Abstract
Heavy alcohol exposure produces profound damage to the developing central nervous system (CNS) as well as the adult brain. Children with fetal alcohol spectrum disorders (FASD) have a variety of cognitive, behavioral, and neurological impairments. FASD currently represents the leading cause of mental retardation. Excessive alcohol consumption is associated with Wernicke–Korsakoff syndrome (WKS) and neurodegeneration in the adult brain. Although the cellular/molecular mechanism underlying ethanol’s neurotoxicity has not been fully understood, it is generally believed that oxidative stress plays an important role. Identification of neuroprotective agents that can ameliorate ethanol neurotoxicity is an important step for developing preventive/therapeutic strategies. Targeting ethanol-induced oxidative stress using natural antioxidants is an attractive approach. Anthocyanins, a large subgroup of flavonoids present in many vegetables and fruits, are safe and potent antioxidants. They exhibit diverse potential health benefits including cardioprotection, anti-atherosclerotic activity, anti-cancer, anti-diabetic, and anti-inflammation properties. Anthocyanins can cross the blood–brain barrier and distribute in the CNS. Recent studies indicate that anthocyanins represent novel neuroprotective agents and may be beneficial in ameliorating ethanol neurotoxicity. In this review, we discuss the evidence and potential of anthocyanins in alleviating ethanol-induced damage to the CNS. Furthermore, we discuss possible underlying mechanisms as well as future research approaches necessary to establish the therapeutic role of anthocyanins.
Keywords: Antioxidants, Brain, Cell death, Cyanidin glucosides, Neuroprotection, Reactive oxygen species
Introduction
Excessive alcohol consumption causes profound damage to human organ systems including the liver, brain, heart, pancreas, lungs, endocrine, and immune systems, as well as bone and skeletal muscles. The central nervous system (CNS) is particularly sensitive to alcohol exposure. Alcohol is a neuroteratogen and alcohol consumption during pregnancy may cause Fetal Alcohol Spectrum Disorders (FASD), among which, fetal alcohol syndrome (FAS) is the most severe form, characterized by growth deficiency, mental retardation, CNS malformation, and craniofacial deficits (Caprara et al. 2007; Riley and McGee 2005). The detrimental effects of ethanol on the developing CNS include reduction of neurogenesis, inhibition of differentiation, disturbance of neuron migration, alteration of cell–cell interaction, and induction of apoptosis (Goodlett and Horn 2001; Guerri 1998; Luo and Miller 1998; Olney et al. 2000; West 1994). These alcohol-induced structural alterations in the developing brain underlie many of the behavioral deficits observed in FASD. Chronic binge alcohol exposure may induce neurodegeneration in the adult brain and is associated with neuro-cognitive deficits and Wernicke–Korsakoff Syndrome (WKS) (Harper 2009; Obernier et al. 2002; Pfefferbaum et al. 2007). A variety of mechanisms have been proposed for ethanol neurotoxicity; it is generally accepted that oxidative stress is a major one (Bleich et al. 2000; Bondy 1992; Bondy and Guo 1994; Chen et al. 2008; Crews and Nixon 2009; Haorah et al. 2008; Nordmann 1987; Ramachandran et al. 2003; Sandhir and Kaur 2006; Sun and Sun 2001). Ethanol readily crosses the blood–brain barrier (BBB) and is metabolized in the brain by enzymes, such as catalase, alcohol dehydrogenase, or ethanol-inducible cytochrome P450. This process produces reactive oxygen species (ROS) which includes superoxide free radicals, hydrogen peroxide, and hydroxyl radicals (Haorah et al. 2008; Zakhari 2006). Disturbance of cellular normal redox state by excessive ROS leads to oxidative stress which causes cellular damage (Bondy 1992; Hampton and Orrenius 1998). The CNS is particularly susceptible to oxidative stress due to its high oxygen consumption rate, elevated levels of polyunsaturated fatty acids, and relatively low content of antioxidative enzymes (Cohen-Kerem and Koren 2003). A tremendous research effort has been made to identify potential neuroprotective agents that can ameliorate ethanol-induced CNS damage, as it is an important initial step for a developing therapeutic strategy. Targeting ethanol-induced ROS and oxidative stress is a logical approach. Natural antioxidants extracted from plants or fruits are attractive candidates for scavenging ROS and alleviating ethanol neurotoxicity due to their safety and tolerance by oral administration. Anthocyanins are potent antioxidants and present in various vegetables and fruits especially edible berries. They exhibit a wide range of antioxidant protection and therapeutic benefits including cardioprotective, anti-inflammatory, anti-diabetic, and anti-carcinogenic properties (Zafra-Stone et al. 2007). In this review, we discuss the recent progress of the research in anthocyanins’ neuroprotective effects; particularly we focus on the beneficial potentials of anthocyanins in ameliorating ethanol-induced CNS damage. First, we discuss the approaches of using antioxidants to treat ethanol-induced neuronal damage.
Approaches Using Antioxidants to Alleviate Ethanol Neurotoxicity
Since oxidative stress is a putative mechanism underlying ethanol neurotoxicity, using antioxidants to ameliorate oxidative stress and alleviate ethanol neurotoxicity is a logical approach. This approach has shown some successes in both in vivo and in vitro models. For example, in a mouse model of FAS, treatment of antioxidants blocks ethanol-induced reduction of glutathione levels and prevents ethanol-induced fetal growth restriction, microcephaly, and fetal death (Toso et al. 2007). In a binge-alcohol administrated model, treatment of antioxidants significantly reduces ethanol-induced neurodegeneration in the dentate gyrus and entorhinal cortex of rats (Hamelink et al. 2005; Vaudry et al. 2005). In a model of chronic ethanol exposure, administration of melatonin, an antioxidant, reverses both the ethanol-induced increase in lipid peroxidation, and the decline in glutathione levels in the hippocampus, cerebral cortex, and cerebellum of young and aged rats (Baydas and Tuzcu 2005). Marino et al. (2004) showed that ethanol induces oxidative stress and neuronal loss in the hippocampus of rat pups; the alterations are accompanied by impaired spatial navigation. Vitamin E, a commonly used antioxidant, alleviates ethanol-induced oxidative stress and neuronal loss, but fails to improve spatial learning deficit. These results suggest that while oxidative stress may contribute to ethanol neurotoxicity, antioxidant protection is not sufficient to prevent the behavioral impairments (Marino et al. 2004). Administration of the antioxidant ebselen (Eb) has been found to block chronic ethanol-induced inhibition of neurogenesis (Herrera et al. 2003). In cell culture models, vitamin E ameliorates ethanol-induced neuronal apoptosis and reduction in neurotrophin secretion in cultured rat cerebellar granule cells (Heaton et al. 2004a, b). Vitamin E and antioxidant β-carotene protect cultured hippocampal neurons against ethanol-induced cell death (Mitchell et al. 1999). Similarly, antioxidant α-lipoic acid is demonstrated to prevent ethanol-induced intracellular protein oxidation, glutathione depletion, and cell death in mouse hippocampal HT722 cells (Pirlich et al. 2002).
However, some studies suggest that oxidative stress may not be the principal mechanism of ethanol neurotoxicity, and antioxidants are not effective in blunting ethanol-induced damage to the CNS. For example, Kane et al. (2008) showed that ethanol does not induce rat cerebellar granule neurons to produce ROS and cause deterioration of the mitochondrial membrane potential. Antioxidants (N-acetylcysteine, U83836E, and melatonin) fail to protect ethanol-induced Purkinje cell loss in the developing rat cerebellum due to alcohol administration (Edwards et al. 2002; Grisel and Chen 2005; Pierce et al. 2006). Tran et al. (2005) showed that vitamin E does not protect against ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Crews et al. investigated the neuroprotective effect of antioxidants using a binge-ethanol exposure model. Among four antioxidants (butylated hydroxytoluene, ebselen, vitamin E, and blueberry extract) tested, only butylated hydroxytoluene reverses binge-ethanol induced brain damage and inhibition of neurogenesis (Crews et al. 2006). Lack of effectiveness of some antioxidants remains a conundrum. The bioavailability of antioxidants may underlie the discrepancies between studies and lack of effectiveness of some antioxidants.
Neuroprotective Potential of Anthocyanins
Anthocyanins are a group of over 500 water-soluble natural pigments responsible for the red, blue, and purple colors of many flowers, vegetables, and fruits (Ghosh and Konishi 2007; McGhie and Walton 2007; Milbury et al. 2002). Anthocyanins belong to a larger group of compounds collectively known as flavonoids, which are a subgroup of an even larger group of compounds known as polyphenols (Duthie et al. 2000). Polyphenols are generally divided into tannins, lignins, and flavonoids (Cantos et al. 2002; Nichenametla et al. 2006; Scalbert and Williamson 2000). Flavonoids are the most abundant polyphenolic compounds in foods and can be classified into flavanols, flavones, flavonols, flavanones, isoflavones, and anthocyanins (Matkowski et al. 2008; Wang and Ho 2009). Flavonoids have diverse health beneficial effects including neuroprotection against a variety of insults to the brain (Dajas et al. 2003a, b; Ha et al. 2003; Huang et al. 2001; Rivero-Perez et al. 2008; Tapiero et al. 2002). Flavonoids have also been implicated in remedying the aging process of the brain (Andres-Lacueva et al. 2005; Bastianetto and Quirion 2002). Some health benefits of flavonoids appear to be attributed to their antioxidant property. Anthocyanins are probably the most potent antioxidants among flavonoids. The occurrence of anthocyanins is widespread in plants and fruits, and these compounds are consumed as part of a normal diet. Consumption was thought to be as much as 180–255 mg/day in the USA, a value that far exceeds the consumption of most other flavonoids (McGhie and Walton 2007). Berry fruits are rich sources of dietary anthocyanins and can contain 10s to 100s of milligrams of anthocyanins in a single serving (McGhie and Walton 2007; Siriwoharn et al. 2004).
Bioavailability of Anthocyanins
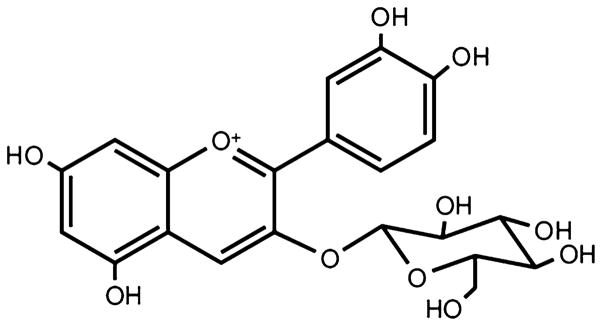
Anthocyanins, exemplified by cyanidin-3-glucoside (C3G, Fig. 1), exist as glycosides and acylglycosides of anthocyanidins and are varied with different hydroxyl or methoxyl substitutions in their basic structure, flavylium. Based on the number and position of hydroxyl groups on the flavan nucleus, most natural anthocyanins can be classified as the glycosides of cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin (Wang and Stoner 2008). Cyanidin is the most abundant anthocyanin in the edible part of plants (Ghosh and Konishi 2007). Anthocyanins are reactive compounds and only stable in low pH. In the presence of oxygen or light, or increase in temperature or pH, anthocyanins are degraded (Walton et al. 2006a). Glycosylation increases the structural stability and water solubility of anthocyanins (Zafra-Stone et al. 2007). Anthocyanins present mainly in food as mono-glycosides of the six main aglycones, but in some foods contain acylated anthocyanins (da Costa et al. 2000).
Fig. 1.
The structure of cyanidin-3-glucoside (C3G)
Most animal and human studies have found that anthocyanins are absorbed mainly in their intact glycosidic form, and rapidly reach the circulatory system within 0.25–2 h (McGhie and Walton 2007; Miyazawa et al. 1999). After oral administration, anthocyanins can be directly absorbed from the gastrointestinal tract (mainly in the stomach and small intestine) (Felgines et al. 2006; Talavera et al. 2005). A recent study shows that the absorption of black raspberry anthocyanins in small intestine tissues of rats reaches 7.5% of the administered dose, much higher than reported bioavailability of these pigments (He et al. 2009). The low pH in the stomach helps to stabilize anthocyanins from degradation (Felgines et al. 2006). After absorbed in the stomach and jejunum, anthocyanins and their glycosides enter the circulatory system, and a portion of absorbed anthocyanins are metabolized (Talavera et al. 2004, 2005). Anthocyanins and their metabolites are promptly distributed to the organs such as the liver, lungs, heart, kidney, and brain (Marczylo et al. 2009; Talavera et al. 2005). In contrast to the stomach and jejunum, anthocyanins in the large intestines are mostly degraded and modified due to the neutral pH and the existence of gut microflora (McGhie and Walton 2007). The metabolism of anthocyanins is complex, as it occurs in the distinct physiochemical environments of different organs and tissues.
The bioavailability of anthocyanins is consistently low, although it varies among studies and the sources of anthocyanins (McGhie and Walton 2007). Measurement of urinary excretion has often been used to assess bioavailability. Generally less than 1% of orally administered anthocyanins are recovered in the urine. Frank et al. (2003, 2005, 2007) investigated the pharmacokinetic parameters and the bioavailability of several dietary anthocyanins following consumption of red wine, red grape juice, or elderberry in healthy human subjects. The urinary recovery of original anthocyanins and their related metabolites range from 0.39 to 0.06%. In a phase I clinical trial, Stoner et al. determined the safety and tolerability of 45 g/day of freeze-dried black raspberries (BRB) administered orally for 1 week. Their results indicate that 45 g of freeze-dried BRB daily in healthy subjects are well tolerated and result in quantifiable specific anthocyanins (C3G, cyanidin-3-sambubioside, cyanidin-3-rutinoside, and cyanidin-3-xylo-sylrutinoside) and ellagic acid in the plasma and urine (Stoner et al. 2005). It has been reported that a significant increase in plasma anthocyanin levels and antioxidant capacity is observed following consumption of cranberry juice, blood orange juice, acai juice, and pulp in healthy human subjects (Mertens-Talcott et al. 2008; Pedersen et al. 2000; Riso et al. 2005).
Cyanidin-3-glucoside (C3G) is a major antioxidant anthocyanin component in many edible berries and pigmented fruits. C3G is well-studied due to its anti-carcinogenic potential. A recent study investigates the pharmacokinetics and the metabolism of C3G in mice (Marczylo et al. 2009). In that study, C57BL6J mice received C3G by either gavage at 500 mg/kg or tail vein injection (iv) at 1 mg/kg. Peak concentrations of anthocyanins occur within 30 min after administration. The predominant flavonoid species after oral administration was C3G, whilst after iv dosing the majority of anthocyanins was C3G metabolites. Anthocyanin peak levels after oral administration of C3G reach 25 μM in plasma. After oral or iv administration, C3G half-lives in the different biofluids and tissues range from 0.7 to 1.8 h and 0.3 to 0.7 h, respectively. The finding indicates that C3G levels can be achieved at an order of magnitude consistent with pharmacological activity (Marczylo et al. 2009). It is believed that protocatechuic acid (PCA) is the major metabolic product of C3G (Seeram et al. 2001; Tsuda et al. 1996, 1999). The peak C3G concentration was detected in plasma within 30 min after it is orally administered to rats; the peak concentration of PCA is reached within 60 min, and is eight times higher than the peak concentration of intact C3G (Tsuda et al. 1999). A study verified that PCA is the major human metabolite of C3G (Vitaglione et al. 2007). However, Marczylo et al. (2009) showed that the major metabolites of C3G are products of methylation and glucuronidation in mice.
It appears that anthocyanins are able to cross the BBB and reach the brain. Passamonti et al. (2005) showed that the intact anthocyanins are detected not only in the plasma, but also in the brain minutes after its introduction into the rat stomach. This finding is supported by two other independent studies. In one study in which rats are fed either a blueberry supplementation or a control diet for 8–10 weeks, several anthocyanins (cyanidin-3-O-beta-galac-toside, cyanidin-3-O-beta-glucoside, cyanidin-3-O-beta-arabinose, malvidin-3-O-beta-galactoside, malvidin-3-O-beta-glucoside, malvidin-3-O-beta-arabinose, peonidin-3-O-beta-arabinose, and delphinidin-3-O-beta-galactoside) are found in the cerebellum, cortex, hippocampus, or striatum of rats, but not in the controls (Andres-Lacueva et al. 2005). Another study shows that the concentration of anthocyanins in the brain is higher than that in the plasma after rats are fed with a blackberry anthocyanins diet for 15 days (Talavera et al. 2005). The results obtained from animal experiments are verified by in vitro studies showing that anthocyanins are able to cross the BBB in a cell culture model (Youdim et al. 2003, 2004). Furthermore, at the cellular level, the evidence of intracellular distribution of anthocyanins indicates they are able to cross the cell membrane and be taken up by the cells (Youdim et al. 2000). The mechanisms for the absorption of anthocyanins and their crossing the cell membrane, however, are unclear. Walton et al. (2006b) suggested that an active transportation mechanism is involved in anthocyanins’ absorption. It is important to note that ethanol does not affect the absorption of anthocyanins in rat intestine (Andlauer et al. 2003), supporting the applicability of anthocyanins for treating ethanol-induced disorders.
Anthocyanin’s Protection Against Ethanol Toxicity
Flavonoid-mediated neuroprotection against ethanol toxicity has been documented in animal and cell culture models. For example, supplementation of flavonoids in the diet significantly decreased ethanol-induced damage to the brain and liver (La et al. 1999). Flavonoids are shown to prevent ethanol-induced apoptosis in cultured fetal rhombencephalic neurons (Antonio and Druse 2008). Oral administration of grape seed flavonoids protect cells from ethanol-induced DNA damage in the mouse cerebellum and hippocampus (Guo et al. 2007). Flavonoids extracted from pine bark protected cultured cerebellum granule cells isolated from 9-day-old rat pups from ethanol-induced neuron death; the protection was accompanied by reduced ROS production and upregulation of expression/activity of copper/zinc superoxide dismutase (Cu/Zn SOD) as well as the glutathione peroxidase/reductase system (Siler-Marsiglio et al. 2004). Alcohol causes oxidative stress and lipid peroxidation, resulting in lipofuscin formation in the cerebellum and hippocampus of rats. Oral supplementation with flavonoids isolated from grape seeds reduces ethanol-induced oxidative stress and prevents ethanol-induced neuronal lipid peroxidation and lipofuscin formation (Assuncao et al. 2007a; De et al. 2004). Ethanol treatment during gestation has the greatest impact on spatial working memory; the addition of flavonoids to the ethanol liquid diet ameliorates ethanol-induced learning deficits (Neese et al. 2004). Chronic ethanol administration to young mice produces an increase in lipid peroxidation, and a decline in forebrain total glutathione (GSH), SOD, and catalase levels, which are significantly reversed by the co-administration of flavonoids (Singh et al. 2003). Flavonoid compounds suppress voluntary alcohol consumption in alcohol-preferring rats (Lin and Li 1998). Grape flavonoids reverse the decrease in synaptic protein function in rat brain caused by chronic ethanol consumption (Sun et al. 1999).
Compared to other flavonoids, anthocyanins are more powerful hydrogen-donating antioxidants due to their capability of electron delocalization and resonating structures formation (Ghosh and Konishi 2007; Zafra-Stone et al. 2007). Anthocyanins show a broad spectrum of beneficial health properties including cardioprotective, vision improving, anti-obesity, anti-inflammatory, and anti-diabetic activities (Ghosh and Konishi 2007; Karlsen et al. 2007; Mink et al. 2007; Sasaki et al. 2007; Toufektsian et al. 2008; Tsuda 2008). Anthocyanins also display neuroprotective and brain benefiting properties. For example, it has been reported that berry anthocyanins are beneficial in reducing age-associated oxidative stress (Zafra-Stone et al. 2007) and improving cognitive brain function (Bagchi et al. 2004; Barros et al. 2006; Hou 2003). Anthocyanins improve learning and memory of rats with estrogen deficits (Varadinova et al. 2009). Anthocyanins reduce the brain infarct volume and apoptotic cell numbers in a rat cerebral ischemia model (Shin et al. 2006). Anthocyanins provide significant protection against oxidative stress-induced lipid peroxidation and DNA fragmentation in mice brain (Bagchi et al. 1998; Dani et al. 2007).
A number of studies show that anthocyanins are beneficial for treating ethanol toxicity. It is reported that chronic exposure to red wine flavonoids (6 months) antagonizes ethanol-induced lipid peroxidation, and prevents ethanol-mediated reduction of glutathione and antioxidant enzymes in adult rat hippocampus (Assuncao et al. 2007b). Furthermore, red wine flavonoids improve ethanol-induced decline in hippocampal-dependent spatial memory (Assuncao et al. 2007b). Using similar regimen, they further demonstrate that red wine flavonoids protect rat cerebellum from ethanol’s toxicity by modulation of cerebellum oxidative status (Assuncao et al. 2008). Since anthocyanins are the main phenolic compounds of red wine and the most important components responsible for the antioxidant capacity of red wine (Rivero-Perez et al. 2008), anthocyanins likely account for most of the neuroprotective effects observed in these studies.
Among anthocyanins, cyanidin glucosides tend to have a higher antioxidant capacity than peonidin or malvidin glucosides (Prior 2003). C3G receives much attention and is well-studied because of its therapeutic potential as an anti-cancer agent. C3G exhibits chemopreventive and chemotherapeutic properties in various models of carcinogenesis and tumor development (Chen et al. 2005, 2006; Ding et al. 2006; Fukamachi et al. 2008; Shih et al. 2005; Zhang et al. 2005, 2008). Other beneficial health properties of C3G include anti-diabetics (Guo et al. 2008; Sasaki et al. 2007), anti-inflammation (Kim and Park 2006; Xia et al. 2006), anti-atherogenic activity (Xia et al. 2006), and anti-obesity (Tsuda et al. 2003). C3G offers neuroprotection against cerebral ischemia in a mouse model (Kang et al. 2006). In cultured neuronal cells, C3G and its metabolites protect cells against ROS-induced mitochondria damage and DNA fragmentation; C3G also ameliorates amyloid-beta peptide-induced toxicity (Tarozzi et al. 2007, 2008).
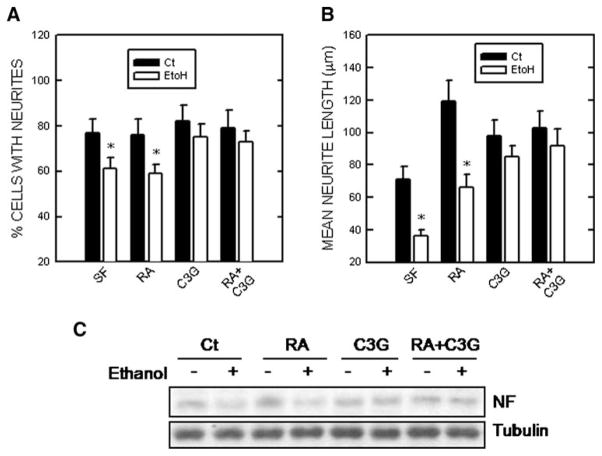
Li et al. reported that oral administration of C3G (4–8 mg/kg of body weight) significantly increases the levels of glutathione and the activities of radical scavenging enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase in rat gastric tissues. C3G also reduces the ethanol-induced increase of lipid peroxidation and free radicals as well as gastric lesions (Li et al. 2008). In an in vitro model, we demonstrate that ethanol inhibits neuronal differentiation of N2a neuroblastoma cells, which is evident by the repression of neurite outgrowth and the expression of neurofilament proteins (Chen et al. 2009). C3G is able to induce neuronal differentiation in N2a cells. More importantly, C3G scavenges ethanol-elicited ROS production and reverses ethanol-induced inhibition of neurite outgrowth and the expression of neurofilament proteins (Fig. 2). However, other antioxidants such as NAC or GSH-MEE do not antagonize ethanol’s effect on neuronal differentiation, although they do reduce ethanol-induced ROS production.
Fig. 2.
Effect of ethanol on neurite outgrowth. N2a cells grown in a serum free (SF) medium were treated with retinoic acid (RA, 5 μM), cyanidin-3-glucoside (C3G, 5 μM) or both (5 μM of each) in the absence or presence of ethanol (400 mg/dl) for 48 h. a The percentage of cells baring neurites was calculated. b The average length of neurites was determined. *denotes significant difference from non-ethanol-treated controls. c Cell lysates were collected and the expression of light chain neurofilament (NF) was determined by immunoblotting. The experiment was replicated three times. From (Chen et al. 2009)
Mechanisms of Anthocyanin-Mediated Protection
Anthocyanins are effective at reducing ethanol-induced ROS production (Chen et al. 2009; Li et al. 2008). The antioxidant property of anthocyanins plays an important role in their neuroprotection. In addition to being ROS scavengers, anthocyanins affect diverse cellular activities; these include modulating the activity of transcription factors, gene expression, intracellular cell signaling, and protein degradation (Ding et al. 2006; Dreiseitel et al. 2008; Fukamachi et al. 2008; Karlsen et al. 2007; Sasaki et al. 2007; Tsuda et al. 2004, 2005, 2006; Xu et al. 2004). The anthocyanin-induced alterations in cellular activity may mediate some of anthocyanins actions. For example, it is demonstrated that anthocyanins’ protection against cerebral ischemia in rat brain may be mediated by their inhibition of c-jun N-terminal kinase (JNK) and p53 (Shin et al. 2006).
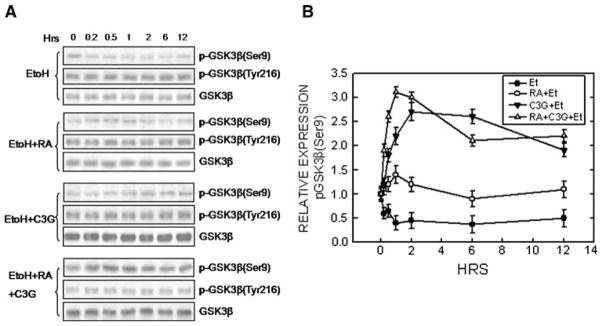
We show that C3G inhibits the activity of glycogen synthase kinase 3β (GSK3β), a multifunctional serine/ threonine kinase (Chen et al. 2009). GSK3β negatively regulates neurite outgrowth of N2a cells; inhibition of GSK3β activity induces neurite outgrowth, while over-expression of a constitutively active S9A GSK3β mutant prevented neurite outgrowth. We demonstrate that ethanol inhibits neurite outgrowth of N2a cells by activating GSK3β through the dephosphorylation of GSK3β at serine 9 (Ser9). C3G antagonizes ethanol’s inhibition of neuronal differentiation through promoting pGSK3β (Ser 9) (Fig. 3). It appears that the effect of C3G on GSK3β is independent of its antioxidant activity (Chen et al. 2009). The mechanisms underlying C3G regulation of GSK3β activity are unclear. Regardless how C3G regulates GSK3β, inhibition of C3G may have important implications; our recent study suggests that GSK3β is involved in ethanol-induced CNS damage (Liu et al. 2009).
Fig. 3.
Effect of ethanol on GSK3β phosphorylation. a N2a cells grown in a serum free (SF) medium were treated with C3G (5 μM) in the absence or presence of ethanol (400 mg/dl) for specified times. Cell lysates were collected and the expression of phosphorylated GSK3β [p-GSK3β(Ser9) and p-GSK3β(Tyr216)] and total GSK3β was examined with immunoblotting. The experiment was replicated three times. c The relative amounts of p-GSK3β(Ser9) were measured by densitometry and normalized to the expression of total GSK3β. Each data point (mean ± SEM) is the mean of three independent experiments. From (Chen et al. 2009)
Summary and Research Direction
Anthocyanins are a group of potent natural antioxidants. Anthocyanins are present in a large quantity in many edible berries and pigmented fruits, and are consumed as part of a normal diet. Oral administration of freeze-dried black raspberries (containing a high concentration of anthocyanins) is well tolerated in healthy adults and results in quantifiable anthocyanins and their metabolites in the plasma. Animal studies indicate that anthocyanin levels in plasma and tissues can be achieved at an order of magnitude consistent with their pharmacological activity after oral administration. Anthocyanins appear to be able to cross the BBB and distribute in the brain, and their neuroprotective effects have been documented in vitro and in vivo. Although studies on anthocyanin’s protection against ethanol neurotoxicity are limited, available evidence indicates that anthocyanins and their metabolites are able to scavenge ethanol-induced ROS production and ameliorate ethanol-induced damage to the CNS. They alleviate the adverse effects of ethanol, such as lipid peroxidation, inhibition of neuronal differentiation, and spatial memory deficits. Anthocyanins represent a novel and safe group of natural antioxidants that may have a therapeutic potential in treating ethanol neurotoxicity. To further evaluate the therapeutic potential, several issues need to be carefully considered.
First, previous studies have shown that anthocyanins are able to cross the BBB and distribute in the cerebellum, cortex, hippocampus, or striatum, the brain areas that are susceptible to ethanol exposure, after oral administration in rats. Further study needs to quantify the concentration of anthocyanins in the brain and determine whether there is a correlation between their distribution, antioxidant property, and neuroprotection.
Second, a systematic study is needed to determine whether anthocyanins or their metabolites attribute to the beneficial effect since most anthocyanins have a short half life in vivo. For example, Tsuda et al. (1999) suggest the metabolites of C3G rather than C3G itself contribute to C3G’s bioactivity. The aglycon cyanidin (CY) and protocatechuic acid (PCA) are primary metabolites of C3G. Tarozzi et al. (2007, 2008) demonstrate that CY and PCA offer better protection against H2O2-induced mitochondrial function loss and DNA fragmentation in SH-SY5Y neuroblastoma cells than C3G, while C3G is more effective than CY and PCA in blocking amyloid-beta induced cell death of SH-SY5Y cells. If a specific anthocyanin or its metabolite is identified as a more effective neuroprotective agent, it is necessary to modify its structure to enhance its stability.
Third, anthocyanins may act in synergy. Bagchi et al. (2004) show that a combination of six different berry extracts display more potent anti-angiogenic, antioxidant, and anti-carcinogenic properties than a single berry extract. We have shown that purified C3G is sufficient to alleviate ethanol-induced inhibition of neuronal differentiation. It is unknown whether C3G in combination with other anthocyanins offer better protection.
Fourth, the antioxidant property of anthocyanins clearly plays an important role in neuroprotection. However, reduction of ROS may not fully explain their neuroprotective property. In addition to its role as a ROS scavenger, anthocyanins, such as C3G, may regulate specific cellular activities. Our study indicates that C3G causes GSK3β inhibition which antagonizes ethanol’s effect on neurite outgrowth. The modulation of GSK3β activity by C3G appears to be independent of its antioxidant property (Chen et al. 2009). Palfi et al. (2009) suggests that the cardioprotective effect of red wine anthocyanins may be mediated by the activation of the Akt pathway and repression of the phosphorylation of PKCα/βII. A supplementation with a blueberry diet improves the performance of aged animals in spatial working memory tasks; the benefits may result from the effects of flavonoids on the ERK-CREB-BDNF pathway (Williams et al. 2008). Long-term dietary treatment with blueberry extract has been shown to reverse cognitive deficits and improve motor performance of senescent rat and to decrease ROS in striatum; however, the decrease in ROS does not appear to completely explain the effect of the blueberry extract (Lau et al. 2005). Recently, neuroinflammation has been proposed as a neurotoxic mechanism in alcoholism (Crews and Nixon 2009; Sullivan and Zahr 2008). Anthocyanins have an anti-inflammatory property. Whether anthocyanins can suppress ethanol-induced neuroinflammation warrants further research. Elucidation of the mechanisms underlying anthocyanin’s effect on these cellular events will be helpful for the development of therapeutic strategy.
Acknowledgments
We thank Kimberly Bower for reading this manuscript. This research was supported by grants from the National Institutes of Health (AA015407 and AA017226).
References
- Andlauer W, Stumpf C, Frank K, Furst P. Absorption and metabolism of anthocyanin cyanidin-3-glucoside in the isolated rat small intestine is not influenced by ethanol. Eur J Nutr. 2003;42:217–223. doi: 10.1007/s00394-003-0417-3. [DOI] [PubMed] [Google Scholar]
- Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- Antonio AM, Druse MJ. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008;1204:16–23. doi: 10.1016/j.brainres.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assuncao M, De FV, Paula-Barbosa M. Grape seed flavonol, but not Port wine, prevent ethanol-induced neuronal lipofuscin formation. Brain Res. 2007a;1129:72–80. doi: 10.1016/j.brainres.2006.10.044. [DOI] [PubMed] [Google Scholar]
- Assuncao M, Santos-Marques MJ, De FM, Carvalho F, Andrade JP, Lukoyanov NV, Paula-Barbosa MM. Red wine antioxidants protect hippocampal neurons against ethanol-induced damage: a biochemical, morphological and behavioral study. Neuroscience. 2007b;146:1581–1592. doi: 10.1016/j.neuroscience.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Assuncao M, Santos-Marques MJ, De FV, Paula-Barbosa MM, Carvalho F. Modulation of rat cerebellum oxidative status by prolonged red wine consumption. Addict Biol. 2008;13:337–344. doi: 10.1111/j.1369-1600.2008.00103.x. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Garg A, Krohn RL, Bagchi M, Bagchi DJ, Balmoori J, Stohs SJ. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol. 1998;30:771–776. doi: 10.1016/s0306-3623(97)00332-7. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Sen CK, Bagchi M, Atalay M. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry (Mosc) 2004;69:75–80. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- Barros D, Amaral OB, Izquierdo I, Geracitano L, do Carmo Bassols RM, Henriques AT, Ramirez MR. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol Biochem Behav. 2006;84:229–234. doi: 10.1016/j.pbb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Quirion R. Natural extracts as possible protective agents of brain aging. Neurobiol Aging. 2002;23:891–897. doi: 10.1016/s0197-4580(02)00024-6. [DOI] [PubMed] [Google Scholar]
- Baydas G, Tuzcu M. Protective effects of melatonin against ethanol-induced reactive gliosis in hippocampus and cortex of young and aged rats. Exp Neurol. 2005;194:175–181. doi: 10.1016/j.expneurol.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Bleich S, Spilker K, Kurth C, Degner D, Quintela-Schneider M, Javaheripour K, Ruther E, Kornhuber J, Wiltfang J. Oxidative stress and an altered methionine metabolism in alcoholism. Neurosci Lett. 2000;293:171–174. doi: 10.1016/s0304-3940(00)01505-6. [DOI] [PubMed] [Google Scholar]
- Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63:231–241. doi: 10.1016/0378-4274(92)90086-y. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Guo SX. Effect of ethanol treatment on indices of cumulative oxidative stress. Eur J Pharmacol. 1994;270:349–355. doi: 10.1016/0926-6917(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Cantos E, Espin JC, Tomas-Barberan FA. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. J Agric Food Chem. 2002;50:5691–5696. doi: 10.1021/jf0204102. [DOI] [PubMed] [Google Scholar]
- Caprara DL, Nash K, Greenbaum R, Rovet J, Koren G. Novel approaches to the diagnosis of fetal alcohol spectrum disorder. Neurosci Biobehav Rev. 2007;31:254–260. doi: 10.1016/j.neubiorev.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Chen PN, Chu SC, Chiou HL, Chiang CL, Yang SF, Hsieh YS. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr Cancer. 2005;53:232–243. doi: 10.1207/s15327914nc5302_12. [DOI] [PubMed] [Google Scholar]
- Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang CL, Hsieh YS. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006;235:248–259. doi: 10.1016/j.canlet.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Chen G, Ma C, Bower KA, Shi X, Ke Z, Luo J. Ethanol promotes endoplasmic reticulum stress-induced neuronal death: involvement of oxidative stress. J Neurosci Res. 2008;86:937–946. doi: 10.1002/jnr.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Bower KA, Xu M, Ding M, Shi X, Ke ZJ, Luo J. Cyanidin-3-glucoside reverses ethanol-induced inhibition of neurite outgrowth: role of glycogen synthase kinase 3 Beta. Neurotox Res. 2009;15:321–331. doi: 10.1007/s12640-009-9036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kerem R, Koren G. Antioxidants and fetal protection against ethanol teratogenicity. I. Review of the experimental data and implications to humans. Neurotoxicol Teratol. 2003;25:1–9. doi: 10.1016/s0892-0362(02)00324-0. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- da Costa CT, Horton D, Margolis SA. Analysis of anthocyanins in foods by liquid chromatography, liquid chromatography–mass spectrometry and capillary electrophoresis. J Chromatogr A. 2000;881:403–410. doi: 10.1016/s0021-9673(00)00328-9. [DOI] [PubMed] [Google Scholar]
- Dajas F, Rivera F, Blasina F, Arredondo F, Echeverry C, Lafon L, Morquio A, Heizen H. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox Res. 2003a;5:425–432. doi: 10.1007/BF03033172. [DOI] [PubMed] [Google Scholar]
- Dajas F, Rivera-Megret F, Blasina F, Arredondo F, Abin-Carriquiry JA, Costa G, Echeverry C, Lafon L, Heizen H, Ferreira M, Morquio A. Neuroprotection by flavonoids. Braz J Med Biol Res. 2003b;36:1613–1620. doi: 10.1590/s0100-879x2003001200002. [DOI] [PubMed] [Google Scholar]
- Dani C, Oliboni LS, Vanderlinde R, Bonatto D, Salvador M, Henriques JA. Phenolic content and antioxidant activities of white and purple juices manufactured with organically- or conventionally-produced grapes. Food Chem Toxicol. 2007;45:2574–2580. doi: 10.1016/j.fct.2007.06.022. [DOI] [PubMed] [Google Scholar]
- De FV, da Silva PP, Assuncao M, Cadete-Leite A, Andrade JP, Paula-Barbosa MM. Flavonoids from grape seeds prevent increased alcohol-induced neuronal lipofuscin formation. Alcohol Alcohol. 2004;39:303–311. doi: 10.1093/alcalc/agh069. [DOI] [PubMed] [Google Scholar]
- Ding M, Feng R, Wang SY, Bowman L, Lu Y, Qian Y, Castranova V, Jiang BH, Shi X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemo-therapeutic activity. J Biol Chem. 2006;281:17359–17368. doi: 10.1074/jbc.M600861200. [DOI] [PubMed] [Google Scholar]
- Dreiseitel A, Schreier P, Oehme A, Locher S, Rogler G, Piberger H, Hajak G, Sand PG. Inhibition of proteasome activity by anthocyanins and anthocyanidins. Biochem Biophys Res Commun. 2008;372:57–61. doi: 10.1016/j.bbrc.2008.04.140. [DOI] [PubMed] [Google Scholar]
- Duthie GG, Duthie SJ, Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- Edwards RB, Manzana EJ, Chen WJ. Melatonin (an antioxidant) does not ameliorate alcohol-induced Purkinje cell loss in the developing cerebellum. Alcohol Clin Exp Res. 2002;26:1003–1009. doi: 10.1097/01.ALC.0000021148.70836.75. [DOI] [PubMed] [Google Scholar]
- Felgines C, Talavera S, Texier O, Besson C, Fogliano V, Lamaison JL, la FL, Galvano G, Remesy C, Galvano F. Absorption and metabolism of red orange juice anthocyanins in rats. Br J Nutr. 2006;95:898–904. [Google Scholar]
- Frank T, Netzel M, Strass G, Bitsch R, Bitsch I. Bioavailability of anthocyanidin-3-glucosides following consumption of red wine and red grape juice. Can J Physiol Pharmacol. 2003;81:423–435. doi: 10.1139/y03-038. [DOI] [PubMed] [Google Scholar]
- Frank T, Sonntag S, Strass G, Bitsch I, Bitsch R, Netzel M. Urinary pharmacokinetics of cyanidin glycosides in healthy young men following consumption of elderberry juice. Int J Clin Pharmacol Res. 2005;25:47–56. [PubMed] [Google Scholar]
- Frank T, Janssen M, Netzet G, Christian B, Bitsch I, Netzel M. Absorption and excretion of elderberry (Sambucus nigra L.) anthocyanins in healthy humans. Methods Find Exp Clin Pharmacol. 2007;29:525–533. doi: 10.1358/mf.2007.29.8.1116309. [DOI] [PubMed] [Google Scholar]
- Fukamachi K, Imada T, Ohshima Y, Xu J, Tsuda H. Purple corn color suppresses Ras protein level and inhibits 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis in the rat. Cancer Sci. 2008;99:1841–1846. doi: 10.1111/j.1349-7006.2008.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr. 2007;16:200–208. [PubMed] [Google Scholar]
- Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25:175–184. [PMC free article] [PubMed] [Google Scholar]
- Grisel JJ, Chen WJ. Antioxidant pretreatment does not ameliorate alcohol-induced Purkinje cell loss in the developing rat cerebellum. Alcohol Clin Exp Res. 2005;29:1223–1229. doi: 10.1097/01.alc.0000171932.13148.cf. [DOI] [PubMed] [Google Scholar]
- Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- Guo L, Wang LH, Sun B, Yang JY, Zhao YQ, Dong YX, Spranger MI, Wu CF. Direct in vivo evidence of protective effects of grape seed procyanidin fractions and other antioxidants against ethanol-induced oxidative DNA damage in mouse brain cells. J Agric Food Chem. 2007;55:5881–5891. doi: 10.1021/jf070440a. [DOI] [PubMed] [Google Scholar]
- Guo H, Ling W, Wang Q, Liu C, Hu Y, Xia M. Cyanidin 3-glucoside protects 3T3-L1 adipocytes against. Biochem Pharmacol. 2008;75:1393–1401. doi: 10.1016/j.bcp.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Ha HJ, Kwon YS, Park SM, Shin T, Park JH, Kim HC, Kwon MS, Wie MB. Quercetin attenuates oxygen–glucose deprivation- and excitotoxin-induced neurotoxicity in primary cortical cell cultures. Biol Pharm Bull. 2003;26:544–546. doi: 10.1248/bpb.26.544. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther. 2005;314:780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton MB, Orrenius S. Redox regulation of apoptotic cell death. Biofactors. 1998;8:1–5. doi: 10.1002/biof.5520080101. [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45:1542–1550. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- He J, Wallace TC, Keatley KE, Failla ML, Giusti MM. Stability of black raspberry anthocyanins in the digestive tract lumen and transport efficiency into gastric and small intestinal tissues in the rat. J Agric Food Chem. 2009;57:3141–3148. doi: 10.1021/jf900567t. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Madorsky I, Paiva M, Siler-Marsiglio KI. Ethanol-induced reduction of neurotrophin secretion in neonatal rat cerebellar granule cells is mitigated by vitamin E. Neurosci Lett. 2004a;370:51–54. doi: 10.1016/j.neulet.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Madorsky I, Paiva M, Siler-Marsiglio KI. Vitamin E amelioration of ethanol neurotoxicity involves modulation of apoptosis-related protein levels in neonatal rat cerebellar granule cells. Brain Res Dev Brain Res. 2004b;150:117–124. doi: 10.1016/j.devbrainres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou DX. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med. 2003;3:149–159. doi: 10.2174/1566524033361555. [DOI] [PubMed] [Google Scholar]
- Huang SS, Tsai MC, Chih CL, Hung LM, Tsai SK. Resveratrol reduction of infarct size in Long-Evans rats subjected to focal cerebral ischemia. Life Sci. 2001;69:1057–1065. doi: 10.1016/s0024-3205(01)01195-x. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Chang JY, Roberson PK, Garg TK, Han L. Ethanol exposure of neonatal rats does not increase biomarkers of oxidative stress in isolated cerebellar granule neurons. Alcohol. 2008;42:29–36. doi: 10.1016/j.alcohol.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Hur JY, Kim HB, Ryu JH, Kim SY. Neuroprotective effects of the cyanidin-3-O-beta-D-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci Lett. 2006;391:122–126. doi: 10.1016/j.neulet.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Karlsen A, Retterstol L, Laake P, Paur I, Kjolsrud-Bohn S, Sandvik L, Blomhoff R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. 2007;137:1951–1954. doi: 10.1093/jn/137.8.1951. [DOI] [PubMed] [Google Scholar]
- Kim AJ, Park S. Mulberry extract supplements ameliorate the inflammation-related hematological parameters in carrageenan-induced arthritic rats. J Med Food. 2006;9:431–435. doi: 10.1089/jmf.2006.9.431. [DOI] [PubMed] [Google Scholar]
- La GL, Wang M, Watkins R, Ortiz D, Sanchez ME, Konst J, Lee C, Reyes E. Protective effects of the flavonoid mixture, silymarin, on fetal rat brain and liver. J Ethnopharmacol. 1999;65:53–61. doi: 10.1016/s0378-8741(98)00144-5. [DOI] [PubMed] [Google Scholar]
- Lau FC, Shukitt-Hale B, Joseph JA. The beneficial effects of fruit polyphenols on brain aging. Neurobiol Aging. 2005;26(suppl 1):128–132. doi: 10.1016/j.neurobiolaging.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Li CY, Xu HD, Zhao BT, Chang HI, Rhee HI. Gastroprotective effect of cyanidin 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol. 2008;42:683–687. doi: 10.1016/j.alcohol.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Lin RC, Li TK. Effects of isoflavones on alcohol pharmacokinetics and alcohol-drinking behavior in rats. Am J Clin Nutr. 1998;68:1512S–1515S. doi: 10.1093/ajcn/68.6.1512S. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen G, Ma C, Bower KA, Xu M, Fan Z, Shi X, Ke ZJ, Luo J. Overexpression of glycogen synthase kinase 3beta sensitizes neuronal cells to ethanol toxicity. J Neurosci Res. 2009 doi: 10.1002/jnr.22098. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Miller MW. Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev. 1998;27:157–167. doi: 10.1016/s0165-0173(98)00009-5. [DOI] [PubMed] [Google Scholar]
- Marczylo TH, Cooke D, Brown K, Steward WP, Gescher AJ. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice. Cancer Chemother Pharmacol. 2009 doi: 10.1007/s00280-009-0996-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. Int J Dev Neurosci. 2004;22:363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Matkowski A, Zielinska S, Oszmianski J, Lamer-Zarawska E. Antioxidant activity of extracts from leaves and roots of Salvia miltiorrhiza Bunge, S. przewalskii Maxim., and S. verticillata L. Bioresour Technol. 2008;99:7892–7896. doi: 10.1016/j.biortech.2008.02.013. [DOI] [PubMed] [Google Scholar]
- McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, Pacheco-Palencia LA, Meibohm B, Talcott ST, Derendorf H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem. 2008;56:7796–7802. doi: 10.1021/jf8007037. [DOI] [PubMed] [Google Scholar]
- Milbury PE, Cao G, Prior RL, Blumberg J. Bioavailablility of elderberry anthocyanins. Mech Ageing Dev. 2002;123:997–1006. doi: 10.1016/s0047-6374(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- Mitchell JJ, Paiva M, Heaton MB. Vitamin E and beta-carotene protect against ethanol combined with ischemia in an embryonic rat hippocampal culture model of fetal alcohol syndrome. Neurosci Lett. 1999;263:189–192. doi: 10.1016/s0304-3940(99)00144-5. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Nakagawa K, Kudo M, Muraishi K, Someya K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. J Agric Food Chem. 1999;47:1083–1091. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- Neese S, La GL, Trujillo E, Romero D. The effects of ethanol and silymarin treatment during gestation on spatial working memory. BMC Complement Altern Med. 2004;4:4. doi: 10.1186/1472-6882-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006;46:161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- Nordmann R. Oxidative stress from alcohol in the brain. Alcohol Alcohol Suppl. 1987;1:75–82. [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–521. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- Palfi A, Bartha E, Copf L, Mark L, Gallyas F, Jr, Veres B, Kalman E, Pajor L, Toth K, Ohmacht R, Sumegi B. Alcohol-free red wine inhibits isoproterenol-induced cardiac remodeling in rats by the regulation of Akt1 and protein kinase C alpha/beta II. J Nutr Biochem. 2009;20:418–425. doi: 10.1016/j.jnutbio.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. Fast access of some grape pigments to the brain. J Agric Food Chem. 2005;53:7029–7034. doi: 10.1021/jf050565k. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Kyle J, Jenkinson AM, Gardner PT, McPhail DB, Duthie GG. Effects of blueberry and cranberry juice consumption on the plasma antioxidant capacity of healthy female volunteers. Eur J Clin Nutr. 2000;54:405–408. doi: 10.1038/sj.ejcn.1600972. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Bell RL, Sullivan EV. Development and resolution of brain lesions caused by pyrithiamine- and dietary-induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: a longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2007;32:1159–1177. doi: 10.1038/sj.npp.1301107. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Cook CC, Hinson JA, Light KE. Are oxidative mechanisms primary in ethanol induced Purkinje neuron death of the neonatal rat? Neurosci Lett. 2006;400:130–134. doi: 10.1016/j.neulet.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Pirlich M, Kiok K, Sandig G, Lochs H, Grune T. Alpha-lipoic acid prevents ethanol-induced protein oxidation in mouse hippocampal HT22 cells. Neurosci Lett. 2002;328:93–96. doi: 10.1016/s0304-3940(02)00415-9. [DOI] [PubMed] [Google Scholar]
- Prior RL. Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr. 2003;78:570S–578S. doi: 10.1093/ajcn/78.3.570S. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74:577–588. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Riso P, Visioli F, Gardana C, Grande S, Brusamolino A, Galvano F, Galvano G, Porrini M. Effects of blood orange juice intake on antioxidant bioavailability and on different markers related to oxidative stress. J Agric Food Chem. 2005;53:941–947. doi: 10.1021/jf0485234. [DOI] [PubMed] [Google Scholar]
- Rivero-Perez MD, Muniz P, Gonzalez-Sanjose ML. Contribution of anthocyanin fraction to the antioxidant properties of wine. Food Chem Toxicol. 2008;46:2815–2822. doi: 10.1016/j.fct.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Kaur K. Influence of ethanol on methanol-induced oxidative stress and neurobehavioral deficits. J Biochem Mol Toxicol. 2006;20:247–254. doi: 10.1002/jbt.20141. [DOI] [PubMed] [Google Scholar]
- Sasaki R, Nishimura N, Hoshino H, Isa Y, Kadowaki M, Ichi T, Tanaka A, Nishiumi S, Fukuda I, Ashida H, Horio F, Tsuda T. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007;74:1619–1627. doi: 10.1016/j.bcp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Bourquin LD, Nair MG. Degradation products of cyanidin glycosides from tart cherries and their bioactivities. J Agric Food Chem. 2001;49:4924–4929. doi: 10.1021/jf0107508. [DOI] [PubMed] [Google Scholar]
- Shih PH, Yeh CT, Yen GC. Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. Food Chem Toxicol. 2005;43:1557–1566. doi: 10.1016/j.fct.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Shin WH, Park SJ, Kim EJ. Protective effect of anthocyanins in middle cerebral artery occlusion and reperfusion model of cerebral ischemia in rats. Life Sci. 2006;79:130–137. doi: 10.1016/j.lfs.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Shaw G, Heaton MB. Pycnogenol and vitamin E inhibit ethanol-induced apoptosis in rat cerebellar granule cells. J Neurobiol. 2004;59:261–271. doi: 10.1002/neu.10311. [DOI] [PubMed] [Google Scholar]
- Singh A, Naidu PS, Kulkarni SK. Reversal of aging and chronic ethanol-induced cognitive dysfunction by quercetin a bioflavonoid. Free Radic Res. 2003;37:1245–1252. doi: 10.1080/10715760310001616014. [DOI] [PubMed] [Google Scholar]
- Siriwoharn T, Wrolstad RE, Finn CE, Pereira CB. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J Agric Food Chem. 2004;52:8021–8030. doi: 10.1021/jf048619y. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Sardo C, Apseloff G, Mullet D, Wargo W, Pound V, Singh A, Sanders J, Aziz R, Casto B, Sun X. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J Clin Pharmacol. 2005;45:1153–1164. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM. Neuroinflammation as a neurotoxic mechanism in alcoholism: commentary on “Increased MCP-1 and microglia in various regions of human alcoholic brain”. Exp Neurol. 2008;213:10–17. doi: 10.1016/j.expneurol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AY, Sun GY. Ethanol and oxidative mechanisms in the brain. J Biomed Sci. 2001;8:37–43. doi: 10.1007/BF02255969. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xia J, Draczynska-Lusiak B, Simonyi A, Sun AY. Grape polyphenols protect neurodegenerative changes induced by chronic ethanol administration. Neuroreport. 1999;10:93–96. doi: 10.1097/00001756-199901180-00018. [DOI] [PubMed] [Google Scholar]
- Talavera S, Felgines C, Texier O, Besson C, Manach C, Lamaison JL, Remesy C. Anthocyanins are efficiently absorbed from the small intestine in rats. J Nutr. 2004;134:2275–2279. doi: 10.1093/jn/134.9.2275. [DOI] [PubMed] [Google Scholar]
- Talavera S, Felgines C, Texier O, Besson C, Gil-Izquierdo A, Lamaison JL, Remesy C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J Agric Food Chem. 2005;53:3902–3908. doi: 10.1021/jf050145v. [DOI] [PubMed] [Google Scholar]
- Tapiero H, Tew KD, Ba GN, Mathe G. Polyphenols: do they play a role in the prevention of human pathologies? Biomed Pharmacother. 2002;56:200–207. doi: 10.1016/s0753-3322(02)00178-6. [DOI] [PubMed] [Google Scholar]
- Tarozzi A, Morroni F, Hrelia S, Angeloni C, Marchesi A, Cantelli-Forti G, Hrelia P. Neuroprotective effects of anthocyanins and their in vivo metabolites in SH-SY5Y cells. Neurosci Lett. 2007;424:36–40. doi: 10.1016/j.neulet.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Tarozzi A, Merlicco A, Morroni F, Franco F, Cantelli-Forti G, Teti G, Falconi M, Hrelia P. Cyanidin 3-O-glucopyranoside protects and rescues SH-SY5Y cells against amyloid-beta peptide-induced toxicity. Neuroreport. 2008;19:1483–1486. doi: 10.1097/WNR.0b013e32830fe4b8. [DOI] [PubMed] [Google Scholar]
- Toso L, Roberson R, Abebe D, Spong CY. Neuroprotective peptides prevent some alcohol-induced alteration in gamma-aminobutyric acid A-beta3, which plays a role in cleft lip and palate and learning in fetal alcohol syndrome. Am J Obstet Gynecol. 2007;196:259. doi: 10.1016/j.ajog.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Toufektsian MC, de LM, Nagy N, Salen P, Donati MB, Giordano L, Mock HP, Peterek S, Matros A, Petroni K, Pilu R, Rotilio D, Tonelli C, de LJ, Boucher F, Martin C. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J Nutr. 2008;138:747–752. doi: 10.1093/jn/138.4.747. [DOI] [PubMed] [Google Scholar]
- Tran TD, Jackson HD, Horn KH, Goodlett CR. Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Alcohol Clin Exp Res. 2005;29:117–129. doi: 10.1097/01.alc.0000150004.53870.e1. [DOI] [PubMed] [Google Scholar]
- Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J Agric Food Chem. 2008;56:642–646. doi: 10.1021/jf073113b. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Ohshima K, Kawakishi S, Osawa T. Oxidation products of cyanidin 3-O-beta-D-glucoside with a free radical initiator. Lipids. 1996;31:1259–1263. doi: 10.1007/BF02587910. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Horio F, Osawa T. Absorption and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett. 1999;449:179–182. doi: 10.1016/s0014-5793(99)00407-x. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Ueno Y, Aoki H, Koda T, Horio F, Takahashi N, Kawada T, Osawa T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem Biophys Res Commun. 2004;316:149–157. doi: 10.1016/j.bbrc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Ueno Y, Kojo H, Yoshikawa T, Osawa T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim Biophys Acta. 2005;1733:137–147. doi: 10.1016/j.bbalip.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Ueno Y, Yoshikawa T, Kojo H, Osawa T. Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem Pharmacol. 2006;71:1184–1197. doi: 10.1016/j.bcp.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Varadinova MG, Docheva-Drenska DI, Boyadjieva NI. Effects of anthocyanins on learning and memory of ovariectomized rats. Menopause. 2009;16:345–349. doi: 10.1097/gme.0b013e3181847619. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Hamelink C, Damadzic R, Eskay RL, Gonzalez B, Eiden LE. Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides. 2005;26:2518–2524. doi: 10.1016/j.peptides.2005.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007;137:2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- Walton MC, Lentle RG, Reynolds GW, Kruger MC, McGhie TK. Anthocyanin absorption and antioxidant status in pigs. J Agric Food Chem. 2006a;54:7940–7946. doi: 10.1021/jf061527j. [DOI] [PubMed] [Google Scholar]
- Walton MC, McGhie TK, Reynolds GW, Hendriks WH. The flavonol quercetin-3-glucoside inhibits cyanidin-3-glucoside absorption in vitro. J Agric Food Chem. 2006b;54:4913–4920. doi: 10.1021/jf0607922. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ho CT. Metabolism of flavonoids. Forum Nutr. 2009;61:64–74. doi: 10.1159/000212739. [DOI] [PubMed] [Google Scholar]
- Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR. Recent findings on the mechanisms by which alcohol damages the developing nervous system. Alcohol Alcohol Suppl. 1994;2:395–399. [PubMed] [Google Scholar]
- Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Xia X, Ling W, Ma J, Xia M, Hou M, Wang Q, Zhu H, Tang Z. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. J Nutr. 2006;136:2220–2225. doi: 10.1093/jn/136.8.2220. [DOI] [PubMed] [Google Scholar]
- Xu JW, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension. 2004;44:217–222. doi: 10.1161/01.HYP.0000135868.38343.c6. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Martin A, Joseph JA. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic Biol Med. 2000;29:51–60. doi: 10.1016/s0891-5849(00)00329-4. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood–brain barrier: in vitro studies. J Neurochem. 2003;85:180–192. doi: 10.1046/j.1471-4159.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic Biol Med. 2004;36:592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Vareed SK, Nair MG. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005;76:1465–1472. doi: 10.1016/j.lfs.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Seeram NP, Lee R, Feng L, Heber D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J Agric Food Chem. 2008;56:670–675. doi: 10.1021/jf071989c. [DOI] [PubMed] [Google Scholar]