located in the acceptor splice site of intron 4 and therefore is predicted to affect splicing. Including the mutation in this infant, 41 different NAGS mutations have been described including 25 (~ 60%) missense, 4 (~ 10%) nonsense, 8 (~ 20%) frameshift, and 4 (~ 10%) splice-site mutations. Other than the missense mutation p.W484R, which was found in homozygous status in 6 families and heterozygous status in one, other mutations are private as they were observed in only one or two families (Table 2).
Table 2.
Mutations in the NAGS gene.
| NAGS gene mutation | Homozygous | Heterozygous |
|---|---|---|
| Missense mutations | ||
| c.499A > G (p.M167V) | 1 | |
| c.518T > A (p.V173E) | 1 | |
| c.598T > C (p.C200R) | 2 | |
| c.603G > C (p.K201N) | 1 | |
| c.779C > T (p.P260L) | 1 | |
| c.791C > T (p.T264M) | 1 | |
| c.835G > A (p.A279T) | 1 | |
| c.872T > A (p.I291N) | 1 | |
| c.929T > C (p.V310A) | 1 | |
| c.935T > C (p.L312P) | 1 | |
| c.1048G > A (p.V350I) | 1 | |
| c.1172T > G (p.L391R) | 1 | |
| c.1192A > T (p.S398C) | 1 | |
| c.1228T > C (p.S410P) | 2 | |
| c.1241G > C (p.R414P) | 1 | |
| c.1289T > C (p.L430P) | 2 | |
| c.1292C > T (p.T431I) | 2 | |
| c.1298A > G (p.E433G) | 1 | |
| c.1299G > C (p.E433D) | 1 | |
| c.1326C > G (p.L442V) | 1 | |
| c.1370G > A (p.G457D) | 1 | |
| c.1450T > C (p.W484R) | 6 | 1 |
| c.1526G > A (p.R509Q) | 1 | |
| c.1535A > G (p.Y512C) | 1 | |
| c.1552G > A (p.A518T) | 2 | |
| Nonsense mutations | ||
| c.971G > A (p.W324⁎) | 2 | |
| c.991C > T (p.Q324⁎) | 1 | |
| c.1264G > T (p.E422⁎) | 1 | |
| c.1494G > A (p.W498⁎) | 1 | |
| Frameshift mutations | ||
| c.278delC (p.93Qfs⁎18) | 1 | |
| c.545delC (p.A182Vfs⁎23) | 2 | |
| c.1025delG (p.R342Pfs⁎50) | 1 | |
| c.1036dupC (p.H346Pfs⁎10) | 1 | |
| c.1307dupC (p.T439Hfs⁎52) | 1 | |
| c.1313dupG (p.T439Hfs⁎52) | 1 | |
| c.1313delG (p.G438Afs⁎10) | 1 | |
| c.1464_1465del (p.H488Qfs⁎2) | 1 | |
| Splice-site mutations | ||
| c.916-2A > T | 1 | |
| c.1097-2A > T | 1 | |
| c.1097-1G > C | 1 | |
| c.1451+5G>A | 1 | |
| Total | 33 | 21 |
Second mutation was not found in three individuals in whom only one heterozygous mutation was identified.
In contrast to the treatment of other urea cycle disorders that includes low-protein diet with essential amino acid supplements, arginine or citrulline supplementation, and the use of ammonia scavengers; treatment of NAGS deficiency is with only N-carbamylglutamate, a stable structural analogous of NAG that activates CPS1 [26]. Therefore, NAGS deficiency is the only urea cycle disorder that can be specifically and effectively treated. Using stable isotope studies, N-carbamylglutamate supplementation was shown to restore ureagenesis in NAGS deficiency [6]. N-carbamylglutamate successfully rescues neonates with NAGS deficiency when present with hyperammonemia and its long-term chronic use corrects the metabolic defects in these individuals and eliminates the need for additional medications or dietary modifications [1]. Although mono-therapy with N-carbamylglutamate is the treatment of choice in NAGS deficiency, some affected individuals receiving N-carbamylglutamate may experience breakthrough hyperammonemia during episodes of acute illness or metabolic decompensation; therefore, protein restriction and additional drugs may be needed during these episodes [16]. The standard N-carbamylglutamate maintenance dosage is 100–200 mg/kg/day (given in 3–4 doses); however it can be adjusted individually by progressive down-titration to the minimum dose required (as low as 10–15 mg/kg) to maintain normal ammonia levels [16]. The infant reported here showed dramatic response to N-carbamylglutamate and remained only on this medication. During the initial hospitalization, using the N-carbamylglutamate with dialysis and other medications made it difficult to appreciate the contribution of N-carbamylglutamate. However, during the second hospitalization, it was obvious how this medication resulted in normalizing the ammonia in just few hours. Although N-carbamylglutamate typically normalizes ammonia in hours [12], [25], a delayed response or no response has been reported in some neonates with NAGS deficiency [19].
In conclusion, NAGS deficiency is a rare urea cycle disorder that has a broad phenotypic spectrum. Typically, it presents with neonatal hyperammonemia; however, late-onset with variable manifestations can occur. Therefore, maintaining a high index of suspicion is needed for early diagnosis. The treatment of choice in NAGS deficiency is N-carbamylglutamate monotherapy which can normalize ammonia in few hours.
Acknowledgment
We thank Dr. Michael Segal for reading an earlier version of this manuscript.
References
- 1.Ah Mew N., Caldovic L. N-acetylglutamate synthase deficiency: an insight into the genetics, epidemiology, pathophysiology, and treatment. Appl. Clin. Genet. 2011;4:127–135. doi: 10.2147/TACG.S12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann C., Krähenbühl S., Colombo J.P., Schubiger G., Jaggi K.H., Tönz O. N-acetylglutamate synthetase deficiency: a disorder of ammonia detoxication. N. Engl. J. Med. 1981;304:543. doi: 10.1056/NEJM198102263040918. [DOI] [PubMed] [Google Scholar]
- 3.Caldovic L., Tuchman M. N-acetylglutamate and its changing role through evolution. Biochem. J. 2003;372:279–290. doi: 10.1042/BJ20030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldovic L., Morizono H., Gracia Panglao M., Gallegos R., Yu X., Shi D., Malamy M.H., Allewell N.M., Tuchman M. Cloning and expression of the human N-acetylglutamate synthase gene. Biochem. Biophys. Res. Commun. 2002;299:581–586. doi: 10.1016/s0006-291x(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 5.Caldovic L., Morizono H., Panglao M.G., Cheng S.F., Packman S., Tuchman M. Null mutations in the N-acetylglutamate synthase gene associated with acute neonatal disease and hyperammonemia. Hum. Genet. 2003;112:364–368. doi: 10.1007/s00439-003-0909-5. [DOI] [PubMed] [Google Scholar]
- 6.Caldovic L., Morizono H., Daikhin Y., Nissim I., McCarter R.J., Yudkoff M., Tuchman M. Restoration of ureagenesis in N-acetylglutamate synthase deficiency by N-carbamylglutamate. J. Pediatr. 2004;145:552–554. doi: 10.1016/j.jpeds.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 7.Caldovic L., Morizono H., Panglao M.G., Lopez G.Y., Shi D., Summar M.L., Tuchman M. Late onset N-acetylglutamate synthase deficiency caused by hypomorphic alleles. Hum. Mutat. 2005;25:293–298. doi: 10.1002/humu.20146. [DOI] [PubMed] [Google Scholar]
- 8.Caldovic L., Morizono H., Tuchman M. Mutations and polymorphisms in the human N-acetylglutamate synthase (NAGS) gene. Hum. Mutat. 2007;28:754–759. doi: 10.1002/humu.20518. [DOI] [PubMed] [Google Scholar]
- 9.Cartagena A., Prasad A.N., Rupar C.A., Strong M., Tuchman M., Ah Mew N., Prasad C. Recurrent encephalopathy: NAGS (N-acetylglutamate synthase) deficiency in adults. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2013;40:3–9. doi: 10.1017/s0317167100012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elpeleg O., Shaag A., Ben-Shalom E., Schmid T., Bachmann C. N-acetylglutamate synthase deficiency and the treatment of hyperammonemic encephalopathy. Ann. Neurol. 2002;52:845–849. doi: 10.1002/ana.10406. [DOI] [PubMed] [Google Scholar]
- 11.Gessler P., Buchal P., Schwenk H.U., Wermuth B. Favourable long-term outcome after immediate treatment of neonatal hyperammonemia due to N-acetylglutamate synthase deficiency. Eur. J. Pediatr. 2010;169:197–199. doi: 10.1007/s00431-009-1006-0. [DOI] [PubMed] [Google Scholar]
- 12.Guffon N., Schiff M., Cheillan D., Wermuth B., Häberle J., Vianey-Saban C. Neonatal hyperammonemia: the N-carbamoyl-L-glutamic acid test. J. Pediatr. 2005;147:260–262. doi: 10.1016/j.jpeds.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 13.Häberle J., Koch H.G. Genetic approach to prenatal diagnosis in urea cycle defects. Prenat. Diagn. 2004;24:378–383. doi: 10.1002/pd.884. [DOI] [PubMed] [Google Scholar]
- 14.Häberle J., Denecke J., Schmidt E., Koch H.G. Diagnosis of N-acetylglutamate synthase deficiency by use of cultured fibroblasts and avoidance of nonsense-mediated mRNA decay. J. Inherit. Metab. Dis. 2003;26:601–605. doi: 10.1023/a:1025912417548. [DOI] [PubMed] [Google Scholar]
- 15.Häberle J., Schmidt E., Pauli S., Kreuder J.G., Plecko B., Galler A., Wermuth B., Harms E., Koch H.G. Mutation analysis in patients with N-acetylglutamate synthase deficiency. Hum. Mutat. 2003;21:593–597. doi: 10.1002/humu.10216. [DOI] [PubMed] [Google Scholar]
- 16.Häberle J., Boddaert N., Burlina A., Chakrapani A., Dixon M., Huemer M., Karall D., Martinelli D., Crespo P.S., Santer R. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J. Rare Dis. 2012;7:32. doi: 10.1186/1750-1172-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J.H., Kim Y.-M., Lee B.H., Cho J.H., Kim G.-H., Choi J.-H., Yoo H.-W. Short-term efficacy of N-carbamylglutamate in a patient with N-acetylglutamate synthase deficiency. J. Hum. Genet. 2015;60:395–397. doi: 10.1038/jhg.2015.30. [DOI] [PubMed] [Google Scholar]
- 18.van de Logt A.-E., Kluijtmans L.A.J., Huigen M.C.D.G., Janssen M.C.H. Hyperammonemia due to adult-onset N-acetylglutamate synthase deficiency. JIMD Rep. 2016 doi: 10.1007/8904_2016_565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordenström A., Halldin M., Hallberg B., Alm J. A trial with N-carbamylglutamate may not detect all patients with NAGS deficiency and neonatal onset. J. Inherit. Metab. Dis. 2007;30:400. doi: 10.1007/s10545-007-0454-9. [DOI] [PubMed] [Google Scholar]
- 20.Rubio V., Britton H.G., Grisolia S. Mitochondrial carbamoyl phosphate synthetase activity in the absence of N-acetyl-L-glutamate. Mechanism of activation by this cofactor. Eur. J. Biochem. FEBS. 1983;134:337–343. doi: 10.1111/j.1432-1033.1983.tb07572.x. [DOI] [PubMed] [Google Scholar]
- 21.Sancho-Vaello E., Marco-Marín C., Gougeard N., Fernández-Murga L., Rüfenacht V., Mustedanagic M., Rubio V., Häberle J. Understanding N-acetyl-L-glutamate synthase deficiency: mutational spectrum, impact of clinical mutations on enzyme functionality, and structural considerations. Hum. Mutat. 2016;37:679–694. doi: 10.1002/humu.22995. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt E., Nuoffer J.-M., Häberle J., Pauli S., Guffon N., Vianey-Saban C., Wermuth B., Koch H.G. Identification of novel mutations of the human N-acetylglutamate synthase gene and their functional investigation by expression studies. Biochim. Biophys. Acta. 2005;1740:54–59. doi: 10.1016/j.bbadis.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Summar, M.L., Koelker, S., Freedenberg, D., Le Mons, C., Haberle, J., Lee, H.-S., Kirmse, B., European Registry and Network for Intoxication Type Metabolic Diseases (E-IMD), and Members of the Urea Cycle Disorders Consortium (UCDC). The incidence of urea cycle disorders. Mol. Genet. Metab. 110, 179–180. [DOI] [PMC free article] [PubMed]
- 24.Tuchman M., Caldovic L., Daikhin Y., Horyn O., Nissim I., Nissim I., Korson M., Burton B., Yudkoff M. N-carbamylglutamate markedly enhances ureagenesis in N-acetylglutamate deficiency and propionic acidemia as measured by isotopic incorporation and blood biomarkers. Pediatr. Res. 2008;64:213–217. doi: 10.1203/PDR.0b013e318179454b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Leynseele A., Jansen A., Goyens P., Martens G., Peeters S., Jonckheere A., De Meirleir L. Early treatment of a child with NAGS deficiency using N-carbamyl glutamate results in a normal neurological outcome. Eur. J. Pediatr. 2014;173:1635–1638. doi: 10.1007/s00431-013-2205-2. [DOI] [PubMed] [Google Scholar]
- 26.Wijburg F.A., Nassogne M.-C. Disorders of the urea cycle and related enzymes. In: Saudubray J.-M., van den Berghe G., Walter J.H., editors. Inborn Metabolic Diseases. Springer; Berlin Heidelberg: 2012. pp. 297–310. [Google Scholar]