Abstract
Milk-derived bioactive peptides have been identified as potential ingredients of health-promoting functional foods. These bioactive peptides are targeted at diet-related chronic diseases especially the non-communicable diseases viz., obesity, cardiovascular diseases and diabetes. Peptides derived from the milk of cow, goat, sheep, buffalo and camel exert multifunctional properties, including anti-microbial, immune modulatory, anti-oxidant, inhibitory effect on enzymes, anti-thrombotic, and antagonistic activities against various toxic agents. Majority of those regulate immunological, gastrointestinal, hormonal and neurological responses, thereby playing a vital role in the prevention of cancer, osteoporosis, hypertension and other disorders as discussed in this review. For the commercial production of such novel bioactive peptides large scale technologies based on membrane separation and ion exchange chromatography methods have been developed. Separation and identification of those peptides and their pharmacodynamic parameters are necessary to transfer their potent functional properties into food applications. The present review summarizes the preliminary classes of bioactive milk-derived peptides along with their physiological functions, general characteristics and potential applications in health-care.
Keywords: Milk, Bioactive peptides, Production, Purification, Healthcare
1. Introduction
Milk contains approximately 3.5% protein of which 80% are casein and 20% whey proteins. Caseins have been classified as α-, β- and k-caseins. Whey contains β-lactoglobulin, α-lactalbumin and several minor proteins with different biological activities such as enzymes, mineral-binding properties and immunoglobulins. The multifunctional properties of biologically active milk peptides are increasingly acknowledged. It could show a positive impact on human physiology and metabolism either, directly or through enzymatic hydrolysis in vivo or in vitro (Kitts and Weiler, 2003). The activity of peptides is based on their inherent amino acid composition and sequence. The size of bioactive peptide sequences known to possess multifunctional properties may vary from two to twenty amino acid residues (Meisel and Fitzgerald, 2003).
Biologically active peptides in the protein sequence are defined as fragments that remain inactive in precursor protein sequences, but when released by the action of proteolytic enzymes, they may interact with selected receptors and regulate the body’s physiological functions. The effect exerted by such peptides may be positive or negative (Schlimme and Meisel, 1995, Meisel and Bockelmann, 1999). Protease enzymes are naturally occurring in food products, such as milk plasmin, hydrolyze proteins and release bioactive fragments during processing or storage. Many types of bacteria applied in the production of fermented food products and occurring naturally in the gastrointestinal tract are capable of producing biologically active peptides. Cheese contains phospho peptides which are further proteolyzed in the process of cheese ripening, leading to the formation of various ACE inhibitors (Saito et al., 2000).
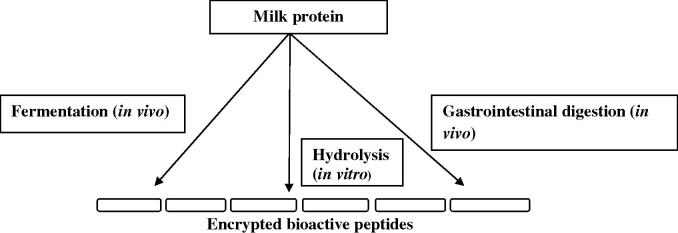
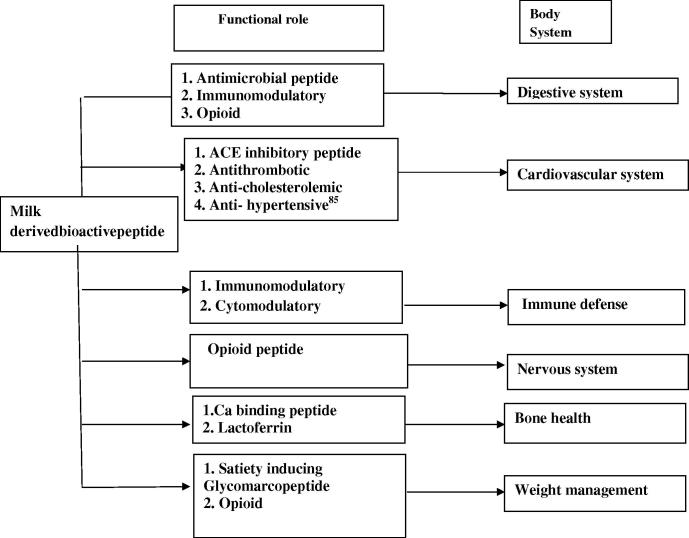
Biologically active peptides derived from milk are initially found in inactive form within the sequence of the precursor molecules but it can be released in three ways; (i) enzymatic hydrolysis with digestive enzymes like pepsin, trypsin, chymotrypsin etc; (ii) fermentation of milk with proteolytic starter cultures; (iii) proteolysis by enzymes derived from proteolytic microorganisms (Fig. 1) (Korhonen and Pihlanto, 2003). Once these bioactive peptides are liberated, they may serve to influence numerous physiological responses including cardiovascular, digestive, endocrine, immune and neurological activity etc. (Fig. 2). Because of such physiological versatility, milk-derived bioactive peptides have drawn the attention of many researchers worldwide in order to formulate several potential drugs with nutraceutical supplement properties, health promoting functional foods or other pharmaceutical products (Korhonen and Pihlanto, 2003, FitzGerald and Meisel, 2003). General characteristics of the primary classes of bioactive milk peptides are discussed in this review.
Figure 1.
Possible mechanisms for the release of bioactive peptides from dietary milk proteins.
Figure 2.
Role of milk-derived bioactive peptides in the body system.
2. Derivation of bioactive peptides
Milk peptides are derived from milk proteins by enzymatic breakdown by digestive enzymes or by the proteinase enzymes produced by lactobacilli during the fermentation of milk (Jauhiainen and Korpela, 2007). Milk-derived bioactive peptides are usually comprised of 2–20 amino acids and become active after release from the precursor protein where they are encrypted either by digestion or proteolysis both in vivo or in vitro (Fig. 1).
2.1. Gastrointestinal digestion (in vivo)
Bioactive peptides may be released in vivo during gastrointestinal digestion by the action of digestive enzymes like pepsin, trypsin or chymotrypsin. Dietary proteins undergo denaturation in the presence of hydrochloric acid (HCl) secreted by the parietal cells of the stomach. This acid activates pepsinogen and converts it into its active form, pepsin. Pepsin acts on proteins to metabolise them to amino acids. Gastrointestinal digestion permits the consequent action of the enzymes present in the small intestine such as pepsin, trypsin or chymotrypsin, which are responsible for protein hydrolysis (Korhonen and Pihlanto, 2003). Several bioactive peptides (viz., anti-bacterial, immunomodulatory, anti-hypertensive and opioid peptides) are known to be released from casein and/or whey proteins by gastrointestinal digestion (Meisel and FitzGerald, 2003, Yamamoto et al., 2003, FitzGerald et al., 2004, Gobbetti et al., 2002, Gobbetti et al., 2004). Some other proteolytic enzymes such as alcalase, thermolysin, may be utilized with pepsin and trypsin in order to simulate gastrointestinal digestion. They have also been employed to release various bioactive peptides, including CCPs (Mcdonagh and Fitzgerald, 1998), ACE inhibitory (Vermeirssen et al., 2004), anti-bacterial (Mohanty et al., 2014), anti-oxidative (Suetsuna et al., 2000, Rival et al., 2001), immunomodulatory (Gauthier et al., 2006) and opioid peptides (Pihlanto-Leppala et al., 1994, Pihlanto-Leppala et al., 1996).
2.2. Microbial fermentation (in vitro)
Several lactic acid bacteria (LAB) (e.g. Lactococcus lactis, Lactobacillus helveticus) have been reported to release bioactive peptides by the process of fermentation. This system consists of a number of distinct intracellular peptidases including endo-peptidases, amino-peptidases, di-peptidases, and tri-peptidases (Christensen et al., 1999). Recent studies have reviewed the production of various bioactive peptides including antimicrobial, immunomodulatory, antioxidative and ACE-inhibitory through microbial proteolysis (Gobbetti et al., 2004, Korhonen and Pihlanto, 2003). The release of bioactive peptides by fermentation of milk using different proteolytic microorganisms or proteolytic enzymes derived from such microorganisms has been summarized in Table 1.
Table 1.
Bioactive peptides released from milk proteins by various microorganisms.
| Microorganism | Precursor protein | Peptide sequence | Bioactivity |
|---|---|---|---|
| L. rhamnosus + digestion with pepsin | β-cn | Asp-Lys-Ile-His-Pro-Phe, Tyr-Gln-Glu-Pro- Val-Leu | ACE inhibitory |
| Lactobacillus helveticus | β-cn, κ-cn | Val-Pro-Pro, Ile-Pro-Pro | ACE inhibitory, antihypertensive |
| Lactobacillus GG enzymes + pepsin and trypsin | β-cn, as1-cn | Tyr-Pro-Phe-Pro, Ala-Val-Pro-Tyr-Pro-Gln Arg, Thr-Thr-Met-Pro-Leu-Trp | Opioid, ACE-inhibitory, immune-stimulatory |
| Lactobacillus delbrueckii subsp., bulgaricus IFO13953 | κ-cn | Ala-Arg-His-Pro-His-Pro-His-Leu-Ser-Phe-met | Antioxidative |
| Kluyveromyces marxianus var. | β-lg | Tyr-Leu-Leu-Phe | ACE-inhibitory |
| Lactobacillus helveticus CP90 proteinase | β-cn | Lys-Val-Leu-Pro-Val-Pro-(Glu) | ACE-inhibitory |
2.3. Enzymatic activity
The most common way to produce bioactive peptides from milk is through enzymatic hydrolysis of the whole protein molecules. Digestive enzymes and combinations of different proteinases including alcalase, chymotrypsin, pepsin and thermolysin as well as enzymes from bacterial and fungal sources have also been utilized to generate bioactive peptides from various proteins.
3. Bioactive peptides and their role in human health
3.1. Antimicrobial peptides
Antimicrobial bioactive peptides derived from milk have been reported to inhibit many Gram positive and Gram negative pathogens including Escherichia coli MTCC82, Aeromonas hydrophila ATCC7966, Salmonella typhi MTCC3216, Bacillus cereus ATCC10702, Salmonella typhimurium SB300, S. enteritidis 125109, Staphylococcus aureus MTCC 96 (Mohanty et al., 2014) and control many microbial infections. In a similar way chymosin digested casein releases caseicidin peptide that exhibits antimicrobial activity against Staphylococcus spp., Sarcina spp., Bacillus subtilis, Streptococcus pyogenes (Lahov and Regelson, 1996). Several such peptides have been detected and some of them are listed in Table 2. A cationic fragment of casein, casocidin-I, is able to inhibit growth of E. coli and S. Carnosus (Zucht et al., 1995) where as two other peptides are isolated from the same casein, namely f183–207 and f164–179 also able to inhibit pathogens (Recio and Visser, 1999). Lactoferrampin, isolated as a fragment of lactoferrin displays inhibitory activity against Streptococcus mutans, E. coli, B. subtilis and Pseudomonas aeruginosa (Van der Kraan et al., 2004). Researchers have recognized new antibacterial peptides from a chymosin, trypsin digest of αs2-CN bovine, namely, Isracidine, which has a strong protective effect against S. aureus, S. pyogenes and Listeria monocytogenes (Lahov and Regelson, 1996). Glyco-macropeptide (GMP) and caseinomacropeptide (CMP) are formed after a specific cleavage of casein by chymosin (Farrell et al., 2004). Caseinomacropeptide (CMP) may have an inhibitory activity against S. mutans and E. coli whereas GMP modulates the gut microflora (Manso and López-Fandiño, 2004). Isfracidin and lactoferricin B are effective against Candida albicans (Bellamy et al., 1993, Lahov and Regelson, 1996). Lactoferrin and its derivatives show the antibacterial activity in vitro against various pathogens, e.g. Clostridium perfringens, C. albicans, Haemophilus influenzae, Helicobacter pylori, L. monocytogenes, P. aeruginosa, Salmonella enteritidis, S. aureus, Vibrio cholerae as well as antiviral activity against hepatitis C,G and B virus HIV-1, poliovirus, rotavirus and herpes simplex virus (Farnaud and Evans, 2003, Pan et al., 2007).
Table 2.
Anti-microbial peptides derived from milk and their target microorganisms.
| Milk peptides | Protease | Pathogens |
|---|---|---|
| Isracidin αs1-CN (f1–23) | Chymosin, chymotrypsin | Staphylococcus aureus |
| Casecidin αs1 and κ-CN | Chymosin, chymotrypsin | Staphylococcus, Bacillus subtilis, Diplococcus pneumonia, Streptococcus pyogenes |
| Lactoferricin B and Lactoferrin (f 17–41) | Pepsin | Bacillus, E. coli, Candida albicans, Listeria, Streptococci, Klebsiella, Staphylococci, Proteus, Pseudomonas, Salmonella |
| β-Casein derived peptides | Trypsin and chymotrypsin | Enterococcus faecium, Bacillus megaterium |
3.2. Immunomodulatory peptides
Glycopeptides, hormones and peptide fragments of immunoglobulins are usually considered as immunomodulatory peptides that regulate cell-mediated and humoral immune functions. Later on, several other peptides were reported from bovine β-casein, which were responsible for phagocytizes in humans and inhibited Klebsiella pneumoniae infection in mice in vivo (Migliore-Samour and Jollès, 1988). More recently many cyto-chemical studies indicate that the immunomodulatory bioactive peptides derived from both casein and whey proteins are related to the stimulation and proliferation of human lymphocytes, macrophage phagocytic activity, antibody synthesis and cytokine regulation (Clare et al., 2003, Gill et al., 2000). Cytomodulatory peptides produced from casein may inhibit cancer cell growth by stimulating the activity of immune competent cells (Meisel and Fitzgerald, 2003). Glycomacropeptide (GMP) and its derivatives have been revealed to be essential immunomodulatory functions including immune suppressive effects on the production of IgG antibodies (Monnai et al., 1998, Manso and López-Fandiño, 2004). Lactoferrin is digested to form Lactoferricin B which directly binds to neutrophils and show an opsonin like activity. Other peptides such as f(63–680) and f(191–193) from bovine β-casein may affect phagocytizes in humans in vitro (Migliore-Samour and Jollès, 1988) where as some other peptides from κ-casein and α-lactalbumin are used in immune therapy of human immune deficiency virus infection (Hadden, 1991). Caseinomacropeptide (CMP) promotes the growth of bifidobacteria or lactobacilli that inhibit enteric infection (Bruck et al., 2003).
3.3. Anti-hypertensive peptides or angiotensin-converting enzyme (ACE) inhibitory peptides
ACE is a peptidyl di-peptidase enzyme having the capacity to cleave the carboxyl terminal end of the substrate that may regulate an increase in blood pressure by converting angiotensin I to an active peptide hormone angiotensin II. This stimulates the release of aldosterone, as a result of which sodium concentration becomes high and blood pressure goes up. But antihypertensive peptide is able to inhibit ACE to control increase of blood pressure (Korhonen and Pihlanto, 2007). ACE inhibitors are di- or tri-peptides containing proline, lysine or arginine at their C-terminal end. Bioactive amino acid sequence displaying antihypertensive activity is mainly isolated from bovine and human caseins. Whey proteins derived by the activity of lactic acid bacteria like L. helveticus, L. lactis are resistant to the digestive tract endo-peptidases, therefore, can be easily absorbed to the blood stream (Saito et al., 2000). ACE inhibitory peptides such as β-casein, κ-casein have been isolated from enzymatic digest of sour milk proteins αs1 and β-CN (Bracquart and Lorient, 1979). In addition to casein derived peptide, ACE inhibitory peptides such as α-lactorphin and β-lactorphin are also generated from whey proteins α-lactalbumin and lactoglobulin, respectively (Maruyama and Suzuki, 1982, Maruyama et al., 1985, Maruyama et al., 1987). The peptides Glu-Met-Pro-Phe-Pro-Lys and Tyr-Pro-Val-Glu-Pro-Phe-Thr-Glu originate from the casein sequences, f(108–113) and f(114–121); the latter showed an in vitro inhibition effect upon ACE (Perpetuo et al., 2003). ACE inhibitor peptides are food derived natural preventives used to control hypertension and could lead to a decrease in the requirement of medicines which exert strong side effects.
3.4. Opioid peptides
Opioid peptides are opioid receptor ligands which are encrypted from bovine and human β-casein enzymatically in vitro (Brantl, 1984) and also found to be present in the endocrine, nervous and immune systems as well as the gastrointestinal tract of mammals. They interact with their endogenous ligands and with exogenous and/or antagonist opioids and may influence the central or peripheral nervous systems which are involved in hypotension, lack of appetite, fluctuating body temperature and alteration of sexual behaviors (Molina and Abumrad, 1994, Dziuba et al., 1999). Endogenous opioid agonist peptides may regulate the growth and function of cells involved in the central nervous system whereas β-casomorphins are transported across mucosal membranes of neonates that regulate physiological responses resulting in calmness and sleep in infants (Calvo et al., 2000, Sturner and Chang, 1988). On other hand, β-casomorphin interacts with opiate receptors in the serosal side of the intestinal epithelium and plays a crucial role in certain activities like regulation of electrolyte transport, insulin secretion and food absorption (Tome and Debabbi, 1998). Opioid antagonists are able to suppress the agonist activity of enkephalin. Two most useful agonistic opioid peptides known as Serorphine and Casoxin C have been isolated from f(399–404) fragments of bovine serum albumin and bovine κ-CN receptor respectively (Meisel and Fitzgerald, 2000). There are several accumulating evidences suggesting that two bovine casoxins (casoxins A and B) are opioid receptor ligands that have relatively low antagonistic potency (Meisel, 1998). Casoxins A and B correspond to amino-acid sequences within bovine k-casein; casoxin A is accounted for by f(35–41) of k-casein (i.e. Tyr-Pro-Ser-Tyr-Gly-Leu-Asn) corresponds to, whereas casoxin B corresponds to f58–61 of k-casein (i.e. Tyr-Pro-Tyr-Tyr). Lastly, casoxin C is a potent opioid antagonist peptide of k-casein f(25–34) (i.e. Tyr-Ile-Pro-Ile-Gln-Tyr-Val-Leu-Ser-Arg), possesses the highest biological potency (Xu, 1998). Presently, data suggest that casomorphins, as opioid ligands, exert anti-secretory action (Daniel et al., 1990), stimulate analgesic behavior (Matthies et al., 1984) and endocrine responses such as secretion of insulin and somatostatin (Meisel and Schlimme, 1990).
3.5. Antioxidant peptides
Several milk peptides also play a regulatory role in oxidative metabolism which is essential for the survival of cells and causes oxidative changes by producing free radicals. But when an excess of free radicals is released, they oxidize cellular protein, membrane lipid, DNA, and enzymes that cause shutting down of cellular respiration and mediate injuries including atherosclerosis, diabetes, rheumatoid arthritis and oxidative DNA-damage leading to cancer (Abuja and Albertini, 2001, Halliwell, 2000, Halliwell and Whiteman, 2004). Moreover, milk-derived anti-oxidative peptides are comprised of five to eleven hydrophobic amino acids including proline, histidine, tyrosine or tryptophan in sequence which are widely distributed among caseins, soybean and gelatine in hydrolysis by proteolytic enzymes (Korhonen and Pihlanto, 2003) as shown in Table 3. They may function by scavenging or preventing the formation of radicals (Cervato et al., 1999, Wong and Kitts, 2003), particularly, free radicals released from casein peptides may influence scavenging activity (Suetsuna et al., 2000, Rival et al., 2001) and also inhibit enzymatic and non-enzymatic lipid peroxidation. Extensive researches on anti-oxidative peptides have revealed that, the artificial antioxidants provide strong antioxidant activity against several oxidation systems. Because of their strong side effects on human physiology and metabolism, these are restricted in some countries and natural antioxidants have therefore been developed from plants (Okada and Okada, 1998). Naturally occurring vitamins (E and C), beta-carotene, and enzymatic systems, mainly superoxide dismutase, catalase and glutathione peroxidase have anti-oxidative activities (Lindmark-Mansson and Kesson, 2000).
Table 3.
Anti-oxidative peptides derived from milk proteins.
| Protein source | Enzyme | Peptide sequence | Antioxidative activity | References |
|---|---|---|---|---|
| Casein | Trypsin | Val-Lys-Glu-Ala-Met-Ala-Pro-Lys | Inhibition of enzymatic and non-enzymatic lipid peroxidation | Suetsuna et al. (2000) |
| Casein | Pepsin | Tyr-Phe-Tyr-Pro-Glu-Leu | Radical scavenging activity | Rival et al. (2001) |
| β-Lactoglobulin (β-lg) | Corolase | Trp-Tyr-Ser-Leu-Ala-Met-Ala-Ala-Ser-Asp-Ile Trp-Tyr-Ser-Leu-Ala-Met-Ala-Ala-Ser-Asp-Ile Tyr-Val-Glu-Glu-Leu | Radical scavenging activity |
4. Conclusion
Bioactive peptides have attracted the interest of researchers as a health promoting functional food. Yet there is limited work done in this area due to lack of advanced technologies, enriched products and molecular approaches. There is an urgent need to focus on developing novel facilities including advanced proteomics approaches, recombinant enzyme technologies and microbial fermentation, to study the various impacts of bioactive peptides on expression of genes and also to optimize the nutritional and health effects of these compounds. Consequently allergenicity, toxicity and stability of its biological functions during gastrointestinal digestion should be tested in formulation of products incorporated with bioactive peptides. Moreover, preliminary beneficial effects of milk derived bioactive peptides on target diseases should be considered carefully before it can be formulated as chemotherapeutical agents or may try to use them directly in their viable condition. Hence separation and identification of these peptides and their pharmaco-dynamic parameters are necessary to transfer their potent functional properties into food and clinical applications. Scientific researches and industrial development in the direction of searching novel bioactive peptides promise to formulate several drugs and health beneficial functional foods.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abuja P., Albertini R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin. Chim. Acta. 2001;306:1–17. doi: 10.1016/s0009-8981(01)00393-x. [DOI] [PubMed] [Google Scholar]
- Bellamy W.R., Wakabayashi H., Takase M., Kawase K., Shimamura S., Tomita M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 1993;182:97–105. doi: 10.1007/BF00189377. [DOI] [PubMed] [Google Scholar]
- Bracquart P., Lorient D. Etude des acides aminés sur la croissance de Streptococcus thermophilus. Milchwissenschaft. 1979;32:221–224. [Google Scholar]
- Brantl V. Novel opioid peptides derived from human beta-casein: human beta-casomorphins. Eur. J. Pharmacol. 1984;106:213–1214. doi: 10.1016/0014-2999(84)90702-7. [DOI] [PubMed] [Google Scholar]
- Bruck W.M., Graverholt G., Gibson G.R. A two-stage continuous culture system to study the effect of supplemental α-lactalbumin and glycomacropeptide on mixed cultures of human gut bacteria challenged with enteropathogenic Escherichia coli and Salmonella serotype typhimurium. J. Appl. Microbiol. 2003;95:44–53. doi: 10.1046/j.1365-2672.2003.01959.x. [DOI] [PubMed] [Google Scholar]
- Calvo C.F., Cesselin F., Gelman M., Glowinski J. Identification of an opioid peptide secreted by rat embryonic mixed brain cells as a promoter of macrophage migration. Eur. J. Neurosci. 2000;12:2676–2684. doi: 10.1046/j.1460-9568.2000.00145.x. [DOI] [PubMed] [Google Scholar]
- Cervato G., Cazzola R., Cestaro B. Studies on the antioxidant activity of milk caseins. Int. J. Food Sci. Nutr. 1999;50:291–294. doi: 10.1080/096374899101175. [DOI] [PubMed] [Google Scholar]
- Christensen J.E., Dudley E.G., Pederson J.A., Steele J.L. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1999;76:217–246. [PubMed] [Google Scholar]
- Clare D.A., Catignani G.L., Swaisgood H.E. Biodefense properties of milk: the role of antimicrobial proteins and peptides. Curr. Pharm. Des. 2003;9:1239–1255. doi: 10.2174/1381612033454874. [DOI] [PubMed] [Google Scholar]
- Daniel H., Vohwinkel M., Rehner G. Effect of casein and beta-casomorphins on gastrointestinal motility in rats. J. Nutr. 1990;3:252–257. doi: 10.1093/jn/120.3.252. [DOI] [PubMed] [Google Scholar]
- Dziuba J., Minkiewicz P., Nalecz D., Iwaniak A. Database of biologically active peptide sequences. Nahrung. 1999;43:190–195. doi: 10.1002/(SICI)1521-3803(19990601)43:3<190::AID-FOOD190>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Farnadu S., Evans R.W. Lactoferrin – a multifunctional protein with antimicrobial properties. Mol. Immunol. 2003;40:395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- Farrell H.M., Jimenez-Flores R., Bleck G.T., Brown E.M., Butler J.E., Creamer L.K., Hicks C.L., Hollar C.M., Ng-Kwai-Hang K.F., Swaisgood H.E. Nomenclature of the proteins of cows’ milk–sixth revision. J. Dairy Sci. 2004;87:1641–1674. doi: 10.3168/jds.S0022-0302(04)73319-6. [DOI] [PubMed] [Google Scholar]
- FitzGerald R.J., Meisel H. Milk protein hydrolysates and bioactive peptides. Adv. Dairy Chem. 2003;3:675–698. [Google Scholar]
- FitzGerald R.J., Murray B.A., Walsh D.J. Hypotensive peptides from milk proteins. J. Nutr. 2004;134:980–988. doi: 10.1093/jn/134.4.980S. [DOI] [PubMed] [Google Scholar]
- Gauthier S.F., Pouliot Y., Maubois J.L. Growth factors from bovine milk and colostrum: composition, extraction and biological activities. Lait. 2006;86:99–125. [Google Scholar]
- Gill H.S., Doull F., Rutherfurd K.J., Cross M.L. Immunoregulatory peptides in bovine milk. Brit. J. Nutr. 2000;84:111–117. doi: 10.1017/s0007114500002336. [DOI] [PubMed] [Google Scholar]
- Gobbetti M., Stepaniak L., De Angelis M., Corsetti A., DiCagno R. Latent bioactive peptides in milk proteins: proteolytic activation and significance in dairy processing. Crit. Rev. Food Sci. Nutr. 2002;42:223–239. doi: 10.1080/10408690290825538. [DOI] [PubMed] [Google Scholar]
- Gobbetti M., Minervini F., Rizzello C.G. Angiotensin I converting-enzyme-inhibitory and antimicrobial bioactive peptides. Int. J. Dairy Technol. 2004;57:172–188. [Google Scholar]
- Hadden J.W. Immunotherapy of human immunodeficiency virus infection. Trends Pharmacol. Sci. 1991;12:107–111. doi: 10.1016/0165-6147(91)90517-v. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Lipid peroxidation, antioxidants and cardiovascular disease: how should we move forward? Cardiovasc. Res. 2000;47:410–418. doi: 10.1016/s0008-6363(00)00097-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhiainen T., Korpela R. Milk peptides and blood pressure. J. Nutr. 2007;137:825–829. doi: 10.1093/jn/137.3.825S. [DOI] [PubMed] [Google Scholar]
- Kitts D.D., Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003;9:1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- Korhonen H., Pihlanto A. Food-derived bioactive peptides opportunities for designing future foods. Curr. Pharm. Des. 2003;9:1297–1308. doi: 10.2174/1381612033454892. [DOI] [PubMed] [Google Scholar]
- Korhonen H., Pihlanto A. Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr. Pharm. Design. 2007;13:829–8434. doi: 10.2174/138161207780363112. [DOI] [PubMed] [Google Scholar]
- Lahov E., Regelson W. Antibacterial and immunostimulating casein-derived substances from milk: caseicidin, isracidin peptides. Food Chem. Toxicol. 1996;34:131–145. doi: 10.1016/0278-6915(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Lindmark-Mansson H., Kesson B. Antioxidative factors in milk. Br. J. Nutr. 2000;84:103–110. doi: 10.1017/s0007114500002324. [DOI] [PubMed] [Google Scholar]
- Manso M.A., López-Fandiño R. κ-casein macropeptides from cheese whey physicochemical, biological, nutritional, and technological features for possible use. Food Res. Int. 2004;20:329–355. [Google Scholar]
- Maruyama S., Suzuki H. A peptide inhibitor of angiotensin I-converting enzyme in the tryptic hydrolysate of casein. Agric. Biol. Chem. 1982;46:1393–1394. [Google Scholar]
- Maruyama S., Nakagomi K., Tomizuka N., Suzuki H. Angiotensin I-converting enzyme inhibitor derived from an enzymatic hydrolysate of casein. II. Isolation and bradykinin-potentiating activity on the uterus and the ileum of rats. Agric. Biol. Chem. 1985;49:1405–1409. [Google Scholar]
- Maruyama S., Mitachi H., Awaya J., Kurono M., Tonizuka N., Suzuki H. Angiotensin I-converting enzyme inhibitory activity of the C-terminal hexapeptide of αs1-casein. Agric. Biol. Chem. 1987;51:2557–2561. [Google Scholar]
- Matthies H., Stark H., Hartrodt B., Ruethrich H.L., Spieler H.T., Barth A., Neubert K. Derivatives of beta-casomorphins with high analgesic potency. Peptides. 1984;5:463–470. doi: 10.1016/0196-9781(84)90070-6. [DOI] [PubMed] [Google Scholar]
- McDonagh D., FitzGerald R.J. Production of caseinophosphopeptides (CPPs) from sodium caseinate using a range of commercial protease preparations. Int. Dairy J. 1998;8:39–45. [Google Scholar]
- Meisel H. Overview on milk protein-derived peptides. Int. Dairy J. 1998;8:363–373. [Google Scholar]
- Meisel H., Bockelmann W. Bioactive peptides encrypted in milk proteins: proteolytic activation and thropho-functional properties. Antonie Van Leeuwenhoek. 1999;76:207–215. [PubMed] [Google Scholar]
- Meisel H., FitzGerald R.J. Opioid peptides encrypted in milk protein sequences. Brit. J. Nutr. 2000;84:27–31. doi: 10.1017/s000711450000221x. [DOI] [PubMed] [Google Scholar]
- Meisel H., FitzGerald R.J. Biofunctional peptides from milk proteins: mineral binding and cytomodulatory effects. Curr. Pharm. Des. 2003;9:1289–1295. doi: 10.2174/1381612033454847. [DOI] [PubMed] [Google Scholar]
- Meisel H., Schlimme E. Milk proteins: precursors of bioactive peptides. Trends Food Sci. Technol. 1990;1:41–43. [Google Scholar]
- Migliore-Samour D., Jollès P. Casein Prohormone with an immunomodulating role for the newborn. Experientia. 1988;44:188–193. doi: 10.1007/BF01941703. [DOI] [PubMed] [Google Scholar]
- Mohanty D.P., Tripathy P., Mohapatra S., Samantaray D.P. Bioactive potential assessment of antibacterial peptide produced by Lactobacillus isolated from milk and milk products. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:72–80. [Google Scholar]
- Molina P.E., Abumrad N.N. Metabolic effects of opiates and opioid peptides. Adv. Neuroimmunol. 1994;4:105–116. doi: 10.1016/s0960-5428(05)80005-1. [DOI] [PubMed] [Google Scholar]
- Monnai M., Horimoto Y., Otani H. Immunomodificatory effect of dietary bovine κ-caseinoglycopeptide on serum antibody levels and proliferative responses of lymphocytes in mice. Milchwissenschaft. 1998;53:129–132. [Google Scholar]
- Okada Y., Okada M. Scavenging effect of water soluble proteins in broad beans on free radicals and active oxygen species. J. Agric. Food Chem. 1998;46:401–406. doi: 10.1021/jf970470l. [DOI] [PubMed] [Google Scholar]
- Pan Y., Rowney M., Guo P., Hobman P. Biological properties of lactoferrin: an overview. Aust. J. Dairy Technol. 2007;62:31–42. [Google Scholar]
- Perpetuo E.A., Juliano L., Lebrun I. Biochemical and pharmacological aspects of two bradykinin-potentiating peptides obtained from tryptic hydrolysis of casein. J. Protein Chem. 2003;22:601–606. doi: 10.1023/b:jopc.0000008724.98339.ff. [DOI] [PubMed] [Google Scholar]
- Pihlanto-Leppala A., Antila P., Mantsala P., Hellman J. Opioid peptides produced by in vitro proteolysis of bovine caseins. Int. Dairy J. 1994;41:291–301. [Google Scholar]
- Pihlanto-Leppala A., Koskinen P., Paakkari I., Tupasela T., Korhonen H. Opioid whey protein peptides obtained by membrane filtration. IDF Bull. 1996;311:36–38. [Google Scholar]
- Recio I., Visser S. Two ion-exchange methods for the isolation of antibacterial peptides from lactoferrin in situ enzymatic hydrolysis on an ion-exchange membrane. J. Chromatogr. 1999;831:191–201. doi: 10.1016/s0021-9673(98)00950-9. [DOI] [PubMed] [Google Scholar]
- Rival S.G., Boeriu C.G., Wichers H.J. Caseins and casein hydrolysates. 2. Antioxidative properties Peroral calcium dosage of infants. Acta Med. Scand. 2001;55:247–255. [Google Scholar]
- Saito T., Nakamura T., Kitazawa H., Kawai Y., Itoh T. Isolation and structural analysis of antihypertensive peptides that exist naturally in gouda cheese. J. Dairy Sci. 2000;83:1434–1440. doi: 10.3168/jds.S0022-0302(00)75013-2. [DOI] [PubMed] [Google Scholar]
- Schlimme E., Meisel H. Bioactive peptides derived from milk proteins. Structural, physiological and analytical aspects. Nahrung. 1995;39:1–20. doi: 10.1002/food.19950390102. [DOI] [PubMed] [Google Scholar]
- Sturner R.A., Chang K.J. Opioid peptide content in infant formulas. Pediatr. Res. 1988;23:4–10. [Google Scholar]
- Suetsuna K., Ukeda H., Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000;11:128–131. doi: 10.1016/s0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Tome D., Debabbi H. Physiological effects of milk protein components. Int. Dairy J. 1998;8:383–392. [Google Scholar]
- Van der Kraan M.I., Nazmi K., Teeken A., Groenink J., vant Hof W., Veerman E.C., Bolscher J.G., Nieuw Amerongen A.V. Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol. Chem. 2004;386:137–142. doi: 10.1515/BC.2005.017. [DOI] [PubMed] [Google Scholar]
- Vermeirssen V., Van C.J., Verstraete W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004;92:357–366. doi: 10.1079/bjn20041189. [DOI] [PubMed] [Google Scholar]
- Wong P.Y.Y., Kitts D.D. Chemistry of butter milk solid antioxidant activity. J. Dairy Sci. 2003;86:1541–1547. doi: 10.3168/jds.S0022-0302(03)73739-4. [DOI] [PubMed] [Google Scholar]
- Xu R.J. Bioactive peptides in milk and their biological and health implications. Food Rev. Int. 1998;14:1–16. [Google Scholar]
- Yamamoto N., Ejiri M., Mizuno S. Biogenic peptides and their potential use. Curr. Pharm. Des. 2003;9:1345–1355. doi: 10.2174/1381612033454801. [DOI] [PubMed] [Google Scholar]
- Zucht H.D., Raida M., Adermann K., Magert H.J., Forssman W.G. Casocidin-I: a casein αs2-derived peptide exhibits antibacterial activity. FEBS Lett. 1995;372:185–188. doi: 10.1016/0014-5793(95)00974-e. [DOI] [PubMed] [Google Scholar]