Abstract
Purpose
To determine age-related changes in corneal viscoelastic properties in healthy individuals.
Methods
This observational cross-sectional study was performed at the Department of Ophthalmology, Imam Khomeini Hospital, Ahvaz, Iran and included 302 healthy individuals in 6 age decades (range: 10–69 years). After complete ocular examination, corneal viscoelastic properties were measured by ocular response analyzer and central corneal thickness (CCT) by an ultrasonic pachymeter. Our main outcome measures were corneal viscoelastic properties in different age groups.
Results
Corneal hysteresis (CH) and corneal resistance factor (CRF) showed a significant negative correlation with age (P < 0.001 for both, r = −0.353 and r = −0.246, respectively). Female gender had significantly higher CH (P = 0.017) and CRF (P = 0.019). CH and CRF were significantly correlated (P < 0.001, r = 0.821). CCT showed a biphasic pattern with significantly higher thicknesses before 20 and after 50 years of age. CH and CRF were significantly correlated with CCT (P < 0.001 for both, r = 0.21 and r = 0.26, respectively) and intraocular pressure (IOP) (P < 0.001 for both, r = −0.474 and r = 0.598, respectively). Corneal-compensated IOP (IOPcc) was significantly higher after age 40 compared to age group <20 (p < 0.045). Goldmann-correlated IOP (IOPg) was significantly correlated with CCT (P = 0.001, r = 0.193), while IOPcc showed no correlation with CCT (P = 0.265, r = 0.062). CH was significantly higher in hyperopic eyes compared to emmetropic eyes (P = 0.009) and myopic eye (P < 0.001).
Conclusions
In this study, there was a decrease in CH and CRF with an increase in age. Hyperopia and female gender are associated with higher CH and CRF. CCT is higher toward the extremes of life and is significantly correlated with CH and CRF.
Keywords: Corneal hysteresis, Corneal resistance factor, Ocular response analyzer, Aging
Introduction
The viscoelastic properties of the cornea were first described by Freidenwald in 1937.1
However, in vivo measurement of corneal biomechanical properties was only possible in 2005 by the Ocular Response Analyzer (ORA) (Reichert Inc., Depew, New York).2
The instrument measures corneal hysteresis (CH) and corneal resistance factor (CRF) as the markers of corneal viscoelastic properties. Technically, CH is the difference between inward and outward applanation pressures created by an air puff and is an indicator of viscous properties of the cornea. However, CRF is a measurement of corneal resistance to deformation and indicates elastic properties of the cornea. Corneal properties other than central corneal thickness (CCT) have several implications especially in glaucoma3 and corneal refractive surgery.4
Corneal viscoelastic properties depend on the structure and organization of collagen fibrils and extracellular matrix (ECM). Collagen fibers are responsible for strength and elasticity of the cornea while the ECM is responsible for the viscous properties. Thus, changes in collagen fibers affect CRF and changes in ECM affect CH. Structural changes with age include an increase in collagen fiber diameter as a result of an increase in number of collagen molecules and expansion of intermolecular space.5 Ex vivo measurements of corneal biomechanical properties have shown progressive stiffening of the cornea with age.6, 7, 8, 9
However, the results of in vivo measurements of corneal biomechanics are inconclusive.10 Some studies have shown no change by age,11, 12, 13, 14 while some report a decrease in CH and CRF.15, 16, 17, 18, 19
This study was conducted to evaluate the corneal viscoelastic properties in different age groups.
Methods
This cross-sectional study was performed at the Department of Ophthalmology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The study protocol was approved by the local ethics committee and adhered to the tenets of Declaration of Helsinki. Informed consent was obtained from all patients.
In this observational cross-sectional study, 302 healthy individuals aged 10–69 years were evaluated. Exclusion criteria included any history of ocular or systemic diseases including diabetes, hypertension, hyperlipidemia, glaucoma, ocular surgery, keratoconus, ocular or systemic medications including corticosteroids, corneal astigmatism >2 D, severe dry eye, pregnancy, and contact lens use within 1 month of the study. After a complete ocular examination, corneal viscoelastic properties were measured using ORA and CCT measured using an ultrasonic pachymeter (Pachymeter SP 3000, Tomey, Nagoya, Japan). In case of ocular disease, the sound eye and otherwise the right eye was chosen for the study. Individuals were put into 6 groups: Group 1 (10–19 years), Group 2 (20–29 years), Group 3 (30–39 years), Group 4 (40–49 years), Group 5 (50–59 years), and Group 6 (60–69 years). Our main outcome measure was corneal viscoelastic properties at different age groups. Second outcome measure was the effects of sex and refractive errors on corneal biomechanics.
ORA is an air puff tonometer that measures the corneal response to a steady air pulse. It makes two applanation measurements: a force-in applanation which has been attributed to the dampening effects of the cornea and a force-out applanation that occurs at a lower pressure than the first. The difference between the two pressures is CH and indicates viscous properties of the cornea, while CRF shows the elastic properties of the cornea. Therefore, these parameters may affect intraocular pressure (IOP) measurements more than CCT alone. The instrument also reports Goldman-correlated IOP (IOPg) and its corneal-compensated counterpart (IOPcc).
ORA parameters including CH, CRF, IOPg, and IOPcc, as well as CCT, and spherical equivalent refractive error were considered for analysis. Refractive errors of −1 to 1 D were considered as emmetropia.
Statistical analysis
To obtain a power of 0.8 and α = 0.05, sample size of 36 cases was calculated for each age decade.
Statistical analyses were performed using SPSS software v.20 (SPSS Inc. Chicago, IL). Pearson correlation coefficient and partial correlation were used in analyses. In addition, to compare data, we used one way analysis of variance (ANOVA). Two-by-two comparisons were done using Tukey test. P values <0.05 were considered significant.
Results
Overall, 302 healthy individuals were enrolled in the study. Table 1 shows demographic data and participants' characteristics.
Table 1.
Participants' characteristics and corneal viscoelastic properties in healthy individuals.
| Age group (year) | N | Age (year) (M ± SD) | Gender M (%) | CH (mmHg) (M ± SD) (Range) | CRF (mmHg) (Range) | CCT (μ) (Range) | IOPg (mmHg) (Range) | IOPcc (mmHg) (Range) |
|---|---|---|---|---|---|---|---|---|
| 10–19 | 56 | 13.1 ± 3.1 | 28 (50) | 12.51 ± 1.8 (7.6–16.2) | 12.10 ± 1.9 (6.7–16.2) | 559.5 ± 42.5 (420–633) | 15.1 ± 3.5 (8.8–24.8) | 13.4 ± 3.5 (6.6–20.7) |
| 20–29 | 53 | 24.5 ± 2.8 | 23 (43.4) | 10.96 ± 1.5 (6.6–15.6) | 10.24 ± 1.9 (6.5–14.1) | 535.2 ± 28.3 (435–588) | 13.3 ± 3.5 (5.5–20.9) | 13.5 ± 2.9 (5.8–19) |
| 30–39 | 61 | 33.6 ± 2.8 | 26 (42.6) | 10.98 ± 1.5 (6.5–13.9) | 10.28 ± 1.7 (7.3–13.9) | 541.4 ± 33.5 (450–616) | 13.3 ± 3.2 (6.6–22.3) | 13.5 ± 3 (7.3–22.2) |
| 40–49 | 45 | 43.2 ± 2.4 | 25 (55.6) | 10.74 ± 1.7 (7.5–13.8) | 10.62 ± 1.8 (6.7–13.4) | 544.9 ± 41.7 (409–616) | 15.2 ± 3.8 (7.2–23.1) | 15.4 ± 3.9 (8.7–24.5) |
| 50–59 | 36 | 54.1 ± 2.6 | 13 (36.1) | 10.90 ± 1.6 (7.7–14.5) | 10.33 ± 1.9 (7–14.2) | 568.1 ± 31.9 (497–650) | 13.7 ± 3.5 (7.7–21.5) | 13.9 ± 3.3 (7.2–22) |
| 60–69 | 51 | 64 ± 2.9 | 29 (56.9) | 10.32 ± 1.9 (6.2–14.1) | 10.22 ± 1.8 (7.9–16.5) | 568.2 ± 26.9 (489–603) | 14.8 ± 4 (6.7–25.5) | 15.1 ± 4.4 (6.1–25.9) |
| Pa | <0.001 | <0.001 | <0.001 | 0.008 | 0.007 | |||
| Multiple comparisonsb | Group 1 & other groups <0.001 | Group 1 & other groups <0.001 | Groups 1&2 5&2,3,4 6&2,3,4 <0.05 |
Groups 1&2,3 (0.007) 3&4 (0.007) |
Groups 1&4 0.045 |
M, male; CH, corneal hysteresis; CRF, corneal resistance factor; CCT, central corneal thickness; IOPg, Goldman-correlated intraocular pressure; IOPcc, corneal-compensated IOP.
Based on ANOVA.
Based on Tukey test.
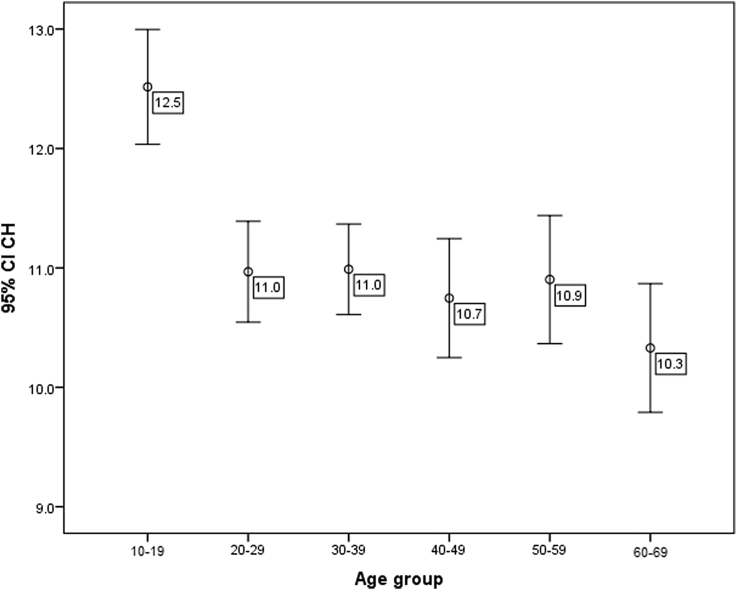
CH and CRF were significantly different among the age groups (P < 0.001, ANOVA). Group 1 (10–19 years old) showed significantly higher CH and CRF compared to all other age groups (P < 0.001) while other groups were not significantly different from each other (All P values >0.05, Tukey test) (Table 1, Fig. 1). CH and CRF were highly correlated (P < 0.001, r = 0.821).
Fig. 1.
Corneal hysteresis (CH) changes with age. CH is significantly higher under age 20 and decreases over time. CI, Confidence interval.
Age showed a significant negative correlation with CH (P < 0.001, r = −0.353) and CRF (P < 0.001, r = −0.326). Female gender had significantly higher CH (11.35 vs 10.85, P = 0.017) and CRF (10.9 vs 10.4, P = 0.019, independent t-test).
After adjustment for gender, CCT, IOP, and refractive error, age remained significantly correlated with CH and CRF (P < 0.001 for both, r = −0.398 and r = −0.372, respectively).
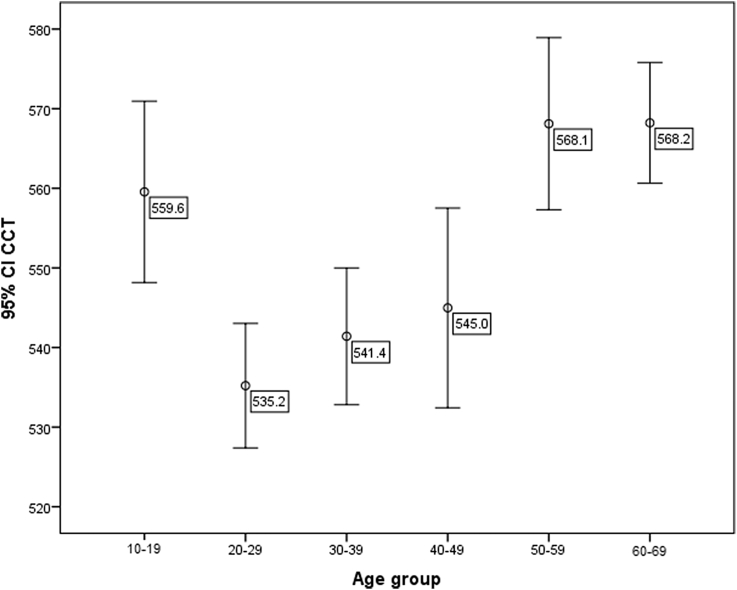
CCT showed a biphasic pattern in which Groups 1, 5, and 6 had significantly higher CCTs compared to other groups (Table 1, Fig. 2). CH and CRF showed significant positive correlation with CCT (P < 0.001 for both, r = 0.21 and r = 0.26, respectively).
Fig. 2.
Central corneal thickness (CCT) shows a biphasic pattern with higher thicknesses before age 20 and after 50. CI, Confidence interval.
IOPcc was significantly higher in Group 4 compared to Group 1 (P = 0.045). IOPg was significantly higher in Group 1 (compared to Groups 2 and 3, P = 0.007) and Group 4 (compared to Group 3, P = 0.007). IOPg and IOPcc were significantly correlated (P < 0.001, r = 0.845) and were not significantly different (P = 0.148). IOPg (but not IOPcc) was significantly correlated with CCT (P = 0.001, r = 0.193) and CRF (P < 0.001, r = 0.598), while IOPcc was negatively correlated with CH (P < 0.001, r = −0.474).
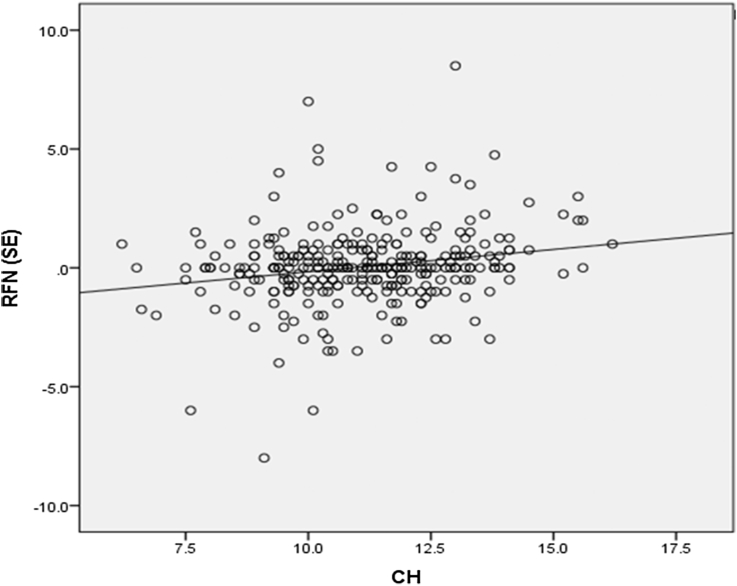
The groups were not significantly different in terms of refractive error. (P = 0.055, ANOVA) CH and CRF showed a significant positive correlation with refractive error (P = 0.001, r = 0.187 and P = 0.007, r = 0.157, respectively, Fig. 3). CH and CRF were significantly higher in hyperopes compared to emmetropes and myopes (Table 2).
Fig. 3.
Corneal hysteresis (CH) shows a significant correlation with refraction in spherical equivalent [RFN (SE)], (P = 0.001, r = 0.187).
Table 2.
Corneal biomechanics and refractive state of the eye in healthy individuals.
| Refractive error | N | Spherical equivalent M ± SD (D) (Range) | CH (mmHg) (Range) | CRF (mmHg) (Range) |
|---|---|---|---|---|
| Myopia (1) | 42 | −3 ± 2.5 (<−1 to −8) | 10.49 ± 1.74 (6.6–13.7) | 10.16 ± 1.87 (6.5–13.9) |
| Emmetropia (2) | 215 | 0.001 ± 0.6 (−1 to 1) | 11.06 ± 1.73 (6.2–16.2) | 10.58 ± 1.89 (6.7–16.2) |
| Hyperopia (3) | 45 | 2.5 ± 1.6 (>1 to 8) | 11.92 ± 1.94 (7.7–15.6) | 11.55 ± 2.05 (7.4–16.5) |
|
Pa 3&2 3&1 |
0.009 0.001 |
0.006 0.002 |
CH, corneal hysteresis; CRF, corneal resistance factor; D, Diopter.
Based on Tukey test.
Discussion
This study showed a significant decrease in corneal response parameters CH and CRF measured with the ORA with age.
Corneal properties including thickness, hydration, elasticity, viscosity, and possibly other unrevealed factors can affect corneal behavior during IOP measurements3 and after refractive surgery.4 Corneal viscoelastic properties are provided by collagen fibers, and glycosaminoglycans and proteoglycans forming the ground substance. Abnormal corneal viscoelastic properties are seen in keratoconus and post laser keratectasia.2, 15
Aging induces structural changes in the cornea including an increase in collagen fiber diameter as a result of an increase in the number of collagen molecules in collagen fibrils and expansion of intermolecular space due to glycation-induced cross-linking.5 Ex vivo studies have shown significant stiffening of the human cornea with age.6, 7, 8, 9 This has been attributed to non-enzymatic cross-linking of the stromal collagen fibrils.7 However, the relationship between corneal stiffening and age is not linear,6, 7 and the stiffness increases by a factor of approximately two between the ages of 20 and 100 years.8
Indirect clinical evidence also suggests that the cornea stiffens with age. For example, keratoconus is a disease of youth, the incidence of which decreases with age.20 Additionally, youth is a risk factor for both post laser keratectasia21 and keratoconus progression.22, 23
Based on the above-mentioned evidence, an increase in corneal stiffening with in vivo measurements is expected as well.
In vivo measurement of corneal biomechanics has been possible since 2005 with ORA, and for years it was the only commercially available instrument. ORA has shown lower CH and CRF in keratoconus and Fuchs endothelial dystrophy as expected.2, 15 However, the results of studies on age-related changes in corneal biomechanics are not consistent. Some studies on children have shown no correlation between age and CH11, 12 or CRF11 with similar or slightly higher CH and CRF values in children compared to adult studies. On the other hand, several studies have reported a decrease in CH and CRF with age.15, 16, 17, 18, 19, 24
Ortiz et al in a study on 165 normal eyes showed significantly lower CH in the age group 60–80 years compared to age group 9–14 years.15 Kotecha et al reported an IOP-independent biomechanical property of the cornea (corneal constant factor; CCF) that increased with thicker CCT and decreased with greater age.16 Their study group consisted of 105 ocular hypertensives and normal individuals with a mean age of 60 years. Some of these studies suffer from a small sample size, limited age range, and mixed study population.10 In another study on 204 normal eyes with a mean age of 46.7 years (range: 19–89 years), a significant decrease in CH and CRF was observed with age in the absence of significant changes in CCT or IOP.18 Our study showed a decrease in CH and CRF with age as well. We observed significantly higher CH and CRF in the younger age group and a significant negative correlation with age. The measured values were similar to those reported elsewhere.10, 11, 12
Most studies evaluating the effect of CCT on corneal viscoelastic properties have reported a positive correlation between CCT and CH,10, 11, 13, 14, 16, 19, 24 or CRF.10, 11, 14, 19, 24
In the present study, CCT showed a biphasic pattern with higher values in the young and elderly and had significant correlation with CH and CRF. In Group 1, higher CH and CRF are in agreement with higher CCT. However, considering structural changes in the elderly, lower CH and higher CCT can be explained by changes in ECM and corneal hydration by aging as the elderly have poorer corneal hydration control compared to the young.25
Thus, while the increase in collagen cross-linking causes corneal stiffening, increased water content of the cornea causes a reduction in CH and CRF. Clinically, it is possible to postulate that low CH accompanied by low CCT is attributed to collagen weakness like keratoconus, while low CH with high CCT is more likely the result of ground substance changes such as hydration. Additionally, instruments have inherent limitations that may cause such contradictions.
Measuring corneal biomechanics by a new ultra-high-speed Scheimpflug camera (Oculus Corvis ST, Scheimpflug Technology; Wetzlar, Germany) has shown significant corneal stiffening by age manifested in an increase in highest concavity time (i.e. time from starting until highest concavity is reached).26
Several studies have shown that a negative correlation exists between CH and IOP.10, 11, 13, 19 In our study, we observed a significant negative correlation between IOPcc and CH, and a significant positive correlation between IOPg and CRF. Although we observed a significantly higher IOPcc in age Group 4 compared to Group 1, the difference does not seem to be clinically important. IOPcc was not significantly different from IOPg measurements. IOPg was significantly correlated with CCT while IOPcc was not. Therefore, IOPcc might be a better indicator of real IOP than IOPg.
It has been reported that CH is significantly lower in high myopes14, 24, 27 and higher in hyperopes compared to normal individuals.24 We observed significantly higher CH and CRF in hyperopes compared to myopes and emmetropes as well.
One limitation of our study is lack of data on axial length and keratometry. However, by excluding high astigmatism we tried to overcome this limitation. Additionally, axial length is correlated with refractive error which was included in the study.
Some strengths of our study are the large sample size and that we enrolled individuals with a wide range of age, without previous surgery, ocular pathology or systemic disease, and evaluated corneal viscoelastic changes in 6 decades.
In conclusion, corneal viscoelastic properties decrease with age and show a positive correlation with CCT. Age-related decrease in CH and CRF may be due to increased corneal hydration with age. Female gender and hyperopes have higher CH and CRF. IOPcc is not correlated to CCT and may be a better indicator of real IOP.
Footnotes
This paper is based on the thesis done by Dr. Roghayeh Bidar.
Financial support: This study was financially supported by Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The sponsor had no role in the design or conduct of this research. None of the authors have a financial interest to disclose.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Woo S.L., Kobayashi A.S., Lawrence C., Schlegel W.A. Mathematical model of the corneo-scleral shell as applied to intraocular pressure-volume relations and applanation tonometry. Ann Biomed Eng. 1972;1:87–98. doi: 10.1007/BF02363420. [DOI] [PubMed] [Google Scholar]
- 2.Luce D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Liu J., Roberts C.J. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31:146–155. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Alastrué V., Calvo B., Peña E., Doblaré M. Biomechanical modeling of refractive corneal surgery. J Biomech Eng. 2006;128:150–160. doi: 10.1115/1.2132368. [DOI] [PubMed] [Google Scholar]
- 5.Daxer A., Misof K., Grabner B., Ettl A., Fratzl P. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci. 1998;39:644–648. [PubMed] [Google Scholar]
- 6.Elsheikh A., Geraghty B., Rama P., Campanelli M., Meek K.M. Characterization of age-related variation in corneal biomechanical properties. J R Soc Interf. 2010;7:1475–1485. doi: 10.1098/rsif.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsheikh A., Wang D., Brown M., Rama P., Campanelli M., Pye D. Assessment of corneal biomechanical properties and their variation with age. Curr Eye Res. 2007;32:11–19. doi: 10.1080/02713680601077145. [DOI] [PubMed] [Google Scholar]
- 8.Knox Cartwright N.E., Tyrer J.R., Marshall J. Age-related differences in the elasticity of the human cornea. Invest Ophthalmol Vis Sci. 2011;52:4324–4329. doi: 10.1167/iovs.09-4798. [DOI] [PubMed] [Google Scholar]
- 9.Randleman J.B., Dawson D.G., Grossniklaus H.E., McCarey B.E., Edelhauser H.F. Depth- dependent cohesive tensile strength in human donor corneas: implications for refractive surgery. J Refract Surg. 2008;24:S85–S89. doi: 10.3928/1081597X-20080101-15. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Porta N., Fernandes P., Queiros A., Salgado-Borges J., Parafita-Mato M., González-Méijome J.M. Corneal biomechanical properties in different ocular conditions and new measurement techniques. ISRN Ophthalmol. 2014:724546. doi: 10.1155/2014/724546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim L., Gazzard G., Chan Y.H. Cornea biomechanical characteristics and their correlates with refractive error in Singaporean children. Invest Ophthalmol Vis Sci. 2008;49:3852–3857. doi: 10.1167/iovs.07-1670. [DOI] [PubMed] [Google Scholar]
- 12.Kirwan C., O'Keefe M., Lanigan B. Corneal hysteresis and intraocular pressure measurement in children using the reichert ocular response analyzer. Am J Ophthalmol. 2006;142:990–992. doi: 10.1016/j.ajo.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya K., Hagishima M., Fujimura F., Shimizu K. Factors affecting corneal hysteresis in normal eyes. Graefes Arch Clin Exp Ophthalmol. 2008;246:1491–1494. doi: 10.1007/s00417-008-0864-x. [DOI] [PubMed] [Google Scholar]
- 14.Shen M., Fan F., Xue A., Wang J., Zhou X., Lu F. Biomechanical properties of the cornea in high myopia. Vis Res. 2008;48:2167–2171. doi: 10.1016/j.visres.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz D., Piñero D., Shabayek M.H., Arnalich-Montiel F., Alió J.L. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33:1371–1375. doi: 10.1016/j.jcrs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Kotecha A., Elsheikh A., Roberts C.R., Zhu H., Garway-Heath D.F. Corneal thickness- and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Invest Ophthalmol Vis Sci. 2006;47:5337–5347. doi: 10.1167/iovs.06-0557. [DOI] [PubMed] [Google Scholar]
- 17.Kida T., Liu J.H.K., Weinreb R.N. Effects of aging on corneal biomechanical properties and their impact on 24-hour measurement of intraocular pressure. Am J Ophthalmol. 2008;146:567–572. doi: 10.1016/j.ajo.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamiya K., Shimizu K., Ohmoto F. Effect of aging on corneal biomechanical parameters using the ocular response analyzer. J Refract Surg. 2009;25:888–893. doi: 10.3928/1081597X-20090917-10. [DOI] [PubMed] [Google Scholar]
- 19.Narayanaswamy A., Chung R.S., Wu R.Y. Determinants of corneal biomechanical properties in an adult Chinese population. Ophthalmology. 2011;118:1253–1259. doi: 10.1016/j.ophtha.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Ertan A., Muftuoglu O. Keratoconus clinical findings according to different age and gender groups. Cornea. 2008;27:1109–1113. doi: 10.1097/ICO.0b013e31817f815a. [DOI] [PubMed] [Google Scholar]
- 21.Randleman J.B., Woodward M., Lynn M.J., Stulting R.D. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37–50. doi: 10.1016/j.ophtha.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 22.McMahon T.T., Edrington T.B., Szczotka-Flynn L., Olafsson H.E., Davis L.J., Schechtman K.B. CLEK Study Group. Longitudinal changes in corneal curvature in keratoconus. Cornea. 2006;25:296–305. doi: 10.1097/01.ico.0000178728.57435.df. [DOI] [PubMed] [Google Scholar]
- 23.Wagner H., Barr J.T., Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study: methods and findings to date. Cont Lens Anterior Eye. 2007;30:223–232. doi: 10.1016/j.clae.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bueno-Gimeno I., España-Gregori E., Gene-Sampedro A., Lanzagorta-Aresti A., Piñero-Llorens D.P. Relationship among corneal biomechanics, refractive error, and axial length. Optom Vis Sci. 2014;91:507–513. doi: 10.1097/OPX.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 25.Polse K.A., Brand R., Mandell R., Vastine D., Demartini D., Flom R. Age differences in corneal hydration control. Invest Ophthalmol Vis Sci. 1989;30:392–399. [PubMed] [Google Scholar]
- 26.Valbon B.F., Ambrósio R., Jr., Fontes B.M., Alves M.R. Effects of age on corneal deformation by non-contact tonometry integrated with an ultra-high-speed (UHS) Scheimpflug camera. Arq Bras Oftalmol. 2013;76:229–232. doi: 10.1590/s0004-27492013000400008. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Z., Shen M., Mao G. Association between corneal biomechanical properties and myopia in Chinese subjects. Eye. 2011;25:1083–1089. doi: 10.1038/eye.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]