Abstract
Tremorogenic β-carboline alkaloids are present in foodstuffs and beverages. Acute exposure to β-carboline derivatives causes severe tremor; however, the disposition of these dietary contaminants remains unclear. This study was performed to evaluate toxicokinetics of harmane and harmine, two major β-carboline alkaloids, in rats. Blood concentrations of both toxicants were quantified by high-performance liquid chromatography (HPLC). Following an intravenous injection (0.5 mg/ kg), the concentration–time profiles of harmane or harmine fit well with a two-compartment model. While both compounds had comparable elimination t1/ 2β (24 and 26 min for harmane and harmine, respectively), the systemic clearance (CLs) for harmine (103.2 ml/ kg/ml) was two times greater than that for harmane (52.2 ml/ kg/ml). Accordingly, the area under the blood concentration–time curve (AUC) in harmane-treated rats was 2.7-fold greater than that in harmine-treated rats. Harmine appeared to distribute to tissues better than harmane, with a larger volume of distribution (Vd) (3.9 and 1.6 L/ kg for harmine and harmane, respectively). After an oral dose (20 mg/ kg), the absolute bioavailability (F) was 19% for harmane and 3% for harmine. Harmane was absorbed more slowly (lower Ka), yet more completely (higher Cmax, AUC, and F) than harmine. An oral administration of harmane resulted in blood harmine whose formation accounted for 13% of the ingested harmane, indicating a biotransformation of harmane to harmine. These results suggest that harmane is absorbed into the systemic circulation more completely than harmine. Upon entering the body, harmane can be metabolized to form harmine; the latter may better distribute to the tissue compartment.
Essential tremor, one of the most common neurological diseases, is 20 times more prevalent than Parkinson’s disease, making it the most common tremor disorder (Louis et al., 1998; Rautakorpi et al., 1982). There are kindreds with an autosomal dominantly inherited form of essential tremor, and in small number, linkage has been demonstrated to regions of chromosomes 2p and 3q (Gulcher et al., 1997; Higgins et al., 1997). In most clinical series, however, sporadic forms of the disease account for more than 50% of essential tremor cases, arguing for the presence of non-genetic, environmental causes of this disease (Louis, 2001).
While the specific environmental factors associated with essential tremor remain unknown, a number of β-carboline alkaloids have been proposed as possible risk factors for this disease (Louis, 2001). The tremorogenic β-carbolines include harmane, harmine, harmaline, ibogaine, and others. These β-carbolines are present in common plant-derived food-stuffs (e.g., wheat, rice, corn, barley, soybeans, rye, grapes, mushrooms, and vinegar), plant-derived beverages (e.g., wine, beer, whisky, brandy, and sake), and plant-derived inhaled substances (e.g., tobacco) (Adachi et al., 1991; Poindexter & Carpenter, 1962; Takeuchi et al., 1973). Some of these substances have also been isolated from animal tissues such as beef and sardines (Adachi et al., 1991; Rommelspacher et al., 1982).
Exposure of laboratory animals (including mice, cats, rabbits, and monkeys) to these chemicals results in an acute action tremor (Du et al., 1997; Fuentes & Longo, 1971; O’Hearn & Molliver, 1997). Moreover, human volunteers exposed to harmine display neurological side effects including an acute coarse tremor (Lewin, 1928; Pennes & Hoch, 1957). Because of their natural presence in the food chain, there appears to be a risk associated with exposure to these β-carbolines from dietary sources, smoking, and consumption of alcoholic beverages. In fact, the occurrence of β-carbolines in human blood and excreta under normal physiological conditions has been reported in the literature (Allen et al., 1980; Bidder et al., 1979; Rommelspacher et al., 1980). Although there have been extensive studies of the tremorogenic properties of β-carboline alkaloids, few toxicokinetic studies have been performed to elucidate the disposition properties of β-carboline derivatives in laboratory animals. Zetler et al. (1972, 1974) studied brain distribution and pharmacokinetics of several β-carbolines after an intravenous (iv) injection using a nonspecific fluorescence spectroscopic method, yet the time courses of these alkaloids in blood following oral doses were not investigated. Thus, the critical toxicokinetic parameters of β-carbolines such as oral bioavailability, rate of elimination, volume of distribution, and systemic clearance were largely unknown. Lack of such information limits the interpretation of the results of a variety of toxicological studies conducted in animals as well as humans.
In the current study, a newly developed high-performance liquid chromatography (HPLC) method (Zheng et al., 2000a) was used to investigate the toxicokinetics of harmane and harmine, two of the major β-carboline derivatives, following iv or oral administration to rats. An important aspect of this study was determination of the time dependency of blood concentrations of harmane or harmine and their absolute bioavailability after oral ingestion. In addition, the biotransformation between harmane and harmine in this species was investigated.
MATERIALS AND METHODS
Chemicals
Chemicals were obtained from the following sources: potassium phosphates, ethyl acetate, methyl t-butyl ether, and methanol from Fisher Scientific (Pittsburgh, PA); harmane (1-methyl-9H-pyrido[3,4-β]indole, molecular weight [MW] 182.2) and harmine (7-methoxy-1-methyl-9H-pyrido[3,4-β]-indole, MW 212.3) from Sigma (St. Louis, MO). All reagents were of analytical grade, HPLC grade, or the best available pharmaceutical grade.
Animals
Male Sprague-Dawley rats were purchased from Hilltop and weighed 200–250 g (2 mo old) at the time of the experiments. The animals were housed in a temperature-controlled, 12:12-h light/dark room, and were allowed free access to tap water and food (Teklad 4% mouse–rat diet, Teklad, Madison, WI). For the oral dosing study, animals were fasted for 12 h prior to administration of harmane or harmine.
Administration of Harmane or Harmine to Animals
For the iv dosing study, harmane or harmine was dissolved in 0.1 N H2SO4 as a stock solution (4 mg/ml). The stock solution was diluted with 0.9% NaCl to the final concentration of 0.1 mg/ml and injected via the tail vein at a dose of 0.5 mg/kg (2.7 and 2.4 μmol/kg for harmane and harmine, respectively) over approximately 5 s. For the oral dosing study, harmane or harmine was dissolved in corn oil (4 mg/ml) and administered by a single gavage at a dose of 20 mg/kg (110 and 94 μmol/kg for harmane and harmine, respectively). This dose regimen was chosen because it produced detectable blood concentration–time profiles, as determined from our preliminary experiments.
Collection of Blood Samples
At the appropriate times, rats were subjected to light anesthesia with ether. Blood samples (0.1–0.5 ml) were collected from the orbital sinus through a heparin-pretreated glass capillary tube and transferred to an Eppendorf tube. For the iv dosing study, blood was collected at 1, 3, 5, 10, 15, 30, 60, 90, and 120 min following iv injection. For the oral dosing study, blood was collected at 3, 5, 10, 15, 30, 60, 90, 120, and 240 min following oral gavage. All blood samples were processed immediately for extraction and HPLC analyses as described later.
Sample Preparation
A novel HPLC method for quantifying harmine and harmane in blood has been previously reported (Zheng et al., 2000a). In brief, 1 volume (0.1–0.5 ml) of whole blood was mixed with half a volume (0.05–0.25 ml) of 1 M NaOH. Following vortex for 30 s, the samples were placed on a horizontal rotator and shaken at room temperature for 30 min. An aliquot (0.5 ml) of the extraction solution consisting of ethyl acetate and methyl t-butyl ether (2:98, v:v) was added to the tube. The tube was then vigorously shaken by hand for 1–2 min, followed by shaking on a horizontal rotor at room temperature for 45 min. After centrifugation at 3000 × g for 10 min, the upper organic phase was separated and transferred to another Eppendorf tube. The extraction procedure was repeated two additional times. The organic phase was combined and evaporated under nitrogen to dryness. The samples were reconstructed in 0.25 ml of methanol. After centrifugation at 3000 × g for 10 min, the supernatant were transferred to autosampler vials with sealed caps for HPLC analysis.
HPLC Analysis
A Perkin-Elmer model LC-250 binary liquid chromatographic system equipped with an LC-600 autosampler and an LS-40 fluorescence detector was used for separation and quantitation. Separation was accomplished using an ion-interaction, reversed-phase Econosphere C18 column (ODS2, 5 μm, 250 × 4.6 mm) attached to a Spherisorb guard column (ODS2, 5 μm, 10 × 4.6 mm). Both analytical and guard columns were purchased from Alltech (Deerfield, IL). An isocratic mobile phase consisted of 17.5 mM potassium phosphate buffer, pH 6.5 (equal molar concentration of both monobasic and dibasic potassium salts), and methanol (30:70, v:v). A 50-μl aliquot of sample extracts was injected and the separation was performed at room temperature at a flow rate of 1 ml/min. The detector was set at an excitation wavelength of 300 nm and an emission wavelength of 435 nm. A Macintosh computer equipped with Mac Integrator II (Rainin) was used to collect and analyze the data.
The identity of harmane and harmine on HPLC chromatographs has been previously clarified (Zheng et al., 2000a). Retention times were about 7–8 min for harmane and 10–11 min for harmine. The detection limit at 0.5 ml blood volume for harmane was 4.2 ng/ml and for harmine was 1.6 ng/ ml. The intraday precision, measured as a coefficient of variation (CV) at 15 ng/ml, was less than 7% for harmane and 3% for harmine. The interday precision was 11% for harmane and 10% for harmine. The absolute recovery from rat blood was 56% and 64% for harmane and harmine, respectively.
Toxicokinetic Analysis
Toxicokinetic data were evaluated in the same way as previously described by this group of investigators (Zheng et al., 1993, 1994, 2000b). Each individual data set was evaluated by a pharmacokinetic data analysis program PKAnalyst (MicroMath, Inc., Salt Lake City, UT). For the iv dosing study, the model that best described the observed data was a two-compartment model with first-order elimination from the central compartment (Gibaldi & Perrier, 1982) and represented by the following biexponential equation:
| (1) |
where C(t) is the plasma concentration at time t, and A and B are the multiexponential coefficients. Values of α and β represent the initial-phase disposition rate constant and terminal-phase disposition rate constant, respectively. PKAnalyst was used to generate the best-fit critical toxicokinetic parameters for each animal, including α, A, B, elimination rate constant (k10), half-life of initial (distribution) phase (t1/2α), half-life of terminal (elimination) phase (t1/2β), intercompartmental transfer rate constants (k12 and k21), and the total area under the blood concentration–time curve (AUC). The systemic or total body clearance (CLs) was computed by dividing the iv dose by AUC. The total volume of distribution (Vd) was calculated from the relationship Vd = CLs/β, and the apparent volume of the central compartment (Vc) was determined by the equation Vc = Doseiv/(A + B).
For the oral dosing studies, the blood concentration–time data were well fit by a one-compartment model with first-order input and represented by the following biexponential equation (Gibaldi & Perrier, 1982):
| (2) |
where C(t) is the blood concentration at time t, and A is the multiexponential coefficient. Ke and Ka represent the elimination rate constant and absorption rate constant, respectively. All parameters including A, Ke, Ka, absorption half-life (t1/2a), elimination half-life (t1/2e), maximum blood concentration (Cmax), time to reach the maximum blood concentration (Tmax), and AUC were computed by PKAnalyst. The absolute oral bioavailability (F) of harmane or harmine was estimated from the ratio of AUCpo/AUCiv and corrected for differences in doses.
In some cases, the metabolism of harmane to harmine was identified following a single oral dose of harmane. The fraction metabolized (Fm) was then calculated using the equation as follows (Gibaldi & Perrier, 1982):
| (3) |
where AUCHI′ is the area under the curve of harmine (metabolite) concentration in blood versus time from zero time to infinity after an iv dose of harmane (as a parent compound), and AUCHI is the total area under the harmine concentration-time curve after an equimolar iv dose of harmine. DHA and DHI represent molar doses of harmane and harmine, respectively.
Statistics
All data are presented as mean ± SE. Statistical analysis for comparison of two means was performed using one-way analysis of variance (ANOVA). In all cases, a probability level of p < .05 was considered as the criterion of significance.
RESULTS
Intravenous Dose of Harmane or Harmine
Figure 1A delineates the blood concentration–time profiles of harmane in rats, as well as a representation of the model fitted to the observed data, after an iv bolus injection of harmane at 0.5 mg/kg in Sprague-Dawley rats. The blood concentration–time profile of harmane followed the biexponential equation:
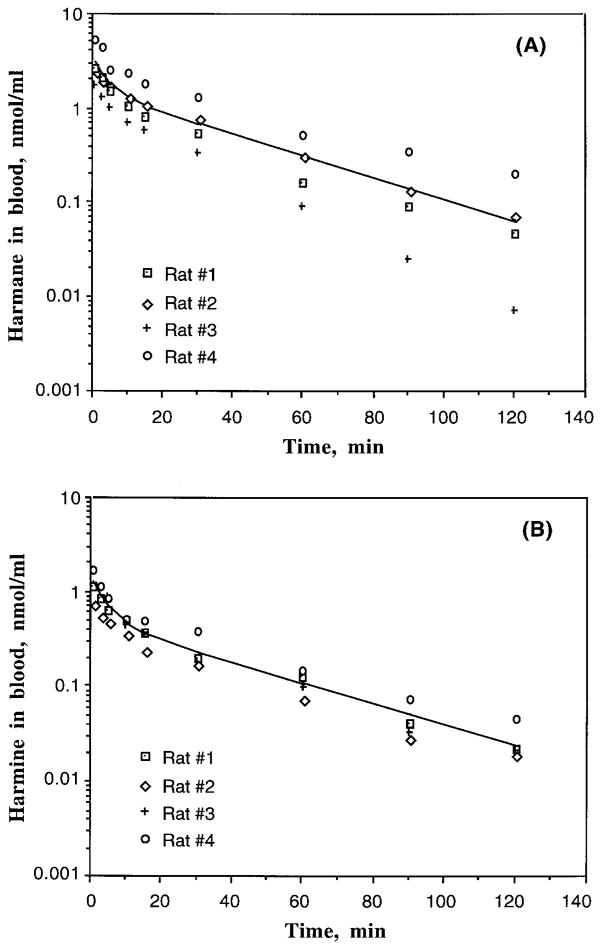
FIGURE 1.
Blood concentration–time profiles of harmane or harmine in male Sprague-Dawley rats following an iv administration of 0.5 mg/kg of either harmane or harmine. Blood concentrations of harmane and harmine were determined by HPLC after sample extraction. The lines indicate the best fit of a two-compartment model with first-order elimination from the central compartment to the observed data: (A) harmane in rat blood (n = 4); (B) harmine in rat blood (n = 4).
This kinetic model, with first-order elimination from the central compartment, provided a good fit to the individual observed data. The toxicokinetic parameters generated from this model are presented in Table 1. Harmane was rapidly eliminated from the blood with a fast first-order initial disposition t1/2α of 2 min and a slower terminal elimination t1/2β of 24 min. By 3 h, the concentrations of harmane in most blood samples were below the detection limit. While the total volume of distribution (Vd) of harmane was 1.63 L/kg, the central volume of distribution (Vc) was only 0.75 L/kg, suggesting a distribution of harmane to the peripheral compartment following iv dose administration. The total body clearance (CLs) of harmane was nearly equivalent to rat hepatic blood flow, which is between 52 and 80 ml/kg/min (Hollinger, 1995). This indicates that the liver may play a role in elimination of harmane.
TABLE 1.
Toxicokinetic Parameters of Harmane and Harmine in Sprague-Dawley Rats Following a Single Intravenous Injection (0.5 mg/kg)
| Parameters | Harmane (n = 4) | Harmine (n = 4) |
|---|---|---|
| A (nmol/ml) | 2.507 ± 0.660 | 1.032 ± 0.212 |
| α (min−1) | 0.385 ± 0.093 | 0.267 ± 0.028 |
| B (nmol/ml) | 1.598 ± 0.330 | 0.528 ± 0.079 |
| β (min−1) | 0.030 ± 0.004 | 0.026 ± 0.001 |
| t1/2α (min) | 2.070 ± 0.380 | 2.700 ± 0.320 |
| t1/2β (min) | 24.44 ± 2.670 | 26.47 ± 0.930 |
| k21 (min−1) | 0.163 ± 0.023 | 0.114 ± 0.019 |
| k10 (min−1) | 0.071 ± 0.017 | 0.064 ± 0.005 |
| k12 (min−1) | 0.181 ± 0.062 | 0.115 ± 0.014 |
| AUC (nmol-min/ml) | 66.76 ± 19.74 | 24.62 ± 3.890a |
| CLs (ml/kg/min) | 52.24 ± 13.80 | 103.2 ± 16.41a |
| Vc (L/kg) | 0.750 ± 0.130 | 1.640 ± 0.290a |
| Vd (L/kg) | 1.630 ± 0.320 | 3.890 ± 0.540a |
Note. Male rats were administered iv bolus dose of harmine or harmane (0.5 mg/kg). Parameters were computed from the blood concentration–time curves of each animal by a two-compartment model. Data represent the mean ± SE, n = 4.
Significant at p < .05, compared to values in the harmane group.
After an iv bolus injection of harmine, the blood concentration–time profile of harmine can be described by the following biexponential equation:
Similar to harmane, a two-compartment model with first-order elimination from the central compartment gave the best fit to the observed data (Figure 1B). While the initial and terminal disposition rate constants of harmine were comparable to those of harmane, the values of Vc and Vd from the harmine study were approximately two times higher, significantly higher than those in the harmane study (Table 1). The observation implies that harmine may be better distributed to the tissue compartment than harmane. By comparison with systemic clearance, harmine was cleared from the blood two times faster, significantly faster than harmane (Table 1). Furthermore, the AUC of harmine was nearly three times less than that of harmane. The combination of larger tissue distribution and faster body clearance of harmine may explain its smaller AUC as compared to harmane.
Oral Dose of Harmane or Harmine
Following a single oral dose of harmane at 20 mg/kg, harmane appeared rapidly in blood (Figure 2A). The blood concentration–time profiles of harmane in animals followed a one-compartment model with first-order input and first-order elimination from the central compartment:
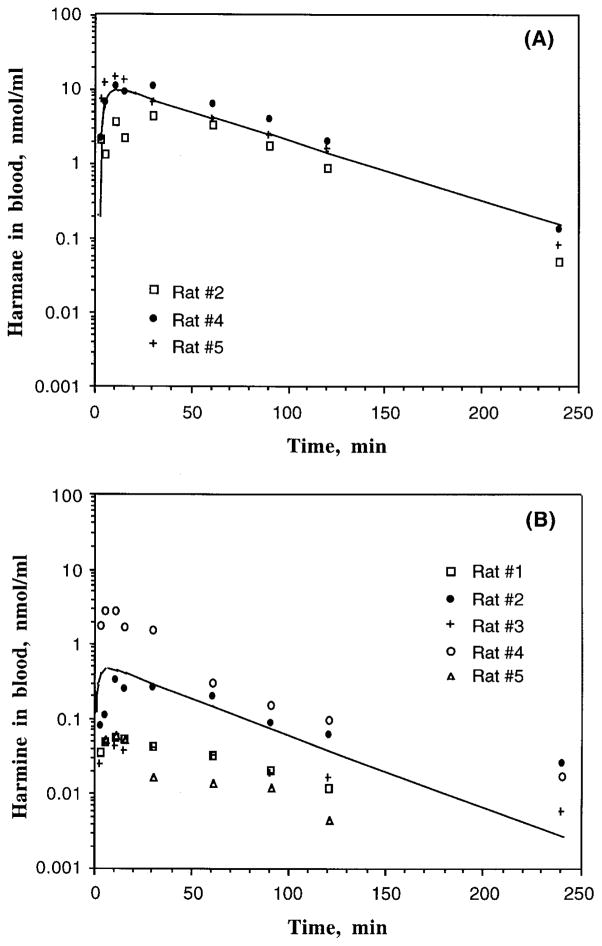
FIGURE 2.
Blood concentration–time profiles of harmane or harmine in male Sprague-Dawley rats following a single oral dose of either harmane or harmine at 20 mg/kg by oral gavage in corn oil. Blood concentrations of harmane and harmine were determined by HPLC after sample extraction. The lines indicate the best fit of a one-compartment model with first-order absorption to the observed data: (A) harmane in rat blood (n = 3); (B) harmine in rat blood (n = 5).
The average Cmax of harmane was achieved at about 20 min following oral dosing. Thereafter, the concentrations of harmane in blood declined rapidly and the terminal phase followed first-order elimination kinetics with a t1/2e of 29 min (Table 2). Oral administration of harmane did not greatly change the terminal t1/2e as compared to rats receiving an iv injection (t1/2β = 24 min, Table 1). The absolute oral bioavailability (F) of harmane following a single oral gavage was 19%, although the linearity of oral bioavailability within the dose range of 0.5–20 mg/kg is uncertain. Interestingly, harmine as a metabolite was identified in the blood of two animals receiving the oral dose of harmane. This metabolism of harmane to harmine is described in the following section.
TABLE 2.
Toxicokinetic Parameters of Harmane and Harmine in Sprague-Dawley Rats Following a Single Oral Gavage (20 mg/kg)
| Parameters | Harmane (n = 3) | Harmine (n = 5) |
|---|---|---|
| A (nmol/ml) | 24.47 ± 3.191 | 0.897 ± 0.730 |
| Ka (min−1) | 0.128 ± 0.073 | 0.705 ± 0.372a |
| Ke (min−1) | 0.024 ± 0.001 | 0.020 ± 0.005 |
| t1/2a (min) | 9.890 ± 4.330 | 2.680 ± 1.310a |
| t1/2e (min) | 29.21 ± 1.520 | 47.13 ± 13.63 |
| Tmax (min) | 21.47 ± 6.180 | 9.700 ± 3.490a |
| Cmax (nmol/ml) | 9.588 ± 2.763 | 0.601 ± 0.495a |
| AUC (nmol-min/ml) | 647.1 ± 155.1 | 25.61 ± 16.78a |
| F (%) | 19.1 ± 3.8 | 3.1 ± 2.5a |
Note. Male rats were administered with oral dose of harmane and harmine (20 mg/kg by gavage in corn oil). Parameters were computed from the blood concentration–time curves of each animal by a one-compartment model with the first-order absorption. Data represent the mean ± SE, n = 3 for harmane and n = 5 for harmine.
Significant at p < .05, compared to values in the harmane group.
The blood concentration–time profiles of harmine in rats after a single oral gavage can also be described by a one-compartment model as follows (Figure 2B):
Harmine appeared to be more rapidly absorbed from the gastrointestinal tract than harmane, with an absorption t1/2a of 3 min as compared with t1/2a of 10 min in the harmane study (Table 2). The time to achieve Cmax was much faster following oral dose of harmine (average Tmax = 9.7 min) than that following oral ingestion of harmane (average Tmax = 21.5 min); however, the average Cmax of harmine (0.60 nmol/ml) was 16 times less than that of harmane (9.6 nmol/ml). Harmine had a longer terminal elimination t1/2e than harmane. The absolute oral bioavailability of harmine was only 3.1%, about 6 times less than that of harmane. These observations suggest a rapid, yet poor bioavailability of harmine in comparison with harmane. Consequently, the AUC of harmine was 25 times less than that of harmane (Table 2). It is worth noting that the blood concentration–time profiles of harmine varied greatly among the tested animals.
Biotransformation of Harmane to Harmine Following Oral Dose
Of a total of seven animals (five in experiments and two in preliminary studies) receiving oral administration of harmane, harmine was identified in the blood samples of two animals. Figure 3 depicts a typical HPLC chromatogram, showing the peaks that correspond to parent harmane and metabolite harmine. The metabolite harmine appeared to have a longer elimination t1/2e than the parent harmane (Figure 4). The times to achieve the Cmax of harmane and harmine in the rats who metabolized harmane to harmine (Tmax = 3 min for harmane and 5 min for harmine, Table 3) were much shorter than those in rats who did not metabolize harmane (Tmax 21 min for harmane and 10 min for harmine, Table 2). It is notable that the average Cmax of harmane in rats capable of metabolizing harmane (21 nmol/ml) was about two times higher than that in rats lacking the ability to metabolize harmane (9.6 nmol/ml).
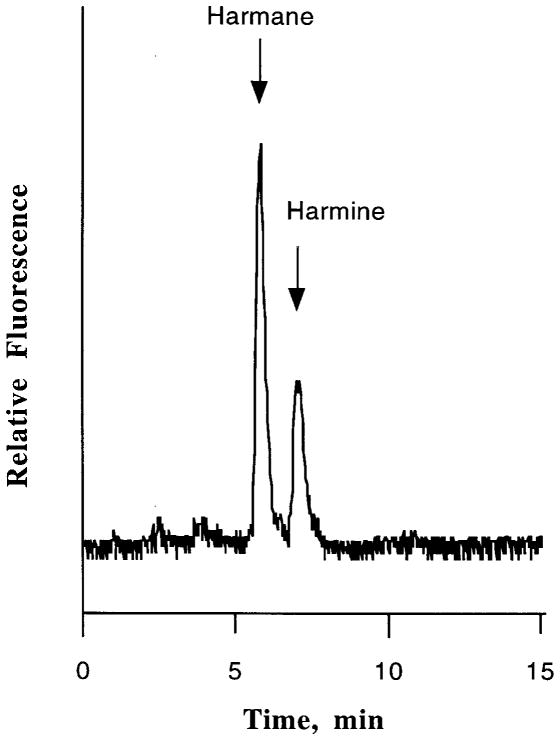
FIGURE 3.
A typical HPLC chromatogram of harmane and its metabolite harmine in rat blood following a single oral dose of harmane (20 mg/kg). The data represent a 10-min blood sample collected from rat 1.
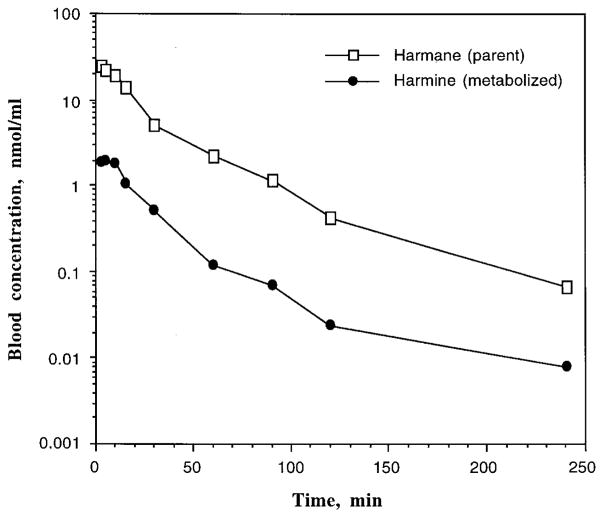
FIGURE 4.
Blood concentration–time profiles of harmane (parent compound) and harmine (metabolite) of rat 1 following a single oral dose of harmane at 20 mg/kg by gavage.
TABLE 3.
Toxicokinetic Parameters of Harmane (Parent Comound Drug) and Harmine (Metabolite) in Sprague-Dawley Rats Following a Single Oral Gavage of Harmane (20 mg/kg)
| Parameters | Harmane | Harmine |
|---|---|---|
| Ke (min−1) | 0.235 | 0.020 |
| t1/2e (min) | 29.66 | 39.68 |
| Tmax (min) | 3 | 5 |
| Cmax (nmol/ml) | 20.79 | 1.045 |
| AUC (nmol-min/ml) | 557.8 | 28.94 |
| Fm (%) | 13.3 |
Note. Biotransformation of harmane to harmine occurred in only two rats that were administered with oral dose of harmane (20 mg/kg by gavage in corn oil). Data represent the average values from two animals.
Based on the data from a single dose of harmane and harmine in the same species, it is possible to estimate Fm, the fraction of a dose of harmane that is converted to a metabolite harmine, using Eq. (3), where AUCHI′ was 152.3 nmol-min/ml (= 28.9/19%, adjusted for oral bioavailability of harmane), DHA was 109.8 μmol/kg (i.e., 20 mg/kg), and AUCHI was 24.6 nmol-min/ml after an iv injection of harmine at a dose (DHI) of 2.36 μmol/kg (i.e., 0.5 mg/kg). This calculation yielded Fm of 13.3%. In other words, once harmane entered the body of these two rats, about 13% of the dosed harmane was metabolized to harmine.
DISCUSSION
As part of a study of the environmental causes of essential tremor, the present experiments were intended to define the toxicokinetic properties of harmane and harmine, two tremorogenic β-carboline derivatives, in animals, as little such information is available in the literature. These estimates should further our understanding of the disposition of these alkaloids in vivo and allow interpretation of the time course of neurotoxic consequences possibly induced by dietary exposure to these naturally occurring tremorogenic compounds.
Following iv bolus administration to rats, harmane and harmine share several toxicokinetic similarities: (1) The blood concentrations of both chemicals declined rapidly upon their entering the systemic circulation; (2) both compounds had comparable terminal elimination t1/2β, although harmine was cleared from the blood two times more rapidly than harmane; and (3) both chemicals possessed a Vc and Vd exceeding the total blood volume (58 ml/kg) or the whole-body water volume (132 ml) of experimental animals, suggesting a tissue distribution and possible accumulation of these chemicals in rats. Differences, however, indeed exist between harmane and harmine with regard to other toxicokinetic properties. For example, at a similar molar dose for iv injection, harmine exhibited a much smaller AUC than did harmane. Noticeably also, the values of Vc and Vd in the harmine study were about two times larger than those in the harmane study. From a chemical point of view, the pyrido[3,4-β]indole structure of harmane can be converted to harmine by introducing an additional methoxyl group in the ring structure. Inclusion of such a group would increase the lipophilicity of the chemical and thereby increase the partitioning of the chemical to the peripheral compartment. This could explain a relatively larger volume of distribution of harmine than harmane in tested rats. Hence, harmine appears to be more prone to accumulation in tissues than harmane.
A smaller AUC of harmine in comparison to harmane could also result from its faster clearance from the blood. Since the systemic clearance of harmane (52 ml/kg/min) closely approximates hepatic blood flow (52–80 ml/kg/min), the hepatic extraction ratio (the ratio of hepatic clearance/ hepatic blood flow) of harmane would approach 1, assuming that the liver clears all harmane molecules present in the systemic circulation. Thus, it seems possible that the liver may serve as the major organ in the metabolism and elimination of harmane. Harmine, on the other hand, had a clearance (103 ml/kg/min) that was much greater than the hepatic blood flow. Thus, in addition to the hepatic clearance, harmine may undergo extrahepatic: metabolic processes that are different from those of harmane, although the exact pathways are unknown.
After oral dose administration, harmine entered the blood circulation much more rapidly, yet reached a blood Cmax much lower than harmane. Given the higher lipophilicity of harmine, a faster rate of absorption for harmine than for harmane is expected. However, the bioavailability of harmine is surprisingly low, about six times less than harmane. Previous work by Tweedie and Burke (1987) demonstrated that harmine can be metabolized primarily to 6-hydroxyl-7-methoxyharman, in a 3-methylcholanthrene (MC)-induced mouse liver microsomal preparation. In the same in vitro model, harmine can be converted, to a lesser extent, to 3- or 4-hydroxy-7-methoxyharmane. These authors also found that the further biotransformation of harmine can result in the formation of a number of unidentified metabolites. In the current study, a rather extensive variation in blood concentration–time profiles of harmine following oral dose was noted. Thus, it seems likely that the extensive hepatic metabolism, serving as the first-pass effect, may contribute to the low bioavailability of harmine.
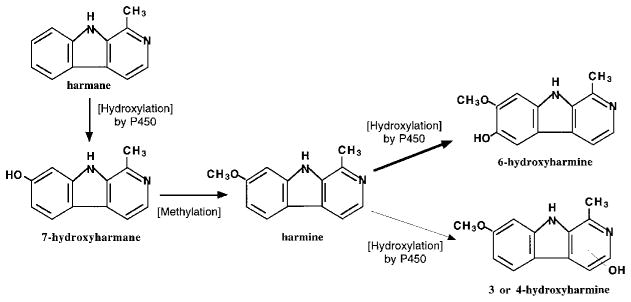
First-pass metabolism may also exist in animals having received an oral dose of harmane. This is evidenced by the fact that harmine was a major metabolite in rat blood by HPLC analyses, accounting for 13% of the administered harmane. The appearance of harmine in blood following the oral dose, but not the iv dose, of harmane suggests that the parent harmane must experience substantial hepatic biotransformation prior to entering the systemic circulation. Based on this and other studies, a metabolic pathway of harmane and harmine is proposed in Figure 5. Harmane, upon being absorbed into the body, undergoes a first-pass metabolism by the liver cytochrome P-450 system, presumably the CYP2A family, to produce a 7-hydroxyl metabolite, which is methylated to generate harmine. A portion of harmine enters the blood circulation as substantiated in the present study, while the other portion of harmine is further catalyzed by the P-450 enzyme for hydroxylation (or by biliary elimination). Since the methylation of 7-hydroxyharmane increases the lipophilicity, harmine in the body may be favorably distributed to the peripheral tissue compartment. The question of why the formation of harmine after oral dose of harmane occurred only in certain but not all tested animals remains unanswered. It is possible that a large variation in the absorption of harmane may contribute to this phenomenon. It is also possible that multiple processes in harmane and harmine metabolism may augment the variations in drug metabolism by individual animals.
FIGURE 5.
Proposed metabolic pathway for harmane and harmine. Harmane, upon being absorbed, undergoes first-pass metabolism by the liver cytochrome P-450 system to produce the 7-hydroxyl metabolite, which is further methylated to form harmine. A portion of harmine enters the blood circulation as shown in Figures 3 and 4, while the other portion of harmine is hydroxylated by P-450 enzyme.
In summary, the toxicokinetics of tremorogenic harmane and harmine after a single iv injection follow a two-compartment disposition model. Harmine, with its higher lipophilicity and a larger volume of distribution, appears to distribute to the peripheral compartment more favorably than harmane. Following oral dose administration, both harmane and harmine undergo first-pass metabolism. Harmane is metabolized to form harmine; the latter is further converted to other hydroxylated products. Extensive hepatic metabolism may contribute to the low bioavailability of harmine after oral ingestion. The clinical significance of these findings requires further exploration.
Acknowledgments
This research was supported in part by National Institute of Environmental Health Sciences grants P30-ES09089 (E. D. Louis, W. Zheng) and RO1-ES08146 (W. Zheng), and National Institute of Neurological Disorders and Stroke grant RO1-NS39422 (E. D. Louis).
Contributor Information
Yongbiao Guan, Division of Environmental Health Sciences, School of Public Health, Columbia University, New York, New York, USA.
Elan D. Louis, Gertrude H. Sergievsky Center and Department of Neurology, College of Physicians and Surgeons, Columbia University, New York, New York, USA
Wei Zheng, Division of Environmental Health Sciences, School of Public Health, and Department of Pharmacology, College of Physicians and Surgeons, Columbia University, New York, New York, USA.
References
- Adachi J, Mizoi Y, Naito T, Yamamoto K, Fujiwara S, Ninomiya I. Determination of β-carbolines in foodstuffs by high performance liquid chromatography and high performance liquid chromatograph-mass spectrometry. J Chromatogr. 1991;538:331–339. doi: 10.1016/s0021-9673(01)88854-3. [DOI] [PubMed] [Google Scholar]
- Allen RF, Beck O, Borg S, Skroder R. Analysis of 1-methyl-1,2,3,4-tetrahydro-β-carboline in human urine and cerebrospinal fluid by gas chromatography–mass spectrometry. Eur J Mass Spectrom. 1980;1:171–177. [Google Scholar]
- Bidder TA, Shoemaker DW, Boettger HG, Evans M, Cummins JT. Harmane in human platelets. Life Sci. 1979;25:157–164. doi: 10.1016/0024-3205(79)90387-4. [DOI] [PubMed] [Google Scholar]
- Du W, Aloyo VJ, Harvey JA. Harmaline competitively inhibits [3H]MK-801 binding to the NMDA receptor in rabbit brain. Brain Res. 1997;770:26–29. doi: 10.1016/s0006-8993(97)00606-9. [DOI] [PubMed] [Google Scholar]
- Fuentes LA, Longo VG. An investigation on the central effects of harmine, harmaline and related B-carbolines. Neuropharmacology. 1971;10:15–23. doi: 10.1016/0028-3908(71)90004-9. [DOI] [PubMed] [Google Scholar]
- Gibaldi M, Perrier D. Pharmacokinetics. New York: Marcel Dekker; 1982. pp. 33–40.pp. 84–109.pp. 409–417. [Google Scholar]
- Gulcher JR, Jonsson P, Kong A, Kristjansson K, Frigge ML, Karason A, Einarsdottir IE, Stefansson H, Einarsdottir AS, Sigurthoardottir S, Baldursson S, Bjornsdottir S, Hrafnkelsdottir SM, Jakobsson F, Benedickz J, Stefansson K. Mapping of a familial essential tremor gene, FET1, to chromosome 3q13. Nature Genet. 1997;17:84–87. doi: 10.1038/ng0997-84. [DOI] [PubMed] [Google Scholar]
- Higgins JJ, Pho LT, Nee LE. A gene (ETM) for essential tremor maps to chromosome 2p22–p25. Movement Disord. 1997;12:859–864. doi: 10.1002/mds.870120605. [DOI] [PubMed] [Google Scholar]
- Hollinger MA. Tables of toxicological importance. In: Derelanko MJ, Hollinger MA, editors. CRC handbook of toxicology. New York: CRC Press; 1995. pp. 799–813. [Google Scholar]
- Lewin L. Untersuchungen Uber Banisteria caapi sp. Arch Exp Pathol Pharmacol. 1928;129:133–149. [Google Scholar]
- Louis ED. Etiology of essential tremor: Should we be searching for environmental causes? Movement Disord. 2001 doi: 10.1002/mds.1183. in press. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder?: Estimates of the prevalence of essential tremor throughout the world. Movement Disord. 1998;13:5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J Neurosci. 1997;17:8828–8841. doi: 10.1523/JNEUROSCI.17-22-08828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennes HH, Hoch PH. Psychotomimetics, clinical and theoretical considerations: Harmine, Win-2299 and Nalline. Am J Psychiat. 1957;113:885–892. doi: 10.1176/ajp.113.10.887. [DOI] [PubMed] [Google Scholar]
- Poindexter EH, Carpenter RD. The isolation of harmane and norharmane from tobacco and cigarette smoke. Phytochemistry. 1962;1:215–221. [Google Scholar]
- Rautakorpi I, Takala J, Martilla RJ, Sievers K, Rinne UK. Essential tremor in a Finnish population. Acta Neurol Scand. 1982;66:58–67. doi: 10.1111/j.1600-0404.1982.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Strauss S, Lindemann J. Excretion of tetrahydroharmane and harmane into the urine of man and rat after load with ethanol. FEBS Lett. 1980;109:209–212. doi: 10.1016/0014-5793(80)81088-x. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Barbey M, Strauss S, Greiner B, Fahndrich E. Is there a correlation between the concentration of β-carbolines and their pharmacodynamic effect? In: Bloom F, Barchas J, Sandler M, Usdin E, editors. Beta-carbolines and tetrahydroisoquinolines. New York: Alan R. Liss; 1982. pp. 41–55. [PubMed] [Google Scholar]
- Takeuchi T, Ogawa K, Linuma H, Suda H, Ukita K. Monoamine oxidase inhibitors isolated from fermented broths. J Antibiot. 1973;26:162–167. doi: 10.7164/antibiotics.26.162. [DOI] [PubMed] [Google Scholar]
- Tweedie DJ, Burke MD. Metabolism of the beta-carbolines, harmine and harmol, by liver microsomes from phenobarbitone- or 3-methylcholanthrene-treated mice. Identification and quantitation of two novel harmine metabolites. Drug Metab Dispos. 1987;15:74–81. [PubMed] [Google Scholar]
- Zetler G, Singbartl G, Schlosser L. Cerebral pharmacokinetics of tremor-producing harmala and iboga alkaloids. Pharmacology. 1972;7:237–248. doi: 10.1159/000136294. [DOI] [PubMed] [Google Scholar]
- Zetler G, Back G, Iven H. Pharmacokinetics in the rat of the hallucinogenic alkaloids harmine and harmaline. Naunyn-Schmiedeberg’s Arch Pharmacol. 1974;285:273–292. doi: 10.1007/BF00498996. [DOI] [PubMed] [Google Scholar]
- Zheng W, Winter SM, Mayersohn M, Bishop JM, Sipes IG. Toxicokinetics of sulfasalazine (salicylazosulfapyridine) and its metabolites in B6C3F1 mice. Drug Metab Dispos. 1993;21:1091–1097. [PubMed] [Google Scholar]
- Zheng W, Winter MS, Kattnig MJ, Carter DE, Sipes IG. Tissue distribution and elimination of indium phosphide in male Fischer 344 rats following oral and intratracheal administration of indium phosphide. J Toxicol Environ Health. 1994;43:483–494. doi: 10.1080/15287399409531936. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang S, Guan Y, Louis ED. Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal Biochem. 2000a;279:125–129. doi: 10.1006/abio.1999.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Kim H, Zhao Q. Comparative toxicokinetics of manganese chloride and methylcyclo-pentadienyl Mn tricarbonyl in male Sprague-Dawley rats. Toxicol Sci. 2000b;54:295–301. doi: 10.1093/toxsci/54.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]