Abstract
The objectives of this study were to determine best conditions for the extraction of phenolic compounds from fresh, frozen and lyophilized basil leaves. The acetone mixtures with the highest addition of acetic acid extracted most of the phenolic compounds when fresh and freeze-dried material have been used. The three times procedure was more effective than once shaking procedure in most of the extracts obtained from fresh basil leaves – unlike the extracts derived from frozen material. Surprisingly, there were not any significant differences in the content of phenolics between the two used procedures in the case of lyophilized basil leaves used for extraction. Additionally, the positive correlation between the phenolic compounds content and antioxidant activity of the studied extracts has been noted. It is concluded that the acetone mixtures were more effective than the methanol ones for polyphenol extraction. The number of extraction steps in most of the cases was also a statistically significant factor affecting the yield of phenolic extraction as well as antioxidant potential of basil leaf extracts.
Keywords: Solvent extraction, Basil, Total phenolic content, Antiradical activity, Reducing power
1. Introduction
Plant foods provide abundant natural bioactive compounds which have many proved health-promoting activities like antioxidant, antibacterial, antihypertensive, anti-inflammatory etc. Phenolic compounds are one of the most widely occurring groups of phytochemicals, are of considerable physiological and morphological importance in plants. Additionally, there are many reports that this group of phytochemicals possesses biological activity. The antioxidant activity of polyphenols is due to their ability to scavenge free radicals, donate hydrogen atoms or electron, or chelate metal cations (Amarowicz et al., 2004, Balasundram et al., 2006). The presence of electron-donating and electron-withdrawing substituents in the ring structure of phenolics as well as the number and arrangement of the hydroxyl groups determines their antioxidant potential (Zhang et al., 2003). Additionally, a huge number of phenolic compounds which have been reported in the literature, show differences in possible biochemical modification (glycosylation, acetylation, manolynation, esterification to organic acids etc.) (Dai and Mumper, 2010, Balasundram et al., 2006).
Differences in the structure of phenolic compounds also determine their solubility in solvents of different polarity. Therefore type of extraction solvent as well as the isolation procedures may have a significant impact on the yield of extraction polyphenols from plants material. There are some reports concerning optimization of extraction conditions of phenolic compound content and antioxidant activities of some plant foods but as some researches indicated optimal procedure is usually different for different plant matrices (Rababah et al., 2010, Pellegrini et al., 2007).
In the present study basil (Ocimum basilicum L.) was selected as model food matrices as it is one of the most common herbs consumed as spice and is a rich source of phenolic compounds especially phenolic acids (like rosmarinic acid, chicoric acid, vanillic acid, p-coumaric acid, benzoic acid, hydroxybenzoic acid, syringic acid, ferulic acid, protocatechuic acid, caffeic acid and gentisic acid), flavonol-glycosides and anthocyanins (Lee and Scagel, 2010, Tarchoune et al., 2012).
The aim of the present work was to determine best conditions for extraction of phenolic compounds from fresh, frozen and lyophilized basil leaves.
2. Materials and methods
2.1. Chemicals
Folin–Ciocalteau reagent, DPPH (1,1-diphenyl-2-picrylhydrazyl), Trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) were purchased from Sigma–Aldrich Company, USA. Any other chemicals were of analytical grade.
2.2. Materials
Basil plants (O. basilicum L.) were purchased at commercial maturity from a local store. One proportion of the samples after weighing was frozen and the other one was freeze-dried.
2.2.1. Sample preparation
Two extraction ways were used for sample preparation:
-
(a)
Fresh material (2 g) (or an appropriate amount of frozen or lyophilized material) was ground in a mortar and pestle with 20 mL of an appropriate solvent mixture (see Table 1) and the polyphenols were extracted for 1 h at 4 °C, then centrifuged at 9000g for 30 min. and adjusted to 50 mL of final volume with used solvent – this was an extract of polyphenols labeled as once shaking.
-
(b)
2 g of fresh material or optional respectively to the weight of the quantity of frozen or lyophilized material was ground in a mortar and pestle with 15 mL of an appropriate solvent mixture (see Table 1) and the polyphenols were extracted for 20 min at 4 °C, then centrifuged at 9000g for 30 min. – this procedure was repeated three times and the supernatants were pooled and adjusted to 50 mL of final volume with used solvent – this was an extract of polyphenols labeled as three times shaking.
Table 1.
Composition of solvents used for phenolic compound extraction.
| Number of solvent mixture | Solvent composition |
|---|---|
| I | Acetone/water/acetic acid (70/28/2, v/v/v) |
| II | Acetone/water/acetic acid (70/29.5/0.5, v/v/v) |
| III | Acetone/water/acetic acid (70/29.8/0.2, v/v/v) |
| IV | Methanol/water (50/50, v/v) |
| V | Methanol/water/acetic acid (50/49.5/0.5, v/v/v) |
| VI | Methanol/acetic acid (99.5/0.5, v/v) |
2.3. Determination of total phenolic compounds (TPC)
The amount of total phenolics was determined using Folin–Ciocalteau reagent (Singleton et al., 1974). To 0.5 mL of the sample, 0.5 mL H2O, 2 mL Folin–Ciocalteau reagent (1:5 H2O) was added, after 3 min, 10 mL of 10% (w/v) Na2CO3 and the contents were mixed and allowed to stand for 30 min. Absorbance at 725 nm was measured in a UV–Vis spectrophotometer. The amount of total phenolics was calculated as gallic acid equivalent (GAE) in mg per g of fresh weight (FW).
2.4. Determination of free radical scavenging activity
The free radical scavenging activity was measured using DPPH (1,1-diphenyl-2-picrylhydrazyl) – according to Brand-Williams et al. (1995) as the source of the free radicals. For the DPPH assay, the 80 μL of methanolic extracts was mixed with 1.92 mL 6 × 10−5 M solution of DPPH• in methanol. Absorbance at 515 nm was measured immediately and after 2.5 mins of incubation. The affinity of the test material to quench DPPH free radicals was evaluated according to the equation:
AC – absorbance of control at 0 min, AA – absorbance of sample after 2.5 min.
The antiradical activity was related to Trolox (an analog of vitamin E) and expressed as mM of Trolox per gram of fresh weight (FW) (TEAC, Trolox equivalent antioxidant activity).
2.5. Determination of reducing power (RP)
Reducing power was determined by the method of Oyaizu (1986). A 0.5 mL of extract was mixed with 0.5 mL (200 mM) of sodium phosphate buffer (pH 6.6) and 0.5 mL potassium ferricyanide (1% v/v) and samples were incubated by 20 min at 50 °C. After that, 0.5 mL of TCA (10% v/v) was added and the samples were centrifuged at 9000g by 10 min. Upper layer (1 mL) of supernatant was mixed with 1 mL of distilled water and 0.2 mL of ferric chloride (0.1% v/v). The absorbance was subsequently measured at 700 nm in the spectrophotometer. The reducing power was related to Trolox (an analog of vitamin E) and expressed as μM of Trolox per gram of fresh weight (FW) (TEAC, Trolox equivalent antioxidant activity).
2.6. Statistical analysis
The experiments were conducted three times and all the determinations were performed in triplicate. Statistical analysis was performed using STATISTICA 7.0 for mean comparison using Tukey’s test at the significance level P < 0.05. Data were also evaluated using Pearson’s correlation coefficients to identify relationships between phenolic contents and antioxidant activities of basil leaves.
3. Results and discussion
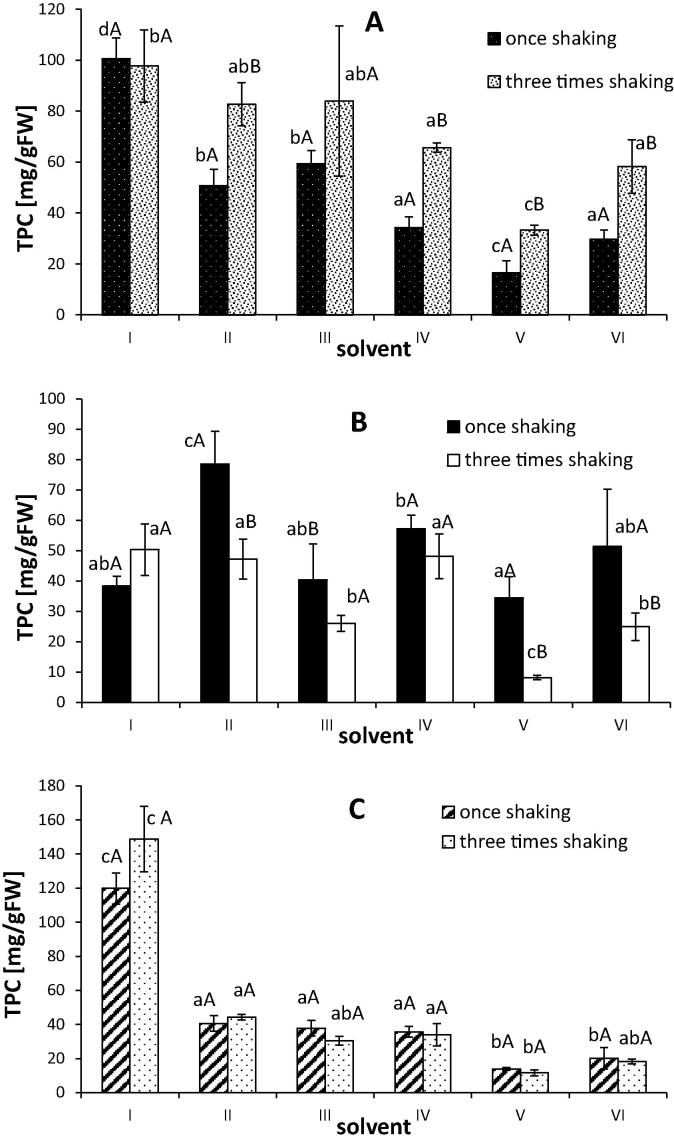
Phytochemicals, including phenolic compounds, present in many herbs have received much attention in recent years due to their many health benefits, including antioxidant and anti-inflammatory activities. For this reason there are interests in using some herbs not only as culinary products but also as a supplement to functional products. Therefore, the study on the stability of some bioactive compounds in the plant material during some processing (drying, freezing, etc.) or preparation of plant extracts would be useful in the selection of the bioactive compounds extraction procedure (Harbourne et al., 2013). For extracting phenolic compounds typically from plant material previously used solvents are methanol, ethanol, acetone and ethyl acetate (Alothman et al., 2009, Lafka et al., 2007). In the present study for extracting phenolic compounds from basil leaves the combination of methanol, acetone and acetic acid at different ratios were used (Table 1). The composition of the extracting solvents significantly (P < 0.05) affected the measured polyphenolic content. The acetone mixtures with the highest addition of acetic acid (solvent I: acetone/water/acetic acid (70/28/2, v/v/v)) extracted most of the phenolic compounds when fresh and freeze-dried material had been used (Fig. 1A and C), whereas the acetone/water/acetic acid (70/29.5/0.5, v/v/v) mixtures appeared to be the best for extracting phenolics from frozen used matrices. It is well known that solvent polarity will play a key role in increasing phenolic solubility and acetone–water mixtures which were very effective solvents in our study are good solvent systems for the extraction of polar antioxidants (Naczk and Shahidi, 2006, Alothman et al., 2009). In a study conducted by Michiels et al. (2012) acetone-based mixtures were also more effective solvents than the methanol-based mixtures for phenolic extraction yields from fruits and vegetables. However, in Tomsone et al. (2012) the best solvents for phenolic extraction from horseradish roots were ethanol and ethanol/water solutions have been reported (Tomsone et al., 2012). Additionally, the maximum polyphenolic extraction yield (48.7 ± 0.7 g GAE/100 g extract) was obtained in the methanol extract of Bauhinia vahlii followed by acetone, hot water and chloroform extracts (Sowndhararajan and Kang, 2013). So, as other researchers suggested (Luthria, 2008, Michiels et al., 2012) that selection of the most efficient solvent for phenolic compounds extraction must depend on the used food matrices.
Figure 1.
Total phenolic content in extracts of fresh (A), frozen (B) and lyophilized (C) basil leaves. I–VI – different solvents used for extraction (see Table 1). Different lower case letters in the same types of material indicate significant differences (P < 0.05). Different capital letters in the same solvent used indicate significant difference among a number of extraction steps (P < 0.05).
Among factors affecting the efficiency of the extraction, the number of extractions should be considered. In this present study, two extraction procedures: once shaking for 30 min and three times shaking for 20 min were performed. Although in the case of solvent I and III the content of phenolic compounds in extracts from fresh material obtained by the different procedure was not significantly (P ⩾ 0.05) different when we used other solvents (II, IV, V and VI) the three times procedure was more effective (Fig. 1A). Contrary, in the case of frozen basil, once shaking procedure led to a larger yield than three times as solvents no II, IV, V and VI were used (Fig. 1B). Surprisingly, there were not significant (P ⩾ 0.05) differences in the content of phenolics between the two used procedures in the case of lyophilized basil leaves used for extraction (Fig. 1C). According to Michiels et al. (2012) the optimal time and procedures of phenolics extraction from plant matrices like fruit (apple) and vegetables (broccoli and leek) was a single 60-min extraction but it depends on the plant because for example in the case of orange the two-step extraction (40 + 20 min) showed the best results.
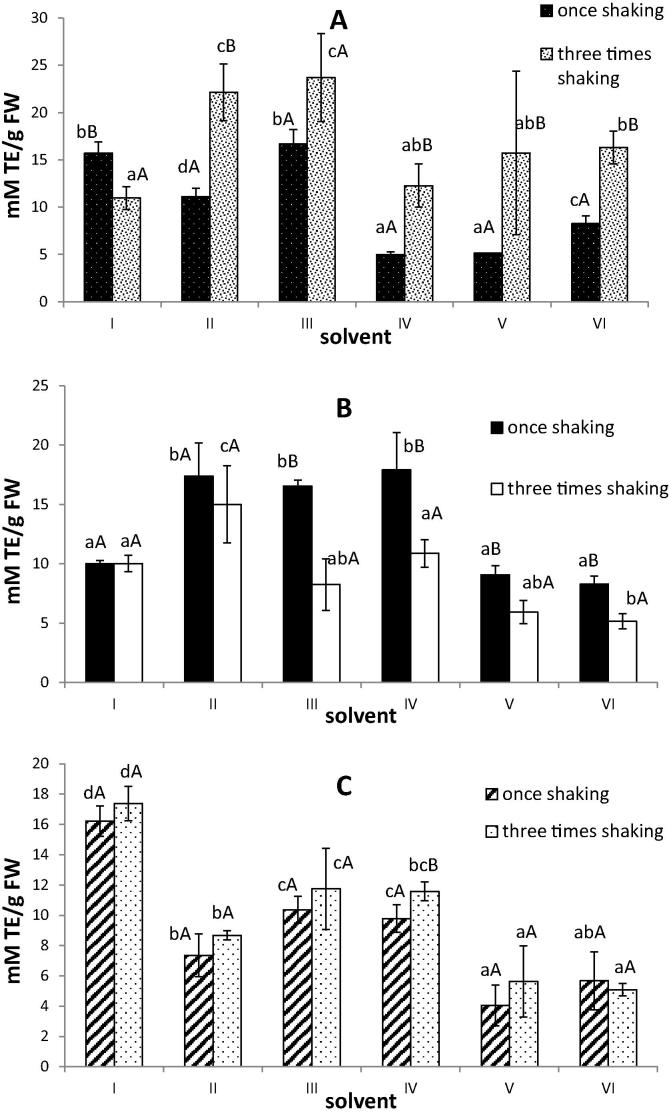
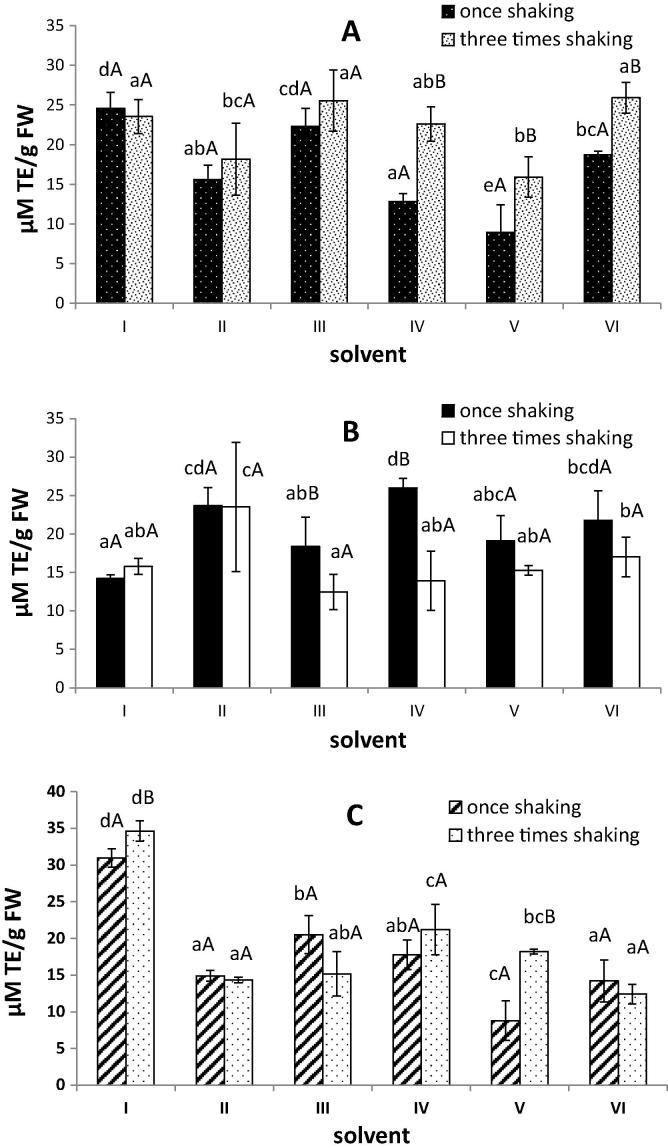
It is well known that plant phenolic compounds can play an important role in the shaping of the biological properties of the plant including antioxidant properties (Pandey and Rizvi, 2009). In the presented study the antioxidant activity was determined with the use of two methods – ability to quench free radicals and reducing power. In the case of lyophilized basil the highest antioxidant potential measured with both used methods in the extracts obtained using the solvent no I (acetone/water/acetic acid (70/28/2, v/v/v)) was observed (Fig. 2C and 3C). These results were correlated with the highest polyphenolic contents (Fig. 1C). Similarly, mixtures of acetone and water were the most effective for total phenolic extraction as well as for producing extracts from lyophilized banana peel with a high antioxidant capacity (González-Montelongo et al., 2010). On the contrary, in the work conducted by Sowndhararajan and Kang (2013) the methanolic extract of B. vahlii leaves has a stronger antioxidant potential than those of acetone, water and chloroform extracts. In our study the highest antiradical activity of extracts from fresh material for II and III solvents was determined (Fig. 2A and 3A).
Figure 2.
Antiradical activity of extracts of fresh (A), frozen (B) and lyophilized (C) basil leaves. I–VI – different solvents used for extraction (see Table 1). Different lower case letters in the same types of material indicate significant difference (P < 0.05). Different capital letters in the same solvent used indicate significant difference among a number of extraction steps (P < 0.05).
Figure 3.
Reducing power of extracts of fresh (A), frozen (B) and lyophilized (C) basil leaves. I–VI – different solvents used for extraction (see Table 1). Different lower case letters in the same types of material indicate significant difference (P < 0.05). Different capital letters in the same solvent used indicate significant difference among a number of extraction steps (P < 0.05).
Comparing the extraction procedure (once shaking for 30 min. and three times shaking for 20 min every time) used in these studies no differences in antioxidant activity in the case of lyophilized basil leaves (except the solvent No. V) should be noted (Fig. 2C and 3C). This is consistent with the results regarding the content of polyphenols in the studied extracts (Fig 1C). When we used fresh basil leaves for preparing extracts, the antioxidant activities measured by the DPPH and RP assays were higher for three times shaking procedure than for once shaking – these results also confirmed the trends relating to the content of phenolic compounds. One exception was extracts obtained using acetone/water/acetic acid (70/28/2, v/v/v) mixture, for which there were no differences in antioxidant activities (Fig 2A and 3A). The obtained results may be due to the fact that in the case of three times shaking procedure the time during which the extraction solvent and matrix are in contact was longer. Our results corresponded with the study conducted by Michiels et al. (2012) in which the DPPH antioxidant capacity of extracts from apple increased with the extraction time. Although in the same study increasing the extraction time had no effect or small negative effect on the ORAC (Oxygen Radical Antioxidant Capacity) values for extracts from apple and leek.
It is well known that the antioxidant potential of the plant material usually appears to correlate with the phenolic content (Kevers et al., 2007). The analyses in the present work of correlation between the phenolic content and antioxidant activity measured in the two assays varied with the material used (Table 2). The highest positive correlation for the extract from lyophilized material has been noted (R2 = 0.880 and 0.908 for the relationship between the content of phenolics and the DPPH or RP, respectively). It should also be noted that in the case of other types of materials (fresh and frozen) the correlations between the antioxidant activities and the phenolic contents were also positive and statistically significant (P < 0.05), but not high (Table 2). Similarly, in the work conducted by Michiels et al. (2012) the correlation between the phenolic content and antioxidant capacity varied from matrix to matrix – it was positive and high for apple and broccoli (between phenolic content and DPPH) as well as for orange (between phenolic content and ORAC). However, for orange (between phenolic content and DPPH) and for broccoli (between phenolic content and ORAC) it was positive, but not significant (P ⩾ 0.05) (Michiels et al., 2012).
Table 2.
Pearson’s correlations among phenolic compound content and antioxidant activities (DPPH and RP) of basil leaves.
| Phenolics/DPPH | Phenolics/RP | |
|---|---|---|
| Fresh material | 0.619 | 0.763 |
| Frozen material | 0.742 | 0.629 |
| Lyophilized material | 0.880 | 0.908 |
4. Conclusion
The optimal conditions for polyphenol extraction from basil leaves were significantly different depending on the types of plant material (fresh, frozen or lyophilized) used. Generally, the combination of acetone, water and acetic acid seem to be more effective than the combination of methanol, water and acetic acid. However, the influence of the number of extraction steps was different for extracts from fresh, frozen and lyophilized basil leaves. It should be also noted that polyphenol contents were positively and statistically significantly correlated with the antioxidant activity of the studied extracts.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alothman M., Bhat R., Karim A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. [Google Scholar]
- Amarowicz R., Pegg R.B., Rahimi-Moghaddam P., Barl B., Weil J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. [Google Scholar]
- Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. [Google Scholar]
- Brand-Williams W., Cuvelier E., Berset C.M. Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- Dai J., Mumper R.J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Montelongo R., Lobo M.G., González M. Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds activity depends on the type and polarity of the extracting solvents the isolation procedures. Food Chem. 2010;119:1030–1039. [Google Scholar]
- Harbourne N., Marete E., Jacquier J.C., O’Riordan D. Stability of phytochemicals as sources of anti-inflammatory nutraceuticals in beverages — A review. Food Res. Int. 2013;50:480–486. [Google Scholar]
- Kevers C., Falkowski M., Tabart J., Defraigne J.O., Dommes J., Pincemail J. Evolution of antioxidant capacity during storage of selected fruits and vegetables. J. Agric. Food Chem. 2007;55(21):8596–8603. doi: 10.1021/jf071736j. [DOI] [PubMed] [Google Scholar]
- Lafka T.I., Sinanoglou V., Lazos E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007;104(3):1206–1214. [Google Scholar]
- Lee J., Scagel C.F. Chicoric acid levels in commercial basil (Ocimum basilicum) and Echinacea purpurea products. J. Funct. Foods. 2010;2:77–84. [Google Scholar]
- Luthria D.L. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008;107:745–752. [Google Scholar]
- Michiels J.A., Kevers C., Pincemail J., Defraigne J.O., Dommes J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012;130:986–993. [Google Scholar]
- Naczk M., Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J. Pharm. Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction- antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44(6):307–315. [Google Scholar]
- Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longevity. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini N., Colombi B., Salvatore S., Brenna O.V., Galaverna G., Del Rio D., Bianchi M., Bennett R., Brighenti F. Evaluation of antioxidant capacity of some fruit and vegetable foods: efficiency of extraction of a sequence of solvents. J. Sci. Food Agric. 2007;87:103–111. [Google Scholar]
- Rababah T.M., Banat F., Rababah A., Ereifej K., Yang W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J. Food Sci. 2010;75(7):C626–C632. doi: 10.1111/j.1750-3841.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1974;299:152–178. [Google Scholar]
- Sowndhararajan K., Kang S.C. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J. Biol. Sci. 2013;20:319–325. doi: 10.1016/j.sjbs.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarchoune I., Sgherrii C., Baâtour O., Izzo R., Lachaâ M., Navari-Izzo F., Ouerghi Z. Phenolic acids and total antioxidant activity in Ocimum basilicum L. grown under Na2SO4 medium. J. Med. Plants Res. 2012;6(48):5868–5875. [Google Scholar]
- Tomsone L., Kruma Z., Galoburda R. Comparison of different solvents and extraction methods for isolation of phenolic compounds from horseradish roots (Armoracia rusticana) World Acad. Sci. Eng. Technol. 2012;64:903–908. [Google Scholar]
- Zhang H.-Y., Sun Y.-M., Wang X.-L. Substituent effects on O–H bond dissociation enthalpies and ionization potentials of catechols: a DFT study and its implications in the rational design of phenolic antioxidants and elucidation of structure–activity relationships for flavonoid antioxidants. Chem. Eur. J. 2003;9(2):502–508. doi: 10.1002/chem.200390052. [DOI] [PubMed] [Google Scholar]