Abstract
Background
Increased rate of infections in diabetes mellitus (DM) is an accepted fact. Pathophysiologically, several tasks of the immune system could be involved including polymorphonuclear (PMN) functions.
Objectives
The aim of this research was to evaluate the respiratory burst process of PMNs that is an essential part of phagocytosis, in children with DM.
Patients and Methods
Fifty two children with insulin dependent diabetes and 29 non-diabetic children were enrolled in this cross sectional study from 2010 to 2011. Nitroblue tetrazolium (NBT) test was done on PMNs taken from their heparinized blood. The resultant data was analyzed by SPSS version 16. P values were considered significant when it was under 0.05.
Results
Mean NBTs were 72.1 ± 15.84 and 94.68 ± 5.31 in diabetics and non-diabetics, respectively (P < 0.001). Using Pearson correlation, there was no significant correlation between the NBT level and age, gender, duration of diabetes, daily insulin usage and blood HbA1C level.
Conclusions
Compared to non-diabetics, respiratory burst process of polymorphonuclears is obviously decreased in diabetic children. This can explain one of the mechanisms involved in the increased rate of infections in DM.
Keywords: Diabetes Mellitus, Neutrophils, Nitrobluetetrazolium, Respiratory Burst
1. Background
Increased rate and severity of infections and sepsis in diabetes mellitus (DM), is an accepted fact (1-3). Patients with DM constitute to 20.1% - 22.7% of all patients with sepsis (4, 5). Distinct infections are more common in these patients, including necrotizing fasciitis, diabetic foot, mucormycosis, emphysematous infections of the gall bladder, kidney and urinary bladder, and invasive otitis externa (6-8). In a year round prospective cohort study, 705 adult patients who had type 1 DM, 6,712 adult patients who had type 2 DM and 18911 control patients who had hypertension without diabetes were compared regarding the incidence of infection. The result documented in patients with type one and two DM had increased risk for urinary tract, skin and mucous membranes, and lower respiratory tract infections (9). The association between DM and tuberculosis was first documented by Persian scientist Avicenna, who lived from 980 through 1,027 (10).
Many aspects of the immune system are investigated in the diabetics and evidences of some disturbance are present now. Impaired chemotaxis and phagocytosis of the monocytes (8, 11) and impaired macrophage function in DM (12), also impairment of the lymphocytes proliferative response to different stimuli of diabetics with poorly controlled disease are presented before (13). Furthermore, an abnormal delayed type hypersensitivity reaction in DM type 1 and type 2 patients is demonstrated (14, 15).
There are conflicting opinions regarding the status of the humoral immunity in DM. Some research finds normal antibody concentrations in patients with DM and good response of them to pneumococcal and intramuscular hepatitis B vaccine (16-18). However, lower immunoglobulin levels are demonstrated in some studies (19, 20). In a study in U.S., no significant association was found between diabetic patients and non-diabetics after immunization with typhoid vaccine (21). Polymorphonuclears (PMNs) are an essential component of the human innate immune system. It is stated that the major functions of PMNs including chemotaxis, adherence, phagocytosis and intracellular killing of microbes, are impaired in diabetic patients that have been attributed to the hyperglycemia, a process that could be reversed by insulin treatment and better metabolic control (9, 22). A couple of studies have illustrated a decrease of microbial killing in the presence of hyperglycemia (23, 24). Controversial findings regarding the effect of hyperglycemia on phagocytosis are published (7, 24-26). Phagocytosis as one of vital PMNs functions has several steps including generation of microbicidal oxidants to kill bacteria in the phagosomes. The microbicidal products, named reactive oxygen species, consist mainly of superoxide anions and hydrogen peroxide that are produced in a process termed “respiratory burst”. This pivotal process involves the enzyme complex known as the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which is responsible for transferring electrons from NADPH to O2, resulting in the formation of superoxide anion. The antimicrobial function of polymorphonuclears is supposed to be inhibited by hyperglycemia, due to G6PD inhibition or alteration of NADPH in the polyol pathway (25).
2. Objectives
Focusing on phagocytosis and specifically “respiratory burst” process of PMNs, this study was designed to assess this important step of microbial killing in diabetic children, and compare it with a control group of non-diabetic children.
3. Patients and Methods
This cross sectional study was accomplished in Mofid children’s hospital, an academic referral center, affiliated to Shahid Beheshti University of Medical Sciences, Tehran, during an eight month period terminating at January 2011. Totally, eighty subjects were enrolled in the study. Fifty two of them were diabetic children and 29 healthy ones.
Five milliliter heparinized blood was collected from each patient and mixed equally with 3% dextran. It is recommended to mix the blood and dextran in a syringe. The mixture was incubated at 37°C for 30 minutes and then the neutrophil enriched plasma was transferred to a 15 mL falcon tube. The plasma was centrifuged for 10 minutes at 1,500 rpm and the pellet was washed with 1 ml of distilled water for 30 seconds and then 5 mL of phosphate buffer saline (PBS) was added to resuspend the cells. The suspension was centrifuged at 1,500 rpm for 10 minutes and the pellet was re-suspended in 1 ml PBS and the number of cells was determined with a hemocytometer. The cells were adjusted to 2.5 × 106 cells/mL.
Fifty microliter of the adjusted cell suspension was added to 50 μL of previously prepared nitrobluetetrazolium (NBT)/PMP reagent. The mixture was incubated for 30 min at 37°C. After incubation, the mixture was centrifuged at 1,500 rpm for 3 minutes. The pellet was collected with a 10 μL sampler and a smear was prepared from the cells in a bullet shape and then stained with Gimsa for 10 minutes. The neutrophils were observed using oil immersion lens and the percentage of the neutrophils which were positive for reduced NBT and formazan crystal, was reported. Staining of all specimens was performed by one person who was expert in NBT test. Analysis of reduction in NBT was assessed in a blinded manner.
4. Results
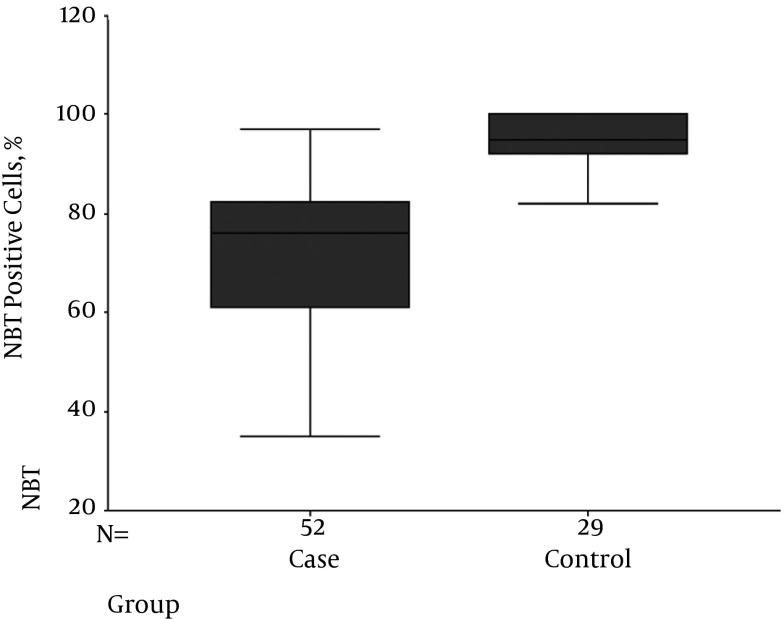
Among patients 44.3% were male and 55.7% female (Table 1). The mean age of diabetics was 102 months (8.5 years) with the youngest being 21 and the eldest 180 months. Mean duration of diabetes was 25 months. The mean age of non-diabetics was 77 months (6.4 years) with the youngest being 13 and the eldest 180 months. Mean NBT in diabetics was 72.1 ± 15.84 and in non-diabetics 94.68 ± 5.31 (P < 0.001) (Figure 1). The mean level of HgA1C was 8.29 and mean daily injected insulin 16.54 units. All the diabetic patients and the non-diabetics had normal blood urea nitrogen and serum creatinine. Using Pearson correlation, there was no significant correlation between the NBT level and age, gender, duration of the disease, daily insulin usage and blood HgA1C level.
Table 1. Sex Distribution of Patients and Control Group.
| Group | Case | Control |
|---|---|---|
| Gender, (%) | ||
| Female | 55.7 | 41.3 |
| Male | 44.3 | 58.7 |
| Mean age, y | 8.5 | 6.4 |
Figure 1. Mean NBT in Diabetics and Non-Diabetics.
5. Discussion
DM is associated with an increased frequency of infections (1). According to Joslinʼs findings in the time previous to insulin era, from a series of 1,000 cases, diabetic coma was usually caused by infection (1, 27). So, infection remains an important cause of death in diabetics (28).
In a separate study, among 1,000 hospitalized patients, 2/3 of bacteremias were found in patients with DM comparing to 1/3 in patients without diabetes. Consistent with these results, diabetic patients developed septic shock in 22% and super infections in 22% of the episodes versus 15.6%, and 11%, respectively of the non-diabetic bacteremic patients (P < 0.05 for all comparisons) (29).
This increased rate of infectious events in DM has persuaded the investigators to assess the capability of different functions of the immune system, including the bactericidal ability of the PMNs, but despite carrying out several researches, there are still conflicting results regarding the bactericidal ability of PMNs in DM.
Spirer et al. (30) showed that the NBT reduction test is normal in well-controlled diabetic patients, and in diabetic acidosis there is a significant decrease in this ability during phagocytosis. According to Pujol-Moix et al.’s (31) study there was no significant difference between the NBT levels of diabetic patients and normal individuals. Furthermore, despite the expectation, NBT reduction did not increase in response to infection in diabetic patients with bacterial infection compared to a corresponding group without infection. Yun Woong et al. (32) assessed NBT and stimulated NBT test with E. coli endotoxin in four groups, including 27 healthy adults and 10 diabetic patients without bacterial infection. The absolute numbers of NBT-positive neutrophils in NBT test in two situations of with and without stimulation were not significantly different between healthy adults and diabetic patients. In a study by Niethammer et al. (33) on 10 diabetic children, the NBT-index and intracellular killing of Candida albicans were normal.
Despite abovementioned researches that are not in favor of changes in intracellular killing capacity in DM, there are several studies that demonstrate the abnormalities of NBT. Walter’s research in 1971 showed that PMNs from normal and non-diabetic individuals had a higher NBT and phagocytic index than those of diabetic persons (34).
In a study on 6 to 18 year old individuals, PMNs of forty normal and thirty diabetic children were assessed for phagocytic and bactericidal activity of PMNs. The duration of diabetes was from 1 to 12 years. The PMNs of diabetic children demonstrated decreased capacity for intracellular killing of bacteria compared to normal individuals (35). In other research, chemotaxis, phagocytosis, adherence, bactericidal activity and NBT reduction capacity were studied in 58 type I diabetic adults. Bactericidal activity in diabetics was decreased compared to non-diabetics (36). Our study as a properly designed research with adequate number of enrolled insulin dependent diabetic children proved the significantly lower bactericidal potential of PMNs of the diabetic children compared to non-diabetic children.
The correlation of hyperglycemia and respiratory burst is yet a matter of controversy. Daoud et al. (24) evaluated the PMN functions of cells isolated from the diabetics and control groups in different concentration of glucose and demonstrated that hypoglycemia lead to alteration in immune cells. In their study, there was a significant reduction (25 to 35 percent) in the respiratory burst activity in patient with diabetes compared to controls.
In other studies, after stabilizing the diabetic state, the activity of the granulocytes improved, but still remained below that of healthy persons (25, 37).
We measured glycosylated hemoglobin HbA1c that is a fraction of hemoglobin to which glucose has been non-enzymatically connected in the bloodstream (Nelson Textbook), as a reliable index of glycemic control of the patient during the approximately previous 120 days. In children, values of 6% - 7.9 % denote a good metabolic control, values of 8.0% - 9.9 %, fair control, and values of 10% or higher, demonstrate a poor control state (38). Mean HbA1C in our patients was 8.29% indicating a fair control state. There was no correlation between the HbA1C level and the NBT activity.
5.1. Conclusion
The respiratory burst phenomenon is obviously decreased in diabetic children, which can explain one of the mechanisms involved in the increased rate of infection in these patients.
Acknowledgments
We would like to thank pediatric infections research center affiliated to Shahid Beheshti University of Medical Sciences, Tehran, for their financial support.
Footnotes
Authors’ Contribution:None declared.
References
- 1.Koh GC, Peacock SJ, van der Poll T, Wiersinga WJ. The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis. 2012;31(4):379–88. doi: 10.1007/s10096-011-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510–3. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 3.Korbel L, Spencer JD. Diabetes mellitus and infection: an evaluation of hospital utilization and management costs in the United States. J Diabetes Complications. 2015;29(2):192–5. doi: 10.1016/j.jdiacomp.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanner O. Intensive versus conventional glucose control in critically ill patients. J Intens Care Soc. 2009;10(3):216–7. doi: 10.1177/175114370901000314. [DOI] [Google Scholar]
- 5.Stegenga ME, Vincent JL, Vail GM, Xie J, Haney DJ, Williams MD, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med. 2010;38(2):539–45. doi: 10.1097/CCM.0b013e3181c02726. [DOI] [PubMed] [Google Scholar]
- 6.Hsu JC, Shen SH, Yang TY, Chen PH, Huang KC, Tsai YH. Necrotizing fasciitis and sepsis caused by Vibrio vulnificus and Klebsiella pneumoniae in diabetic patients. Biomed J. 2015;38(2):136–42. doi: 10.4103/2319-4170.137767. [DOI] [PubMed] [Google Scholar]
- 7.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16 Suppl 1:S27–36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lecube A, Pachon G, Petriz J, Hernandez C, Simo R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One. 2011;6(8):e3989. doi: 10.1371/journal.pone.0023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41(3):281–8. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 10.Oscarsson PN, Silwer H. II Incidence of Pulmonary Tuberculosis among Diabetics. Acta Med Scand. 1958; 161(335):23–48. doi: 10.1111/j.0954-6820.1958.tb04605.x. [DOI] [PubMed] [Google Scholar]
- 11.Dunaeva M, Voo S, van Oosterhoud C, Waltenberger J. Sonic hedgehog is a potent chemoattractant for human monocytes: diabetes mellitus inhibits Sonic hedgehog-induced monocyte chemotaxis. Basic Res Cardiol. 2010;105(1):61–71. doi: 10.1007/s00395-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5(3):e3989. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992;18(3):187–201. [PubMed] [Google Scholar]
- 14.Casey JI, Heeter BJ, Klyshevich KA. Impaired response of lymphocytes of diabetic subjects to antigen of Staphylococcus aureus. J Infect Dis. 1977;136(4):495–501. doi: 10.1093/infdis/136.4.495. [DOI] [PubMed] [Google Scholar]
- 15.MacCuish AC, Urbaniak SJ, Campbell CJ, Duncan LJ, Irvine WJ. Phytohemagglutinin transformation and circulating lymphocyte subpopulations in insulin-dependent diabetic patients. Diabetes. 1974;23(8):708–12. doi: 10.2337/diab.23.8.708. [DOI] [PubMed] [Google Scholar]
- 16.Volti SL, Caruso-Nicoletti M, Biazzo F. Hyporesponsiveness to intradermal administration of hepatitis B vaccine in insulin dependent diabetes mellitus. Arch Dis Child. 1998;78(1):54–7. doi: 10.1136/adc.78.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederman MM, Schiffman G, Rodman HM. Pneumococcal immunization in adult diabetics. Diabetes. 1981;30(2):119–21. doi: 10.2337/diab.30.2.119. [DOI] [PubMed] [Google Scholar]
- 18.Beam TJ, Crigler ED, Goldman JK, Schiffman G. Antibody response to polyvalent pneumococcal polysaccharide vaccine in diabetics. JAMA. 1980;244(23):2621–4. [PubMed] [Google Scholar]
- 19.Schillie SF, Spradling PR, Murphy TV. Immune response of hepatitis B vaccine among persons with diabetes: a systematic review of the literature. Diabetes Care. 2012;35(12):2690–7. doi: 10.2337/dc12-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ocak S, Eskiocak AF. The evaluation of immune responses to hepatitis B vaccination in diabetic and non-diabetic haemodialysis patients and the use of tetanus toxoid. Nephrology (Carlton). 2008;13(6):487–91. doi: 10.1111/j.1440-1797.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- 21.Duderstadt SK, Rose CJ, Real TM, Sabatier JF, Stewart B, Ma G, et al. Vaccination and risk of type 1 diabetes mellitus in active component U.S. Military, 2002-2008. Vaccine. 2012;30(4):813–9. doi: 10.1016/j.vaccine.2011.10.087. [DOI] [PubMed] [Google Scholar]
- 22.Walrand S, Guillet C, Boirie Y, Vasson MP. In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions. J Leukoc Biol. 2004;76(6):1104–10. doi: 10.1189/jlb.0104050. [DOI] [PubMed] [Google Scholar]
- 23.de Souza Ferreira C, Araujo TH, Angelo ML, Pennacchi PC, Okada SS, de Araujo Paula FB, et al. Neutrophil dysfunction induced by hyperglycemia: modulation of myeloperoxidase activity. Cell Biochem Funct. 2012;30(7):604–10. doi: 10.1002/cbf.2840. [DOI] [PubMed] [Google Scholar]
- 24.Daoud AK, Tayyar MA, Fouda IM, Harfeil NA. Effects of diabetes mellitus vs. in vitro hyperglycemia on select immune cell functions. J Immunotoxicol. 2009;6(1):36–41. doi: 10.1080/15476910802604564. [DOI] [PubMed] [Google Scholar]
- 25.Huang J., Xiao Y., Xu A, Zhou Z. Neutrophils in type 1 diabetes. J Diabetes Investig . 2015 doi: 10.1111/jdi.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Restrepo BI, Twahirwa M, Rahbar MH, Schlesinger LS. Phagocytosis via complement or Fc-gamma receptors is compromised in monocytes from type 2 diabetes patients with chronic hyperglycemia. PLoS One. 2014;9(3):e3989. doi: 10.1371/journal.pone.0092977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joslin EP. The treatment of diabetes mellitus: with observations upon the disease based upon thirteen hundred cases. Philadelphia: Lea & Febiger; 1917. [Google Scholar]
- 28.Knapp S. Diabetes and infection: is there a link?--A mini-review. Gerontology. 2013;59(2):99–104. doi: 10.1159/000345107. [DOI] [PubMed] [Google Scholar]
- 29.Carton JA, Maradona JA, Nuno FJ, Fernandez-Alvarez R, Perez-Gonzalez F, Asensi V. Diabetes mellitus and bacteraemia: a comparative study between diabetic and non-diabetic patients. Eur J Med. 1992;1(5):281–7. [PubMed] [Google Scholar]
- 30.Spirer Z, Bogair N. Nitroblue tetrazolium reduction test in diabetes mellitus. J Pediatr . 1973;83(4):685–7. doi: 10.1016/S0022-3476(73)80241-0. [DOI] [PubMed] [Google Scholar]
- 31.Pujol-Moix MN. Nitroblue-tetrazolium reducing capacity of neutrophils in diabetes. N Engl J Med. 1973;289(17):920. doi: 10.1056/nejm197310252891719. [DOI] [PubMed] [Google Scholar]
- 32.Yun Woong K, Jee Sook H, Chong Youl P. Nbt and stimulated nbt test in patients with diabetes mellitus. Korean J Hematol. 1977;12(2):107–15. [Google Scholar]
- 33.Niethammer D, Heinze E, Teller W, Kleihauer E, Wildfeuer A, Haferkamp O. Impairment of granulocyte function in juvenile diabetes. Klin Wochenschr. 1975;53(22):1057–60. doi: 10.1007/BF01614381. [DOI] [PubMed] [Google Scholar]
- 34.Walters MI, Lessler MA, Stevenson TD. Oxidative metabolism of leukocytes from nondiabetic and diabetic patients. J Lab Clin Med. 1971;78(1):158–66. [PubMed] [Google Scholar]
- 35.Dziatkowiak H, Kowalska M, Denys A. Phagocytic and bactericidal activity of granulocytes in diabetic children. Diabetes. 1982;31(12):1041–3. doi: 10.2337/diacare.31.12.1041. [DOI] [PubMed] [Google Scholar]
- 36.Tater D, Tepaut B, Bercovici JP, Youinou P. Polymorphonuclear cell derangements in type I diabetes. Horm Metab Res. 1987;19(12):642–7. doi: 10.1055/s-2007-1011899. [DOI] [PubMed] [Google Scholar]
- 37.Grossmann V, Schmitt VH, Zeller T, Panova-Noeva M, Schulz A, Laubert-Reh D, et al. Profile of the Immune and Inflammatory Response in Individuals With Prediabetes and Type 2 Diabetes. Diabetes Care. 2015;38(7):1356–64. doi: 10.2337/dc14-3008. [DOI] [PubMed] [Google Scholar]
- 38.Svoren BM, Jospe N. Type1 diabetes mellitus (immune mediated). Philadelphia: Saunders Elsevier; 2016. pp. 2763–83. [Google Scholar]