Introduction
Immune checkpoint inhibitors such as ipilimumab (anticytotoxic T-lymphocyte–associated antigen), pembrolizumab, and nivolumab (anti–programmed death receptor 1) represent some of the newest and most promising medications for the treatment of metastatic melanoma.1 As a class, immune checkpoint inhibitors interfere with tumoral suppression of T cells, resulting in a more robust immune response and subsequent benefit in the treatment of melanoma, non–small-cell lung cancer, and potentially other malignancies. Unfortunately, immune checkpoint inhibitors also interfere with suppression of autoimmunity, and several immune-related adverse events have been attributed to these medications.2, 3, 4 Patients receiving programmed death receptor 1 (PD-1) inhibitors have had morbilliform eruptions, vitiligo, pruritus, neutrophilic dermatosis, lichen planus, psoriasis, bullous erythema multiforme, Stevens-Johnson syndrome, and bullous pemphigoid.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Here we present a novel case of a pityriasis lichenoides chronica–like drug eruption developing during pembrolizumab therapy.
Report of a case
A 67-year-old woman with metastatic melanoma, a medical history of hypertension and squamous cell carcinoma of the anus, and a family history of psoriasis was started on pembrolizumab, 2 mg/kg every 3 weeks. After her second infusion, she had dozens of pruritic 3- to 4-mm, red-brown, thin papules with centrally adherent micaceous scale on the forearms and lower legs (Fig 1, A and B). These lesions failed to respond to over-the-counter antihistamines and continued to worsen with each infusion. A punch biopsy was obtained from a representative area on the left lower leg (Fig 1, A and B).
Fig 1.
A and B, Pityriasis lichenoides chronica–like drug eruption. The lower legs show several, 3- to 4-mm, red-brown, thin papules with centrally adherent micaceous scale and intermixed erosions.
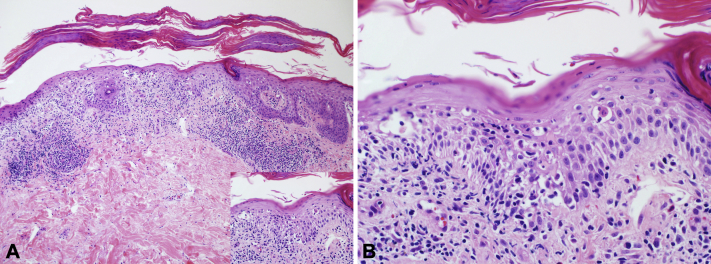
Punch biopsy found a parakeratotic, spongiotic, and focally acanthotic epidermis with exocytosis of cytologically bland-appearing lymphocytes and rare neutrophils. Focal basal layer vacuolar degeneration with interface and perivascular lymphocytic inflammation and extravasated red blood cells were also observed (Fig 2, A and B). Immunohistochemistry found an equal ratio of CD4+:CD8+ cells in the epidermis and a slight CD4 predominance in the dermis.
Fig 2.
A and B, Microscopic examination of stained slides of a lower leg punch biopsy found a diffusely spongiotic epidermis with focal acanthosis dotted by exocytosis of bland-appearing lymphocytes, extravasated red blood cells, and rare neutrophils, surfaced by overlying parakeratosis. Focal basal layer vacuolar degeneration with interface and perivascular lymphocytic inflammation was also observed (A and B, Hematoxylin-eosin stain; original magnifications: A, ×100; B, ×400.)
The combined clinical and histopathologic findings were consistent with a diagnosis of pityriasis lichenoides chronica–like drug eruption. The skin lesions were treated with topical clobetasol with complete clearance within 2 months. About 1 month after clearance of her pityriasis lichenoides chronica–like drug eruption an inflammatory arthritis developed that resulted in discontinuation of pembrolizumab and required treatment with methotrexate. Eight months after discontinuation of pembrolizumab, she has not had a recurrence of her pityriasis lichenoides chronica–like drug eruption but is still on methotrexate for ongoing inflammatory arthritis.
Discussion
Immune checkpoint inhibitors have fundamentally transformed the approach to the treatment of metastatic melanoma and are already being used or investigated as treatments for multiple other malignancies.3 Unfortunately, as well as inducing the immune system to eradicate malignant cells, these medications have resulted in a variety of immune-related adverse events. Interestingly, although PD-1 inhibitors are thought to evoke a primarily cytotoxic T-cell response against malignant cells, the cutaneous immune-related adverse events include both autoantibody (epidermolysis bullosa acquisita and bullous pemphigoid) and psoriasiform eruptions in addition to the more predictable cytotoxic eruptions (morbilliform, vitiligo, lichenoid, Stevens-Johnson syndrome and erythema multiforme).1, 2, 3, 4, 5, 6, 7, 8, 9, 10
To our knowledge, the development of a pityriasis lichenoides chronica–like drug eruption during pembrolizumab therapy has not been reported; however, a potential association may not be surprising, as both psoriasiform and lichenoid dermatitides have occurred during PD-1 therapy.3, 4, 7, 9, 10 In light of the temporal relationship between starting pembrolizumab and the development of a pityriasis lichenoides chronica–like drug eruption and the known association of pembrolizumab with psoriasiform and lichenoid dermatitides, we believe it is most likely that our patient's pityriasis lichenoides chronica–like drug eruption was induced by pembrolizumab. In our case, the patient's pityriasis lichenoides chronica–like drug eruption rapidly improved with high potency topical steroids, allowing continuation of pembrolizumab therapy. Subsequent development of inflammatory arthritis, however, necessitated discontinuation of pembrolizumab and initiation of methotrexate therapy. Because methotrexate is also an effective treatment for pityriasis lichenoides chronica, it is unclear if her continued clearance is secondary to drug discontinuation or if her pityriasis lichenoides chronica–like drug eruption is merely being suppressed by methotrexate. Our case expands the spectrum of cutaneous immune-related adverse events from PD-1 inhibitors and should raise awareness of this potentially treatable side effect of PD-1 inhibitor therapy.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Milhem serves on the advisory boards of BMS, EMDSERONO, Novartis, EISAI, and Genetech. Drs Mutgi, Swick, and Liu have no conflicts to declare.
References
- 1.Robert C., Ribas A., Wolchok J.D. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 2.Sanlorenzo M., Vujic I., Daud A. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol. 2015;151:1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jour G., Glitza I.C., Ellis R.M. Autoimmune Dermatologic Toxicities from Immune Checkpoint Blockade with anti-PD-1 Antibody Therapy: A Report on Bullous Skin Eruptions. J Cutaneous Pathol. 2016;43:688–696. doi: 10.1111/cup.12717. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann L., Forschner A., Loquai C. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Carlos G., Anforth R., Chou S., Clements A., Fernandez-Penas P. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Mel Res. 2015;25(3):265–268. doi: 10.1097/CMR.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 6.Rajakulendran T., Adam D.N. Spotlight on pembrolizumab in the treatment of advanced melanoma. Drug Des Devel Ther. 2015;9:2883–2886. doi: 10.2147/DDDT.S78036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtsuka M., Miura T., Mori T., Ishikawa M., Yamamoto T. Occurrence of Psoriasiform Eruption During Nivolumab Therapy for Primary Oral Mucosal Melanoma. JAMA Dermatol. 2015;151(7):797–799. doi: 10.1001/jamadermatol.2015.0249. [DOI] [PubMed] [Google Scholar]
- 8.Goldinger S.M., Stieger P., Meier B. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2872. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Joseph R.W., Cappel M., Goedjen B. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3(1):18–22. doi: 10.1158/2326-6066.CIR-14-0134. [DOI] [PubMed] [Google Scholar]
- 10.Schaberg K.B., Novoa R.A., Wakelee H.A. Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. J Cutan Pathol. 2016;43(4):339–346. doi: 10.1111/cup.12666. [DOI] [PubMed] [Google Scholar]