Introduction
We report the case of a drug-induced morbilliform eruption associated with amlodipine that mimicked the presentation of CD30+ mycosis fungoides, both clinically and histologically. The rash resolved after discontinuation of the amlodipine, suggesting that the event was a rare CD30+ pseudolymphoma cutaneous reaction to this drug.
Case report
A 76-year-old white woman presented with a 3-month history of a pruritic, diffuse morbilliform eruption consisting of erythematous papules and plaques with cigarette-paper scale coalescing to cover more than 80% body surface area (Fig 1). Based on clinical presentation, the initial differential diagnosis included a drug eruption or urticarial bullous pemphigoid. The patient's amlodipine dose was doubled by her primary care physician 11 months after initially prescribing 5 mg daily, and she was maintained on 2 additional antihypertensive medications, metoprolol and losartan. Two days after adjustment in medications, the patient was seen in the emergency room for exacerbation of the rash on her face. The patient's primary care physician subsequently discontinued amlodipine and initiated nifedipine to control the patient's blood pressure.
Fig 1.
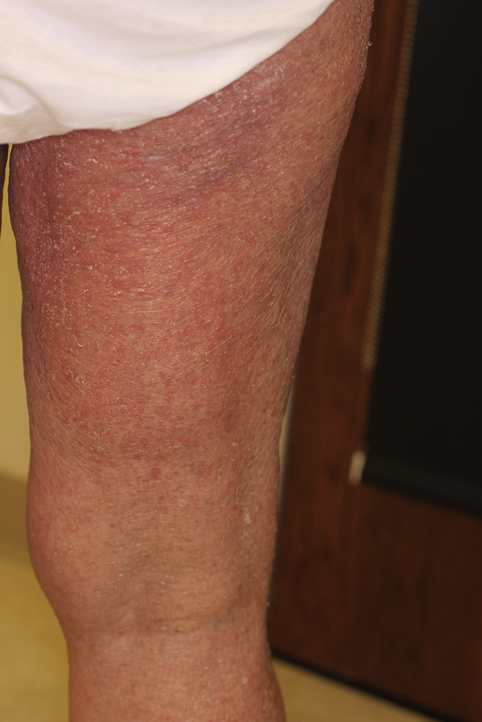
Pruritic erythematous scaly papules coalescing into plaques on the patient's dorsal right thigh.
The patient's medical history was significant for graft-versus-host disease (GVHD) 24 years prior after a bone marrow transplant for chronic myelogenous leukemia, diabetes, and amlodipine-associated flushing and ankle edema. The patient was taking metformin for diabetes. Her chronic myelogenous leukemia has been in remission for the last 24 years after transplant and requires no medication.
Whole-body positron emission tomography–computed tomography found a mildly hypermetabolic subcentimeter left cervical level Ib lymph node with standardized uptake value of a maximum of 1.69. Flow cytometric analysis of peripheral blood showed a slightly elevated CD4/CD8 ratio of 5.4 but otherwise did not show an immunophenotypically abnormal cell population. T-cell receptor γ assay of the blood was positive for a polyclonal T-cell receptor γ gene rearrangement.
A biopsy of the right thigh found superficial perivascular and interstitial dermatitis with atypical lymphocytes. Spongiosis of the overlying epidermis was noted with subcorneal collections of neutrophils along with subtle epidermotropism (Fig 2). Immunostaining found that the large atypical cells were strongly CD30+ and weakly CD3+. CD30 highlighted a portion of the epidermotropic infiltrate (Fig 3). The pathology-based differential diagnoses included lymphomatoid papulosis, lymphomatoid drug reaction, and less likely CD30+-transformed mycosis fungoides. A clonal rearrangement of the T-cell receptor γ gene was detected by polymerase chain reaction in the skin.
Fig 2.

Intact stratum corneum and Pautrier's microabscess within the viable epidermis. Vacuolar interface changes and atypical hyperchromatic lymphocytes in the papillary dermis.
Fig 3.
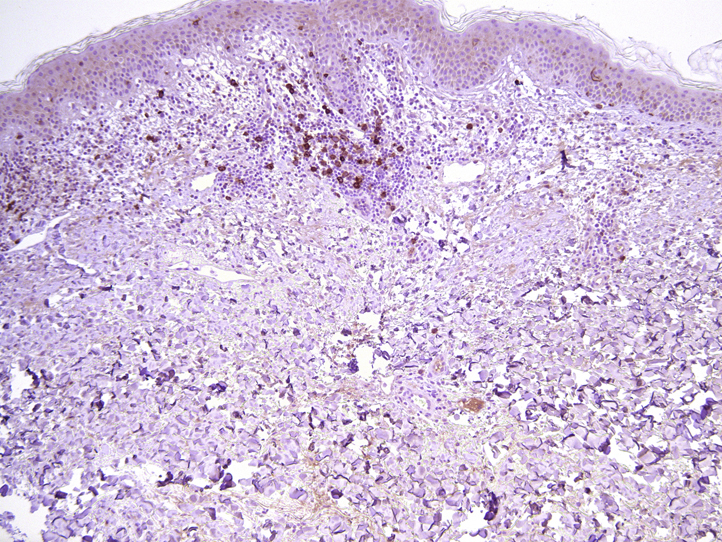
Prominent CD30+ staining is seen both perivascularly at the dermo-epidermal junction and at viable epidermis.
The patient was prescribed mechlorethamine gel 0.016% 3 times a week and showed improvement on her face and axillae, but erythema on her temples persisted. Mechlorethamine was continued for her rash, and nifedipine was discontinued and hydralazine was initiated to control the patient's hypertension. Four weeks later, the violaceous erythema and papules disappeared, and no other signs or symptoms of dermatitis remained (Fig 4). The patient was instructed to discontinue mechlorethamine use at this point. She remains without evidence of lymphoproliferative disease. The patient has not been rechallenged with amlodipine or nifedipine.
Fig 4.
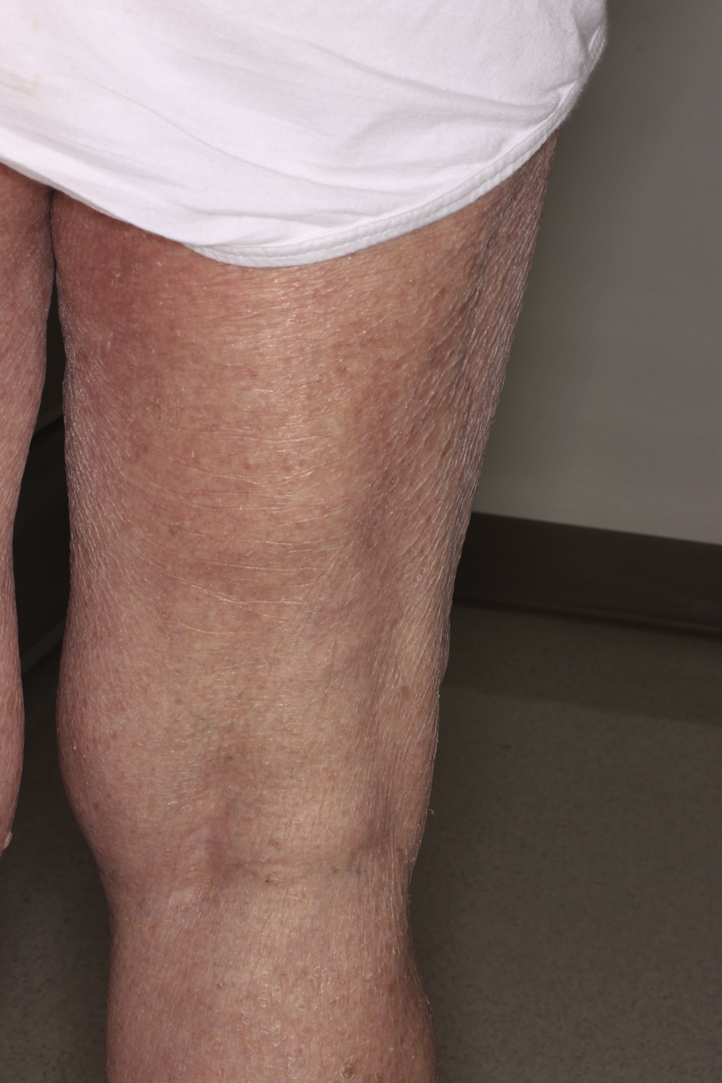
Diffuse mild xerosis after rash on resolution of the original eruption.
Discussion
Cutaneous drug hypersensitivity reactions account for roughly 3% of hospitalizations,1 although this finding may be an underestimation because of variability in the presentation, identification, and reporting of drug-induced cutaneous rashes. Drug-induced reactions usually appear within weeks or months of administration of the offending drug and resolve within 2 months of withdrawal.1, 2 In rare cases, these reactions present as a CD30+ lymphocytic infiltrate that resembles clinical and histologic variants of cutaneous T-cell lymphoma.1
Amlodipine works by blocking the calcium ion channels and inhibiting the actin-myosin complex and cardiac muscle contraction.3 Amlodipine-induced CD30+ drug reactions are reported in the literature, but the mechanism is not well understood.2 It is hypothesized that the implicated drug plays a role in diminishing T-cell suppressor function, which leads to an exaggerated T-helper cell response to various antigens.4 Other antihypertensive drugs implicated in producing atypical cutaneous lymphoid hyperplasia in addition to calcium channel blockers include diuretics, angiotensin-converting enzyme inhibitors, β-blockers and α-antagonists.5
The cutaneous manifestations of pseudolymphoma induced by amlodipine range from diffuse desquamated erythema to more focal papules and annular plaques.6, 7 The diagnosis for our patient was more consistent with a drug-induced pseudolymphoma rather than a nonspecific drug hypersensitivity syndrome. Histologic examination found features of cutaneous T-cell lymphoma, and the cutaneous reaction resolved once the drug was stopped.8 A rare finding, this case had similarities to previously reported cases in which amlodipine induced a pseudolymphomatous CD30+ cutaneous eruption. Kabashima et al9 described a case of a 74-year-old man with a 10-month history of red papules and erythematous plaques who had been taking 5 mg of amlodipine daily for the last 12 months. Upon biopsy, a lymphocytic infiltrate with large irregular hyperchromatic nuclei in the papillary dermis was noted with admixed eosinophils, an elevated CD4+/CD8+ ratio, and a large number of CD30+ cells. Similarly, Fukamachi et al10 described the case of a 70-year-old man with a 4-month history of scaly erythematous plaques primarily on his trunk and extremities who had been taking amlodipine for 1 year. Biopsy found a CD4+ and CD30+ infiltrate with mild hyperkeratosis and a dense lymphocytic infiltrate mixed with eosinophils in the middle to papillary dermis. Both of these CD30+ amlodipine-induced reactions resolved within 3 weeks to 2 months after stopping amlodipine.1, 9, 10
In our patient, an increased ratio of CD4/CD8 and a clonal T-cell population were detected in the blood before the discontinuation of nifedipine. Similar findings were noted in a case presented by Plaza et al,4 in which 15 cases of drug-induced lymphomatoid hypersensitivity with reversible T-cell dyscrasias were found to be positive for a β T-cell–receptor gene rearrangement. The implicated drugs in these cases were nifedipine and amlodipine; a monoclonal population was observed in the case associated with nifedipine.4
In the case presented above, our patient's past GVHD may have increased her susceptibility to this episode of T-cell dyscrasia exacerbated by the administration of amlodipine. Although our patient's GVHD was a remote clinical diagnostic possibility based on her initial presentation, the histologic findings were not compatible with recurrence.
The above case illustrates the ability of amlodipine to induce a CD30+ lymphomatoid drug reaction. Careful correlation of both clinical and histologic data should be made to differentiate a primary lymphocytic proliferative process from a reactive lymphomatoid drug reaction in the setting of CD30 positivity. In patients with similar clinical presentations, this case underscores the importance of carefully considering the patient's medication list to identify any potential iatrogenic etiologies of the cutaneous findings. If amlodipine or any other calcium channel blocker is suspected as the causative agent, the medication should be discontinued and then rechallenged if appropriate, or an alternative antihypertensive therapy should be initiated.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Pulitzer M.P., Nolan K.A., Oshman R.G., Phelps R.G. CD30+ lymphomatoid drug reactions. Am J Dermatopathol. 2013;35(3):343–350. doi: 10.1097/DAD.0b013e31826bc1e5. [DOI] [PubMed] [Google Scholar]
- 2.Stern R., Khalasa J.H. Cutaneous adverse reactions associated with calcium channel blockers. Arch Intern Med. 1989;149:829–832. [PubMed] [Google Scholar]
- 3.Ioulios P., Charalampos M., Efrossini T. The spectrum of cutaneous reactions associated with calcium antagonists: a review of the literature and the possible etiopathogenic mechanisms. Dermatol Online J. 2003;9(5):6. [PubMed] [Google Scholar]
- 4.Plaza J.A., Morrison C., Magro C.M. Assessment of TCR-beta clonality in a diverse group of cutaneous T-Cell infiltrates. J Cutan Pathol. 2008;35:358–365. doi: 10.1111/j.1600-0560.2007.00813.x. [DOI] [PubMed] [Google Scholar]
- 5.Magro C.M., Crowson A.N. Drug-induced immune dysregulation as a cause of atypical cutaneous lymphoid infiltrates: a hypothesis. Hum Pathol. 1996;27(2):125–132. doi: 10.1016/s0046-8177(96)90365-2. [DOI] [PubMed] [Google Scholar]
- 6.Rezakovic S., Pocanic L. Cutaneous adverse drug reactions induced by amlodipine. Cardiol Croat. 2014;9(7-8):314–317. [Google Scholar]
- 7.Valcarel Y., Jimenez R., Hernandez V., Aristegui R., Gil A. Efficacy and safety of amlodipine: a comparative study of antihypertensive patients treated at primary- and specialized-care centres. Clin Drug Invest. 2006;26(3):125–133. doi: 10.2165/00044011-200626030-00002. [DOI] [PubMed] [Google Scholar]
- 8.Callot V., Roujeau J.-C., Bagot M. Drug-induced pseudolymphoma and hypersensitivity syndrome: Two different clinical entities. Arch Dermatol. 1996;132:1315–1321. [PubMed] [Google Scholar]
- 9.Kabashima R., Orimo H., Hino R., Nakashima D., Kabashima K., Tokura Y. CD30-positive T-cell pseudolymphoma induced by amlodipine. JEADV. 2008;22:1522–1524. doi: 10.1111/j.1468-3083.2008.02671.x. [DOI] [PubMed] [Google Scholar]
- 10.Fukamachi S., Sugita K., Sawada Y., Bito T., Nakamura M., Tokura Y. Drug-induced CD30+ T cell pseudolymphoma. Eur J Dermatol. 2009;19:292–294. doi: 10.1684/ejd.2009.0667. [DOI] [PubMed] [Google Scholar]


