Abstract
Aerobic anoxygenic phototrophic (AAP) bacteria are well known to be abundant in estuaries, coastal regions and in the open ocean, but little is known about their activity in any aquatic ecosystem. To explore the activity of AAP bacteria in the Delaware estuary and coastal waters, single-cell 3H-leucine incorporation by these bacteria was examined with a new approach that combines infrared epifluorescence microscopy and microautoradiography. The approach was used on samples from the Delaware coast from August through December and on transects through the Delaware estuary in August and November 2011. The percent of active AAP bacteria was up to twofold higher than the percentage of active cells in the rest of the bacterial community in the estuary. Likewise, the silver grain area around active AAP bacteria in microautoradiography preparations was larger than the area around cells in the rest of the bacterial community, indicating higher rates of leucine consumption by AAP bacteria. The cell size of AAP bacteria was 50% bigger than the size of other bacteria, about the same difference on average as measured for activity. The abundance of AAP bacteria was negatively correlated and their activity positively correlated with light availability in the water column, although light did not affect 3H-leucine incorporation in light–dark experiments. Our results suggest that AAP bacteria are bigger and more active than other bacteria, and likely contribute more to organic carbon fluxes than indicated by their abundance.
Keywords: AAP bacteria, bacteriochlorophyll, carbon cycling, DOC, photoheterotrophy
Introduction
The capacity of photoheterotrophic bacteria to capture energy from sunlight would seem to give them an advantage over strict heterotrophic bacteria using only organic material for energy as well as carbon. This presumed advantage led to the hypothesis that one type of photoheterotroph, aerobic anoxygenic phototrophic (AAP) bacteria, would be most abundant in oligotrophic systems (Kolber et al., 2000), but subsequent work demonstrated that AAP bacteria are abundant in eutrophic estuaries and coastal waters when compared with the open oceans (Schwalbach and Fuhrman, 2005; Jiao et al., 2007; Lami et al., 2007; Waidner and Kirchman, 2007; Ritchie and Johnson, 2012). Light has been shown to positively affect the growth rate and other aspects of AAP bacterial activity in laboratory experiments (Shiba, 1984; Okamura et al., 1986; Holert et al., 2011; Tomasch et al., 2011; Hauruseu and Koblížek, 2012), but it has complex effects on natural microbial communities which include photoheterotrophic microbes (Schwalbach et al., 2005; Straza and Kirchman, 2011; Ruiz-González et al., 2012a; Ruiz-González et al., 2012b). Data on growth-related activity in natural communities would help elucidate the advantages of photoheterotrophy and whether photoheterotrophic microbes change models of carbon cycling (Karl, 2002).
The few studies exploring the activity of AAP bacteria in natural communities have found that these bacteria have higher growth rates than other bacteria. Koblížek and colleagues (Koblížek et al., 2005; Koblížek et al., 2007; Hojerová et al., 2011) have used the turnover of bacteriochlorophyll a (BChl) between night and day to calculate growth rates of AAP bacteria in the Atlantic Ocean, Baltic Sea and the coastal Mediterranean Sea. In the Atlantic Ocean, for example, Koblížek et al. (2007) found growth rates as high as 2 per day for AAP bacteria, about 10-fold higher than bacterial growth rates expected for this system (Ducklow, 2000). Previous studies using other approaches have also found evidence of high-AAP bacterial growth. AAP bacteria grew about twofold faster than the total-bacterial community according to direct count data from manipulation experiments with Mediterranean seawater (Ferrera et al., 2011). Likewise, the frequency of dividing AAP bacterial cells was about threefold higher than the frequency for the entire bacterial community in the South China Sea (Liu et al., 2010), again suggesting much higher growth rates for AAP bacteria. These differences in growth rate are higher than would be predicted based on theoretical calculations (Kirchman and Hanson, 2013). Data on the uptake of leucine and other organic compounds would be useful for exploring AAP bacterial activity, but uptake by AAP bacteria in natural communities cannot be examined with current methods.
A common approach to examine the activity of specific bacterial groups is fluorescence in-situ hybridization combined with microautoradiography (Lee et al., 1999; Ouverney and Fuhrman, 1999; Cottrell and Kirchman, 2000). This approach provides information on bacteria targeted by fluorescence in-situ hybridization probes and on their activity based on consumption of radiolabeled compounds, such as 3H-leucine. However, AAP bacteria are too diverse to be detected by a practical number of fluorescence in-situ hybridization probes for ribosomal RNA (Yutin et al., 2007). Instead, AAP bacteria are identified and enumerated by the infrared (IR) autofluorescence of BChl (Kolber et al., 2001). However, this autofluorescence cannot be used with microautoradiography because BChl does not survive the microautoradiography assay (unpublished data), preventing identification of AAP bacteria after microautoradiography.
In this study we developed a method that combines IR epifluorescence microscopy and microautoradiography and used it to examine the growth-related activity of AAP bacteria over the salinity gradient of the Delaware estuary. The distribution of abundance and activity in the estuary along with light–dark experiments were used to identify factors, such as particle concentrations and light availability, potentially controlling AAP bacterial communities. AAP bacteria can be especially abundant associated with particles in the turbid portions of the Delaware estuary and similar waters in the Mediterranean Sea (Waidner and Kirchman, 2007; Cottrell et al., 2010; Lamy et al., 2011), although there are exceptions (Lami et al., 2009). Light availability would explain why AAP bacterial abundance is highest in the euphotic zone of the oceans and decreases with depth (Cottrell et al., 2006; Sieracki et al., 2006; Salka et al., 2008). Abundance of these bacteria also correlated with light intensity and day length over a year in Mediterranean coastal waters (Ferrera et al., 2013). We hypothesized that AAP bacteria would be more active than the rest of the bacterial community and that their activity would be enhanced by light. We found that AAP bacteria were more active and larger than other bacteria in the estuary. The ecological strategy of these bacteria appears to include being highly active with fast growth rates even if it leads to a large-cell size and higher mortality.
Materials and methods
The data reported here are from two cruises in August and November 2011 and monthly sampling trips at a station, which was previously examined (Campbell et al., 2011), just outside of the Delaware Bay (38° 47.15′ N; 74° 55.76 W). Samples were processed on board the ship or immediately upon returning to the laboratory. Light attenuation was estimated from the intensities of photosynthetically active radiance over a depth profile measured with a Biospherical PNF-210 radiometer (San Diego, CA, USA). The concentrations of Chl, inorganic nutrients (nitrate, ammonium, phosphate and silicate) and dissolved organic carbon were analyzed by standard methods. Total-bacterial production was determined from the incorporation of 3H-leucine using the microcentrifuge method (Kirchman, 2001).
Samples for AAP bacteria-microautoradiography (AAP-MAR) were incubated with 3H-leucine at a final concentration of 20 nM in polycarbonate bottles either under ambient surface light conditions or in the dark (see Results). Since polycarbonate does not allow penetration of light with wavelengths<420 nm (Wulff et al., 1999), these experiments in effect examined bacterial activity exposed to photosynthetically active radiance. The assays used [4,5-3H] leucine with a specific activity of 50–60 Ci mol−1 (Perkin-Elmer, Waltham, MA, USA). Incubations lasted 1 h and were ended with the addition of paraformaldehyde at a final concentration of 2%. An hour after the addition of paraformaldehyde, samples were filtered through black polycarbonate filters (25 mm with a pore size of 0.2 μm). The samples were kept at −80 °C before analysis.
Effect of light on AAP bacterial activity
Three types of experiments were conducted to examine the effect of light on AAP and total-bacterial activity. The first was to compare single-cell activity of communities incubated either in the light or the dark immediately after collection during various times of the day. In the second experiment, samples were collected 4 and 9 h after dusk and then the activity of AAP bacteria and the total-bacterial community was assayed in dark incubations. We hypothesized that AAP bacterial activity would be higher in communities most recently in natural sunlight. The third experiment examined bacterial activity in light and dark incubations by communities collected before dawn. Surface water was placed in 10 l polycarbonate bottles that were either blacked out with heavy-duty aluminum foil or were left uncovered, allowing full exposure to surface sunlight. After 6 h in the light or the dark, 50 mL of sample water from each treatment was incubated with 3H-leucine for 1 h at a final concentration of 20 nM and added with 2% paraformaldehyde. The samples were filtered as described above and then stored frozen at −80 °C until analysis.
Enumeration of AAP bacteria and microautoradiography
The fraction of bacteria active in incorporating 3H-leucine and the silver grain area around active cells were determined by microscopic analysis of cells from the 3H-leucine incubations. A section of the black polycarbonate filter with samples from the 3H-leucine incubations was stained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min and then mounted on a microscope slide. The slide was placed on an automatic microscope stage (Prior Scientific, Rockland, MA, USA) capable of moving in the x-y plane with a precision of 0.2 μm. Before the analysis began, the x-y coordinates of the apex and one other corner of the filter were recorded. These filter corners were used as fixed points for relocating the fields of view after microautoradiography. The abundances of AAP bacteria and all bacteria were determined from images of DAPI, BChl (IR autofluorescence), Chl and phycoerythrin-positive cells as described previously (Cottrell et al., 2006).
After identifying and counting the AAP bacteria, the filter pieces were removed from the microscope slide, dipped in 100% ethanol to remove the microscope immersion oil, and dried. The filter pieces were then analyzed by microautoradiography following published protocols (Cottrell and Kirchman, 2003). Exposure in the emulsion lasted 24 h. The coordinates of the apex and corner of each filter section in the microautoradiography preparation were used to relocate the fields of view identified previously during IR epifluorescence analysis for AAP bacterial abundance (see above). The fields were relocated from the coordinates recorded during the IR epifluorescence analysis relative to the new coordinates in the microautoradiography analysis. As discussed more thoroughly in Supplementary Information, relocation is possible because two properties are the same before and after microautoradiography, regardless of the orientation of the filter piece on the microscope stage: (1) the distance between the field of view and the lower left filter corner, and (2) the angle formed by the line connecting the filter corners and the line between the field of view and lower left corner.
The slide containing the filter section was then moved using the computer-driven mechanical stage to the new calculated coordinates. Once a field was located, the DAPI-stained cells were manually brought into focus, and images of DAPI fluorescence and silver grains were acquired. The silver grain image was acquired using transmitted light (bright field). These steps were repeated until all fields previously analyzed for AAP bacterial abundance were examined. After all fields of view had been analyzed, ImagePro (Media Cybernetics, Rockville, MD, USA) was used to align DAPI images with identified AAP bacteria from before and after the microautoradiography assay. The user determined whether the images were properly aligned based on the cells shared between the two images. If the images matched, then the two DAPI images were merged to create a composite image that accounted for all cells. The MicrobeCounter program (Cottrell and Kirchman, 2003) counts all cells and all AAP cells, and it identifies which cells have silver grains. It also calculates the area of the silver grains around active cells. The current version of the method does not give accurate estimates of the biovolume of active and inactive cells in the AAP bacterial and total communities.
The relative abundance and cell size of AAP bacteria reported here were estimated by standard IR epifluorescence microscopy (Cottrell et al., 2006), similar to the analysis before microautoradiography described above. While it was clear that entire sections of cells on the filter piece were not transferred to the photographic film emulsion, which is common in microautoradiography (Kirchman et al., 1985), all cells in other sections appeared to be transferred intact. There was no evidence of preferential loss of AAP bacteria. The relative abundances of AAP bacteria determined by the standard IR epifluorescence analysis and by AAP-MAR were statistically the same (paired t-test, P>0.05); the difference between the two (standard minus AAP-MAR) was −0.03±0.05% (n=13).
Before parametric statistical analyses, relative AAP bacterial abundance and percentages of active cells were arcsine transformed and silver grain areas were log transformed. The standard deviations and the number of samples reported here are for samples taken at different stations within the estuary or at different times.
Results
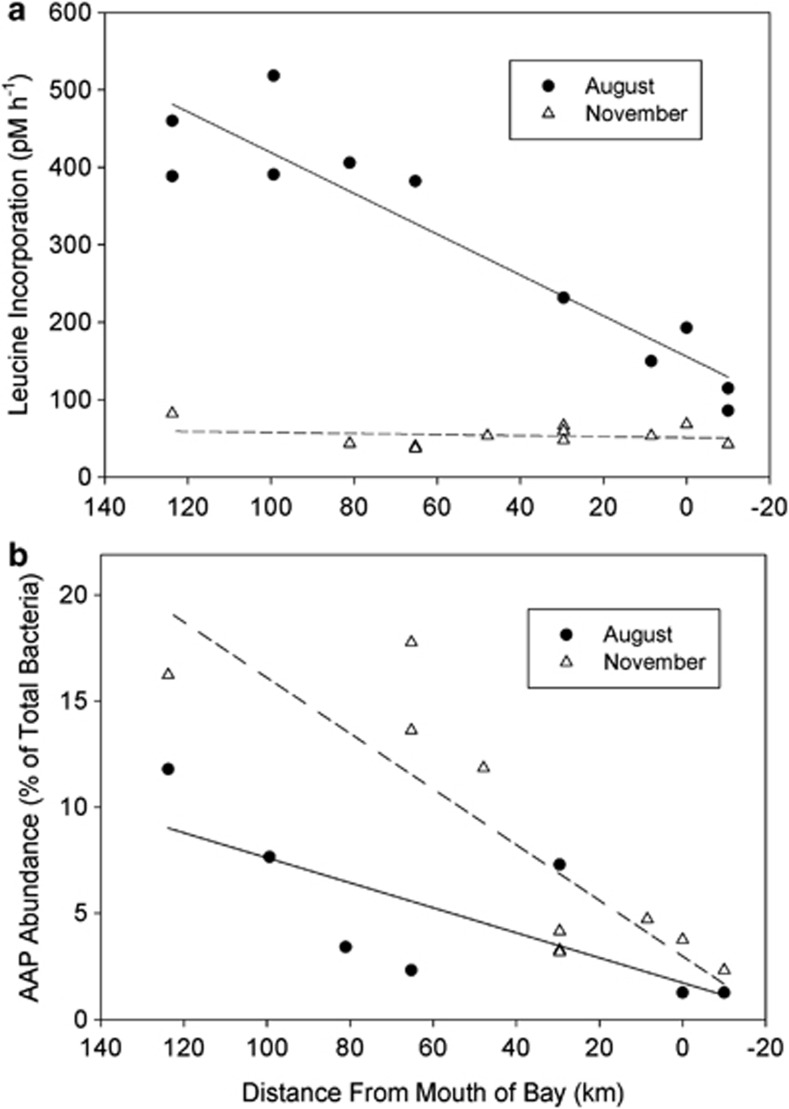
The abundance and activity of AAP bacteria and of the total-bacterial community in the Delaware estuary were examined along with basic biogeochemical properties in August and November 2011. In August total-bacterial abundance was highest near the mouth of the estuary and lowest in brackish water, overall averaging 3.35 × 106 cells per ml (Table 1). In November bacterial abundance did not vary substantially, remaining around 2.15 × 106 cells ml−1 in the entire estuary. In both months salinity increased and light attenuation decreased along transects from brackish water to the mouth of the estuary (Table 1). Chl concentrations was highest in brackish waters, reaching 17 μg l−1 in August and 8.5 μg l−1 in November, whereas it was about twofold lower at the mouth of the estuary. Nutrients also varied throughout the estuary. Nitrate ranged from 132 μM in brackish waters to about 2 μM near the mouth of the estuary during both months (Table 1). Phosphate concentrations were slightly lower at the mouth of the estuary during both months, averaging 0.8 to 1.9 μM in the entire estuary. In August, leucine incorporation was highest in brackish waters and then decreased by about fivefold at the mouth of the estuary (Figure 1a). Incorporation rates were lower in November and did not vary substantially in the estuary.
Table 1. Biogeochemical parameters of the Delaware estuary in August and November 2011.
|
August |
November |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mouth |
Middle |
Brackish |
Mouth |
Middle |
Brackish |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Total bacteria (106 cells ml−1) | 3.35 | 0.6 | 2.78 | 0.6 | 1.87 | 0.9 | 2.13 | 0.8 | 2.13 | 1.0 | 2.19 | 0.7 |
| Salinity | 28.3 | 2.5 | 17.0 | 2.9 | 3.6 | 4.0 | 26.4 | 4.7 | 12.4 | 3.9 | 3.1 | 2.5 |
| Temperature (°C) | 23.8 | 2.4 | 27.2 | 0.4 | 28.1 | 0.2 | 13.3 | 0.4 | 12.3 | 1.0 | 11.8 | 1.0 |
| Light atten (m−1) | −0.8 | 0.2 | −1.25 | 0.04 | −2.7 | 1.4 | −0.69 | 0.1 | −1.6 | 0.6 | −2.9 | 1.6 |
| Secchi (m) | 2.1 | 1.9 | 1.0 | 0.1 | 0.6 | 0.4 | 2.1 | 0.4 | 0.9 | 0.3 | 0.7 | 0.5 |
| Chl a (μg l−1) | 4.0 | 2.1 | 4.6 | 0.9 | 8.9 | 5.1 | 4.8 | 1.2 | 3.1 | 1.0 | 8.5 | 3.1 |
| Nitrate (μM) | 34.0 | 73.4 | 63.3 | 14.3 | 92.7 | 31.6 | 17.4 | 12.4 | 55.0 | 13.8 | 99.7 | 16.7 |
| Ammonium (μM) | 5.0 | 2.3 | 4.1 | 2.2 | 2.4 | 3.2 | 4.2 | 1.4 | 3.5 | 0.7 | 7.0 | 8.9 |
| Phosphate (μM) | 0.9 | 0.6 | 1.5 | 0.5 | 1.9 | 0.2 | 0.8 | 0.3 | 1.5 | 0.1 | 1.4 | 0.1 |
| Silicate (μM) | 33.3 | 41.1 | 48.6 | 40.8 | 14.8 | 7.1 | 21.1 | 13.0 | 35.4 | 12.2 | 51.5 | 51.5 |
Abbreviations: Atten, attenuation coefficient; Chl a, chlorophyll a.
The bay was split into three sections: mouth, 0–40 km from the mouth of the bay; middle, 40–80 km from the mouth of the bay; and brackish, 80–120 km from the mouth of bay. For August: n=10 for mouth; n=6 for middle; and n=7 for brackish. For November: n=11 for mouth; n=7 for middle; and n=7 for brackish.
Figure 1.
Abundance of AAP bacteria (a) and leucine incorporation (b) in the Delaware estuary. The lines were calculated by regression analyses.
The abundance of AAP bacteria varied with location in the estuary and between the two months. Relative abundance of AAP bacteria was highest in brackish waters, reaching 12% in August and 16% in November (Figure 1b). AAP bacterial abundance was lowest at the mouth of the estuary, as low as 1.2% in August and 2.3% in November. AAP bacterial abundance was higher in November than in August (Figure 1b). There was a significant negative correlation between the relative abundance of AAP bacteria and salinity (r=−0.69; P<0.0001; n=26).
Leucine incorporation by AAP bacteria
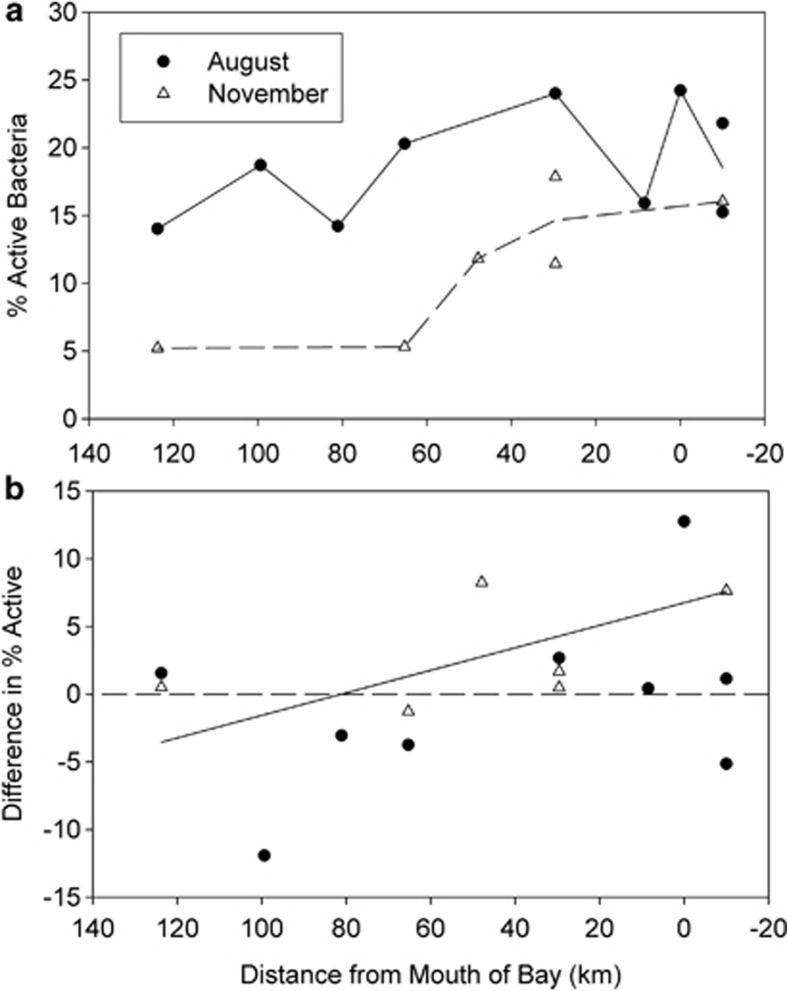
The percent of AAP bacteria and of all bacteria incorporating leucine varied throughout the estuary. The percent of all bacteria that incorporated leucine was 15–25% in August and 5–17% in November (Figure 2a). AAP bacteria were more active than the rest of the community near the mouth of the estuary (Figure 2b). In August, AAP bacteria were up to 13% more active than the rest of the community near the mouth of the estuary (37% of AAP bacteria were active versus 24% of the total community). In November, the AAP bacteria were only 7% more active than the rest of the community near the mouth of the estuary. However, the total-bacterial community was more active than the AAP bacteria in some locations. In brackish waters 120 km from the mouth, there was little difference between the percent activity of AAP bacteria and of the total community in both August and November.
Figure 2.
Percent active bacteria in the Delaware Estuary assayed by single-cell leucine incorporation. (a) The percent active cells in the total-bacterial community. (b) The difference in percent activity between AAP bacteria and the total population. The difference was percent of active AAP bacteria minus percent of active total bacteria. The solid line was determined by a regression analysis of all points (ANOVA; P<0.05; d.f.=26).
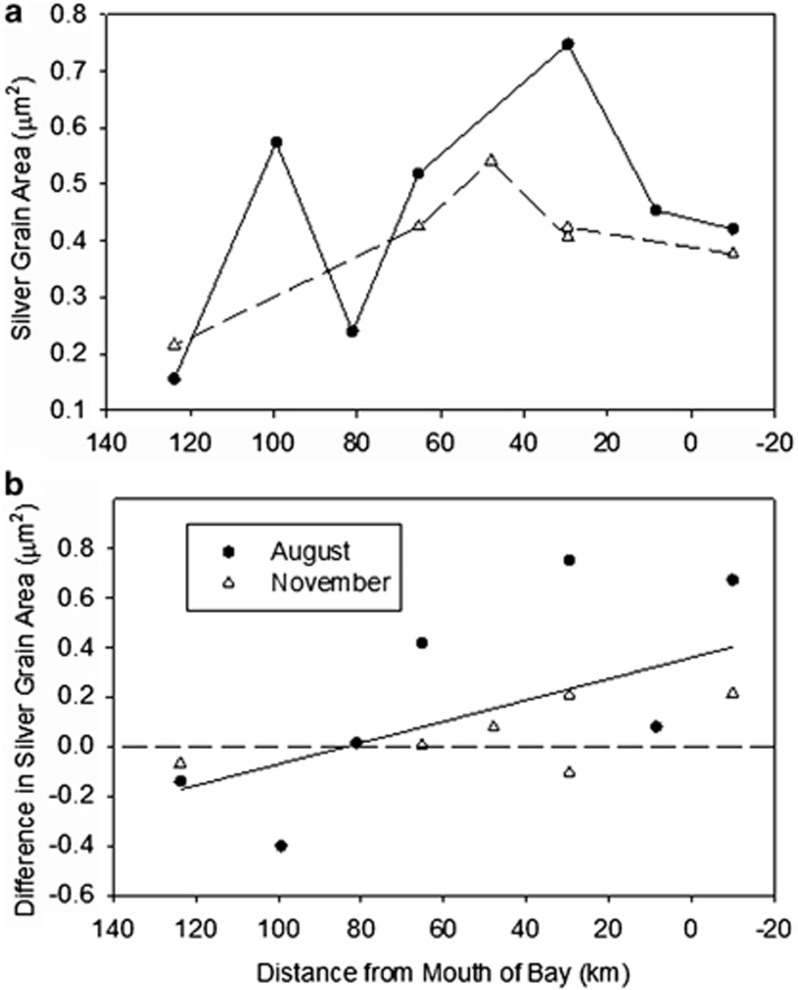
The area of silver grains around active cells created during microautoradiography is a quantitative indication of leucine incorporation (Cottrell and Kirchman, 2003; Sintes and Herndl, 2006). The average silver grain area varied in August from 0.15 to 0.78 μm2 for the total community (Figure 3a). In November the average silver grain area was smaller, ranging from 0.2 to 0.55 μm2. Overall, active AAP bacteria had larger silver grain area than the average active bacterium near the mouth of the estuary (Figure 3b), whereas farther up the estuary (80 to 125 km from the mouth) AAP bacteria did not always have larger silver grain area. Near the mouth of the estuary in August, the silver grain area associated with the active AAP bacteria was up to 0.8 μm2 larger than that with the rest of the active bacteria. In November, the difference in silver grain area was smaller (only 0.2 μm2) than in August.
Figure 3.
Area of silver grains associated with leucine-active bacteria in the Delaware Estuary. (a) The average silver grain area for the entire bacterial community. (b) The difference in silver grain areas for AAP bacteria and the rest of the community. This difference equals the average silver grain area associated with AAP bacteria minus the area for the total-bacterial community at a sample site. The solid line was determined by a regression analysis of all points (ANOVA; P<0.02; d.f.=21).
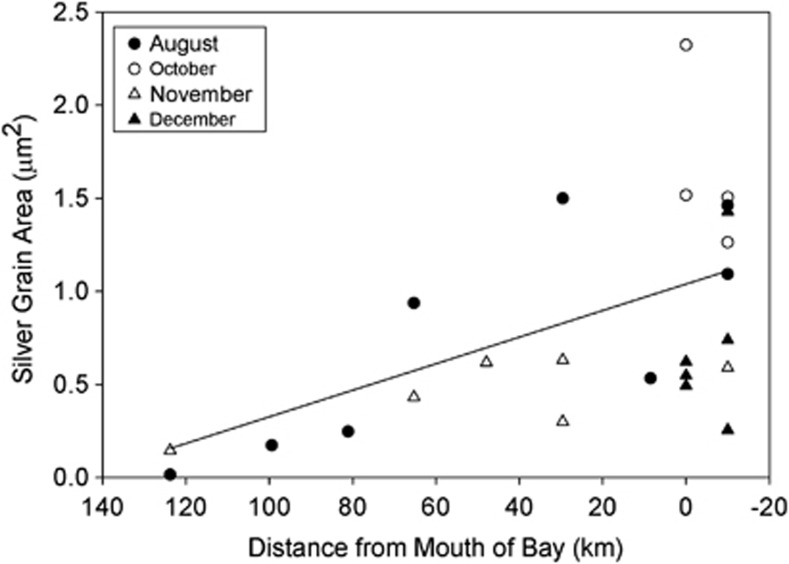
Silver grain area associated with AAP bacteria tended to be larger at the mouth of the estuary, and it was smallest in the brackish waters (Figure 4). The negative relationship between silver grain area and distance was apparent in both August and November. When data from both months were combined, there was a significant negative correlation between distance and the silver grain area of active AAP bacteria (r=−0.50, P<0.02, d.f.=21).
Figure 4.
Silver grain area associated with AAP bacteria active for leucine incorporation in the Delaware estuary. The line was calculated from a regression analysis (ANOVA, P<0.02; d.f =21).
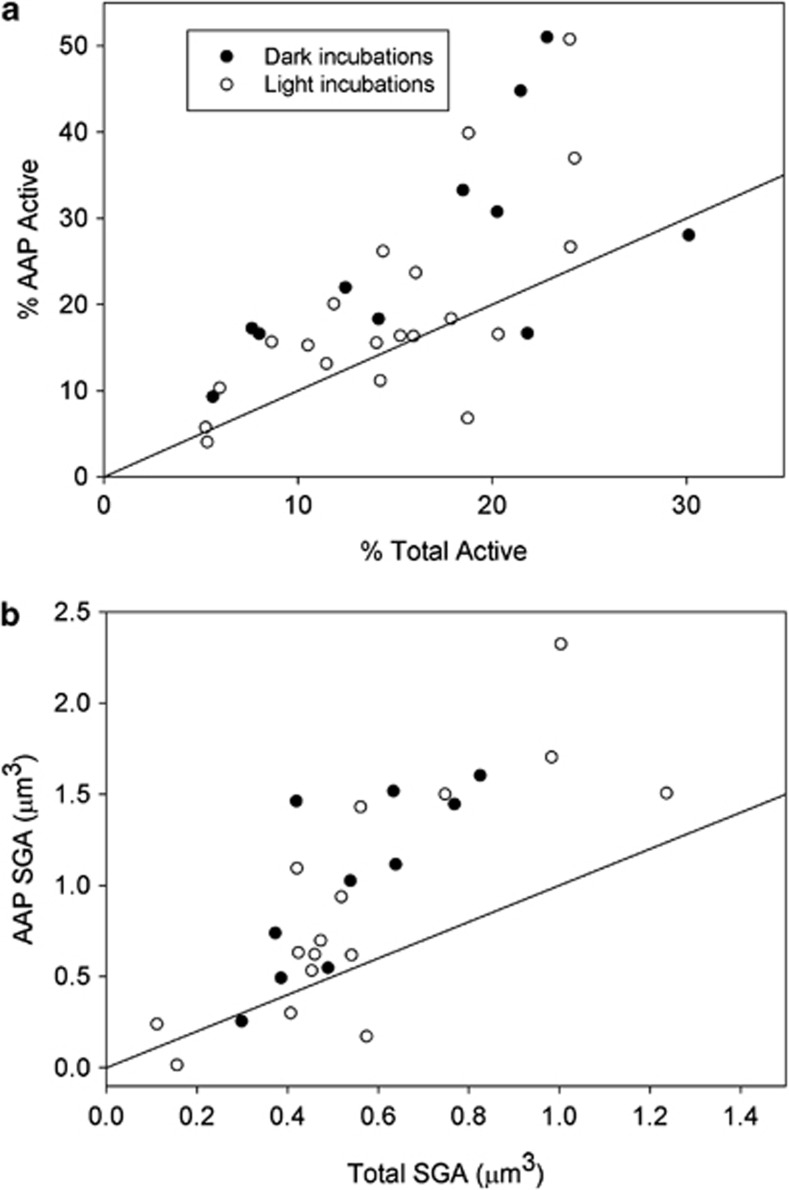
Single-cell leucine incorporation was examined from August to December at the mouth of the estuary and combined with the transect data to give an overall picture of the AAP bacterial activity in these waters (Figure 5). Percent of active AAP bacteria was higher than the percent of active bacteria in 80% of the 31 assays (points about the 1:1 line in Figure 5a). For the entire data set in Figure 5a, the ratio of percent active AAP bacteria to percent active total bacteria was 1.43±0.51 (SD). That is, relative activity of AAP bacteria was about 40% higher than that for the total-bacterial community. There was no difference in percent active cells in light versus dark incubations (Figure 5a).
Figure 5.
Activity of AAP bacteria versus activity of the total-bacterial community determined by single-cell leucine incorporation. (a) Percentage of active cells in the AAP bacterial community versus the percentage for the total-bacterial community in the same sample. (b) Average silver grain area (SGA) associated with active AAP bacteria and the total-bacterial community. The solid line indicates a 1:1 relationship between AAP bacteria and the total community.
A similar analysis was done with the silver grain area around active cells (Figure 5b). As with the percentage data, the silver grain area of AAP bacteria was larger than that for all bacteria in 80% of the comparisons. The ratio of silver grain area for AAP bacteria to total bacteria was 1.60±0.77 (SD). As with the percentage data, light had no effect on silver grain area in these short incubations.
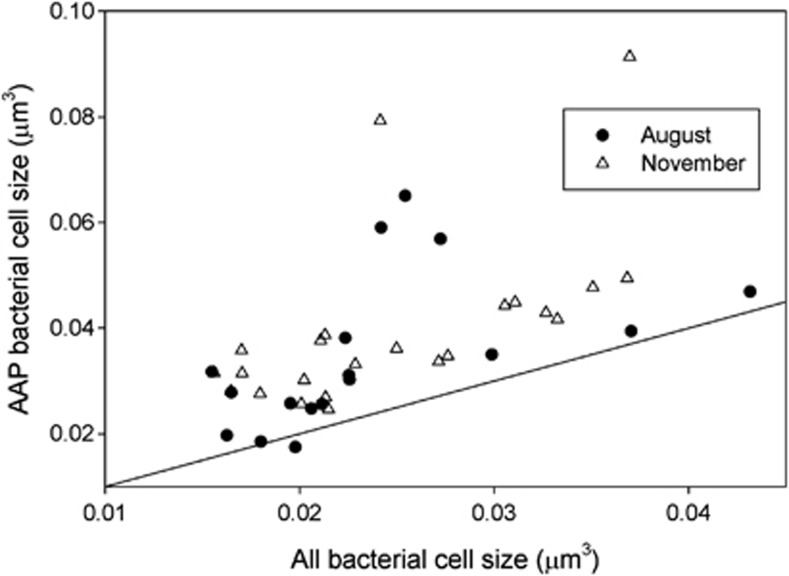
Cell size of AAP bacteria
Biovolumes of AAP and other bacterial cells were examined to determine if the activity of these bacteria was related to cell size. AAP bacteria were overall about 50% bigger than other bacteria according to measurements of DAPI-stained images from standard IR epifluorescence analyses (Figure 6), about the same difference as seen in percent active cells and silver grain area (see above). The difference between AAP and other bacterial cell sizes was highly significant (Wilcoxon signed rank test, P<0.0001, N=40). Cell sizes in August did not differ from those in November (Kruskal–Wallis rank sum test, P>0.05, N=17 and 23 for August and November, respectively). Cell sizes of active- and inactive-AAP bacteria cannot be determined with the current version of the AAP-MAR method.
Figure 6.
Cell size of AAP bacteria versus cell size of all bacteria in the same sample.
Light and AAP bacterial activity
Additional experiments were conducted at two locations in the estuary to determine the effect of light on the single-cell incorporation of leucine by AAP bacteria (Table 2). The AAP bacteria were almost twice as active as the total bacteria in these experiments, in terms of both percent active cells and silver grain area. However, there was no significant difference in activity of AAP bacteria or of cells in the total-bacterial community in the light versus dark incubations. The activity of AAP bacteria and of the total-bacterial community was also similar in dark-incubated samples collected 4 and 9 h after dusk (data not shown).
Table 2. Activity of AAP bacteria (“AAP Bact”) and the total community (“All Bact”) in light and dark experiments.
|
% Active cells |
Silver grain area (μm2) |
|||||
|---|---|---|---|---|---|---|
| All bact | SD | AAP bact | SD | All bact | AAP bact | |
| Exp 1 | ||||||
| Initial | 12.4 | 5.5 | 22.0 | 16.5 | 0.64 | 1.12 |
| Light 6 h | 8.6 | 3.5 | 15.7 | 10.3 | 0.47 | 0.70 |
| Dark 6 h | 20.2 | 11.9 | 30.8 | 16.0 | 0.77 | 1.45 |
| Exp 2 | ||||||
| Initial | 21.5 | 15.5 | 44.8 | 20.9 | 0.54 | 1.03 |
| Light 6 h | 24.0 | 6.6 | 50.8 | 24.5 | 0.98 | 1.70 |
| Dark 6 h | 22.8 | 15.0 | 51.0 | 26.3 | 0.83 | 1.60 |
Abbreviations: AAP, aerobic anoxygenic phototrophic; Exp, experiment.
Seawater was collected before sunrise and incubated for 6 h under natural light or dark conditions. The leucine incorporation by single cells was determined immediately after collection (‘initial') and after exposure to 6 h of light or complete darkness. Experiment 1 was conducted with samples from 63.5 km from the mouth of the bay in 14.4-salinity water on 9 August 2012. Experiment 2 was conducted on water from 10 km from the mouth in 31.2-salinity water on 8 August 2012.
Correlations with light and other environmental properties
Several aspects of AAP bacteria were positively correlated with relative light availability, evident from the negative correlation with light attenuation. There was a negative correlation between the percent of active AAP bacteria and light attenuation (Table 3) and a positive correlation between relative AAP bacterial abundance and light attenuation (r=0.46; P=0.030; n=21). The difference in percent active cells between AAP bacteria and the rest of the bacterial community was also negatively correlated with light attenuation (Table 3). Since the attenuation coefficient increases as the water column becomes more turbid, a negative correlation signifies a positive relationship between AAP bacterial activity and light availability.
Table 3. Correlation between aspects of AAP bacterial activity and environmental parameters.
| % Active AAP bacteria | AAP bacterial SGA | Difference in % active (AAP-total) | Difference in SGA (AAP-total) | |
|---|---|---|---|---|
| Distance to mouth | −0.389 | −0.500* | −0.466* | −0.170 |
| Salinity | 0.378 | 0.444* | 0.233 | −0.049 |
| Temperature | 0.176 | 0.021 | −0.202 | −0.047 |
| Leu incorporation | 0.191 | 0.061 | −0.330 | −0.052 |
| Light attenuation | −0.642* | −0.510 | −0.636** | −0.460 |
| Chlorophyll | −0.274 | −0.449 | −0.494 | −0.624* |
| Nitrate | −0.704** | −0.713** | −0.463 | −0.660* |
| Ammonium | −0.221 | −0.248 | 0.191 | −0.179 |
| Phosphate | −0.467 | −0.583* | −0.463 | −0.514 |
| Silicate | −0.616* | −0.271 | −0.060 | −0.196 |
Abbreviations: AAP, aerobic anoxygenic phototrophic; Leu, leucine; SGA, silver grain areas.
Pearson correlation coefficients were calculated for percent active AAP bacteria, the area of silver grains associated with AAP bacteria, the difference in percent active cells and the difference in silver grain areas (SGA).*P<0.05; **P<0.01.
There were several other significant correlations between AAP bacteria and other environmental properties (Table 3), because the relative abundance decreased and activity of AAP bacteria tended to increase while other environmental properties decreased along transects from brackish waters to the mouth of the estuary.
Discussion
We investigated the incorporation of leucine by AAP bacteria in the Delaware estuary and coastal waters using a new variation of microautoradiography to identify the activity of individual AAP bacterial cells. The impact of light was explored using natural variation of environmental properties in the estuary and in controlled light–dark experiments. We hypothesized that AAP bacteria would be more active than other bacteria throughout the Delaware estuary. We found that on an average AAP bacteria incorporated more leucine than other bacteria, consistent with the hypothesis that phototrophy gives AAP bacteria a metabolic advantage over heterotrophic bacteria.
But AAP bacteria were not always more active than other bacteria in all locations and times in Delaware waters, whereas the evidence from previous studies indicates that AAP bacteria grow substantially faster than other bacteria in marine waters. Studies using the BChl-turnover approach found that growth rates of AAP bacteria were as much as 3 per day (Koblížek et al., 2005; Koblížek et al., 2007; Hojerová et al., 2011), substantially higher than bacterial growth rates expected for these systems (Ducklow, 2000). Similarly, frequency of dividing cell data indicate that growth rates of AAP bacteria are about threefold higher than rates for other bacteria (Liu et al., 2010). In the Delaware estuary, in contrast, AAP bacteria were 40–60% more active than other bacteria in taking up leucine. Our results suggest that phototrophy promotes higher activity by AAP bacteria but not to the degree found by previous studies using other approaches. Theoretical calculations indicate that the energetic advantage of phototrophy for photoheterotrophs is even smaller than our results suggest (Kirchman and Hanson, 2013). The difference may indicate problems with the model, such as the fact that the numbers for the light-harvesting apparatus used in the calculations were assumed to be constant (Kirchman and Hanson, 2013). Other ecological factors, such as top-down controls (see below), may also help explain the variability in AAP bacterial activity not predicted by a model examining only light effects.
There are several possible explanations for the difference between our work and previous studies. The most obvious is the difference in methodology. Incubations in our study were only 1 hour as opposed to the 18–72 h needed to detect the activity of AAP bacteria through the change in BChl a concentrations (Koblížek et al., 2005; Koblížek et al., 2007), whereas no incubation is needed for the frequency of dividing cell method (Hagström et al., 1979; Liu et al., 2010). Another methodological difference is that our approach assumes that AAP activity is adequately traced by the incorporation of 3H-leucine. Leucine may not track all active bacteria, and the relationship between leucine incorporation and actual bacterial growth can differ (del Giorgio and Gasol, 2008). Finally, the differences between our results and previous studies may simply reflect environmental differences among the marine systems examined by these studies.
Several hypotheses may explain our observation that the activity of AAP bacteria varied throughout the estuary, with the lowest activity in brackish waters and the highest in coastal waters near the mouth of the estuary. This pattern is the opposite from that predicted by the hypothesis that AAP bacteria favor particles (Waidner and Kirchman, 2007; Cottrell et al., 2010; Lamy et al., 2011). If particles provided an advantage, then AAP bacterial activity would be higher in brackish waters because of its large-particle load. Another hypothesis is that AAP bacterial activity varies because of grazing. AAP bacteria are potentially easy targets for grazers because they are larger than other bacteria (Sieracki et al. (2006); this study). Although it is unclear if this top-down control varies systematically in the estuary, there seems to be some role for grazing because of the negative relationship between abundance and single-cell leucine incorporation by AAP bacteria; relative AAP bacterial abundance was lowest near the mouth of the estuary where AAP bacterial activity was highest. Dissolved organic carbon and inorganic nutrients (nitrate and phosphate) are other properties that change consistently throughout the estuary (Sharp et al., 2009) and may affect AAP bacterial activity. However, concentrations of these dissolved compounds in the Delaware vary the opposite of AAP bacterial activity, suggesting that total dissolved organic carbon and inorganic nutrients do not explain variation in AAP bacterial activity in the estuary.
The percent of active AAP bacteria did have a negative correlation with light attenuation; activity and light availability were both low in brackish waters and high in coastal waters. The importance of light controlling AAP bacterial activity has been shown by several pure culture studies (Shiba, 1984; Okamura et al., 1986; Holert et al., 2011; Tomasch et al., 2011; Hauruseu and Koblížek, 2012). However, we did not see a significant effect of light in short term incubations. It may be hard to determine an effect of light on photoheterotrophic bacteria in natural microbial communities in a short incubation because of the small amount of energy apparently gained by AAP bacteria via phototrophy (Kirchman and Hanson, 2013). The lack of a light effect in our experiments is consistent with previous work in Delaware and elsewhere in which light effects were complex and often contradictory (Schwalbach et al., 2005; Straza and Kirchman, 2011; Ruiz-González et al., 2012a; Ruiz-González et al., 2012b). Light did stimulate the activity of the NOR5 gammaproteobacterial clade known to contain the AAP bacterial taxa (Ruiz-González et al., 2012a) and of another likely photoheterotroph (SAR11 bacteria) in short term experiments (Gomez-Pereira et al., 2013). In any case, light seems to be the most likely factor, along with top-down factors, affecting AAP bacterial abundance and activity relative to the rest of the bacterial community.
Likewise, phototrophy is also the most likely explanation for why AAP bacteria are bigger than other bacteria in the Delaware estuary (this study) and the North Atlantic Ocean (Sieracki et al., 2006). In both systems, the average cell size of AAP bacteria is about 50–80% bigger than the size of other heterotrophic and photoheterotrophic bacteria, excluding cyanobacteria. AAP bacteria in natural communities appear to be roughly similar in size to cells in another group of photoheterotrophs, those in the cyanobacterial genera Synechococcus and Prochlorococcus (Zubkov et al., 2003; Michelou et al., 2007). It is possible that AAP bacteria are larger than other bacteria because of a facet of bacterial metabolism other than phototrophy and that AAP bacteria take up more leucine per cell simply because they are larger; AAP bacteria and other bacteria are about equally active when leucine incorporation is normalized for biovolume. A more parsimonious explanation is that phototrophy leads to more activity and thus larger cell size. Other types of data at the whole community level indicate that active bacteria are generally larger than inactive ones (del Giorgio and Gasol, 2008). AAP bacteria appear to be an example of a taxon that is active with high-growth rates at the expense of being larger and thus attracting higher mortality.
Our results are useful for exploring the role of phototrophy in the ecophysiology of natural bacterial communities and are important first steps in evaluating the contribution of AAP bacteria to carbon cycling in aquatic systems. Because AAP bacteria are more active, grow faster, and are bigger than other bacteria, they contribute more to bacterial biomass production and potentially to degradation of organic material than their abundance would suggest. In the waters we studied, AAP bacteria could contribute 3% to about 25% of total-bacterial production along the estuarine gradient, given that these organisms made up 2% to 17% of total-bacterial abundance and were about 50% more active than other bacteria. Because of AAP bacteria and other photoheterotrophic microbes, models of carbon cycling for the oceans need to be modified to include a direct effect of light on fluxes of organic material (Karl, 2002). Obviously more work is needed to explore whether AAP bacteria have unique roles in the carbon cycle, such as in consuming some organic compounds more so than others (Hauruseu and Koblížek, 2012) or in using types of organic material less accessible to purely heterotrophic bacteria (Waidner and Kirchman, 2007). These questions and others can now be explored using the approach developed here.
Acknowledgments
We thank Mrina Nikrad, Katie Kalis and Raphäel Lami for help during sampling and Lying Yu for technical assistance. David Kieber provided insights about photochemical processes in the estuary. This work was supported by NSF OCE 1030306 and the Partners University Fund.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Campbell BJ, Yu L, Heidelberg JF, Kirchman DL. (2011). Activity of abundant and rare bacteria in a coastal ocean. Proc Natl Acad Sci USA 108: 12776–12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell MT, Kirchman DL. (2000). Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high- molecular-weight dissolved organic matter. Appl Environ Microbiol 66: 1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell MT, Kirchman DL. (2003). Contribution of major bacterial groups to bacterial biomass production (thymidine and leucine incorporation) in the Delaware Estuary. Limnol Oceanogr 48: 168–178. [Google Scholar]
- Cottrell MT, Mannino A, Kirchman DL. (2006). Aerobic anoxygenic phototrophic bacteria in the Mid-Atlantic Bight and the North Pacific Gyre. Appl Environ Microbiol 72: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell MT, Ras J, Kirchman DL. (2010). Bacteriochlorophyll and community structure of aerobic anoxygenic phototrophic bacteria in a particle-rich estuary. ISME J 4: 945–954. [DOI] [PubMed] [Google Scholar]
- del Giorgio PA, Gasol JM. (2008). Physiological structure and single-cell activity in marine bacterioplankton. In: Kirchman DL (ed) Microbial Ecology of the Ocean 2nd edn. John Wiley & Sons: New York, NY, USA, pp 243–298. [Google Scholar]
- Ducklow H. (2000). Bacterial production and biomass in the oceans. In: Kirchman DL (ed) Microbial Ecology of the Oceans. John Wiley & Sons: New York, NY, USA, pp 85–120. [Google Scholar]
- Ferrera I, Gasol JM, Sebastian M, Hojerová E, Koblížek M. (2011). Comparison of growth rates of aerobic anoxygenic phototrophic bacteria and other bacterioplankton groups in coastal Mediterranean waters. Appl Environ Microbiol 77: 7451–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera I, Borrego CM, Salazar G, Gasol JM. (2013). Marked seasonality of aerobic anoxygenic phototrophic bacteria in the coastal NW Mediterranean Sea as revealed by cell abundance, pigment concentration and pyrosequencing of pufM gene. Environ Microbiol e-pub ahead of print 13 September 2013 doi:10.1111/1462-2920.12278. [DOI] [PubMed]
- Gomez-Pereira PR, Hartmann M, Grob C, Tarran GA, Martin AP, Fuchs BM et al. (2013). Comparable light stimulation of organic nutrient uptake by SAR11 and Prochlorococcus in the North Atlantic subtropical gyre. ISME J 7: 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström Å, Larsson U, Horstedt P, Normark S. (1979). Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol 37: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauruseu D, Koblížek M. (2012). Influence of light on carbon utilization in aerobic anoxygenic phototrophs. Appl Environ Microbiol 78: 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojerová E, Mašín M, Brunet C, Ferrera I, Gasol JM, Koblížek M. (2011). Distribution and growth of aerobic anoxygenic phototrophs in the Mediterranean Sea. Environ Microbiol 13: 2717–2725. [DOI] [PubMed] [Google Scholar]
- Holert J, Hahnke S, Cypionka H. (2011). Influence of light and anoxia on chemiosmotic energy conservation in Dinoroseobacter shibae. Environ Microbio Rep 3: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao N, Zhang Y, Zeng Y, Hong N, Liu R, Chen F et al. (2007). Distinct distribution pattern of abundance and diversity of aerobic anoxygenic phototrophic bacteria in the global ocean. Environ Microbiol 9: 3091–3099. [DOI] [PubMed] [Google Scholar]
- Karl DM. (2002). Microbiological oceanography - Hidden in a sea of microbes. Nature 415: 590–591. [DOI] [PubMed] [Google Scholar]
- Kirchman DL, K'nees E, Hodson RE. (1985). Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol 49: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman DL. (2001). Measuring bacterial biomass production and growth rates from leucine incorporation in natural aquatic environments. In: Paul JH (ed) Marine Microbiology. Academic Press: San Diego, CA, USA, pp 227–237. [Google Scholar]
- Kirchman DL, Hanson TE. (2013). Bioenergetics of photoheterotrophic bacteria in the oceans. Environ Microbiol Rep 5: 188–199. [DOI] [PubMed] [Google Scholar]
- Koblížek M, Ston-Egiert J, Sagan S, Kolber ZS. (2005). Diel changes in bacteriochlorophyll a concentration suggest rapid bacterioplankton cycling in the Baltic Sea. FEMS Microbiol Ecol 51: 353–361. [DOI] [PubMed] [Google Scholar]
- Koblížek M, Masin M, Ras J, Poulton AJ, Prasil O. (2007). Rapid growth rates of aerobic anoxygenic phototrophs in the ocean. Environ Microbiol 9: 2401–2406. [DOI] [PubMed] [Google Scholar]
- Kolber ZS, Van Dover CL, Niederman RA, Falkowski PG. (2000). Bacterial photosynthesis in surface waters of the open ocean. Nature 407: 177–179. [DOI] [PubMed] [Google Scholar]
- Kolber ZS, Plumley FG, Lang AS, Beatty JT, Blankenship RE, Van Dover CL et al. (2001). Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292: 2492–2495. [DOI] [PubMed] [Google Scholar]
- Lami R, Cottrell MT, Ras J, Ulloa O, Obernosterer I, Claustre H et al. (2007). High abundances of aerobic anoxygenic photosynthetic bacteria in the South Pacific Ocean. Appl Environ Microbiol 73: 4198–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lami R, Cuperova Z, Ras J, Lebaron P, Koblížek M. (2009). Distribution of free-living and particle-attached aerobic anoxygenic phototrophic bacteria in marine environments. Aquat Microb Ecol 55: 31–38. [Google Scholar]
- Lamy D, De Carvalho-Maalouf P, Cottrell MT, Lami R, Catala P, Oriol L et al. (2011). Seasonal dynamics of aerobic anoxygenic phototrophs in a Mediterranean coastal lagoon. Aquat Microb Ecol 62: 153–163. [Google Scholar]
- Lee N, Nielsen PH, Andreasen KH, Juretschko S, Nielsen JL, Schleifer KH et al. (1999). Combination of fluorescent in situ hybridization and microautoradiography - a new tool for structure-function analyses in microbial ecology. Appl Environ Microbiol 65: 1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Zhang Y, Jiao N. (2010). Diel variations in frequency of dividing cells and abundance of aerobic anoxygenic phototrophic bacteria in a coral reef system of the South China Sea. Aquat Microb Ecol 58: 303–310. [Google Scholar]
- Michelou VK, Cottrell MT, Kirchman DL. (2007). Light-stimulated bacterial production and amino acid assimilation by cyanobacteria and other microbes in the North Atlantic Ocean. Appl Environ Microbiol 73: 5539–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Mitsumori F, Ito O, Takamiya K, Nishimura M. (1986). Photophosphorylation and oxidative phosphorylation in intact cells and chromatophores of an aerobic photosynthetic bacterium, Erythrobacter sp. strain OCh114. J Bacteriol 168: 1142–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouverney CC, Fuhrman JA. (1999). Combined microautoradiography-16S rRNA probe technique for determination of radioisotope uptake by specific microbial cell types in situ. Appl Environ Microbiol 65: 1746–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie AE, Johnson ZI. (2012). Abundance and genetic diversity of aerobic anoxygenic phototrophic bacteria of coastal regions of the Pacific Ocean. Appl Environ Microbiol 78: 2858–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-González C, Lefort T, Galí M, Montserrat Sala M, Sommaruga R, Simó R et al. (2012. a). Seasonal patterns in the sunlight sensitivity of bacterioplankton from Mediterranean surface coastal waters. FEMS Microbiol Ecol 79: 661–674. [DOI] [PubMed] [Google Scholar]
- Ruiz-González C, Lefort T, Massana R, Simó R, Gasol JM. (2012. b). Diel changes in bulk and single-cell bacterial heterotrophic activity in winter surface waters of the northwestern Mediterranean Sea. Limnol Oceanogr 57: 29–42. [Google Scholar]
- Salka I, Moulisova V, Koblížek M, Jost G, Jurgens K, Labrenz M. (2008). Abundance, depth distribution, and composition of aerobic bacteriochlorophyll a-producing bacteria in four basins of the central Baltic Sea. Appl Environ Microbiol 74: 4398–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbach MS, Brown M, Fuhrman JA. (2005). Impact of light on marine bacterioplankton community structure. Aquat Microb Ecol 39: 235–245. [Google Scholar]
- Schwalbach MS, Fuhrman JA. (2005). Wide-ranging abundances of aerobic anoxygenic phototrophic bacteria in the world ocean revealed by epifluorescence microscopy and quantitative PCR. Limnol Oceanogr 50: 620–628. [Google Scholar]
- Sharp JH, Yoshiyama K, Parker AE, Schwartz MC, Curless SE, Beauregard AY et al. (2009). A biogeochemical view of estuarine eutrophication: Seasonal and spatial trends and correlations in the Delaware Estuary. Estuar Coast 32: 1023–1043. [Google Scholar]
- Shiba T. (1984). Utilization of light energy by the strictly aerobic bacterium Erythrobacter sp OCH 114. J Gen Appl Microbiol 30: 239–244. [Google Scholar]
- Sieracki ME, Gilg IC, Thier EC, Poulton NJ, Goericke R. (2006). Distribution of planktonic aerobic anoxygenic photoheterotrophic bacteria in the northwest Atlantic. Limnol Oceanogr 51: 38–46. [Google Scholar]
- Sintes E, Herndl GJ. (2006). Quantifying substrate uptake by individual cells of marine bacterioplankton by catalyzed reporter deposition fluorescence in situ hybridization combined with microautoradiography. Appl Environ Microbiol 72: 7022–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straza TRA, Kirchman DL. (2011). Single-cell response of bacterial groups to light and other environmental factors in the Delaware Bay, USA. Aquat Microb Ecol 62: 267–277. [Google Scholar]
- Tomasch J, Gohl R, Bunk B, Diez MS, Wagner-Dobler I. (2011). Transcriptional response of the photoheterotrophic marine bacterium Dinoroseobacter shibae to changing light regimes. ISME J 5: 1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidner LA, Kirchman DL. (2007). Aerobic anoxygenic phototrophic bacteria attached to particles in turbid waters of the Delaware and Chesapeake estuaries. Appl Environ Microbiol 73: 3936–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff A, Nilsson C, Sundback K, Wangberg SA, Odmark S. (1999). UV radiation effects on microbenthos—a four month field experiment. Aquat Microb Ecol 19: 269–278. [Google Scholar]
- Yutin N, Suzuki MT, Teeling H, Weber M, Venter JC, Rusch DB et al. (2007). Assessing diversity and biogeography of aerobic anoxygenic phototrophic bacteria in surface waters of the Atlantic and Pacific Oceans using the Global Ocean Sampling expedition metagenomes. Environ Microbiol 9: 1464–1475. [DOI] [PubMed] [Google Scholar]
- Zubkov MV, Fuchs BM, Tarran GA, Burkill PH, Amann R. (2003). High rate of uptake of organic nitrogen compounds by Prochlorococcus cyanobacteria as a key to their dominance in oligotrophic oceanic waters. Appl Environ Microbiol 69: 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.