Abstract
The incessant activity of swimming microorganisms has a direct physical effect on surrounding microscopic objects, leading to enhanced diffusion far beyond the level of Brownian motion with possible influences on the spatial distribution of non-motile planktonic species and particulate drifters. Here we study in detail the effect of eukaryotic flagellates, represented by the green microalga Chlamydomonas reinhardtii, on microparticles. Macro- and microscopic experiments reveal that microorganism-colloid interactions are dominated by rare close encounters leading to large displacements through direct entrainment. Simulations and theoretical modelling show that the ensuing particle dynamics can be understood in terms of a simple jump-diffusion process, combining standard diffusion with Poisson-distributed jumps. This heterogeneous dynamics is likely to depend on generic features of the near-field of swimming microorganisms with front-mounted flagella.
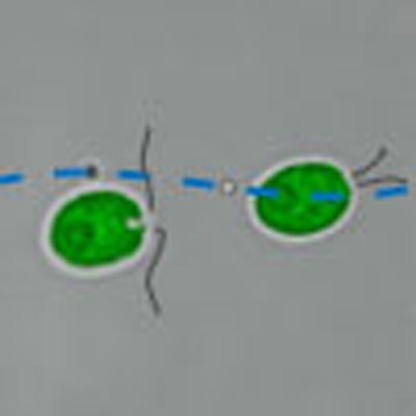 Passive particles surrounded by swimming protists diffuse tens of times faster than their thermal motion, which might have an impact on microscopic predator-prey interaction in nature. Here, Jeanneret et al. investigate its physical origin and identify direct particle entrainment as the dominant feature.
Passive particles surrounded by swimming protists diffuse tens of times faster than their thermal motion, which might have an impact on microscopic predator-prey interaction in nature. Here, Jeanneret et al. investigate its physical origin and identify direct particle entrainment as the dominant feature.
Swimming microorganisms navigate commonly through fluids characterized by a variety of suspended microparticles, with which they inevitably interact. From malarial parasites meandering around densely packed red blood cells and infecting them1, to protists which regulate primary production in oceans and lakes by grazing on sparsely distributed microalgae and bacteria2,3, these interactions can have important biological and ecological implications. Some, like biogenic mixing, are still intensely debated4,5,6. Understanding the basic mechanisms of such interactions is timely, as a vast number of plastic micro- and nanoparticles of anthropogenic origin scattered throughout the world's oceans (up to ∼105 m−3) appear to be easily ingested by microorganisms, which then recycle plastics within the marine food web with currently unknown consequences7. From a physics perspective, these systems are particularly appealing. Abstracted as binary suspensions8,9, where an active, self-propelled species interacts with a passive, thermalized one, they represent a naturally occurring category of out-of-equilibrium stochastic systems driven by energy produced directly within the bulk, rather than transmitted through the system's boundaries. Easily accessible experimentally, and amenable to detailed quantitative modelling10, they are uniquely placed to provide benchmark tests for theories of out-of-equilibrium statistical mechanics11,12,13. Currently best characterized are the so-called bacterial baths. Wu and Libchaber14, and more recently15,16,17,18, showed that colloidal particles within bacterial suspensions perform a persistent random walk leading in bulk to a diffusivity up to ∼10 × the thermal value18. This increase is proportional, at least in the dilute limit, to the product of bacterial speed and concentration, a consequence of random successive single-particle interactions5,19,20,21,22,23. The intrinsic non-equilibrium nature of bacterial baths is also known to lead to other peculiar effects, including motility-induced colloidal pair interactions10 and coupling between enhanced translational and rotational diffusion24.
Microparticles' interactions with the other major class of microorganisms, eukaryotic flagellates, is distinctly less explored. Almost exclusively larger than bacteria (∼10–100 μm), these species probe a new and biologically relevant physical regime, where the microparticles' size is significantly smaller than that of the microorganisms (but see also ref. 25 for the effect of bacterial motion on molecular diffusion). Working with the microalga Chlamydomonas reinhardtii (CR), often studied as model eukaryotic microswimmer, the pioneering study of Leptos et al.26—followed by ref. 27—reported an increase in particle diffusivity of magnitude similar to the bacterial case, as a consequence of loop-like particle trajectories induced by the far-field flow of the algae21,22,28,29. Particle displacements followed a diffusively scaling fat-tailed distribution which, if valid for arbitrary large observation times, would imply a violation of the Central Limit Theorem30. These fat-tailed distributions have indeed been observed in short simulations21,31, but should converge to normal distributions at long times29,31. This prediction, however, has not been tested experimentally.
Here we revisit the behaviour of colloids within a suspension of CR, taken as representative of eukaryotic flagellates, and reveal that their overall dynamics is in fact dominated by rare but dramatic entrainment events. These jumps underpin the surprisingly large diffusivity we observe directly in both sedimentation and collective spreading experiments, ≳40 × greater than values reported previously for the same geometry. The colloids' behaviour, alternating entrainments and periods of standard enhanced diffusion, is fundamentally different from the persistent random walk common with bacterial suspensions. Through microscopic experiments, simulations and analytical modelling we show instead that this dynamics is well captured by a simple jump-diffusion model.
Results
Experiments
A full description of the experimental procedures can be found in the Methods and Supplementary Methods sections. Briefly, wild-type CR strain CC125 was grown axenically in Tris-acetate-phosphate medium32 at 21 °C under continuous fluorescent illumination. Cells were harvested in the exponential phase (∼5 × 106cell ml−1), concentrated by gentle spinning and then resuspended to reach the desired concentration. Polystyrene microparticles (1 μm diameter) were then added to the suspension, and their diffusivity at different CR concentrations (Nc) was measured from three different sets of microfluidic experiments (Supplementary Methods 1): mapping the particles' sedimentation profile at steady state; measuring the relaxation dynamics of an inhomogeneous distribution of particles; and by long timescale direct tracking of individual colloids' dynamics in a thin Hele–Shaw cell. Schematic representations of the experimental setups are shown Supplementary Figs 1 and 2. Except for the sedimentation experiments, the medium was density-matched with the colloids using Percoll33. This increased its viscosity (ηpercoll=(1.5±0.1)ηwater) as reflected in the slower average swimming speed, 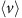 , of the algae. We measured
, of the algae. We measured 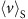 =81.8±3.5 μm s−1,
=81.8±3.5 μm s−1, 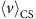 =40.9±3.5 μm s−1 and
=40.9±3.5 μm s−1 and 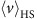 =49.1±2.5 μm s−1 for the sedimentation, spreading and tracking experiments respectively. A colloidal Péclet number for tracer/swimmer interactions can be defined as Pe=vL/D0, where v and L(∼10 μm) are CR's characteristic speed and body length, and D0 the Brownian diffusivity of the colloids. We obtain PeS≃2,000, PeCS≃1,500 and PeHS≃1,750, respectively for the three experiments.
=49.1±2.5 μm s−1 for the sedimentation, spreading and tracking experiments respectively. A colloidal Péclet number for tracer/swimmer interactions can be defined as Pe=vL/D0, where v and L(∼10 μm) are CR's characteristic speed and body length, and D0 the Brownian diffusivity of the colloids. We obtain PeS≃2,000, PeCS≃1,500 and PeHS≃1,750, respectively for the three experiments.
Macroscopic diffusion
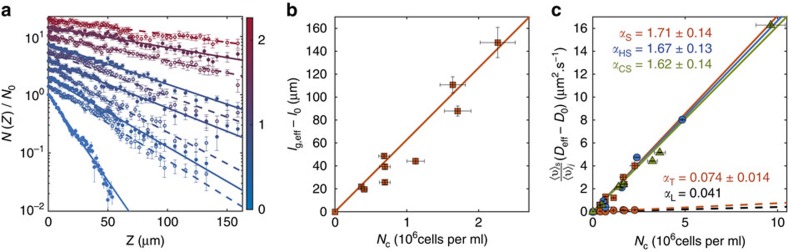
Macroscopic diffusion experiments coarse-grain over the colloids' microscopic dynamics and ensure the direct measurement of their effective transport properties, in the spirit of Jean Perrin's seminal work on sedimentation equilibrium34. We begin by characterizing the colloids' steady-state sedimentation profile at increasing CR concentrations, always within the dilute regime (volume fractions ≲0.15%). The average concentrations probed will always be well below the threshold required to induce bioconvective instabilities within our ∼200 μm-thick sample cells. At the same time the concentration profile is not exactly homogeneous, but slightly decreasing with height, see Supplementary Fig. 3 and Supplementary Note 1. However, as it will be evident from the results, this inhomogeneity did not appear to have appreciable consequences on our measurements. Figure 1a shows that even when the algae are present, the microparticles' distributions are still in excellent agreement with simple exponential profiles, but crucially with different effective gravitational lengths lg,eff (Fig. 1b). The exponential profiles are a standard consequence of the balance between the particles' sedimentation speed vsed, here due to a δρ=50 g l−1 density mismatch, and their effective diffusivity, combining passive and active processes. Boltzmann-like distributions are indeed what should be expected even in these out-of-equilibrium systems, at least for small enough vsed (ref. 35), akin to what has been observed for active colloids alone36. The characteristic length lg,eff, experimentally observed to be proportional to the concentration of algae, allows us to measure the concentration-dependent effective diffusivity as Deff=vsed
lg,eff (Fig. 1c, orange squares, Supplementary Fig. 4 for a close-up at low concentrations). We obtain Deff=D0+αSNc, where D0=0.40±0.01 μm2 s−1 is the thermal diffusivity, the algal concentration Nc is in units of 106cells ml−1, and αS=1.71±0.14(μm2 s−1)/(106cells ml−1) (slopes α will be expressed in these units throughout the paper). Within the same experiments, however, the diffusivity can also be inferred microscopically from direct short-duration tracking of microparticles' trajectories in bulk (mean tracks duration δttracks=2.6 s), as previously done in ref. 26. These measurements return a different estimate, Deff=D0+αTNc (Fig. 1c, orange circles), with a slope αT=0.074±0.014 in reasonable agreement with previous results (αL=0.041 in ref. 26) but more than 40-times smaller than the sedimentation value αS. The surprisingly large value of αS, larger than any previously reported bulk value, calls for an independent verification. It was tested here at the macroscopic level by following the diffusive spreading of a uniform band of density-matched colloids within a microfluidic device filled with a uniform concentration of cells (for experimental details see the Methods section, Supplementary Fig. 5 and Supplementary Methods 2). The band's profile, initially tight around the middle third of a 2-mm-wide, 60-μm-thick channel and running along its full length (∼10 mm), spreads with a characteristically diffusive dynamics which enables to measure directly the effective diffusivity Deff at different Nc values (Supplementary Fig. 5 and Supplementary Methods 2). The results are shown in Fig. 1c (green triangles) after being multiplied by the ratio 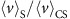 of the cells' swimming speeds to account for their slower motion in the density-matched medium. As before, Deff depends linearly on cell concentration, with a slope αCS=1.62±0.14 in remarkable agreement with the sedimentation value.
of the cells' swimming speeds to account for their slower motion in the density-matched medium. As before, Deff depends linearly on cell concentration, with a slope αCS=1.62±0.14 in remarkable agreement with the sedimentation value.
Figure 1. Effect of swimming algae on particle diffusivity.
(a) Semi-log plot of the normalized density profiles of 1 μm-PS colloids along the gravity direction for different cell concentrations. Full lines are best exponential fits to the data. Curves have been shifted apart along the y axis for clarity. Colour bar: CR concentration Nc in units of 106 cells ml−1. Error bars represent the s.d. (b) Gravitational length lg,eff−l0 as a function of Nc extracted from the fits in a. The solid orange line is the best linear fit to the data, lg,eff−l0=((62.8±5)Nc)μm (Nc in units of 106 cells ml−1). Vertical error bars represent the uncertainty on the fits in a. Horizontal error bars are the standard deviations of cell concentrations. (c) Effective microparticle diffusivity (Deff−D0) as a function of Nc for the three experiments. Diffusivities are rescaled by the ratio of the average CR speeds 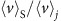 , where j stands for sedimentation (S), spreading (CS) or tracking (HS) experiments. Solid lines are best linear fits to the data. Orange squares: sedimentation experiment (slope αS=1.71±0.14(μm2 s−1)/(106 cells ml−1); all other values in the same units); green triangles: spreading experiment (αCS=1.62±0.14); blue circles: Hele–Shaw experiment (αHS=1.67±0.13). Orange circles and dashed line: direct tracking in the sedimentation experiment (αT=0.074±0.014). Black dashed line: fit to the experimental diffusivity obtained by direct tracking in ref. 26 (αL=0.041). See Supplementary Fig. 5 for a close-up on the low-concentration values. Vertical error bars represent the uncertainty on the fits to obtain the effective diffusivities. Horizontal error bars are the s.d. of the cell concentrations.
, where j stands for sedimentation (S), spreading (CS) or tracking (HS) experiments. Solid lines are best linear fits to the data. Orange squares: sedimentation experiment (slope αS=1.71±0.14(μm2 s−1)/(106 cells ml−1); all other values in the same units); green triangles: spreading experiment (αCS=1.62±0.14); blue circles: Hele–Shaw experiment (αHS=1.67±0.13). Orange circles and dashed line: direct tracking in the sedimentation experiment (αT=0.074±0.014). Black dashed line: fit to the experimental diffusivity obtained by direct tracking in ref. 26 (αL=0.041). See Supplementary Fig. 5 for a close-up on the low-concentration values. Vertical error bars represent the uncertainty on the fits to obtain the effective diffusivities. Horizontal error bars are the s.d. of the cell concentrations.
Microscopic entrainment and diffusion
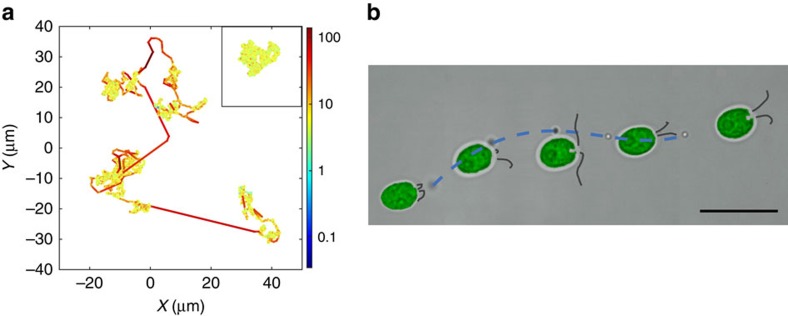
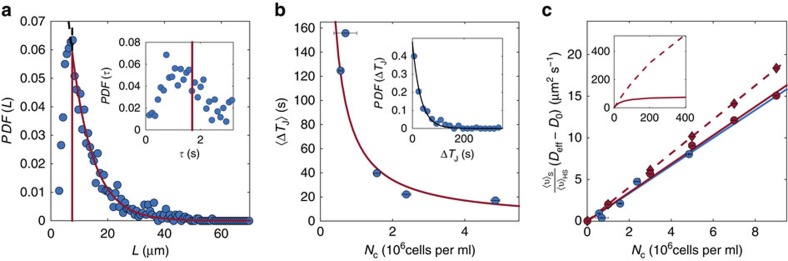
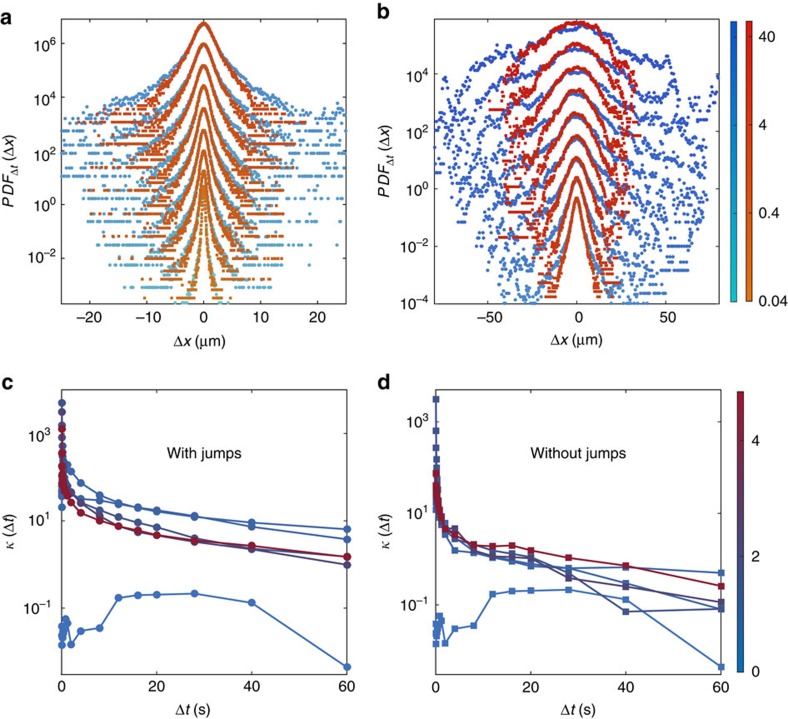
The quantitative agreement between steady-state and time-dependent macroscopic measurements suggests that our understanding of the microscopic interaction between particles and microorganisms, based on the effect of the swimmers' far-field flows29,37,38,39 and leading to αT(<<αS), is missing a key element. The crucial microscopic insight is provided by long-time tracking of the particles at a range of Nc values. This is achieved here by confining the system within a 26 μm-thick Hele–Shaw cell, which allows us to follow individual colloids for ∼200 s. Figure 2a and Supplementary Movie 1 show a typical colloidal trajectory in this confined geometry. The dynamics, which leads to a dramatically larger spreading than simple Brownian motion (Fig. 2a inset), results from the combination of three different effects of well-separated magnitudes. The weakest component is standard Brownian motion, which dominates the dynamics when the algae are more than ∼25 μm (ref. 26) away from the colloid. At closer separation, but before close contact, the far-field flows of the microorganisms induce loop-like trajectories21,22,31,39,40. Originally reported in ref. 26, these loops provide the concentration-dependent contribution to the particles' diffusivity previously estimated as αTNc (ref. 26). Finally, particles within the near field can be occasionally entrained by the algae over distances up to tens of microns (see Fig. 2b), giving rise to the sudden jumps in the trajectory highlighted as red solid lines in Fig. 2a. These jumps, a fundamental feature of the dynamics never observed previously, are the microscopic origin of the term αSNc, the unexpectedly large contribution to the particle diffusivity observed in the macroscopic experiments. This is confirmed directly on Supplementary Movie 2, where we show such an entrainment event taking place during a sedimentation experiment. For our 1 μm-diameter tracers, the jump length L is (mostly) exponentially distributed with a characteristic length LJ=7.5±0.5 μm, above a threshold value LT=7.5±0.5 μm (Fig. 3a) (see Supplementary Methods 3 and Supplementary Fig. 6 for details on the extraction of the jumps from the trajectories). The average displacement is 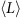 =12.6±0.5 μm, although we did record values of L up to ∼70 μm. The effect of jumps is also clear in the full probability distribution functions of tracer displacements, Fig. 4a,b, which display exponential-like tails much larger than those characteristic of loop-like perturbations only26. Both distributions eventually converge to Gaussians -with different variances- as the observation time increases, a consequence of the Central Limit Theorem already predicted in refs 29, 31, see Fig. 4 and Supplementary Note 2.
=12.6±0.5 μm, although we did record values of L up to ∼70 μm. The effect of jumps is also clear in the full probability distribution functions of tracer displacements, Fig. 4a,b, which display exponential-like tails much larger than those characteristic of loop-like perturbations only26. Both distributions eventually converge to Gaussians -with different variances- as the observation time increases, a consequence of the Central Limit Theorem already predicted in refs 29, 31, see Fig. 4 and Supplementary Note 2.
Figure 2. Microparticle behaviour within the active suspension.
(a) Typical microparticle trajectory (∼210 s) in the Hele–Shaw experiment at Nc=4.84±0.13 × 106 cells ml−1. Colour represents instantaneous speed (colour bar unit: μm s−1). The trajectory shows three types of dynamics: Brownian motion and loop-like perturbations (yellow-green blobs) followed by rare and large jumps (red lines). Inset: representative trajectory of a purely Brownian particle in the same setup, lasting ∼210 s. (b) A representative entrainment event: as the cell swims from the left to the right of the panel, it drives the colloid along the dashed line. Scale bar, 20 μm. The event lasts 1.16 s.
Figure 3. Microscopic characterization of particle dynamics.
(a) Probability distribution function (PDF) of the end-to-end length L of the jumps. Above LT=7.5 μm, the distribution is well fitted by an exponential function with characteristic length LJ=7.5 μm (solid red line). Inset: PDF of the duration 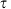 of the jumps. The average is
of the jumps. The average is 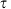 =1.7 s when considering only jumps of length L≥LT (solid red line). (b) Mean time interval between consecutive jumps, 〈ΔTJ〉, as a function of Nc. The red solid line is the hyperbolic fit used in the simulations, 〈ΔTJ〉=((68.2±8)/Nc)s (Nc in units of 106cells ml−1). Horizontal error bars are the standard deviations of cell concentrations. Inset: PDF of the time interval ΔTJ between consecutive jumps at Nc=(1.56±0.10) × 106 cells ml−1. Black solid line: exponential fit with characteristic time (31.9±4)s. Note that these distributions provide a biased measure of the mean waiting time 〈ΔTJ〉, which should be estimated instead from the average number of jump events as discussed in Supplementary Methods 4. (c) Effective diffusivities, Deff−D0, from the Hele–Shaw experiment (blue circles) and from the simulations: red circles/solid red line for
=1.7 s when considering only jumps of length L≥LT (solid red line). (b) Mean time interval between consecutive jumps, 〈ΔTJ〉, as a function of Nc. The red solid line is the hyperbolic fit used in the simulations, 〈ΔTJ〉=((68.2±8)/Nc)s (Nc in units of 106cells ml−1). Horizontal error bars are the standard deviations of cell concentrations. Inset: PDF of the time interval ΔTJ between consecutive jumps at Nc=(1.56±0.10) × 106 cells ml−1. Black solid line: exponential fit with characteristic time (31.9±4)s. Note that these distributions provide a biased measure of the mean waiting time 〈ΔTJ〉, which should be estimated instead from the average number of jump events as discussed in Supplementary Methods 4. (c) Effective diffusivities, Deff−D0, from the Hele–Shaw experiment (blue circles) and from the simulations: red circles/solid red line for 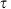 =1.7 s (slope 1.03 × αHS); red diamonds/dashed red line for
=1.7 s (slope 1.03 × αHS); red diamonds/dashed red line for 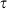 =0.1 s (slope 1.22 × αHS). Vertical error bars represent the uncertainty on the fits to obtain the effective diffusivities. Horizontal error bars are the standard deviations of the cell concentrations. Inset: continuation of the simulated Deff−D0 curves to very high cell concentrations shows saturation to a
=0.1 s (slope 1.22 × αHS). Vertical error bars represent the uncertainty on the fits to obtain the effective diffusivities. Horizontal error bars are the standard deviations of the cell concentrations. Inset: continuation of the simulated Deff−D0 curves to very high cell concentrations shows saturation to a 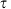 -dependent value.
-dependent value.
Figure 4. Probability distribution functions of particle displacements in the Hele–Shaw experiments.
(a,b) Evolution of the PDF of displacements PDFΔt(Δx) of the colloids with the time interval Δt when considering the jumps (blue curves) or not (red curves) for Nc=(4.84±0.13) × 106 cells ml−1. The curves have been shifted and separated into two figures for clarity. a corresponds to Δt∈[0.04, 0.8]s, while b corresponds to Δt∈[1.2, 48]s (colour bars: Δt in seconds). Both distributions exhibit exponential-like tails at short time which reflect the presence of loop-like perturbations and jumps in the dynamics. (c,d) Modified kurtosis κ of the PDF of displacements as a function of the time interval Δt with/without jumps respectively. The colours code for the cell concentration (unit: 106 cells ml−1). The PDFs with jumps show slower convergence to the Gaussian limit, as the plots in a,b also suggest.
Jumps are fast (Fig. 3a, inset), lasting on average 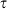 =1.5 s (
=1.5 s (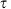 =1.7 s if considering only jumps with L≥LT) but they are rare: as shown in Fig. 2b and Supplementary Movie 3, the algae need to meet a microparticle almost head on in order to entrain it (∼65% of all jumps have initial impact parameter ≤2 μm). As a consequence, even at the highest cell concentration probed, NC≃5 × 106 cells ml−1, the average time between two consecutive jumps is rather long,
=1.7 s if considering only jumps with L≥LT) but they are rare: as shown in Fig. 2b and Supplementary Movie 3, the algae need to meet a microparticle almost head on in order to entrain it (∼65% of all jumps have initial impact parameter ≤2 μm). As a consequence, even at the highest cell concentration probed, NC≃5 × 106 cells ml−1, the average time between two consecutive jumps is rather long, 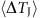 ≳16 s. Because
≳16 s. Because 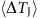 is dictated by the cell–microparticle encounter rate, for a purely random process one would expect
is dictated by the cell–microparticle encounter rate, for a purely random process one would expect 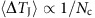 : Fig. 3b shows that this hypothesis is clearly supported experimentally. Further support comes from the analysis of individual inter-jump intervals, which appear to be exponentially distributed as predicted for a simple Poisson process (Fig. 3b inset, see Supplementary Methods 4 for details on the computation of
: Fig. 3b shows that this hypothesis is clearly supported experimentally. Further support comes from the analysis of individual inter-jump intervals, which appear to be exponentially distributed as predicted for a simple Poisson process (Fig. 3b inset, see Supplementary Methods 4 for details on the computation of 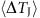 ).
).
Computing the mean-square displacement from these long colloidal trajectories offers a microscopic estimate of the effective particle diffusivity Deff, Supplementary Fig. 7 and Supplementary Note 3. The results are shown in Fig. 1c (blue circles) after multiplication by the swimming speeds ratio 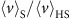 to account for the trivial dependence of the encounter frequency on microalgal speed: Deff is linear with cell concentration, with a slope αHS=1.67±0.13 in excellent quantitative agreement with both macroscopic values. This agreement confirms that the properties of the colloids' microscopic dynamics, which includes important but rare jumps, have indeed been probed appropriately.
to account for the trivial dependence of the encounter frequency on microalgal speed: Deff is linear with cell concentration, with a slope αHS=1.67±0.13 in excellent quantitative agreement with both macroscopic values. This agreement confirms that the properties of the colloids' microscopic dynamics, which includes important but rare jumps, have indeed been probed appropriately.
Numerical simulations
The agreement between the macroscopic and microscopic diffusivity measurements highlights the importance of entrainment events, and suggests a combination of jumps and (far-field-enhanced) diffusion as a plausible minimal model of microparticles' dynamics, at least over the medium-to-long timescales we are interested in. Consequently, we model the two-dimensional projection of the stochastic trajectory of a colloid in the Hele–Shaw experiments, (X(t), Y(t)), as
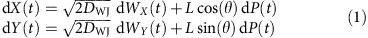 |
This combines standard Wiener processes dWX,Y(t) leading to diffusion with diffusivity DWJ, with a Poisson process for the jumps, dP(t), characterized by the average time interval 〈ΔTJ(Nc)〉 between jumps (Fig. 3b, solid red line). Far-field effects are included at a coarse-grained level by choosing DWJ=D0+αWJNc, where αWJ=0.33±0.05. This is the effective diffusivity, rescaled by 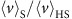 , which is measured from the microscopic experimental tracks when the entrainment events have been removed. Notice that αWJ>αT due to our conservative choice for what constitutes a jump, which in the simulation we draw from an exponential fit to the part of the experimental distribution strictly above LT (Fig. 3a, solid red line). As a result, the compounded effect of shorter jumps is included within the coefficient αWJ. The jumps' orientation θ is uniformly distributed to give an isotropic process, and their duration
, which is measured from the microscopic experimental tracks when the entrainment events have been removed. Notice that αWJ>αT due to our conservative choice for what constitutes a jump, which in the simulation we draw from an exponential fit to the part of the experimental distribution strictly above LT (Fig. 3a, solid red line). As a result, the compounded effect of shorter jumps is included within the coefficient αWJ. The jumps' orientation θ is uniformly distributed to give an isotropic process, and their duration 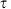 =1.7 s is constant.
=1.7 s is constant.
This dynamics, simulated with a simple acceptance-rejection method (see Methods section), produces trajectories very similar to the experimental, Supplementary Fig. 8 and Supplementary Note 4. The motion is characterized by an effective diffusivity in excellent agreement with the values from the microscopic experiments (see Fig. 3c, red and blue circles respectively), and ≳5 × larger than DWJ at the corresponding cell concentration. The simulations, then, highlight the striking influence that jumps have on particle dynamics, despite their rarity. At the same time, they allow us to explore easily parameter values that have not yet been probed experimentally. For example, Fig. 3c (red diamonds and dashed red line) shows that within the experimental range of cell concentrations, even a drastic reduction of the entrainment duration 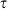 to 0.1 s has only a minimal effect on particle diffusivity. The exact value of
to 0.1 s has only a minimal effect on particle diffusivity. The exact value of 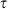 , however, will have a major influence as soon as
, however, will have a major influence as soon as 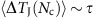 , leading to a plateau in the effective diffusivity as the cell concentration grows above a threshold
, leading to a plateau in the effective diffusivity as the cell concentration grows above a threshold 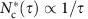 (Fig. 3c inset), at least as long as collective effects do not modify our single-particle picture.
(Fig. 3c inset), at least as long as collective effects do not modify our single-particle picture.
Analytical theory
The experimental and simulation results can be tied together further through a simple continuum theory for the dynamics of microparticles, which rationalizes the dependence of the effective diffusivity Deff(Nc) on the concentration of algae. Here we extend to two dimensions the one-dimensional approach developed in ref. 41. Briefly, we consider two populations whose densities at position (x, y) and time t are ρd(x, y, t) and ρb(x, y, t, φ), corresponding respectively to particles diffusing with diffusivity DWJ, and to particles moving ballistically with a constant velocity u in the direction φ. Particles switch from diffusion to directed motion and vice versa with constant transition rates λd(=1/〈ΔTJ〉) and λb(=1/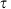 ) respectively. The system then obeys the following set of equations:
) respectively. The system then obeys the following set of equations:
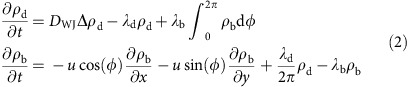 |
which can be easily solved by Fourier–Laplace transform, Supplementary Note 5, yielding the time evolution of the particles' mean-square displacement and hence their diffusivity. At long timescales this is given by
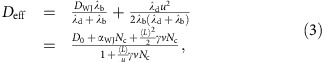 |
where we wrote DWJ=D0+αWJNc, λb=u/〈L〉 and λd=γvNc as the frequency of entrainment events is proportional to the product of the speed v and concentration Nc of microorganisms, also called ‘active flux'16. The quantity γ can be interpreted as a cross-section for entrainment: as an alga swims past, the tracer will be entrained -with a given probability- if it is contained within a region of area γ around the line of motion of the alga19,21. For our Hele–Shaw experiments, using 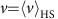 and the experimental values of 〈ΔTJ〉 (Fig. 3b) we obtain γ=299±35 mm2 (respectively γ=299±35 μm2 if Nc is expressed in units of cells ml−1 rather than 106cells ml−1). At low concentrations, where λd<<λb (or equally
and the experimental values of 〈ΔTJ〉 (Fig. 3b) we obtain γ=299±35 mm2 (respectively γ=299±35 μm2 if Nc is expressed in units of cells ml−1 rather than 106cells ml−1). At low concentrations, where λd<<λb (or equally 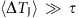 ), Deff becomes
), Deff becomes
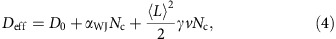 |
which is independent of the jumps' duration 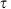 , here equivalent to independence on u, as suggested by the simulations. Equation (4) recovers the clear division between thermal, far-field and entrainment contributions to the diffusivity that we previously discussed in the context of microscopic experiments. Notice that the contribution from the jumps, by far the most important in our experiments, is simply what should be expected if we interpreted the colloidal trajectory as a freely jointed chain where bonds with exponentially distributed length of mean 〈L〉 are added at a rate λd=γvNc.
, here equivalent to independence on u, as suggested by the simulations. Equation (4) recovers the clear division between thermal, far-field and entrainment contributions to the diffusivity that we previously discussed in the context of microscopic experiments. Notice that the contribution from the jumps, by far the most important in our experiments, is simply what should be expected if we interpreted the colloidal trajectory as a freely jointed chain where bonds with exponentially distributed length of mean 〈L〉 are added at a rate λd=γvNc.
The coefficient αWJ, representing far-field effects, is proportional to the speed v of the microalgae21,26,29, leading to an overall contribution (Deff−D0) which scales with the active flux vNc, as originally predicted in ref. 19. Figure 1c shows indeed that the diffusivity curves from different experiments collapse when rescaled by the corresponding velocities. In turn, then, the distribution of jump lengths should be independent of the average velocity of the microorganisms, as expected at low Reynolds numbers. Within the model, however, this proportionality is limited to sufficiently low cell concentrations. As Nc increases and 〈ΔTJ〉 becomes closer to 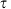 , nonlinearities become important, and eventually Deff plateaus to a
, nonlinearities become important, and eventually Deff plateaus to a 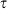 -dependent value as seen in the simulations (Fig. 3c, inset) and well captured by the present model, Supplementary Fig. 9 and Supplementary Note 5.
-dependent value as seen in the simulations (Fig. 3c, inset) and well captured by the present model, Supplementary Fig. 9 and Supplementary Note 5.
Finally, the short timescale limit of the particles' mean-square displacement returns Deff=xdDWJ, where xd is the fraction of particles that are in the diffusing state. Estimating 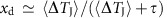 , we can assume xd≃1 within the whole range of cell concentrations probed experimentally. Short timescale tracking of microparticles, then, will inevitably return Deff≃DWJ rather than the full expression in equation (4). This is the reason for the small diffusivity reported in ref. 26, which we also observe from direct short-duration tracking of microparticles in the sedimentation experiments.
, we can assume xd≃1 within the whole range of cell concentrations probed experimentally. Short timescale tracking of microparticles, then, will inevitably return Deff≃DWJ rather than the full expression in equation (4). This is the reason for the small diffusivity reported in ref. 26, which we also observe from direct short-duration tracking of microparticles in the sedimentation experiments.
Discussion
Proposed theoretically either as a consequence of microparticle capture within ‘wake bubbles'21 or as a consequence of Darwin drift28, particle entrainment by microorganisms was expected to provide at best a contribution similar to that observed for to far-field loops42 and most likely much smaller22. At the same time, lack of experimental evidence for entrainment questioned not just its importance for particle–microswimmer interactions, but its existence as well.
Here we show experimentally not only that entrainment of microparticles by microorganisms exists but also that these rare but large events can dominate particle dynamics, leading in the present case to a diffusivity more than 40 × larger than previously reported. Simulation and analytical results support a jump-diffusion process as a good minimal model for the medium-to-long timescales dynamics of the colloids. The simplified continuum model we discuss provides a theoretical support for the observed dependence of the experimental diffusivities on the active flux vNc, already introduced in the bacterial context16,17,43. At the same time, it clarifies that long-duration particle tracking is necessary to sample correctly the microscopic dynamics and recover the real long timescale impact of microorganisms.
Although we cannot yet pinpoint the specific mechanism leading to entrainment, the structure of the near-field flow is likely to play a crucial role. The entrainment we observe requires almost head-on collisions, which is likely to be facilitated by the type of stagnation point found (on average) in front of the cell37,38. After reaching the cell apex, the entrained particles slide down the sides of the cell body along a high-shear region almost co-moving with the microorganism, and are eventually left behind having spent slightly more than half of the jump in the front part of the cell (54±9%). The no-slip boundary on the bodies of microorganisms, and the stagnation points in their flow fields, have in fact been argued to play a major role in large microparticle displacements5,21,29, which are seen here to dominate the effective diffusivity. Our results support these conjectures.
Eukaryotic microswimmers with multiple front-mounted flagella will share much of the near-field flow structure found in Chlamydomonas: entrainment is then likely to be a generic feature of this whole class of microorganisms. Several of these species are predators2,3, and prey on cells of size similar to our plastic particles. Front-mounted flagella, then, would spontaneously lead to contact with the prey at a predictable location on the cell body within easy reach of the flagella, and therefore facilitate the ingestion of both natural preys and environmental microplastics.
Methods
Cell culture
Cultures of CR strain CC125 were grown axenically in a Tris-Acetate-Phosphate medium32 at 21 °C under continuous fluorescent illumination (100 μE m−2 s−1, OSRAM Fluora). Cells were harvested at ∼5 × 106cells ml−1 in the exponentially growing phase, then centrifuged at 800 r.p.m. for 10 min and the supernatants replaced by DI-water (sedimentation experiment) or by a Percoll solution (tracking and spreading experiments; Percoll Plus, Sigma) already containing the desired concentration of PS colloids (Polybead Microspheres, diameter d=1±0.02 μm).
Microfluidics and microscopy
The Percoll solution (38.5% vol/vol) was made to density-match the beads for the Hele–Shaw and spreading experiments, while preserving the Newtonian nature of the flows33. The appropriate solution was injected into either 185 μm (sedimentation experiment) or 26 μm (tracking experiment) thick Polydimethylsiloxane-based microfluidic channels previously passivated with 0.15%w/w BSA solution in water. The microfluidic devices were then sealed using photocurable glue (Norland NOA-68) to prevent evaporation. Regarding the spreading experiment, the band of colloids was initiated in a three-arms fork-shape channel (60 μm thick) by injecting at the same flow-rate (using a PHD 2,000 Harvard Apparatus syringe pump and high-precision Hamilton 50 and 100 μl gas-tight syringes) the CR+beads Percoll solution in the central arm and the CR Percoll solution only in the two sided arms. Colloids were observed under either bright-field (sedimentation experiment) or phase contrast (tracking and spreading experiments) illumination on a Nikon TE2000-U inverted microscope. A long-pass filter (cutoff wavelength 765 nm) was added to the optical path to prevent phototactic response of the cells. Stacks of 200 images at 60 × magnification were acquired at 10 f.p.s. (camera Pike F-100B, AVT) layer by layer by manually moving the plane of focus in order to reconstruct the density profiles for the sedimentation experiment. We used a × 40 oil immersion objective (Nikon CFI S Fluor × 40 oil) combined with an extra × 1.5 optovar magnification. The condenser iris was completely opened to minimize the depth of field. Regarding the Hele–Shaw experiment, several movies of 2 × 104 images were recorded at 25 f.p.s. (camera Pike F-100B, AVT) using a 20 × phase contrast objective (Nikon LWD ADL 20 × F). Particles trajectories were then digitized using a standard Matlab particle tracking algorithm (The code can be downloaded at http://people.umass.edu/kilfoil/downloads.html). Finally the spreading dynamics of the band of colloids was probed by recording the system for a few hours at × 10 magnification (Nikon ADL 10 × I) and low frame rate (0.5 f.p.s.) using a Nikon D5000 DSLR camera. The colloids were then featured using the same Matlab particle tracking algorithm in order to reconstruct the density profiles. In all experiments, the concentration of algae was determined in situ by imaging the system under dark-field illumination at low magnification (objective Nikon CFI Plan Achromat UW 2 ×).
Numerical Simulations
The parameters of the simulation correspond to the red lines in Fig. 3a,b. For each algae concentration, 1,000 trajectories of 2,000 s have been simulated using a time-step δt=0.004 s, which is 10 times smaller than the acquisition period in the Hele–Shaw experiment. At each time step a random walk with diffusivity DWJ is performed. To simulate the Poisson process, we draw at each time step a random number in the open interval ]0;1[. If this number is within the centred closed interval [(1−δt/〈ΔTJ〉)/2;(1+δt/〈ΔTJ〉)/2], then we perform a jump that lasts 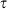 =1.7 s (or 0.1 s) with a length taken out of the distribution PDF(L), along the direction corresponding to θ, which is uniformly distributed in [−π, π[. This technique allows to simulate accurately the Poisson process44. We stress that within the simulation, once the particle has entered a jump, it will escape it after exactly
=1.7 s (or 0.1 s) with a length taken out of the distribution PDF(L), along the direction corresponding to θ, which is uniformly distributed in [−π, π[. This technique allows to simulate accurately the Poisson process44. We stress that within the simulation, once the particle has entered a jump, it will escape it after exactly 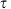 =1.7 s (or 0.1 s). This is done in order to approximate the experiments, which show that the distribution function of jumps' duration is tighter around the mean than that of jumps' lengths. Notice that during the jumps the random walk component is switched off. After the jump has been performed, a new random number is picked in order to choose between a random step or a jump and continue the jump-diffusion process.
=1.7 s (or 0.1 s). This is done in order to approximate the experiments, which show that the distribution function of jumps' duration is tighter around the mean than that of jumps' lengths. Notice that during the jumps the random walk component is switched off. After the jump has been performed, a new random number is picked in order to choose between a random step or a jump and continue the jump-diffusion process.
Data availability
The authors declare that the data supporting the findings of this study are available on request.
Additional information
How to cite this article: Jeanneret, R. et al. Entrainment dominates the interaction of microalgae with micron-sized objects. Nat. Commun. 7:12518 doi: 10.1038/ncomms12518 (2016).
Supplementary Material
Supplementary Figure 1-9, Supplementary Notes 1-5, Supplementary Methods and Supplementary References
Dynamics of a single microparticle in the Hele-Shaw experiment taken at 30x magnification, phase contrast, 25fps. The colour shows the instantaneous velocity of the bead (frame-to-frame displacement multiplied by frame rate). Colour code is the same used in Fig 2a of the main text.
Example of an entrainment event during the sedimentation experiment. 60x magnification, bright field, 10fps.
High speed movie of an entrainment event in the Hele-Shaw experiment recorded at 500fps, 100x magnification, bright field. Slowed down 17x.
Footnotes
Author contributions R.J., D.O.P., V.K., M.P. designed the study; R.J. performed the experiments, analytical and numerical modelling; R.J. and M.P. analysed the results; R.J., V.K., M.P. wrote the manuscript.
References
- Heddergott N. et al. Trypanosome motion represents an adaptation to the crowded environment of the verterbrate bloodstream. PLoS Pathog. 8, e1003023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnes D. J. S. et al. Selective feeding behaviour of key free-living protists: avenues for continued study. Aquat. Microb. Ecol. 53, 83–98 (2008). [Google Scholar]
- Wetherbee R. & Andersen R. Flagella of a chrysophycean alga play an active role in prey capture and selection. Direct observations on Epiphysis pulchra using image enhanced video microscopy. Protoplasma 166, 1–7 (1992). [Google Scholar]
- Visser A. W. Biomixing of the oceans? Science 316, 838–839 (2007). [DOI] [PubMed] [Google Scholar]
- Thiffeault J.-L. & Childress S. Stirring by swimming bodies. Phys. Lett. A 374, 3487–3490 (2010). [Google Scholar]
- Katija K. Biogenic inputs to ocean mixing. J. Exp. Biol. 215, 1040–1049 (2012). [DOI] [PubMed] [Google Scholar]
- Wright S. L., Thompson R. C. & Galloway T. S. The physical impact of microplastics on marine organisms: a review. Env. Pol 178, 483–492 (2013). [DOI] [PubMed] [Google Scholar]
- Mallory S. A., Valeriani C. & Cacciuto A. Induced activation of a passive tracer in an active bath. Phys. Rev. E 90, 032309 (2014). [DOI] [PubMed] [Google Scholar]
- Kümmel F., Shabestari P., Lozano C., Volpe G. & Bechinger C. Formation, compression and surface melting of colloidal clusters by active particles. Soft Matter 11, 6187 (2015). [DOI] [PubMed] [Google Scholar]
- Angelani L., Maggi C., Bernardini M. L., Rizzo A. & di Leonardo R. Effective interactions between colloidal particles suspended in a bath of swimming cells. Phys. Rev. Lett. 107, 138302 (2011). [DOI] [PubMed] [Google Scholar]
- Maggi C. et al. Generalized energy equipartition in harmonic oscillators driven by active baths. Phys. Rev. Lett. 113, 238303 (2014). [DOI] [PubMed] [Google Scholar]
- Koumakis N., Maggi C. & di Leonardo R. Directed transport of active particles over asymmetric energy barriers. Soft Matter 10, 5695 (2014). [DOI] [PubMed] [Google Scholar]
- Takatori S. C. & Brady J. F. A theory for the phase behavior of mixtures of active particles. Soft Matter 11, 7920–7931 (2015). [DOI] [PubMed] [Google Scholar]
- Wu X.-L. & Libchaber A. Particle diffusion in a quasi-two-dimensional bacterial bath. Phys. Rev. Lett. 84, 13 (2000). [DOI] [PubMed] [Google Scholar]
- Valeriani C., Li M., Novosel J., Arlt J. & Marenduzzo D. Colloids in a bacterial bath: simulations and experiments. Soft Matter 7, 5228 (2011). [Google Scholar]
- Miño G. L. et al. Enhanced diffusion due to active swimmers at a solid surface. Phys. Rev. Lett. 106, 048102 (2011). [DOI] [PubMed] [Google Scholar]
- Jepson A., Martinez V. A., Schwarz-Linek J., Morozov A. & Poon W. C. K. Enhanced diffusion of non swimmers in a three-dimensional bath of motile bacteria. Phys. Rev. E 88, 041002 (R) (2013). [DOI] [PubMed] [Google Scholar]
- Patteson A. E., Gopinath A., Purohit P. K. & Arratia P. E. Particle diffusion in active fluids is non-monotonic in size. Soft Matter 12, 2365–2372 (2016). [DOI] [PubMed] [Google Scholar]
- Underhill P. T., Hernandez-Ortiz J. P. & Graham M. D. Diffusion and spatial correlations in suspensions of swimming particles. Phys. Rev. Lett. 100, 248101 (2008). [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Locsei J. T. & Pedley T. J. Fluid particle diffusion in a semidilute suspension of model micro-organisms. Phys. Rev. E 82, 021408 (2010). [DOI] [PubMed] [Google Scholar]
- Lin Z., Thiffeault J.-L. & Childress S. Stirring by squirmers. J. Fluid Mech. 669, 167–177 (2011). [Google Scholar]
- Morozov A. & Marenduzzo D. Enhanced diffusion of tracer particles in dilute bacterial suspensions. Soft Matter 10, 2748–2758 (2014). [DOI] [PubMed] [Google Scholar]
- Kasyap T. V., Koch D. L. & Wu M. Hydrodynamic tracer diffusion in suspensions of swimming bacteria. Phys. Fluids 26, 081901 (2014). [Google Scholar]
- Peng Y. et al. Diffusion of ellipsoids in bacterial suspensions. Phys. Rev. Lett. 116, 068303 (2016). [DOI] [PubMed] [Google Scholar]
- Kim M. & Breuer K. Enhanced diffusion due to motile bacteria. Phys. Fluids 16, L78–L81 (2004). [Google Scholar]
- Leptos K. C., Guasto J. S., Gollub J. P., Pesci A. I. & Goldstein R. E. Dynamics of enhanced tracer diffusion in suspensions of swimming eukaryotic microorganisms. Phys. Rev. Lett. 103, 198103 (2009). [DOI] [PubMed] [Google Scholar]
- Kurtuldu H., Guasto J. S., Johnson K. A. & Gollub J. P. Enhancement of biomixing by swimming algal cells in two-dimensional films. Proc. Natl Acad. Sci. USA 108, 10391–10395 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkin D. O. & Yeomans J. M. Fluid mixing by curved trajectories of microswimmers. Phys. Rev. Lett. 111, 188101 (2013). [DOI] [PubMed] [Google Scholar]
- Thiffeault J.-L. Distribution of particle displacements due to swimming microorganisms. Phys. Rev. E 92, 023023 (2015). [DOI] [PubMed] [Google Scholar]
- Zaid I. M., Dunkel J. & Yeomans J. M. Lévy fluctuations and mixing in dilute suspensions of algae and bacteria. J. R. Soc. Interface 8, 1314–1331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkin D. O. & Yeomans J. M. Stirring by swimmers in confined microenvironments. J. Stat. Mech. 4, P04030 (2014). [Google Scholar]
- Rochaix J. D., Mayfield S., Goldschmidt-Clermont M. & Erickson J. M. in Plant Molecular Biology: A Practical Approach ed. Schaw C. H. 253–275IRL Press (1988). [Google Scholar]
- Gachelin J. et al. Non-Newtonian viscosity of Escherichia coli suspensions. Phys. Rev. Lett. 110, 268103 (2013). [DOI] [PubMed] [Google Scholar]
- Perrin J. Molecular reality. Ann. Chim. Phys. 8, 1 (1909). [Google Scholar]
- Tailleur J. & Cates M. E. Sedimentation, trapping, and rectification of dilute bacteria. Eur. Phys. Lett. 86, 60002 (2009). [Google Scholar]
- Palacci J., Cottin-Bizonne C., Ybert C. & Bocquet L. Sedimentation and effective temperature of active colloidal suspensions. Phys. Rev. Lett. 105, 088304 (2010). [DOI] [PubMed] [Google Scholar]
- Drescher K., Goldstein R. E., Michel N., Polin M. & Tuval I. Direct measurement of the flow field around swimming microorganisms. Phys. Rev. Lett. 105, 168101 (2010). [DOI] [PubMed] [Google Scholar]
- Guasto J. S., Johnson K. A. & Gollub J. P. Oscillatory flows induced by microorganisms swimming in two dimensions. Phys. Rev. Lett. 105, 168102 (2010). [DOI] [PubMed] [Google Scholar]
- Dunkel J., Putz V. B., Zaid I. M. & Yeomans J. M. Swimmer-tracer scattering at low Reynolds number. Soft Matter 6, 4268–4276 (2010). [Google Scholar]
- Mathjissen A. J. T. M., Pushkin D. O. & Yeomans J. M. Tracer trajectories and displacement due to a micro-swimmer near a surface. J. Fluid Mech. 773, 498–519 (2015). [Google Scholar]
- Méndez V., Campos D. & Bartumeus F. Stochastic Foundations in Movement Ecology Ch. 3.3 Springer Complexity (2014). [Google Scholar]
- Pushkin D. O., Shum H. & Yeomans J. M. Fluid transport by individual microswimmers. J. Fluid Mech. 726, 5–25 (2013). [Google Scholar]
- Miño G. L., Dunstan J., Rousselet A., CleŽment E. & Soto R. Induced diffusion of tracers in a bacterial suspension: theory and experiments. J. Fluid Mech. 729, 423–444 (2013). [Google Scholar]
- Hanson F. B. Applied Stochastic Processes and Control for Jump-Diffusions: Modeling, Analysis, and Computation Ch. 1 SIAM (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1-9, Supplementary Notes 1-5, Supplementary Methods and Supplementary References
Dynamics of a single microparticle in the Hele-Shaw experiment taken at 30x magnification, phase contrast, 25fps. The colour shows the instantaneous velocity of the bead (frame-to-frame displacement multiplied by frame rate). Colour code is the same used in Fig 2a of the main text.
Example of an entrainment event during the sedimentation experiment. 60x magnification, bright field, 10fps.
High speed movie of an entrainment event in the Hele-Shaw experiment recorded at 500fps, 100x magnification, bright field. Slowed down 17x.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available on request.