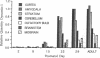Abstract
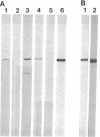
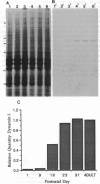
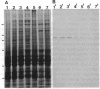
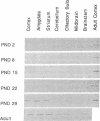
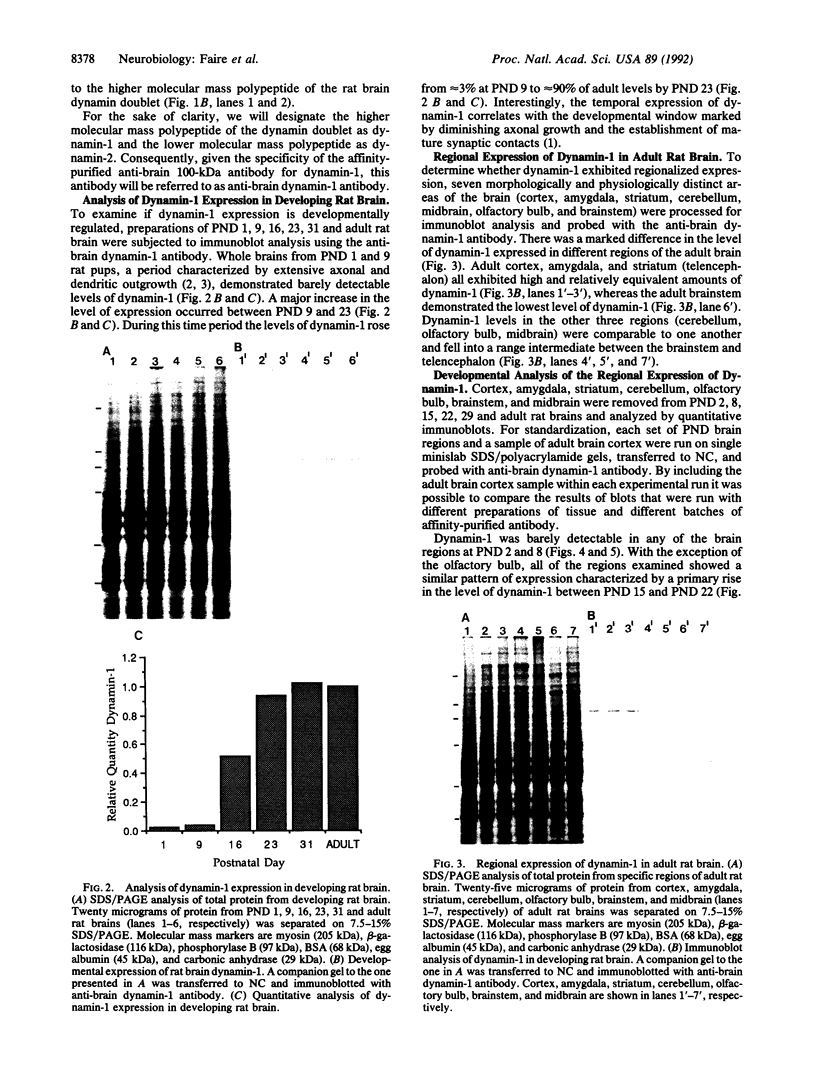
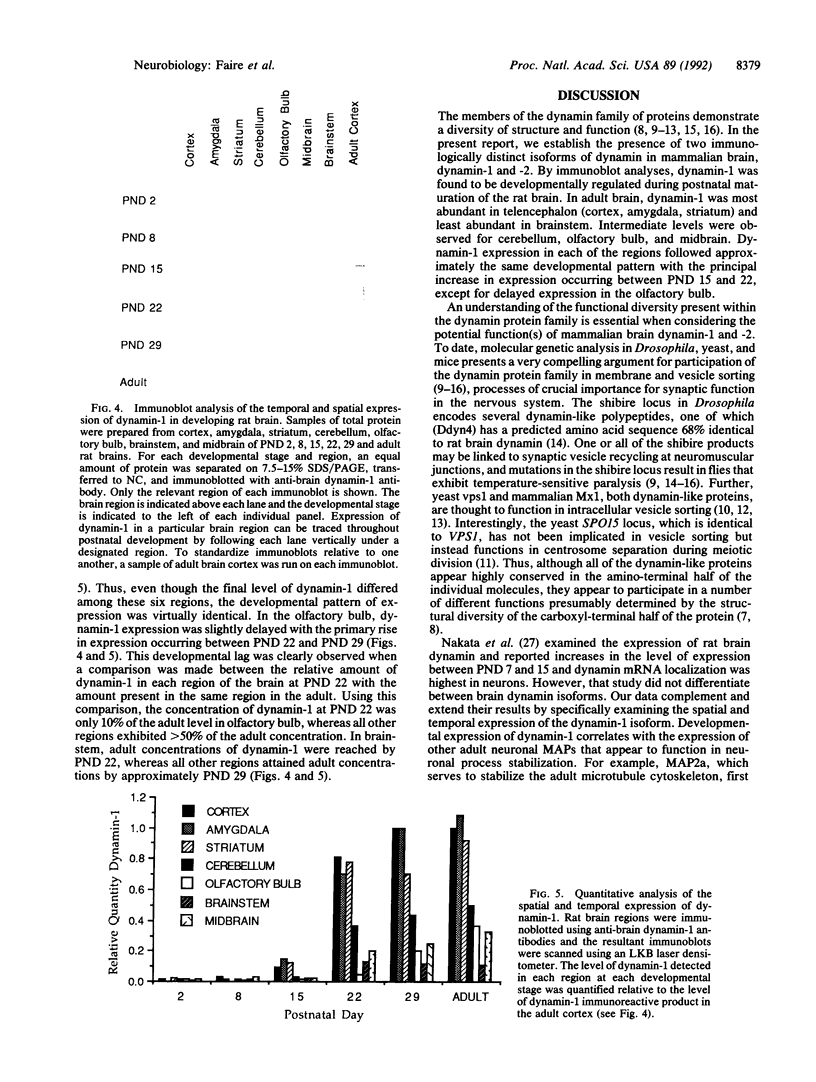
In adult rat brain, the microtubule-associated protein dynamin is composed of a closely spaced polypeptide doublet of approximately 100 kDa. Using an antibody preparation that is monospecific for dynamin-1 (the higher molecular mass isoform) we examined the temporal and regional expression of dynamin-1 in developing rat brain. Analysis of whole rat brain homogenates established that prior to postnatal day 9, dynamin-1 was present only at very low levels and thereafter its expression steadily increased with adult levels being attained by postnatal day 23. In individual regions of the brain, dynamin-1 levels were highest in cortex, amygdala, and striatum, significantly lower in olfactory bulb, cerebellum, and midbrain, and lowest in brainstem. During postnatal development, each of the regions exhibited approximately the same time course of protein expression except for a slight lag in expression in olfactory bulb. The spatial and temporal patterns of expression of dynamin-1 correlate with the establishment and/or maintenance of mature neuronal structure and function rather than dendritic or axonal outgrowth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Meier E. Mx proteins: antiviral proteins by chance or by necessity? New Biol. 1990 Oct;2(10):851–857. [PubMed] [Google Scholar]
- Arnheiter H., Skuntz S., Noteborn M., Chang S., Meier E. Transgenic mice with intracellular immunity to influenza virus. Cell. 1990 Jul 13;62(1):51–61. doi: 10.1016/0092-8674(90)90239-b. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Obar R. A., Schroeder C. C., Austin T. W., Poodry C. A., Wadsworth S. C., Vallee R. B. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991 Jun 13;351(6327):583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Dani J. W., Armstrong D. M., Benowitz L. I. Mapping the development of the rat brain by GAP-43 immunocytochemistry. Neuroscience. 1991;40(1):277–287. doi: 10.1016/0306-4522(91)90190-y. [DOI] [PubMed] [Google Scholar]
- Dubreuil R., Byers T. J., Branton D., Goldstein L. S., Kiehart D. P. Drosophilia spectrin. I. Characterization of the purified protein. J Cell Biol. 1987 Nov;105(5):2095–2102. doi: 10.1083/jcb.105.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979 Dec;18(4):1053–1063. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- Hattori T., McGeer P. L. Synaptogenesis in the corpus striatum of infant rat. Exp Neurol. 1973 Jan;38(1):70–79. doi: 10.1016/0014-4886(73)90008-3. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Ikeda K. Reversible blockage of membrane retrieval and endocytosis in the garland cell of the temperature-sensitive mutant of Drosophila melanogaster, shibirets1. J Cell Biol. 1983 Aug;97(2):499–507. doi: 10.1083/jcb.97.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Nakata T., Iwamoto A., Noda Y., Takemura R., Yoshikura H., Hirokawa N. Predominant and developmentally regulated expression of dynamin in neurons. Neuron. 1991 Sep;7(3):461–469. doi: 10.1016/0896-6273(91)90298-e. [DOI] [PubMed] [Google Scholar]
- Nunez J. Differential expression of microtubule components during brain development. Dev Neurosci. 1986;8(3):125–141. doi: 10.1159/000112248. [DOI] [PubMed] [Google Scholar]
- Obar R. A., Collins C. A., Hammarback J. A., Shpetner H. S., Vallee R. B. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990 Sep 20;347(6290):256–261. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Poodry C. A., Edgar L. Reversible alteration in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. J Cell Biol. 1979 Jun;81(3):520–527. doi: 10.1083/jcb.81.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer B. M. Some aspects of the neuronal cytoskeleton in development. Eur J Morphol. 1990;28(2-4):347–378. [PubMed] [Google Scholar]
- Riederer B., Matus A. Differential expression of distinct microtubule-associated proteins during brain development. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6006–6009. doi: 10.1073/pnas.82.17.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. H., Raymond C. K., Gilbert T., O'Hara P. J., Stevens T. H. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 1990 Jun 15;61(6):1063–1074. doi: 10.1016/0092-8674(90)90070-u. [DOI] [PubMed] [Google Scholar]
- Scaife R., Margolis R. L. Biochemical and immunochemical analysis of rat brain dynamin interaction with microtubules and organelles in vivo and in vitro. J Cell Biol. 1990 Dec;111(6 Pt 2):3023–3033. doi: 10.1083/jcb.111.6.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A., Sheetz M. P. Functions of microtubule-based motors. Annu Rev Physiol. 1991;53:629–652. doi: 10.1146/annurev.ph.53.030191.003213. [DOI] [PubMed] [Google Scholar]
- Shpetner H. S., Vallee R. B. Dynamin is a GTPase stimulated to high levels of activity by microtubules. Nature. 1992 Feb 20;355(6362):733–735. doi: 10.1038/355733a0. [DOI] [PubMed] [Google Scholar]
- Shpetner H. S., Vallee R. B. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989 Nov 3;59(3):421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Tepper J. M., Trent F., Nakamura S. Postnatal development of the electrical activity of rat nigrostriatal dopaminergic neurons. Brain Res Dev Brain Res. 1990 Jun 1;54(1):21–33. doi: 10.1016/0165-3806(90)90061-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P. The roles of microtubule-associated proteins in brain morphogenesis: a review. Brain Res Brain Res Rev. 1990 May-Aug;15(2):101–120. doi: 10.1016/0165-0173(90)90013-e. [DOI] [PubMed] [Google Scholar]
- Viereck C., Tucker R. P., Matus A. The adult rat olfactory system expresses microtubule-associated proteins found in the developing brain. J Neurosci. 1989 Oct;9(10):3547–3557. doi: 10.1523/JNEUROSCI.09-10-03547.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. J., Ritchie T. C., Coulter J. D. Distribution and developmental expression of the nerve terminal protein NT75 in the rat cerebellum. J Comp Neurol. 1991 Feb 22;304(4):530–543. doi: 10.1002/cne.903040403. [DOI] [PubMed] [Google Scholar]
- Yeh E., Driscoll R., Coltrera M., Olins A., Bloom K. A dynamin-like protein encoded by the yeast sporulation gene SPO15. Nature. 1991 Feb 21;349(6311):713–715. doi: 10.1038/349713a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Meyerowitz E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991 May 30;351(6325):411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]