Highlights
-
•
Zinc and copper are essential trace elements required for cell function.
-
•
Nutrient Immunity restricts zinc and copper access and mediates toxicity.
-
•
Divergent fungi integrate zinc and copper responsive regulons for pathogenesis.
Abstract
All organisms must secure essential trace nutrients, including iron, zinc, manganese and copper for survival and proliferation. However, these very nutrients are also highly toxic if present at elevated levels. Mammalian immunity has harnessed both the essentiality and toxicity of micronutrients to defend against microbial invasion — processes known collectively as ‘nutritional immunity’. Therefore, pathogenic microbes must possess highly effective micronutrient assimilation and detoxification mechanisms to survive and proliferate within the infected host. In this review we compare and contrast the micronutrient homeostatic mechanisms of Cryptococcus and Candida — yeasts which, despite ancient evolutionary divergence, account for over a million life-threatening infections per year. We focus on two emerging arenas within the host–pathogen battle for essential trace metals: adaptive responses to zinc limitation and copper availability.
Current Opinion in Microbiology 2016, 32:128–134
This review comes from a themed issue on Host-microbe interactions: fungi
Edited by Elaine Bignell and Bart PHJ Thomma
For a complete overview see the Issue and the Editorial
Available online 18th June 2016
http://dx.doi.org/10.1016/j.mib.2016.05.013
1369-5274/© 2016 The Author(s). Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
The concept of nutritional immunity, in terms of host-driven iron sequestration, has been appreciated for decades: our bodies maintain extremely low iron cation levels via intracellular sequestration (ferritin, haemoglobin, the hepcidin axis) and expression of extracellular high-affinity iron-binding proteins (transferrin, lactoferrin). Successful pathogens have in turn evolved effective assimilation mechanisms (high-affinity transporters, siderophores and specialised binding proteins) [1]. However, additional layers of nutritional immunity have been revealed in recent years: micronutrient restriction is not limited to iron, with host zinc and manganese sequestration playing key roles in controlling infection [2]. Moreover, in certain host niches, pathogens can be exposed to potentially toxic levels of iron, zinc and copper. Therefore, beyond high-affinity iron uptake systems, microbial pathogens must also possess highly effective homeostatic mechanisms (both assimilation and detoxification) for other trace metals within their hosts [2].
Host nutritional immunity during fungal infection and the pathogen assimilation pathways employed in counterattack have been extensively discussed in a number of recent reviews [3, 4, 5, 6]. In this mini-review we focus on two emerging areas: the adaptive responses of Candida and Cryptococcus species to changes in environmental zinc and copper.
Divergent virulence factor intercalation of the zinc regulon
Zinc is the second most abundant transition metal in the human body but, like iron, its availability to microbial pathogens is strictly limited. Zinc restriction is further compounded during periods of inflammation due to the action of calprotectin. Calprotectin constitutes approximately half the cytoplasmic protein content of neutrophils, is a dominant component of NETs (neutrophil extracellular traps) and elicits antifungal activity via zinc chelation [7]. Calprotectin is a heterodimer of S100A8 and S100A9, members of the S100 family of low molecular weight proteins. Antifungal activity of another S100 family member, psoriasin (S100A7) has recently been reported to elicit antifungal activity via zinc-sequestration [8•], suggesting that multiple members of this protein family may have similar functions. Furthermore, host cell internalisation and subcellular compartmentalisation further limit accessibility to extracellular and intracellular pathogens, respectively. For example, activated, Histoplasma-infected macrophages shuttle zinc to their Golgi, restricting fungal proliferation and promoting clearance [9].
Interestingly, contemporary pathogenic fungal species appear to simultaneously exhibit remarkably similar and divergent responses to changes in zinc availability. First described in the model yeast Saccharomyces cerevisiae, Zap1 is a zinc finger transcription factor that regulates the expression of zinc transporter-encoding genes and is essential for maintaining zinc homeostasis. Under zinc limitation, Zap1 exhibits positive auto-regulation, rapidly amplifying its own transcription and subsequent protein levels and positively regulating the expression of zinc transporter-encoding genes, including ZRT1, ZRT2 and ZRT3. For a detailed discussion of S. cerevisiae Zap1 function, readers are directed to an excellent recent review by Wilson and Bird [10].
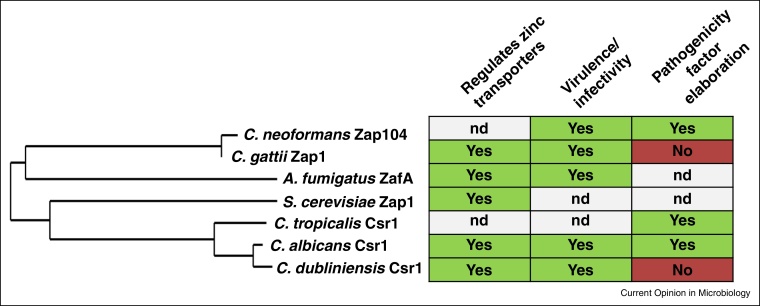
It would appear that Zap1 orthologues function as conserved master regulators of zinc homeostasis in fungi: in the pathogenic fungal species Candida albicans, Candida dubliniensis, Aspergillus fumigatus, and Cryptococcus deuterogattii, Zap1 orthologues (also known as Csr1 in Candida, ZafA in Aspergillus) have been directly demonstrated to regulate the expression of zinc transporter-encoding genes. Given the evolutionary divergence of these species, it is plausible that orthologues of Zap1 regulate zinc homeostasis in many (if not all) fungi [11, 12•, 13, 14]. Underscoring the importance of fungal zinc homeostasis during infection, each fungal pathogen tested to date exhibits virulence attenuation or decreased infectivity upon deletion of their respective ZAP1 orthologue in relevant animal models of infection (Figure 1).
Figure 1.
Phylogenetic and functional relationships of Zap1 orthologues in fungi. The predicted amino acid sequences of Zap1 orthologues were downloaded from FungiDB, Aspergillus Genome Database, Saccharomyces Genome Database and Candida Genome Database and aligned using Phylogeny.fr. Note that in all reported cases, Zap1 regulates the expression of zinc transporters and is required for virulence or infectivity in animal models of infection, but that confirmed pathogenicity factor expression is species-specific.
Despite this apparent conservation of function as a regulator of zinc homeostasis and virulence, there may be differences in the Zap1-regulons of different contemporary fungal species. Cryptococcus neoformans and C. deuterogattii are closely related species that express three major virulence factors for infectivity: capsule formation, melanin production and growth capacity at 37 °C. Interestingly, while C. deuterogattii ZAP1 is dispensable for capsule and melanin production [14], C. neoformans cells lacking the ZAP1 orthologue (ZAP104) are deficient in capsule, melanin, and a/α cell fusion, suggesting rewiring [15, 16•]. While we cannot rule out that C. deuterogattii Zap1 and zinc homeostasis may influence melanin and capsule formation under conditions not examined by Schneider et al. [14], Liu et al. and Jung et al. independently demonstrate ZAP104 involvement in virulence factor expression (melanin and capsule synthesis) in C. neoformans [14, 15, 16•]. These three studies together suggest that Zap1-mediated zinc homeostasis in C. neoformans, but not C. deuterogattii, may impinge on virulence factor (capsule/melanin) expression, and raise questions about the reasons for phenotypic differences. An examination of Zap1 and Zap104 protein sequences immediately reveals structural differences: where the Zap1 N-terminus encodes a single zinc-binding domain, Zap104 encodes two [14]. Whether these structural differences lead to differing sensitivities to zinc availability or differences in promoter binding remain to be explored.
In the Candida genus, Zap1 function has been investigated in three relatively closely related species [17, 18]: C. albicans, C. dubliniensis and C. tropicalis. Despite diverging from basidiomycetes approximately 500 mya [19], C. albicans, like C. neoformans, appears to regulate major virulence attributes (hyphal morphogenesis, adhesion and biofilm maturation) via Zap1 (note that Zap1 in Candida is also known by the common name Csr1). Kim et al. [20] first reported that ZAP1 deletion in C. albicans precluded hyphal morphogenesis under a range of environmental conditions. Moreover, overexpression of ZRT1 or ZRT2 (encoding the two predicted plasma membrane zinc transporters of C. albicans) promoted filamentous growth of the zap1Δ mutant on certain media, suggesting that the morphogenic defect may be due to perturbed cellular zinc homeostasis rather than direct Zap1-regulation of hypha-formation.
A recent study of C. dubliniensis found that, in this close relative of C. albicans, Zap1 also positively regulates ZRT1, ZRT2 and PRA1 [12•], reinforcing the concept that Zap1 is the universal regulator of zinc assimilation in the fungal kingdom (Figure 1). However, unlike C. albicans, the C. dubliniensis zap1Δ mutant was not defective for hypha formation. On the other hand, Zap1 does regulate filamentation in C. tropicalis [21].
Using a powerful combination of large-scale mutant library screening, transcriptional profiling, chromatin immunoprecipitation, regulatory network analysis and infection modelling, the group of Aaron Mitchell found that Zap1 regulates extracellular matrix production by C. albicans biofilms and governs the expression of adhesin molecules. The zap1Δ mutant efficiently formed biofilms; however, these structures produced highly elevated levels of β-glucan, indicating that Zap1 negatively regulates extracellular matrix production — a key attribute in biofilm maturation [11]. Zap1 occupies ZRT1, ZRT2 and PRA1 promoters and positively regulates their transcription, in agreement with Zap1 acting as the master regulator of zinc homeostasis in C. albicans. However, overexpression of these zinc assimilation factors in the zap1Δ mutant had no effect on β-glucan production, suggesting that cellular zinc homeostasis per se may not directly regulate extracellular matrix synthesis, and that Zap1 possesses additional regulatory function [11]. Indeed, Zap1 was also found to be required for the regulation of genes involved in adhesion — another key element of biofilm formation [22]. Despite this regulatory role, the zap1Δ mutant itself exhibited wild type levels of adhesion. Therefore, to test whether Zap1 functions redundantly in adherence regulation, these authors tested the effect of ZAP1 overexpression in strains lacking other transcription factors important for adhesion. This approach demonstrated that ZAP1 overexpression restored the adhesive capacity of multiple adhesion-defective transcription factor mutants. This was most likely due to Zap1-dependent transcriptional induction of CSTAR (cell surface targets of adherence regulators) genes, as ZAP1 overexpression was found to increase the expression of these target genes in mutants lacking three direct regulators of adhesion (zcf28Δ, try2Δ and try3Δ). Therefore, in addition to zinc assimilation, C. albicans appears to have intercalated key virulence attributes — adhesion and biofilm maturation — into its Zap1 regulon. It should be noted that, in these studies, zinc limited media was not used, and Zap1 may regulate different subsets of genes, depending on the environmental zinc status.
In summary, it would appear that beyond zinc homeostasis, the Zap1 transcription factor plays different roles in different fungal species and, at least in C. neoformans and C. albicans, these are directly associated with the expression of ‘classical’ pathogenicity factors. So why might fungal pathogens place control of key virulence attributes within their Zap1 regulon? One likely possibility is the relative zinc levels found in the natural reservoirs of these yeasts, compared to the infected host. Both arboreal environments and the mammalian digestive tract are relatively high zinc environments, whilst nutritional immunity creates extreme zinc depletion within infected tissues. Hardwiring virulence factor expression into their zinc starvation-responses may contribute to the high pathogenic potential of these species, similar to the situation for iron [23].
Dynamic regulation of response to nutritional copper immunity
In contrast to zinc nutritional immunity, which functions primarily via micronutrient sequestration by the host, copper nutritional immunity appears to represent a highly dynamic system [3, 23]. During C. albicans bloodstream infection, serum copper levels rise early in infection [24]. Upon kidney colonisation, an early spike in copper levels is followed by rapid copper sequestration. For C. neoformans, while the lungs are a high copper environment, alveolar macrophages induce host CTR1 but repress the ATP7A transporter for phagosomal copper compartmentalization, resulting in copper starvation upon engulfment [25]. Consistent with this, C. neoformans requires the copper transporter CTR4 for survival within alveolar macrophages but it is dispensable for growth in the lungs [26••, 27]. Although cerebral spinal fluid (CSF) copper levels are estimated at 100 μM under certain conditions, the CSF is generally copper poor and induces copper transporter expression [27, 28]. Likewise, upon crossing the blood–brain-barrier, the low copper availability of the brain necessitates CTR1 or CTR4 for C. neoformans virulence [27]. Responding to this dynamic environment requires a coordinated transcriptional response on the part of the fungus.
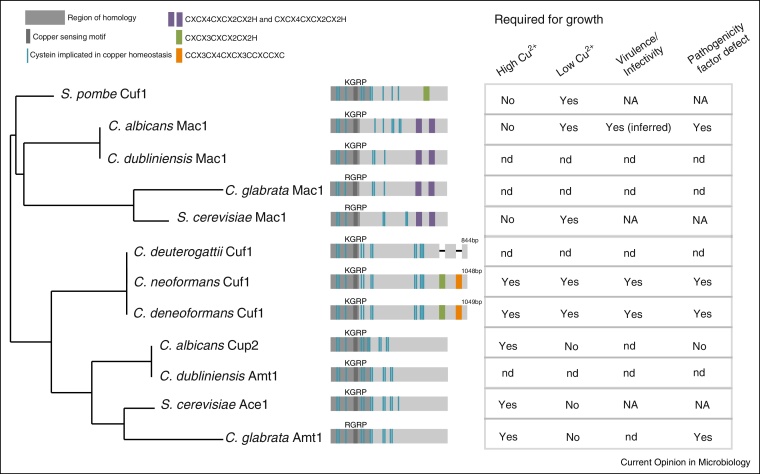
Structurally, fungal copper responsive transcription factors fall into three classes typified by S. cerevisiae Ace1 and Mac1 and S. pombe Cuf1 (Figure 2). All maintain an N-terminal copper responsive (R/K)GRP motif and conserved N-terminus [29•]. In ScAce1 and ScMac1 residues 1–40 encode a zinc-binding domain. ScAce1 residues 40–110 encode CXC and CX2C motifs necessary to coordinate a tetra-copper cluster for binding MRE promoter motifs. Mac1 is characterised instead by dual cysteine rich C-terminal motifs, REP-I (CXCX4CXCX2CX2H) and REP-II (CXCX4CXCX2CX2H), which sense copper and stabilise DNA binding, respectively[30, 31]. Specificity is achieved through division of labour for high and low copper responses: ScMac1 regulates the expression of the CTR1 and CTR2 copper importers in response to copper limitation, while copper toxicity triggers Mac1 degradation and assembly of the ScAce1 tetra-copper cluster, activating DNA-binding and induction of CUP1 and CRS5 metallothioneins and SOD1, which in turn further negatively regulates Mac1 [32, 33, 34]. Likewise, the C. glabrata Ace1 orthologue Amt1 is required for high copper and metallothionein expression and C. glabrata encodes a putative Mac1 homolog with overall structural similarity to ScMac1 [35, 36] (Figure 2).
Figure 2.
Phylogenetic and structural relationships of copper responsive transcription factors in fungi. The predicted amino acid sequences of the indicated orthologs were downloaded from FungiDB, Broad, NCBI, Saccharomyces Genome Database, Pombase, and Candida Genome Database and aligned using Phylogeny.fr. Structural schematics were constructed based on sequence analysis and functional analysis is based on the reported literature, discussed in the text.
SpCuf1 is representative of the third class, with a single, structurally distinct REP motif: CXCX3CXCX2CX2H. SpCuf1 is required for growth on limited copper through binding CuSE motifs in the CTR4 promoter; in the presence of high copper, Cu-REP interaction induces a conformational change masking the N-terminal nuclear localisation signal and inhibiting function [37]. S. pombe lacks metallothineins, but detoxifies copper through a dual function SOD chaperone, PCCS [38].
C. albicans responds to both low and high copper levels via CaMac1, which induces either Cu-SOD1 in high copper or Mn-SOD3 in low copper, along with the CTR1 copper importer; both CaMAC1 and CTR1 are required for hyphal growth, and SOD1 is required for virulence [24, 39, 40, 41]. CaACE1/CUP2 is additionally required for growth on high copper and regulates CUP1 and CRD2 metallothioneins [42, 43]. C. dubliniensis does encode MAC1 and AMT1 homologues that presumably act in a similar fashion, however their targets remain uncharacterised (Figure 2).
Cryptococcus Cuf1 is required for growth on both low and high copper and is structurally distinct from SpCuf1 and ScMac1/Ace1: in addition to the Cuf1 REP motif, a second cysteine-rich repeat (CCX3CX4CXCX3CCXCCXC) is conserved in both C. neoformans and C. deneoformans, estimated to have diverged 18–24 mya [44, 45]. For the outbreak strain C. deuterogattii, Cuf1 sequences in multiple isolates (R265, LA55, RAM5) have undergone truncations that specifically excise the copper responsive REP motifs (Figure 2), although these motifs are maintained in C. decagattii (IND107), C. gattii (EJB2, Ru294, WM276) and C. bacillisporus (CA1873, Ca1280). The relationship between copper and Cuf1 activity has not yet been reported in these organisms.
For both C. neoformans and C. deneoformans, growth in low copper requires CTR1/CTR2 (CNAG_07701) and CTR4 [25, 26••, 46, 47]. While CTR1 is constitutively expressed, CTR4 is specifically induced by low copper in a CUF1-dependent manner. During growth on high copper, CUF1 mediates CMT1 and CMT2 metallothionein expression [46]. Similarly, Lin et al. report that C. deneoformans Mac1/Cuf1 is required for growth on both high and low copper [48]. This dual activity represents a rewiring in comparison to Cuf1 activity in S. pombe, where high copper leads to the sequestration of Cuf1 in the cytoplasm [37]. In addition to growth, copper levels directly influence Cryptococcus virulence through capsule and melanin via Cuf1-independent and Cuf1-dependent mechanisms. In both species, Cuf1 is required for melanin production in a low copper environment, likely because melanin synthesis requires the LAC1 copper-dependent oxidase and a cuf1Δ mutant is unable to acquire sufficient copper from the environment [46, 48, 49, 50]. Lac1 copper loading is mediated by the CCC2 P-type copper transporting ATPase, ATX1 copper chaperone, and the ClC-A chloride channel. However, in copper-replete conditions, Cncuf1Δ melanin defects persist in the absence of growth defects [16•, 47], and the cuf1Δ null is more readily phagocytosed [51]. Although a complete transcriptional analysis of Cuf1 and copper homeostasis during virulence factor induction has not been performed, CTR1 and CTR4, but not CUF1, are induced by the temperature shift sufficient to induce capsule during growth in DMEM [51, 52]. In C. deneoformans, excess copper induces filamentation and is dependent on Ccc2 activity [48]. Interestingly, a C. albicans gpa2Δ/Δ mutant is deficient for CTR1 and FRE7 copper importer expression and inappropriately expresses CRD2, suggesting a role for cAMP/PKA in copper homeostasis and hyphal growth. In C. neoformans, cAMP/PKA regulates melanin and capsule via integration with the pH-responsive transcription factor Rim101 and ESCRT [53, 54]. Cnrim101 and ESCRT mutants vps25 and rim20 are sensitive to low copper, although capsule and immune evasion defects in these mutants are likely due to changes in the cell wall rather than defects in expression [51, 55]. CnCCC2 and ATX1 are also required for growth on low iron, likely due to the interaction between copper and iron uptake [49].
In summary, Candida and Cryptococcus species experience significant shifts in zinc and copper availability upon transitions between commensal or environmental and infective stages. It would appear that certain successful fungal pathogens have hardwired virulence factor expression into their metal ion sensing machinery and may use the metal ion environment of the host as a key signal. A major challenge for the future will be to understand how these signals are integrated, and whether we can therapeutically target these pathways to treat fungal infections.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
ERB is funded by the BBSRC (BB/M014525/1). DW is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 102549/Z/13/Z). We additionally acknowledge the MRC and University of Aberdeen for funding (MR/N006364/1) and the Wellcome Trust Strategic Award for Medical Mycology and Fungal Immunology (097377/Z/11/Z). Finally, we acknowledge FungiDB and the Candida Genome Database [56, 57].
References
- 1.Skaar E.P. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010;6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood M.I., Skaar E.P. Nutritional immunity: transition metals at the pathogen–host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potrykus J., Ballou E.R., Childers D.S., Brown A.J. Conflicting interests in the pathogen–host tug of war: fungal micronutrient scavenging versus mammalian nutritional immunity. PLoS Pathog. 2014;10:e1003910. doi: 10.1371/journal.ppat.1003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian Vignesh K., Landero Figueroa J.A., Porollo A., Caruso J.A., Deepe G.S., Jr. Zinc sequestration: arming phagocyte defense against fungal attack. PLoS Pathog. 2013;9:e1003815. doi: 10.1371/journal.ppat.1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson D., Citiulo F., Hube B. Zinc exploitation by pathogenic fungi. PLoS Pathog. 2012;8:e1003034. doi: 10.1371/journal.ppat.1003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford A., Wilson D. Essential metals at the host–pathogen interface: nutritional immunity and micronutrient assimilation by human fungal pathogens. FEMS Yeast Res. 2015:15. doi: 10.1093/femsyr/fov071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban C.F., Ermert D., Schmid M., Abu-Abed U., Goosmann C. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Hein K.Z., Takahashi H., Tsumori T., Yasui Y., Nanjoh Y. Disulphide-reduced psoriasin is a human apoptosis-inducing broad-spectrum fungicide. Proc Natl Acad Sci U S A. 2015;112:13039–13044. doi: 10.1073/pnas.1511197112. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hein et al. found that another S100A7 protein family member, psoriasin, also elicits antifungal activity via zin sequestration, suggesting that multiple members of this large protein family may contribute to nutritional immunty.
- 9.Subramanian Vignesh K., Landero Figueroa J.A., Porollo A., Caruso J.A., Deepe G.S., Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity. 2013;39:697–710. doi: 10.1016/j.immuni.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson S., Bird A.J. Zinc sensing and regulation in yeast model systems. Arch Biochem Biophys. 2016 doi: 10.1016/j.abb.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobile C.J., Nett J.E., Hernday A.D., Homann O.R., Deneault J.S. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 2009;7:e1000133. doi: 10.1371/journal.pbio.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Bottcher B., Palige K., Jacobsen I.D., Hube B., Brunke S. Csr1/Zap1 maintains zinc homeostasis and influences virulence in Candida dubliniensis but is not coupled to morphogenesis. Eukaryot Cell. 2015;14:661–670. doi: 10.1128/EC.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bottcher et al. reported that, in stark contrast to its close relative, C. albicans, the Zap1 transcription factor of C. dubliniensis does not regulate morphogenesis. This indicates that Zap1 governs different pathogenic properties in very closely realted species.
- 13.Moreno M.A., Ibrahim-Granet O., Vicentefranqueira R., Amich J., Ave P. The regulation of zinc homeostasis by the ZafA transcriptional activator is essential for Aspergillus fumigatus virulence. Mol Microbiol. 2007;64:1182–1197. doi: 10.1111/j.1365-2958.2007.05726.x. [DOI] [PubMed] [Google Scholar]
- 14.Schneider Rde O., Fogaca Nde S., Kmetzsch L., Schrank A., Vainstein M.H. Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii. PLoS One. 2012;7:e43773. doi: 10.1371/journal.pone.0043773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu O.W., Chun C.D., Chow E.D., Chen C., Madhani H.D. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell. 2008;135:174–188. doi: 10.1016/j.cell.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Jung K.W., Yang D.H., Maeng S., Lee K.T., So Y.S. Systematic functional profiling of transcription factor networks in Cryptococcus neoformans. Nat Commun. 2015;6:6757. doi: 10.1038/ncomms7757. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jung et al. performed a comprehensive mutant analysis for 155 predicted C. neoformans zinc-finger transcription factors mutants, reporting the first phenotypic analysis for 85% of these ORFs across 32 conditions, including cell wall, temperature, and chemicals stresses, virulence factor regulation, and impact on virulence in the Galleria mellonella and murine models of infection.
- 17.Butler G., Rasmussen M.D., Lin M.F., Santos M.A., Sakthikumar S. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson A.P., Gamble J.A., Yeomans T., Moran G.P., Saunders D. Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans. Genome Res. 2009;19:2231–2244. doi: 10.1101/gr.097501.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor J.W., Berbee M.L. Dating divergences in the fungal tree of life: review and new analyses. Mycologia. 2006;98:838–849. doi: 10.3852/mycologia.98.6.838. [DOI] [PubMed] [Google Scholar]
- 20.Kim M.J., Kil M., Jung J.H., Kim J. Roles of zinc-responsive transcription factor Csr1 in filamentous growth of the pathogenic yeast Candida albicans. J Microbiol Biotechnol. 2008;18:242–247. [PubMed] [Google Scholar]
- 21.Zhang Q., Tao L., Guan G., Yue H., Liang W. Regulation of filamentation in the human fungal pathogen Candida tropicalis. Mol Microbiol. 2016;99:528–545. doi: 10.1111/mmi.13247. [DOI] [PubMed] [Google Scholar]
- 22.Finkel J.S., Xu W., Huang D., Hill E.M., Desai J.V. Portrait of Candida albicans adherence regulators. PLoS Pathog. 2012;8:e1002525. doi: 10.1371/journal.ppat.1002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronstad J.W., Hu G., Jung W.H. An encapsulation of iron homeostasis and virulence in Cryptococcus neoformans. Trends Microbiol. 2013;21:457–465. doi: 10.1016/j.tim.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samanovic M.I., Ding C., Thiele D.J., Darwin K.H. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe. 2012;11:106–115. doi: 10.1016/j.chom.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C.X., Gleason J.E., Zhang S.X., Bruno V.M., Cormack B.P. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. 2015;112:E5336–E5342. doi: 10.1073/pnas.1513447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Ding C., Festa R.A., Chen Y.-L., Espart A., Palacios Ò. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe. 2013;13:265–276. doi: 10.1016/j.chom.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; C. albicans can defend itself against the oxidative burst of innate immunity in the absence of zinc by utilising a ‘copper-only’ superoxide dismutase, Sod5.
- 27.Waterman S.R., Park Y.D., Raja M., Qiu J., Hammoud D.A. Role of CTR4 in the virulence of Cryptococcus neoformans. mBio. 2012:3. doi: 10.1128/mBio.00285-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun T.S., Ju X., Gao H.L., Wang T., Thiele D.J. Reciprocal functions of Cryptococcus neoformans copper homeostasis machinery during pulmonary infection and meningoencephalitis. Nat Commun. 2014;5:5550. doi: 10.1038/ncomms6550. [DOI] [PubMed] [Google Scholar]
- 29•.Waggoner D.J., Drisaldi B., Bartnikas T.B., Casareno R.L., Prohaska J.R. Brain copper content and cuproenzyme activity do not vary with prion protein expression level. J Biol Chem. 2000;275:7455–7458. doi: 10.1074/jbc.275.11.7455. [DOI] [PubMed] [Google Scholar]; Sun and colleagues demonstrate in vivo the requirement for a dynamic response to changing copper availability during C. neoformans infection and carefully dissect conflicting data regarding Ctr1/4 function. They show that resistance to copper toxicity through deletion of CTR4 leads to increased proliferation in the lung, independent of Cmt1/Cmt2 activity, and that Ctr activity is required for full virulence in the brain.
- 30.Beaudoin J., Labbe S. The fission yeast copper-sensing transcription factor Cuf1 regulates the copper transporter gene expression through an Ace1/Amt1-like recognition sequence. J Biol Chem. 2001;276:15472–15480. doi: 10.1074/jbc.M011256200. [DOI] [PubMed] [Google Scholar]
- 31.Keller G., Gross C., Kelleher M., Winge D.R. Functional independence of the two cysteine-rich activation domains in the yeast Mac1 transcription factor. J Biol Chem. 2000;275:29193–29199. doi: 10.1074/jbc.M001552200. [DOI] [PubMed] [Google Scholar]
- 32.Voutsina A., Fragiadakis G.S., Boutla A., Alexandraki D. The second cysteine-rich domain of Mac1p is a potent transactivator that modulates DNA binding efficiency and functionality of the protein. FEBS Lett. 2001;494:38–43. doi: 10.1016/s0014-5793(01)02298-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Z., Labbe S., Pena M.M., Thiele D.J. Copper differentially regulates the activity and degradation of yeast Mac1 transcription factor. J Biol Chem. 1998;273:1277–1280. doi: 10.1074/jbc.273.3.1277. [DOI] [PubMed] [Google Scholar]
- 34.Winge D. Copper-regulatory domain involved in gene expression. Prog Nucleic Acid Res Mol Biol. 1997;58:165–195. doi: 10.1016/s0079-6603(08)60036-7. [DOI] [PubMed] [Google Scholar]
- 35.Wood L.K., Thiele D.J. Transcriptional activation in yeast in response to copper deficiency involves copper-zinc superoxide dismutase. J Biol Chem. 2009;284:404–413. doi: 10.1074/jbc.M807027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch K.A., Thiele D.J. Autoactivation by a Candida glabrata copper metalloregulatory transcription factor requires critical minor groove interactions. Mol Cell Biol. 1996;16:724–734. doi: 10.1128/mcb.16.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorvaldsen J.L., Sewell A.K., McCowen C.L., Winge D.R. Regulation of metallothionein genes by the ACE1 and AMT1 transcription factors. J Biol Chem. 1993;268:12512–12518. [PubMed] [Google Scholar]
- 38.Beaudoin J., Labbe S. Copper induces cytoplasmic retention of fission yeast transcription factor cuf1. Eukaryot Cell. 2006;5:277–292. doi: 10.1128/EC.5.2.277-292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laliberte J., Whitson L.J., Beaudoin J., Holloway S.P., Hart P.J. The Schizosaccharomyces pombe Pccs protein functions in both copper trafficking and metal detoxification pathways. J Biol Chem. 2004;279:28744–28755. doi: 10.1074/jbc.M403426200. [DOI] [PubMed] [Google Scholar]
- 40.Hwang C.S., Rhie G.E., Oh J.H., Huh W.K., Yim H.S. Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology. 2002;148:3705–3713. doi: 10.1099/00221287-148-11-3705. [DOI] [PubMed] [Google Scholar]
- 41.Marvin M.E., Mason R.P., Cashmore A.M. The CaCTR1 gene is required for high-affinity iron uptake and is transcriptionally controlled by a copper-sensing transactivator encoded by CaMAC1. Microbiology. 2004;150:2197–2208. doi: 10.1099/mic.0.27004-0. [DOI] [PubMed] [Google Scholar]
- 42.Huang G.H., Nie X.Y., Chen J.Y. CaMac1, a Candida albicans copper ion-sensing transcription factor, promotes filamentous and invasive growth in Saccharomyces cerevisiae. Acta Biochim Biophys Sin (Shanghai) 2006;38:213–217. doi: 10.1111/j.1745-7270.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 43.Homann O.R., Dea J., Noble S.M., Johnson A.D. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009;5:e1000783. doi: 10.1371/journal.pgen.1000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz J.A., Olarte K.T., Michalek J.L., Jandu G.S., Michel S.L. Regulation of copper toxicity by Candida albicans GPA2. Eukaryot Cell. 2013;12:954–961. doi: 10.1128/EC.00344-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang N., Liu X., Yang J., Li Z., Pan J. Regulation of copper homeostasis by Cuf1 associates with its subcellular localization in the pathogenic yeast Cryptococcus neoformans H99. FEMS Yeast Res. 2011;11:440–448. doi: 10.1111/j.1567-1364.2011.00733.x. [DOI] [PubMed] [Google Scholar]
- 46.Hagen F., Khayhan K., Theelen B., Kolecka A., Polacheck I. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Ding C., Yin J., Tovar E.M., Fitzpatrick D.A., Higgins D.G. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol Microbiol. 2011;81:1560–1576. doi: 10.1111/j.1365-2958.2011.07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waterman S.R., Hacham M., Hu G., Zhu X., Park Y.D. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J Clin Invest. 2007;117:794–802. doi: 10.1172/JCI30006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin X., Huang J.C., Mitchell T.G., Heitman J. Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATalpha allele enhances filamentation. PLoS Genet. 2006;2:e187. doi: 10.1371/journal.pgen.0020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walton F.J., Idnurm A., Heitman J. Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol Microbiol. 2005;57:1381–1396. doi: 10.1111/j.1365-2958.2005.04779.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhu X., Gibbons J., Zhang S., Williamson P.R. Copper-mediated reversal of defective laccase in a Deltavph1 avirulent mutant of Cryptococcus neoformans. Mol Microbiol. 2003;47:1007–1014. doi: 10.1046/j.1365-2958.2003.03340.x. [DOI] [PubMed] [Google Scholar]
- 52.Chun C.D., Madhani H.D. Ctr2 links copper homeostasis to polysaccharide capsule formation and phagocytosis inhibition in the human fungal pathogen Cryptococcus neoformans. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0012503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haynes B.C., Skowyra M.L., Spencer S.J., Gish S.R., Williams M. Toward an integrated model of capsule regulation in Cryptococcus neoformans. PLoS Pathog. 2011;7:e1002411. doi: 10.1371/journal.ppat.1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Meara T.R., Xu W., Selvig K.M., O’Meara M.J., Mitchell A.P. The Cryptococcus neoformans Rim101 transcription factor directly regulates genes required for adaptation to the host. Mol Cell Biol. 2014;34:673–684. doi: 10.1128/MCB.01359-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Meara T.R., Holmer S.M., Selvig K., Dietrich F., Alspaugh J.A. Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. mBio. 2013:4. doi: 10.1128/mBio.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stajich J.E., Harris T., Brunk B.P., Brestelli J., Fischer S. FungiDB: an integrated functional genomics database for fungi. Nucleic Acids Res. 2012;40:D675–D681. doi: 10.1093/nar/gkr918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inglis D.O., Arnaud M.B., Binkley J., Shah P., Skrzypek M.S. The Candida Genome Database incorporates multiple Candida species: multispecies search and analysis tools with curated gene and protein information for Candida albicans and Candida glabrata. Nucleic Acids Res. 2012;40:D667–D674. doi: 10.1093/nar/gkr945. [DOI] [PMC free article] [PubMed] [Google Scholar]