Abstract
Purpose
To evaluate modes of cataractogenesis in the hypertensive state by using different hypertensive animal models, including fructose, cadmium chloride (CdCl2), Nω-nitro-l-arginine methyl ester (l-NAME), and two-kidney, one clip (2K1C) method.
Methods
Male Sprague–Dawley albino rats (150–180 g) were divided into different groups, each group containing six animals. Hypertension was induced in animals via six weeks administration of fructose (10% solution in drinking water), CdCl2 (0.5 mg/kg/day, i.p.), and l-NAME (20 mg/kg/day, p.o.) in their respective groups and NaCl (0.9% solution in drinking water) in the 2K1C group. The Ramipril-treated group (2 mg/kg/day, orally) served as a standard group for the 2K1C animal model. Blood pressure was measured biweekly using non-invasive blood pressure system. The biochemical parameters in serum and eye lenses were evaluated after six weeks of the experimental protocol.
Results
Hypertensive animal models showed significant induction of systolic and diastolic blood pressure and modulation of oxidative stress through depletion of antioxidants, including glutathione peroxidase, catalase, superoxide dismutase, glutathione, and elevation of malondialdehyde in serum and eye lenses. A significant elevation of ionic contents (Na+ and Ca2+) and reduction of total protein and Ca2+ ATPase activity in eye lenses were observed in all hypertensive animal models except l-NAME when compared with the normal group. The significant restoration of the antioxidants, Malondialdehyde (MDA) total protein, and ionic contents in the eye lenses concomitant with reduction of blood pressure were observed in the ramipril-treated group as compared to the 2K1C animal model. The results indicate that the fructose, CdCl2, and 2K1C models showed pronounced cataractogenic effects in the rat eye lenses.
Conclusion
Based on our findings, it can be concluded that systemic hypertension significantly increases the risk of cataract formation in the rat eyes via modulation of the antioxidant defense mechanism and electrolyte homeostasis.
Keywords: Hypertension; Cataract; Oxidative stress; Fructose; CdCl2; Two-kidney, one clip
Introduction
Cataract is the leading cause of blindness in the world and the most prevalent ocular disease.1 The number of cataract-blind is expected to increase dramatically in coming decades as the number of elderly in the world's population increases. It is suggested that the number of cataract-blind could reach close to 40 million by the year 2025.2 There are several risk factors which are associated with induction of cataractogenesis, such as diabetes, oxidative stress, ultraviolet radiation, age, etc.3 Several epidemiological studies revealed that hypertension is also associated with cataract, but sometimes it is significant1, 4, 5 and sometimes not.6 Their studies, however, lacked the exact exploitation of biological mechanism involved in the entire process regarding the exacerbation of cataract in hypertensive state. Some preclinical studies indicate that systemic hypertension may alter the electrolyte homeostasis through inhibition of Na+ K+ ATPase pump activity in the lens which causes the cataract formation,7 but at present, another possible mechanism involved in cataractogenesis through hypertension is still unclear. In this perspective, we designed an experimental study to evaluate modes of cataractogenesis in the hypertensive state by using different hypertensive animal models, such as fructose, cadmium chloride (CdCl2), Nω-nitro-l-arginine methyl ester (l-NAME), and two-kidney, one clip (2K1C), which have different modes of cellular pathogenesis. In fructose animal model, chronic fructose administration induces hypertension by systemic oxidative stress, sympathetic overactivity, and increased production of vasoconstrictor molecules, viz endothelin-I and angiotensin-II.8 CdCl2 induced hypertension is related to Ca2+ mimicking contractile activity of cadmium ion on vascular smooth muscles,9 oxidative damage, and vascular endothelial dysfunction.10 The 2K1C model elevates the plasma renin activity, which activates the angiotensin II-mediated hypertensive actions,11 and l-NAME modulates the nitric oxide (NO) level in smooth muscles, which lead to elevation of blood pressure.12
Methods
Drugs and chemicals
l-NAME was purchased from Sigma–Aldrich (St. Louis, MO, USA). d-fructose and CdCl2 were purchased from HiMedia chemicals (Mumbai, India). Ramipril was obtained from Cipla Limited (Mumbai, India) as a gift sample. Other chemicals and reagents used were of analytical grade.
Experimental animals
Sprague–Dawley albino male rats (150–180 g) were used for the experimental study and were housed under standard environmental condition (23 ± 2 °C, with 55 ± 5% humidity and 12 h light/dark cycle) according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India, and were fed a standard pellet diet with water ad libitum under hygienic conditions. Animals were habituated to laboratory conditions for at least 48–72 h prior to the experimental protocol to minimize non-specific stress, if any. The protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Institute of Pharmaceutical Science, Guru Ghasidas Vishwavidyalaya, Bilaspur (C.G), India (Reg. No.-994/GO/ERe/S/06/CPCSEA), and the experiments were conducted according to the ethical principles and guidelines provided by CPCSEA, Govt. of India and the Association for Research in Vision and Ophthalmology (ARVO) for animals.
Experimental design
Male Sprague–Dawley albino rats (150–180 g) which were normal regarding the ocular examination and blood pressure at the baseline were randomly selected and divided into six different groups, each group containing six animals. Hypertension was induced in animals via six-week administration of fructose (10% solution in drinking water) in group II,13 CdCl2 (0.5 mg/kg/day, i.p.) in group III,9 l-NAME (20 mg/kg/day, p.o.) in group IV14, and in group V, hypertension was induced by 2K1C animal model.15 The ramipril-treated group (2 mg/kg/day, p.o.) served as standard group (2K1C animal model).16 Group I served as normal control.
The systolic (SBP) and diastolic blood pressure (DBP) in each group were monitored biweekly via non-invasive blood pressure system (NIBP; CODA-08 Channel, Kent scientific, USA), and biochemical parameter in serum and eye lens were determined after six weeks in sacrificed animals.
Surgical procedure for 2K1C model
2K1C was performed on all the rats by anesthetizing with ketamine and xylazine (60:10 mg/kg, i.p.). The kidney was visualized by a left lateral abdominal incision, and the left renal artery and ureter were ligated by a silk thread. The muscle and skin layer (incision site) were sutured with highly sterile suture needles. After surgery, rats were allowed to drink water ad libitum, with no further treatment. All uninephrectomized animals were given 0.9% NaCl in the drinking water for six consecutive weeks.15
Blood collection
Animals were sacrificed after six weeks, blood was collected from each group via cardiac puncture, and serum was separated and stored at 2–8 °C for further biochemical analysis.
Preparation of lens homogenate
The eyeball was isolated from the sacrificed animals. Lenses were dissected via posterior approach, washed with cold saline, and stored with saline at −20 °C until analysis. Lens homogenate was prepared from both lenses of each animal in 10 volumes of 0.1 M potassium phosphate buffer (pH 7). The homogenate was centrifuged at 10,000 rpm for 1 h, and the supernatant was separated and used for biochemical analysis.17
Determination of lenticular opacity
The lenticular opacity of the experimental groups was determined by the photographic method based on the appearance of graph lines through the lens. The eye lenses were dissected via a posterior approach, put on graph paper immediately, and photographed by a digital camera (Sony Cybershot DSC-W810). The graph lines would appear clearly in the transparent lens and cloudy or not visible in the cataractous lens.18
Biochemical parameters
Enzymatic and non-enzymatic antioxidants in serum and lens
Glutathione peroxidase (GPx)
The activity of the GPx was assayed by using the method of Tappel, (1978). Briefly, 0.2 ml of the test sample was reacted with 0.2 ml of 0.4 M tris buffer, 0.1 ml of 10 mM sodium azide, 0.1 ml of 0.2 mM hydrogen peroxide, and 0.2 ml of glutathione. The reaction mixture was incubated at 37 °C for 10 min, and then reaction was arrested by the addition of 0.4 ml of 10% trichloroacetic acid (TCA). The absorbance was read at 340 nm.19
Catalase (CAT)
The CAT activity was monitored at 240 nm for 30 s at 25 °C by using the method of Aebi et al (1984). One unit of CAT is defined as the amount of enzyme required to decompose 1.0 M of hydrogen peroxide into water per minute at pH 7.0 and 25 °C.20
Superoxide dismutase (SOD)
The reaction mixture contained 1 ml of homogenate (10% w/v in 0.25 M sucrose buffer), 1.2 ml of sodium pyrophosphate buffer (0.052 M, pH 8.3), 0.1 ml of 186 μM phenazonium methosulphate, 0.3 ml of 300 μM nitro blue tetrazolium, and 0.2 ml of 780 μM NADH. The reaction mixture was diluted up to 3 ml by distilled water and incubated at 30 °C for 60 s. The reaction was arrested by addition of 1.0 ml glacial acetic acid and stirred vigorously. 4.0 ml of n-butanol was added to the mixture, shaken well, allowed to stand for 10 min, and centrifuged at 2500 rpm for 5 min. Butanol layer was separated out, and absorbance was measured at 560 nm against a butanol blank. Xanthine oxidase enzyme served as the control.21
Glutathione (GSH)
The lens and serum glutathione level were measured using Ellman's reagent (Ellman, 1959). Briefly, the homogenized lens (10% w/v in cold 20 mM EDTA) and serum were deproteinized with 0.5 ml of 10% TCA and centrifuged. The protein free supernatant (0.2 ml) was treated with 4 ml of 0.3 M Na2HPO4 (pH 8.0) and 0.5 ml of 0.04% (w/v) 5, 5’-dithiobis (2-nitrobenzoic acid) (Ellman's reagents). The absorbance was measured at 412 nm. The pure glutathione was used as a standard for establishing the calibration curve.22
Malondialdehyde (MDA) in serum and lens
MDA, an end product of lipid peroxidation, was estimated in the lens homogenate and serum by using the method of Ohkawa (1979). Briefly, 0.2 ml of test sample was reacted with 0.2 ml of 8.1% sodium dodecyl sulphate, 1.5 ml of 20% acetic acid (pH 3.5), and 1.5 ml of 0.81% thiobarbituric acid in succession. The mixture was heated in boiling water for 60 min. After cooling at room temperature, 5 ml of butanol: pyridine (15:1 v/v) solution was added, and the mixture was centrifuged at 5000 rpm for 15 min. The upper organic layer was separated out, and intensity of the resultant pink color was read at 532 nm. Tetra methoxy propane was used as a standard.23
Total protein content in lens
The total protein content was measured by using the method of Lowry et al (1951). Briefly, 0.1 ml of lens homogenate was treated with 4.0 ml of alkaline copper solution and allowed to stand for 10 min, and then 0.4 ml of phenol reagent was added very rapidly, mixed quickly, and incubated at room temperature for 30 min for color development. The absorbance of the resultant color product was measured at 610 nm against blank. Bovine serum albumin was used as a standard for establishing the calibration curve.24
Ca2+ ATPase activity in lens
The Ca2+ ATPase activity was determined by using the method of Rorive and Kleinzeller (1974). Briefly, 0.1 ml of the lens homogenate (10% w/v in 0.25 M sucrose) was treated with 0.2 ml of ATP (40 mM in 0.4 M tris–HCl buffer, pH 7) and incubated at 37 °C in a water bath for 30 min. The enzyme activity was stopped by adding 2 ml of 10% TCA, and 0.2 ml of ATP was added and kept in ice for 20 min. The reaction mixture was centrifuged at 2500 rpm for 10 min, and the supernatant was collected. 3 ml of supernatant was treated with 1 ml of 2.5% ammonium molybdate and 0.4 ml of amino naphthol sulfonic acid. The absorbance of the resultant color product was measured at 680 nm after 20 min.25
Ionic contents (Na+ and Ca2+)
Ionic contents of the serum and lens were estimated by spectrophotometric method using diagnostic kits (Labcare Diagnostics Pvt. Ltd., India).
Statistical analysis
The results were expressed as mean ± standard error of mean (SEM). The significant differences between multiple groups were statistically analyzed by using one-way and two-way analysis of variance (ANOVA). The data were considered statistically significant at p < 0.05. Statistical analysis was performed using Graph Pad Prism 5.0 software (GraphPad software, Inc., USA).
Results
Effects on blood pressure
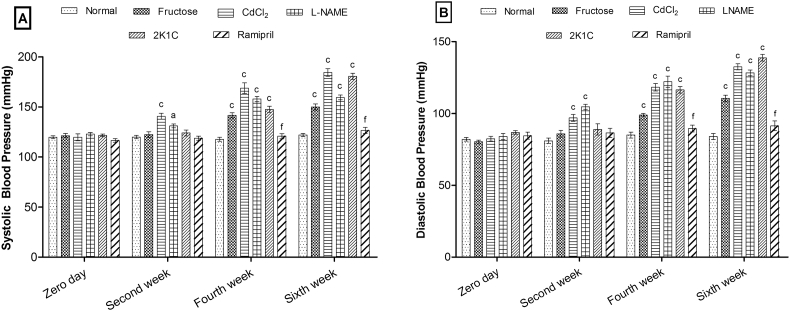
During six weeks of experimental protocol, blood pressure (SBP and DBP) was found to increase significantly in a time-dependent manner in each group (except the ramipril-treated group) as compared to the normal group. CdCl2 and l-NAME models showed a significant increase in SBP (p < 0.001, p < 0.05) and DBP (p < 0.001, p < 0.001) from the second week, respectively, whereas the fructose and 2K1C models showed significant elevation in SBP (p < 0.001) and DBP (p < 0.001) from the fourth week. The ramipril-treated group showed significant (p < 0.001) reduction in SBP and DBP as compared to 2K1C (Fig. 1).
Fig. 1.
Effects of hypertensive animal models on (A) systolic blood pressure and (B) diastolic blood pressure. Values are expressed as mean ± standard error of mean (n = 6), ap < 0.05, bp < 0.01, cp < 0.001 when compared to normal and dp < 0.05, ep < 0.01, fp < 0.001 when compared to 2K1C (two-way ANOVA followed by Bonferroni's post hoc test).
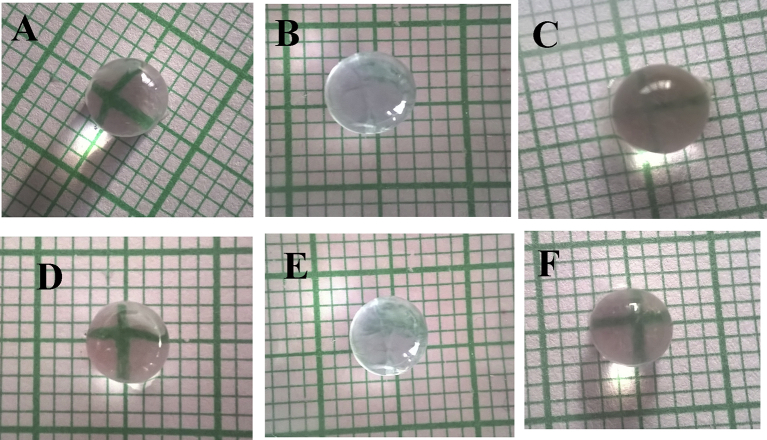
Effects on lens opacity
The eye lenses of hypertensive animals (Fig. 2 B to E) were more opaque than the normal control (Fig. 2 A). The eye lenses of fructose, CdCl2, and 2K1C hypertensive animal models were more opaque than the lenses of the l-NAME animal model. The administration of ramipril to 2K1C hypertensive animals showed substantial reduction in lenticular opacity as compared to 2K1C (Fig. 2 F).
Fig. 2.
Effects on lenticular opacity, (A) Normal, (B) Fructose, (C) CdCl2, (D) l-NAME, (E) 2K1C, and (F) Ramipril.
Effects on biochemical parameters in serum
The significant augmentation in systemic oxidative stress was observed in all hypertensive animal models (Table 1). Fructose, CdCl2, and 2K1C models showed significant (p < 0.001) depletion of serum enzymatic antioxidants (GPx, CAT, and SOD) in serum while l-NAME showed depletion in CAT (p < 0.01) and SOD (p < 0.01) only when compared to normal.
Table 1.
Effects on GPx, CAT, SOD, GSH, and MDA level in serum.
| Group | GPx (μM/min/mg of Hb) | CAT (μM of H2O2 consumed/min/mg of Hb) | SOD (μM/min/mg of Hb) | GSH (μM/ml) | MDA (μM/ml) |
|---|---|---|---|---|---|
| Normal | 5.07 ± 0.33 | 0.53 ± 0.03 | 2.11 ± 0.06 | 2.56 ± 0.14 | 2.63 ± 0.12 |
| Fructose | 2.40 ± 0.19c | 0.34 ± 0.02c | 1.05 ± 0.07c | 1.65 ± 0.14b | 5.04 ± 0.38c |
| CdCl2 | 3.47 ± 0.27c | 0.38 ± 0.02c | 1.35 ± 0.14c | 1.73 ± 0.11b | 3.81 ± 0.24a |
| l-NAME | 4.75 ± 0.21 | 0.41 ± 0.01b | 1.59 ± 0.11b | 2.33 ± 0.16 | 3.43 ± 0.26a |
| 2K1C | 3.48 ± 0.20c | 0.36 ± 0.03c | 1.24 ± 0.13c | 1.17 ± 0.07c | 4.21 ± 0.35b |
| Ramipril | 4.76 ± 0.13e | 0.48 ± 0.02e | 1.89 ± 0.07f | 2.29 ± 0.13f | 2.77 ± 0.17e |
Values are expressed as mean ± standard error of mean (n = 6), ap < 0.05, bp < 0.01, cp < 0.001 when compared to normal and dp < 0.05, ep < 0.01, fp < 0.001 when compared to 2K1C (one-way ANOVA followed by Newman–Keuls post hoc test). CAT: catalase; GPx: glutathione peroxidase; GSH: reduced glutathione; l-NAME: Nω-nitro-l-arginine methyl ester; MDA: Malondialdehyde; SOD: superoxide dismutase; 2K1C: two-kidney, one clip.
The level of non-enzymatic antioxidant (GSH) and an end product of lipid peroxidation, MDA were significantly reduced in fructose (p < 0.01, p < 0.001), CdCl2 (p < 0.01, p < 0.05), l-NAME (p < 0.05, p < 0.05), and 2K1C (p < 0.001, p < 0.01) models, respectively, when compared to normal (Table 1). The ramipril-treated group showed significant elevation in serum GPx (p < 0.01), CAT (p < 0.01), SOD (p < 0.001), and GSH (p < 0.001) level and reduction in serum MDA (p < 0.01) level as compared to the 2K1C group.
Effects on biochemical parameters in eye lenses
The hypertensive animal models showed marked oxidative stress via attenuation in enzymatic and non-enzymatic antioxidants in rat eye lenses (Table 2). The fructose, CdCl2, and 2K1C models showed significant (p < 0.001) reduction in GPx, CAT, SOD, and GSH levels, while l-NAME model showed significant reduction in GPx (p < 0.05), CAT (p < 0.001), and SOD (p < 0.05) only when compared to normal.
Table 2.
Effects on GPx, CAT, SOD, GSH, and MDA level on rat eye lenses.
| Group | GPx (μM/min/mg protein) | CAT (μM of H2O2 consumed/min/mg protein) | SOD (μM/min/mg protein) | GSH (μM/mg protein) | MDA (μM/mg protein) |
|---|---|---|---|---|---|
| Normal | 3.69 ± 0.24 | 0.46 ± 0.02 | 2.74 ± 0.05 | 2.68 ± 0.17 | 0.35 ± 0.023 |
| Fructose | 1.69 ± 0.08c | 0.26 ± 0.01c | 0.82 ± 0.02c | 1.54 ± 0.09c | 0.61 ± 0.020c |
| CdCl2 | 1.14 ± 0.07c | 0.30 ± 0.01c | 1.89 ± 0.04c | 1.14 ± 0.21c | 0.45 ± 0.02a |
| l-NAME | 3.07 ± 0.16a | 0.25 ± 0.03c | 2.52 ± 0.05a | 2.28 ± 0.22 | 0.47 ± 0.01b |
| 2K1C | 1.69 ± 0.04c | 0.18 ± 0.02c | 1.73 ± 0.05c | 0.87 ± 0.04c | 0.64 ± 0.02c |
| Ramipril | 3.42 ± 0.20f | 0.40 ± 0.01f | 2.55 ± 0.05af | 2.35 ± 0.20f | 0.38 ± 0.01f |
Values are expressed as mean ± standard error of mean (n = 6), ap < 0.05, bp < 0.01, cp < 0.001 when compared to normal and dp < 0.05, ep < 0.01, fp < 0.001 when compared to 2K1C (one-way ANOVA followed by Newman–Keuls post hoc test). CAT: catalase; GPx: glutathione peroxidase; GSH: reduced glutathione; l-NAME: Nω-nitro-l-arginine methyl ester; MDA: Malondialdehyde; SOD: superoxide dismutase; 2K1C: two-kidney, one clip.
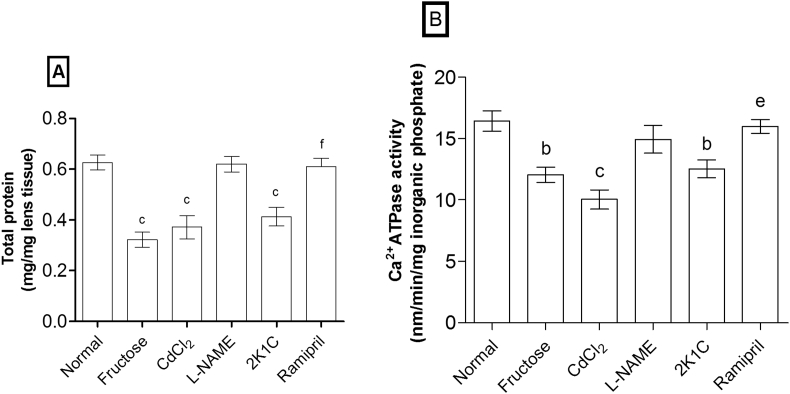
MDA level (Table 2) was significantly increased in fructose (p < 0.001), CdCl2 (p < 0.05), 2K1C (p < 0.001), and l-NAME (p < 0.01) models. The total protein content and Ca2+ ATPase activity in eye lenses were significantly reduced in fructose (p < 0.001, p < 0.01), CdCl2 (p < 0.001, p < 0.001), and 2K1C (p < 0.001, p < 0.01) models, respectively, whereas l-NAME showed non-significant reduction as compared to normal (Fig. 3).
Fig. 3.
Effects on (A) total protein content and (B) Ca2+ ATPase activity in rat eye lenses. Values are expressed as mean ± standard error of mean (n = 6), ap < 0.05, bp < 0.01, cp < 0.001 when compared to normal and dp < 0.05, ep < 0.01, fp < 0.001 when compared to 2K1C (one-way ANOVA followed by Newman–Keuls post hoc test).
The significant (p < 0.001) elevation of serum antioxidants (GPx, CAT, SOD, and GSH) and depletion of MDA level were observed in ramipril-treated rats. Moreover, total protein content (p < 0.001) and Ca2+ ATPase activity (p < 0.01) in eye lenses were significantly increased by ramipril in 2K1C hypertensive animal model.
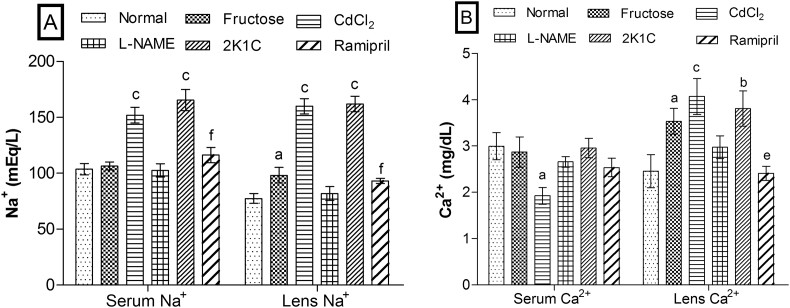
Effects on ionic (Na+ and Ca2+) contents
Ionic contents in serum and lens were altered markedly in CdCl2 and 2K1C models than fructose and l-NAME models (Fig. 4). Serum Na+ level was significantly (p < 0.01) increased in CdCl2 and 2K1C models whereas its level was unaffected in fructose and l-NAME models. Lens Na+ was also significantly increased by fructose (p < 0.05), CdCl2 (p < 0.001), and 2K1C (p < 0.001) while l-NAME failed to show the significant effect as compared to normal. Serum Ca2+ was significantly (p < 0.05) reduced in CdCl2 model only whereas lens Ca2+ was significantly increased in fructose (p < 0.05), CdCl2 (p < 0.001), and 2K1C (p < 0.01) models when compared to normal. The significant restoration of Na+ (p < 0.001) in serum and lens, and Ca2+ (p < 0.01) in the lens was observed in the ramipril-treated group.
Fig. 4.
Effects on (A) Na+ and (B) Ca2+ in serum and eye lenses. Values are expressed as mean ± standard error of mean (n = 6), ap < 0.05, bp < 0.01, cp < 0.001 when compared to normal and dp < 0.05, ep < 0.01, fp < 0.001 when compared to 2K1C (one-way ANOVA followed by Newman–Keuls post hoc test).
Discussion
In the present study the fructose, CdCl2, l-NAME, and 2K1C hypertensive animal models showed significant increase in hypertension in terms of elevation of SBP and DBP during six weeks of the experimental protocol. The mode of induction of hypertension was independent in each animal model.
Numerous studies reported that the reactive oxygen species (ROS) level is increased in the chronic hypertensive state that is indicated by depletion of serum antioxidants and elevation of MDA level. The increased blood pressure markedly produces the ROS through vascular stimulation by mechanical stretch and activation of the renin-angiotensin system and further leads to oxidative stress.26, 27, 28 In our study, we found that chronic hypertension in different animal models significantly augmented the oxidative stress as indicated by depletion of antioxidants (SOD, GPx, CAT, and GSH) and elevation of MDA level in serum. The reduced serum antioxidants via hypertension may further lead to cellular damage of several organs, including the eye lenses via modulation of antioxidant defense mechanism that is confirmed by depleting antioxidants and elevating MDA level in the eye lenses.29, 30
The increased oxidative stress and ionic imbalance in lenses are key mechanisms for the development of cataract; it can modulate the protein content and lenticular opacity.31, 32, 33 The increased ROS in the lens can cause the molecular damage of lens protein and phospholipids leading to lipid peroxidation and depletion of the antioxidant enzymes such as SOD, GPx, and CAT, resulting in elevation of lenticular opacity and cataract formation.34, 35 SOD is a chain-breaking antioxidant. It converts superoxide to H2O2 and scavenges the superoxide anion to form hydrogen peroxide,36 GPx is responsible for degrading the level of H2O2,37 and CAT helps to keep the level of free radicals below toxic levels.38 Thus, enhanced oxidative stress in lens induced by hypertension might be responsible for the risk of cataract formation. Moreover, ramipril, an angiotensin-converting enzyme (ACE) inhibitor administered to the 2K1C-induced hypertensive animals, significantly restored the serum and lens antioxidants and MDA level concomitant with reduction of blood pressure, suggesting that high blood pressure is potentially associated with increased ROS formation in serum and lens and further leads to cataractogenic effects on eye lenses.
The elevated MDA level and reduced GSH level in hypertensive rat lenses also implicated the progression of cataractogenesis in rats. The reduced level of GSH is anticipated to cause the formation of disulphide bonds through sulfhydryl oxidation of lens crystalline and to cause the cross-linkage, which leads to cataract formation39 whereas increased level of MDA, a product of membrane lipid peroxidation, causes the development of lenticular opacity due to cross-links between membrane lipids and proteins.40
The transparency of the lens is also dependent on protein content and intracellular ions, especially Ca2+ and Na+. Accumulation of these ions leads to the aggregation of lens proteins and further reduces the total and soluble proteins, and increases the insoluble proteins, and causes lenticular opacity. The activation of calpain via increased Ca2+ in lens leads to enhanced proteolytic activity in lens epithelial cell that results in digestion of cytoskeletal and junctional proteins and initiates lenticular opacity.32, 33 As a limitation in our study, we estimated the lens total protein content only instead of soluble/insoluble proteins, which are more relevant to the cataractogenesis. In our studies, we found that lens Ca2+ and Na+ were noticeably enhanced, and total protein was markedly reduced in fructose, CdCl2, and 2K1C animal models than the l-NAME animal model. The accumulation of Ca2+ is caused by the reduction of Ca2+ ATPase activity,41 and accumulation of Na+ ion is related to the impairment of Na+K+ ATPase activity.33 The restoration of elevated Ca2+ and Na+ in the eye lenses of the 2K1C hypertensive animals were observed concomitant with the elevation of total protein content in the lens by administration of ramipril, suggesting that reduction of lens protein content might be due to the enhanced level of Ca2+ and Na+ in eye lenses.
The possible mode of cataractogenesis through hypertension might be different for each animal model. Fructose animal model causes marked oxidative stress and ionic imbalance in eye lenses. Additionally, fructose is an end product of polyol pathway of glucose metabolism in the lens and further leads to the formation of ROS and advanced glycation end products (AGE)42, 43 that may exacerbate the cataractogenic effects of hypertension.
CdCl2-induced cataractogenic effects through hypertension might be related to their over intracellular Ca2+ ion-dependent cataractogenic actions that lead to oxidative damage, deregulating ATPase pumps in the eye lenses. In addition, researchers also reported that cadmium (Cd2+) induces the lens epithelial cell death due to enhanced oxidative stress and lipid peroxidation.44 Thus, the observation of cataractogenesis in CdCl2-treated rats may be due to the primary toxicity of the (Cd2+) that may produce oxidative damage to the vascular walls and eye lenses, and further leads to hypertension and cataractogenesis.
The cataractogenic effects of 2K1C hypertensive animal model might be related to the hyperactivity of the ocular renin-angiotensin system. It is established that 2K1C trigger the angiotensin II-mediated actions. The literature revealed that serum angiotensin-II cannot enter the eye,45 but it can modulate the action of ocular renin-angiotensin system46 and trigger the angiotensin II-mediated angiogenic and inflammatory action in the eye through production of vascular endothelial growth factor,47 mitogen-activated protein kinases,48 and AGE,49 and further lead to the development of several blinding ocular disorders like diabetic retinopathy, glaucoma, and macular degenerations.50 In the present study, inhibition of ACE via ramipril showed protective effects against 2K1C-induced hypertension associated cataractogenic action. Moreover, several researchers also found that ACE inhibitors showed beneficial effects in the reduction of cataract through restoration of ionic balance (Na+/K+), free radical scavenging activity, and enhancement of enzymatic and non-enzymatic defense mechanism as well as inhibition of AGE production.51, 52 Therefore, cataractogenic effects in 2K1C hypertensive animal model might be related to the action of ocular renin-angiotensin system that enhances oxidative stress and ionic imbalance in eye lenses and further leads to the development of cataract.
In the present study, l-NAME significantly elevated the blood pressure similar to other animal models but showed minor reduction of serum and lens antioxidants as compared to other animal models. It showed non-significant effects on total protein content and ions level, indicating that l-NAME independently affects the lenticular alterations. It is established that l-NAME produces depletion of the serum NO level and enhances blood pressure14 whereas increased nitrite (metabolite of NO) levels in lens causes the cataractogenesis.53 In addition, Ito et al reported that NO synthase inhibitors (l-NAME) prevented the development of cataract in selenite-treated rats.54 Therefore, it has mild cataractogenic effects and might be related to oxidative stress through hypertension rather than modulation of NO levels via l-NAME.
The risk of cataractogenesis through hypertension is dependent on its pathogenesis. Uncontrolled hypertension may modulate the oxidative stress and electrolyte homeostasis in the eye lenses and further enhance the risk of cataract formation. Fructose might exacerbate the oxidative damage, and CdCl2-induced hypertension might up-regulate Ca2+ activity and oxidative stress. The 2K1C activates the ocular renin-angiotensin system in the eye lenses via uncontrolled hypertension and further leads to cataractogenesis whereas l-NAME independently affects the lenticular alterations unrelated to hypertension. In conclusion, systemic hypertension seems to be a major risk factor that indirectly affects the lens integrity associated with cataract formation.
Footnotes
Funding information: None.
Declaration: The author(s) declare that the present manuscript has not been published, accepted or under editorial review for publication elsewhere.
Conflicts of interest: The authors have no conflict of interest.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Sabanayagam C., Wang J.J., Mitchell P. Metabolic syndrome components and age-related cataract: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2011;52:2397–2404. doi: 10.1167/iovs.10-6373. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S., Pascolini D., Etya’ale D. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 3.Andjelic S., Hawlina M. Cataractogenesis. Zdrav Vestn. 2012;81:I122–I132. [Google Scholar]
- 4.Chopra R., Chander A., Jacob J.J. Ocular associations of metabolic syndrome. Indian J Endocrinol Metabol. 2012;16:S6–S11. doi: 10.4103/2230-8210.94244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X., Lyu D., Dong X., He J., Yao K. Hypertensionion and risk of cataract: a meta-analysis. PLoS One. 2014;9:e114012. doi: 10.1371/journal.pone.0114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekibele C.O., Ashaye A.O., Ajayi B.G.K. Risk factors for visually disabling age related cataracts in Ibadan. Ann Afr Med. 2003;2:27–32. [Google Scholar]
- 7.Rodriguez-Sargent C., Cangiano J.L., Caban G.B., Marrero E., Martinez-Maldonado M. Cataracts and hypertension in salt-sensitive rats. a possible ion transport defect. Hypertension. 1987;9:304–308. doi: 10.1161/01.hyp.9.3.304. [DOI] [PubMed] [Google Scholar]
- 8.Linda T.T., Violet G.Y., John H.M. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009;332:145–159. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 9.Rathod S.P., Shah N., Balaraman R. Antihypertensive effect of dietary calcium and diltiazem, a calcium channel blocker on experimentally induced hypertensive rats. Indian J Pharmacol. 1997;29:99–104. [Google Scholar]
- 10.Sangartit W., Kukongviriyapan U., Donpunha W. Tetrahydrocurcumin protects against cadmium-induced hypertension, raised arterial stiffness and vascular remodeling in mice. PLoS One. 2014;9:e114908. doi: 10.1371/journal.pone.0114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang D.G., Yun Y.G., Ryoo J.H., Lee H.S. Anti-hypertensive effect of water extract of danshen on renovascular hypertension through inhibition of the renin angiotensin system. Am J Chin Med. 2001;30:87–93. doi: 10.1142/S0192415X02000107. [DOI] [PubMed] [Google Scholar]
- 12.Buxton I.L.O., Cheek D.J., Eckman D., Westfall D.P., Sanders K.M., Keef K.D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- 13.Asdaq S.M., Inamdar M.N. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine. 2010;17:1016–1026. doi: 10.1016/j.phymed.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Suo M., Kalliovalkama J., Porsti I. N(G)-nitro-L-arginine methyl ester-induced hypertension and natriuretic peptide gene expression: inhibition by angiotensin II type 1 receptor antagonism. J Cardiovasc Pharmacol. 2002;40:478–486. doi: 10.1097/00005344-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Sharifi A.M., Akbarloo N., Heshmatian B., Ziai A. Alteration of local ACE activity and vascular responsiveness during development of 2K1C renovascular hypertension. Pharmacol Res. 2003;47:201–209. doi: 10.1016/s1043-6618(02)00319-5. [DOI] [PubMed] [Google Scholar]
- 16.Uduman M.S.T.S., Reddy R.B., Punuru P., Chakka G., Karunakaran G. Protective role of ramipril and candesartan against myocardial ischemic reperfusion injury: a biochemical and transmission electron microscopical study. Adv Pharmacol Sci. 2016;2016:4608979. doi: 10.1155/2016/4608979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son H., Kim H., Kwon Y.H. Taurine prevent oxidative damage of high glucose-induced cataractogenesis in isolated rat lensens. J Ntri Sci Vitaminol. 2007;53:324–330. doi: 10.3177/jnsv.53.324. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson J., Marcantonio J.M., Duncan G. A human lens model of cortical cataract: Ca2+-induced protein loss, vimentin cleavage and opacification. Invest Ophthalmol Vis Sci. 2000;41:2255–2261. [PubMed] [Google Scholar]
- 19.Tappel A.L. Glutathione peroxidase and hydroperoxides. Meth Enzymol. 1978;52:506–513. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- 20.Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophy. 1984;21:130–132. [PubMed] [Google Scholar]
- 22.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H., Ohishi N., Yagi K. Assay of lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Lowry C.H., Rosebrough N.J., Farr L., Randall R.J. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Rorive G., Kleinzeller A. Ca2+-activated ATPase from renal tubular cells. Methods Enzymol. 1974;32:303–306. doi: 10.1016/0076-6879(74)32031-9. [DOI] [PubMed] [Google Scholar]
- 26.Hirata Y., Satonaka H. Hypertension and oxidative stress. Jpn Med Assoc J. 2001;44:540–545. [Google Scholar]
- 27.Khanna H.D., Sinha M.K., Khanna S., Tandon R. Oxidative stress in hypertension: association with antihypertensive treatment. Indian J Physiol Pharmacol. 2008;52:283–287. [PubMed] [Google Scholar]
- 28.Vaziri N.D., Wang X.Q., Oveisi F., Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 29.Chang D., Zhang X., Rong S. Serum antioxidative enzymes levels and oxidative stress products in age-related cataract patients. Oxid Med Cell Longev. 2013;2013:587826. doi: 10.1155/2013/587826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oduntan O.A., Mashige K.P. A review of the role of oxidative stress in the pathogenesis of eye diseases. S Afr Optom. 2011;70:191–199. [Google Scholar]
- 31.Bodakhe S.H., Ram A., Verma S., Pandey D.P. Anticataract activity of rhamnocitrin isolated from Bauhinia variegata stem bark. Orient Pharm Exp Med. 2012;12:227–232. [Google Scholar]
- 32.Gupta P.D., Johar K., Vasavada A. Causative and preventive action of calcium in cataractogenesis. Acta Pharmacol Sin. 2004;25:1250–1256. [PubMed] [Google Scholar]
- 33.Mirsamadi M., Nourmohammadi I., Imamian M. Comparative study of serum Na+ and K+ levels in senile cataract patients and normal individuals. Int J Med Sci. 2004;1:165–169. doi: 10.7150/ijms.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsili S., Salganik R.I., Albright C.D. Cataract formation in a strain of rats selected for high oxidative stress. Exp Eye Res. 2004;79(5):595–612. doi: 10.1016/j.exer.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Sulochana K.N., Punitham R., Ramakrishna S. Effect of cigarette smoking on cataract: antioxidant enzymes. Indian J Pharmacol. 2002;34:428–431. [Google Scholar]
- 36.Varma S., Ets T., Richards R.D. Protection against superoxide radicals in rat lens. Ophthalmic Res. 1977;9(6):421–431. [Google Scholar]
- 37.Umamaheswari M., Asokkumar K., Sivashanmugam A.T., Subhadradevi V., Neethu M. Anti-cataract activity of Erythrina stricta against naphthalene induced cataractogenesis in rats. Bangladesh J Pharmacol. 2010;5(2):77–81. [Google Scholar]
- 38.Ozmen D., Ozmen B., Erkin E., Guner I., Habil S., Bayindir O. Lens superoxide dismutase and catalase activities in diabetic cataract. Clin Biochem. 2000;35:69–72. doi: 10.1016/s0009-9120(01)00284-3. [DOI] [PubMed] [Google Scholar]
- 39.Mahfouz M.H., Ghaffar A.A.E., Mohamed M.A., Ghanem H.M. Effects of L-carnitine and cinnamon extract treatment on lens crystallins of rats fed high fructose diet. Am J Biochem Biotech. 2011;7(2):63–69. [Google Scholar]
- 40.Grattagliano I., Vendemiale G., Boscia F., Ferrari T.M., Cardia L. Oxidative retinal products and ocular damages in diabetic patients. Free Radic Biol Med. 1998;25(3):369–372. doi: 10.1016/s0891-5849(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 41.Sai Varsha M.K., Raman T., Manikandan R. Inhibition of diabetic-cataract by vitamin K1 involves modulation of hyperglycemia-induced alterations to lens calcium homeostasis. Exp Eye Res. 2014;128:73–82. doi: 10.1016/j.exer.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Mathebula S.D. Polyol pathway: a possible mechanism of diabetes complications in the eye. Afr Vis Eye Health. 2015;74:5. [Google Scholar]
- 43.Takagi Y., Kashiwagi A., Tanaka Y., Ashahin T., Kikkawa R., Shigeta Y. Significance of fructose-induced protein oxidation and formation of advanced glycation end product. J Diabetes Complications. 1995;9:87–91. doi: 10.1016/1056-8727(94)00022-g. [DOI] [PubMed] [Google Scholar]
- 44.Kalariya N.M., Nair B., Kalariya D.K., Wills N.K., van Kuijk F.J. Cadmium-induced induction of cell death in human lens epithelial cells: implications to smoking associated cataractogenesis. Toxicol Lett. 2010;198(1):56–62. doi: 10.1016/j.toxlet.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Danser A.H., Derkx F.H., Admiraal P.J., Deinum J., De Jong P.T., Schalekamp M.A. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci. 1994;35(3):1008–1018. [PubMed] [Google Scholar]
- 46.Milenkovic V.M., Brockmann M., Meyer C. Regulation of the renin expression in the retinal pigment epithelium by systemic stimuli. Am J Physiol Ren Physiol. 2010;299:F396–F403. doi: 10.1152/ajprenal.00576.2009. [DOI] [PubMed] [Google Scholar]
- 47.Sugiyama T., Okuno T., Fukuhara M. Angiotensin II receptor blocker inhibits abnormal accumulation of advanced glycation end products and retinal damage in a rat model of type 2 diabetes. Exp Eye Res. 2007;85(3):406–412. doi: 10.1016/j.exer.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Pons M., Cousins S.W., Alcazar O., Striker G.E., Marin-Castano M.E. Angiotensin II-induced MMP-2 activity and MMP-14 and basigin protein expression are mediated via angiotensin II receptor type 1- mitogen-activated protein kinase 1 pathway in retinal pigment epithelium. Am J Pathol. 2011;178(6):2665–2681. doi: 10.1016/j.ajpath.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller A.G., Tan G., Binger K.J. Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-i function. Diabetes. 2010;59:3208–3215. doi: 10.2337/db10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurihara T., Ozawa Y., Ishida S., Okano H., Tsubota K. Renin angiotensin system hyperactivation can induce inflammation and retinal neural dysfunction. Int J Inflam. 2012;2012:581695. doi: 10.1155/2012/581695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jablecka A., Czaplicka E., Olszewski J., Bogdanski P., Krauss H., Smolarek I. Influence of selected angiotensin-converting enzyme inhibitors on alloxan-induced diabetic cataract in rabbits. Med Sci Monit. 2009;15(8):BR334–BR338. [PubMed] [Google Scholar]
- 52.Langade D.G., Rao G., Girme R.C., Patki P.S., Bulakh P.M. In vitro prevention by ACE inhibitors of cataract induced by glucose. Indian J Pharmacol. 2006;38(2):107–110. [Google Scholar]
- 53.Ornek K., Karel F., Buyukbingol Z. May nitric oxide molecule have a role in the pathogenesis of human cataract? Exp Eye Res. 2003;76:23–27. doi: 10.1016/s0014-4835(02)00268-3. [DOI] [PubMed] [Google Scholar]
- 54.Ito Y., Nabekura T., Takeda M. Nitric oxide participates in cataract developement in selenite-treated rats. Curr Eye Res. 2001;22(3):215–220. doi: 10.1076/ceyr.22.3.215.5516. [DOI] [PubMed] [Google Scholar]