SUMMARY
BACKGROUND
India has a high burden of active tuberculosis (TB) and human immunodeficiency virus (HIV) infection. Pregnancy increases the risks of developing TB in HIV-infected women. Isoniazid preventive therapy (IPT) reduces progression to TB, but may increase costs and hepatotoxicity. The cost-effectiveness of IPT for HIV-infected pregnant women in India is unknown.
DESIGN
We evaluated the cost-effectiveness of ante-partum IPT among HIV-infected women in India using a decision-analytic model. We compared current practice (no IPT) with: Intervention 1 (IPT regardless of CD4 count) and Intervention 2 (IPT for those with CD4 count ≤ 200 cells/μl). We modeled IPT irrespective of tuberculin skin test (TST) status and TST-driven strategies. Primary outcomes were anticipated costs, disability-adjusted life-years (DALYs) and TB cases.
RESULTS
Both IPT interventions are highly cost-effective compared to no IPT at current willingness-to-pay thresholds (respectively US$178.00 and US$201.00 per DALY averted for Interventions 1 and 2). However, providing IPT irrespective of CD4 count results in the greatest health benefits (21 TB cases averted/1000 patients) compared to current practice. IPT irrespective of TST status was also highly cost-effective compared to TST-driven IPT (respectively US$1027.00 and US$1154.00/DALY averted for Interventions 1 and 2).
CONCLUSION
Antepartum IPT for HIV-infected women is highly cost-effective for TB prevention compared to current practices in India.
Keywords: tuberculosis, economic evaluation, IPT, pregnancy
According to World Health Organization (WHO) estimates, one third of the world’s population is infected with latent tuberculous infection (LTBI). People living with the human immunodeficiency virus (PLHIV) are at increased risk of progressing from LTBI to active tuberculosis (TB) compared to persons without.1,2 Emerging evidence suggests that women of childbearing age are particularly vulnerable to TB during pregnancy, with TB potentially accounting for 6–15% of global maternal mortality.3,4 Furthermore, women in early postpartum may be twice as likely to develop TB as non-pregnant women.5 HIV-infected pregnant women have a more than 10-fold higher risk of developing TB than their HIV-negative counterparts,3 and preventing pregnancy-associated TB among HIV-infected women is a global priority. In addition to overall mortality, HIV-TB co-infection has been shown to increase the risk of mother-to-child transmission of HIV (MTCT) by a factor of 2.5,4 and contribute to poorer birth outcomes, such as premature birth and miscarriage.3,6 Maternal TB also increases rates of perinatal TB and poses risks for increased pediatric morbidity and mortality.3
India has the highest number of incident TB cases, accounting for 26% of global cases, and nearly half of the population has LTBI.2,7 India also has 2.39 million PLHIV,2,8,9 among whom TB accounts for 50% of deaths.7
Isoniazid preventive therapy (IPT) reduces progression to active disease in LTBI patients by 60–90%.10 To reduce progression to active TB, WHO guidelines recommend 6 months of IPT for PLHIV who are tuberculin skin test (TST) positive, and empiric IPT for PLHIV in endemic areas where TST is not feasible.1
Despite the large disease burden, Indian guidelines have not fully adopted IPT due to concerns about toxicity and logistical challenges associated with implementation.8 However, IPT during pregnancy for PLHIV represents an opportunity for a targeted approach to TB prevention, as many women enter the health care system for antenatal care.
The objective of our study was to analyze the cost-effectiveness of antepartum IPT interventions compared to the standard of care (SOC) in India of no IPT.
METHODS
We conducted a cost-effectiveness analysis from a health system perspective with a target population of HIV-infected pregnant women in India using a decision-tree model (Figure 1). We used a 1-year timeframe for the intervention, with an analytic horizon of the patients’ life expectancy. Future costs and disability-adjusted life-years (DALYs) were discounted at 3%.11
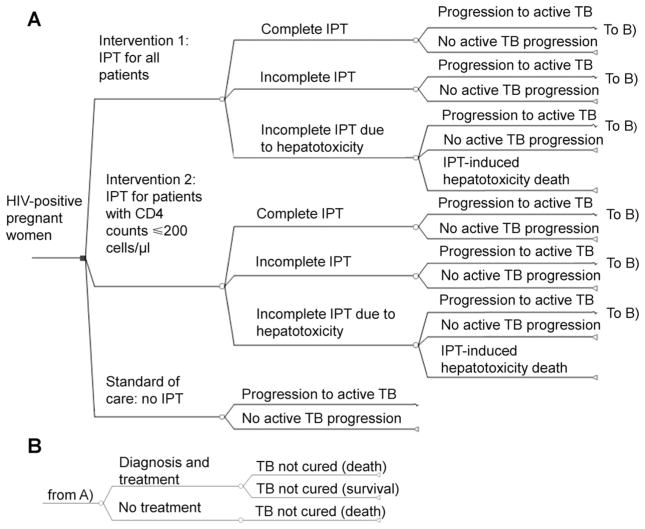
Figure 1.
Schematic diagram of decision analysis model: simplified schematic of decision tree model. The target population was HIV-infected pregnant women. We assumed provisions for ART and knowledge of CD4+T-cell count. In all model arms, the proportion of patients progressing to active TB was affected by baseline latent TB (TST) status, CD4 count, and usage of IPT. In the base-case intervention arms, women are given IPT irrespective of TST status, but we explored TST-driven strategies in secondary analysis. We included progression to active TB among patients with a negative baseline TST (lower proportion than for TST-positive) to account for imperfect test sensitivity and possibility of infection at time points after model entry. For the IPT interventions, we accounted for reduced efficacy with incomplete adherence, and incorporated costs and effects of IPT-induced hepatotoxicity. In all arms, we assumed that patients developing active TB could be diagnosed and treated based on current case-detection and treatment rates in India. HIV-infected pregnant women with undiagnosed and untreated active TB were assumed to die. HIV =human immunodeficiency virus; IPT =isoniazid preventive therapy; TB =tuberculosis; ART =antiretroviral therapy; TST = tuberculin skin test.
Study model
The model was developed and analyzed using TreeAge (TreeAge Software Inc, Williamstown, Massachusetts, USA). Current Indian guidelines endorse lifelong combination antiretroviral therapy (ART) for all pregnant and breastfeeding women, regardless of CD4 count (i.e., Option B+); we assumed all women were on ART upon entering the model.12 The population was stratified according to CD4 count; we incorporated higher risk TB progression among those with CD4 ≤ 200 cells/μl. Three TB prevention strategies were evaluated: 1) Intervention 1: IPT for all HIV-infected pregnant women; 2) Intervention 2: IPT for HIV-infected pregnant women with CD4 count ≤ 200 cells/μl; and 3) SOC: no IPT for HIV-infected pregnant women.
The women were further stratified according to presumed TST status based upon literature estimates, but TST was not included as part of our intervention algorithms in base case.7,10 The proportion progressing to active TB was modified by CD4 count, IPT usage and baseline TST status (Table 1 and Figure 1). Patients with a negative TST could still go on to develop active TB due to a false-negative TST or new TB infection.31 In the base case scenario, patients were given IPT regardless of TST status, but we explored alternative TST-driven treatment strategies in secondary analysis.
Table 1.
Key parameters for cost-effectiveness analysis
| Value | Base case | Low | High | Source |
|---|---|---|---|---|
| Epidemiologic, diagnostic, and treatment parameters | ||||
| Prevalence of LTBI | 0.21 | 0.0875 | 0.85 | 7,10 |
| Proportion of patients with CD4 ≤ 200 cells/μl | 0.10 | 0.001 | 0.775 | 7 |
| Proportion with LTBI progressing to active TB in patients with CD4 > 200 cells/μl (CD4 ≤ 200 cells/μl)* | 0.125 (0.218) | 0.0312 (0.0312) | 0.3 (0.75) | 10,13–16 |
| Relative risk reduction in progression to active TB with 6-month IPT† | 0.63 | 0.20 | 0.94 | 3,10,13,14,16,17 |
| IPT-induced hepatotoxicity in patients with CD4 > 200 cells/μl (patients with CD4 ≤ 200 cells/μl)‡ | 0.025 (0.05) | 0.00625 (0.0125) | 0.01 (0.15) | 13,18–20 |
| Probability of active TB case detection | 0.59 | 0.4 | 0.9 | 2 |
| Probability of cure of active TB with treatment in patients with CD4 > 200 cells/μl (CD4 ≤ 200 cells/μl) | 0.85 (0.76) | 0.212 (0.19) | 1 (1) | 2 |
| IPT and active TB treatment costs (2014 USD)¶ | ||||
| 6 months IPT | 22.77 | 5.69 | 90.00 | 21–23 |
| Mild IPT hepatotoxicity treatment | 35.73 | 8.93 | 62.53 | 21,22 |
| Severe hepatotoxicity treatment | 100.81 | 25.20 | 176.42 | 9,10 |
| Active TB diagnostics | 19.32 | 4.98 | 33.81 | 21,22,24 |
| Active TB treatment | 95.29 | 23.82 | 166.76 | 21–23 |
| Disability weights¶ | ||||
| Living with HIV on ART | 0.053 | 0.034 | 0.079 | 25 |
| Mild IPT-induced hepatotoxicity§ | 0.15 | 0.05 | 0.3 | 25–28 |
| Severe IPT-induced hepatitis§ | 0.6 | 0.1 | 0.9 | 25–28 |
| Active TB disease | 0.399 | 0.267 | 0.547 | 25,28 |
| Active TB treatment | 0.1 | 0.01 | 0.25 | 28 |
| Life expectancies, years¶# | ||||
| HIV on ART with CD4 > 200 cells/μl (CD4 ≤ 200 cells/μl) | 12.9 (6.45) | 3 (1.5) | 30 (15) | Assumption29 |
We modeled progression to active TB within 5 years of model entry. We also incorporated the possibility of tuberculous infection among TST-negative individuals either due to false-negative TST results or to infection after model entry (1% progression to active TB).
Assuming that the 6-month IPT completion percentage was 0.82 in the base case (range 0.205–1.0).10,30 For those who did not complete 6-month IPT, we estimated they received about 3 months of IPT, which resulted in a relative risk reduction of 0.315 in the base case (range 0.10–0.48).3,10,13,14,16,17
IPT-induced hepatotoxicity is defined as grade 3 or higher hepatitis: probability that once the patient has developed IPT-induced hepatitis that it will become ‘severe’. Severe hepatitis was defined as hepatitis grade 5 or higher or hepatitis involving hospitalization resulting in death. In the base case, we estimated that 56% of those with IPT-induced hepatotoxicity developed severe hepatitis (range 1.4–9.8) and 98% mortality due to severe hepatitis (range 24.5–100).19,20
Assuming that there was no disability associated with IPT alone, except through adverse events such as hepatitis or death.
Future costs and disability-adjusted life-years were discounted at 3%.11
Defined as the life expectancy with HIV at the median age of women in the model (assumed to be 25 years old, consistent with literature estimates) in India; we assumed that those who died due to severe hepatitis or active TB disease had a shortened life expectancy of 6 months (range 0.1–1.5 years).7
LTBI=latent tuberculous infection; TB=tuberculosis; IPT=isoniazid preventive therapy; USD=US dollar; HIV=human immunodeficiency virus; ART=antiretroviral therapy; TST = tuberculin skin test.
We incorporated costs and adverse health effects of IPT, including potential for hepatotoxicity and death (Table 1).7,13,18,19,21,22 Among individuals progressing to active TB, we estimated clinical outcomes using Indian case-detection rates and anti-tuberculosis treatment outcomes.
Ethical considerations
This economic evaluation was evaluated by the ethics committee of the Johns Hopkins University, Baltimore, Maryland, USA, and was exempt, as it did not constitute human subjects research.
Epidemiologic, diagnostic and treatment parameters
Key model parameters, values and ranges are shown in Table 1. For parameters with limited data, uncertainty ranges were determined by varying base-case values by ±75%. Probabilistic sensitivity analysis (PSA) using Monte-Carlo simulations was conducted to simultaneously vary all parameters across their ranges (triangular distribution with base-case values used as the mean) to generate 95% uncertainty ranges (UR) for outcome estimates of costs, DALYs, and cost-effectiveness.
LTBI prevalence in HIV-infected pregnant women in India was estimated at 21% based on data on TST positivity in studied cohorts, but varied widely in sensitivity analysis.7,10 Given the uncertainty about the future risk of tuberculous infection or re-infection and the long-term durability of IPT benefit in endemic settings, our model focused on short- and medium-term risks and benefits of IPT;32 without IPT, we assumed that the 5-year risk of progression from LTBI to active TB was 12.5% in patients with CD4 count > 200 cells/μl, with increased risk with lower CD4 count (Table 1).14,15
In the base case, we estimated that 82% of patients would complete 6 months of IPT, with a 63% relative risk reduction in progression to active TB, accounting for the possibility of reinfection after IPT within the time horizon and incomplete IPT efficacy to prevent progression.3,10,13,14,16,17,30
Outcome parameters
Primary model outcomes were estimated costs, active TB cases, deaths (from active TB or severe IPT-induced hepatitis), and DALYs. Cost-effectiveness was represented by incremental cost-effectiveness ratios (ICER), comparing the interventions with SOC and with WHO-suggested country-specific willingness to pay (WTP) thresholds, defined as per-capita Indian gross domestic product (US$1500) per DALY averted.33,34 Alternative thresholds were explored through PSA and expressed as a cost-effectiveness acceptability curve. DALYs were calculated using years of disability accrued from living with HIV, developing IPT-induced hepatitis, developing active TB, and from years of life lost due to TB or drug-related hepatotoxicity (Table 1).
Key costs
Costs were calculated using an ingredients approach based on unit costs (Table 1) derived from published sources and multiplied by expected quantities utilized. We did not incorporate costs associated with ART in any model arms in the base case. Costs of 6 months of IPT included drug and treatment monitoring costs (Table 1).21–23 Those developing isoniazid (INH) related hepatotoxicity incurred additional costs related to out-patient visits and hospitalization costs in cases of severe hepatotoxicity.21,22 The costs of diagnosing and treating active TB included chest X-rays, sputum smears/cultures, 6 months of a standard four-drug regimen, and out-patient follow-up visits. We also estimated net health system costs for a cohort of 49 000 HIV-infected pregnant women per year in India.9
RESULTS
In the base-case scenario, treating all HIV-infected pregnant women with IPT, irrespective of CD4 count (Intervention 1), was projected to lead to the greatest improvement in health outcomes of the three options considered (114 DALYs-averted/1000 persons compared to SOC), but was also the most expensive (US$22.53/person [incremental cost US$20.26/person compared to SOC]; Table 2). Nonetheless, IPT for all HIV-infected pregnant women was highly cost-effective, at an ICER of US$178.00/DALY averted, compared to current SOC. This intervention was also projected to result in improved TB-specific outcomes, with 21 more active TB cases and 10 more deaths due to active TB averted/1000 patients compared to the current SOC.
Table 2.
Costs and effects of IPT compared to standard of care in base case
| Variable | Intervention 1: IPT for all patients (95%UR)* | Intervention 2: IPT for patients with CD4 ≤ 200 cells/μl (95%UR)* | Standard of care: no IPT (95%UR)* |
|---|---|---|---|
| Costs, USD | |||
| Net costs per individual | 22.53 (15.02 to 69.27) | 4.28 (3.76 to 35.02) | 2.27 (1.03 to 10.45) |
| Incremental | 20.26 (10.69 to 65.82) | 2.01 (1.04 to 30.44) | Reference |
| Total health system costs* | 1 103 970 (735 980 to 3 394 280) | 209 720 (184 240 to 1 715 980) | 111 230 (50 470 to 512 050) |
| Incremental | 992 740 (523 810 to 3 225 180) | 98 490 (50 960 to 1 491 560) | Reference |
| Effects | |||
| DALYs per individual | 20.287 (20.105 to 23.614) | 20.391 (20.245 to 20.863) | 20.401 (20.284 to 23.837) |
| Incremental | 0.114 (0.033 to 0.785) | 0.010 (0.002 to 0.319) | Reference |
| Total health system DALYs† | 994 063 (985 145 to 1 157 086) | 999 159 (992 005 to 1 169 287) | 999 649 (993 916 to 1 168 013) |
| Incremental | 5 586 (1 617 to 38 465) | 490 (98 to 15 631) | Reference |
| TB or IPT deaths (deaths/1000 patients)‡ | 11 (8 to 74) | 19 (12 to 107) | 21 (14 to 129) |
| Incremental | 10 (4 to 77) | 2 (1 to 40) | Reference |
| Total health system deaths† | 539 (392 to 3 626) | 931 (588 to 5 243) | 1470 (686 to 6 321) |
| Incremental | 490 (196 to 3 773) | 98 (49 to 1 960) | Reference |
| Active TB (cases/1 000 patients) | 16 (9 to 100) | 34 (17 to 150) | 37 (20 to 184) |
| Incremental | 21 (11 to 112) | 3 (2 to 64) | Reference |
| Total health system active TB cases** | 784 (441 to 4 900) | 1 666 (833 to 7 350) | 1813 (980 to 9 016) |
| Incremental | 1 029 (539 to 5 488) | 147 (98 to 3 136) | Reference |
| Cost-effectiveness (ICERs)§ | |||
| USD/DALY averted | 178 (23 to 1 309) | 201 (19 to 1 197) | Reference |
| USD/DALY averted | 176 (−309 to 343) | Reference | — |
95% URs were calculated based on probabilistic sensitivity analysis using Monte-Carlo simulation methods in which all key parameters are varied across their uncertainty ranges simultaneously.
Total health system costs and effects were calculated based on an estimated population size of 49 000 HIV-infected pregnant women entering the system every year in India. Costs and effects included those associated with IPT usage, but also downstream costs and effects associated with hepatotoxicity, active TB diagnosis and treatment.9
Deaths due to active TB disease or IPT-related hepatotoxicity.
Calculated as incremental costs divided by incremental effects (costs per DALYs averted).
IPT = isoniazid preventive therapy; UR = uncertainty range; USD = US dollar; TB = tuberculosis; DALY = disability-adjusted life-years; ICER = incremental cost-effectiveness ratio.
Treating only pregnant women with CD4 count ≤ 200 cells/μl with IPT (Intervention 2) was projected to cost US$4.28/person, and was also considered cost-effective compared to SOC (ICER US$201.00/DALY averted). However, it was less efficacious and less cost-effective than Intervention 1 (i.e., weakly dominated, Table 2). Nonetheless, Intervention 2 was projected to avert three more active TB cases and two more deaths due to active TB averted/1000 patients compared to SOC.
Given the estimated 49 000 HIV-infected pregnant women in the Indian health system each year, IPT for all women would result in a total of 85 500 DALYs averted, 7500 fewer TB deaths, and 15 750 fewer cases of active TB disease compared to current SOC.
Sensitivity analysis
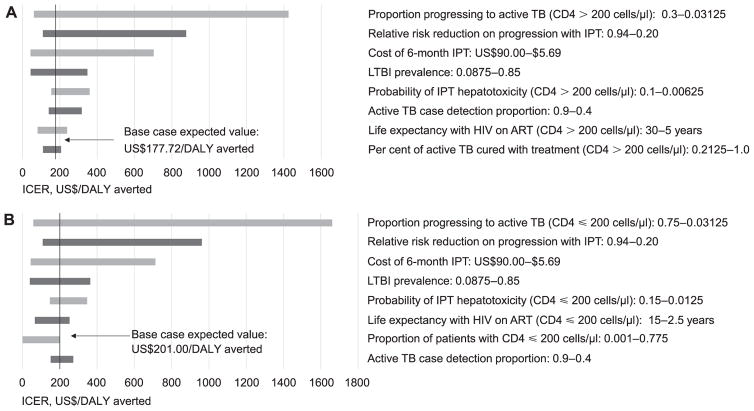
Figure 2 shows those parameters that were found to have the greatest effect on base-case ICERs for both interventions in one-way sensitivity analysis compared to SOC. We did not identify any conditions in which IPT for all HIV-infected pregnant women was not cost-effective; the intervention remained below WTP thresholds, even at lowest estimates of LTBI prevalence and IPT efficacy, or highest estimates of IPT costs (e.g., Intervention 1 is not cost-effective when LTBI prevalence drops below 1% or IPT costs increase above US$190.00; neither scenario is consistent with current parameter estimates in India). In both interventions, the ICER was most sensitive to estimates for the risk of progression from LTBI to active TB. When the risk of progression was reduced to 3%, the ICER increased to US$1402.00/DALY averted in Intervention 1 and US$1661.00/DALY averted in Intervention 2.
Figure 2.
Tornado diagrams showing one-way sensitivity analysis for ICERs of key model parameters in base case for both interventions. A) Intervention 1: IPT for CD4 > 200 cells/μl vs. SOC. B) Intervention 2: IPT for CD4 ≤ 200 cells/μl vs. SOC. Arrows = ICER at base-case estimates for all parameters. Not all parameters tested in the sensitivity analysis are shown. Ranges for parameters are shown next to parameter name. Only the top nine factors affecting ICER are shown. TB = tuberculosis disease; IPT = isoniazid preventive therapy; LTBI = latent tuberculous infection; HIV = human immunodeficiency virus; ART =antiretroviral therapy; DALY = disability-adjusted life-years; ICER = incremental cost-effectiveness ratio; SOC = standard of care.
Base-case ICERs were also sensitive to relative risk reduction of IPT on LTBI progression and cost of IPT. When the relative risk reduction was 20%, the ICER increased to US$867.00/DALY averted in Intervention 1 and US$961.00/DALY averted in Intervention 2. When costs were adjusted to US$90.00, the ICER increased to US$702.00/DALY averted in Intervention 1 and US$713.00/DALY averted in Intervention 2.
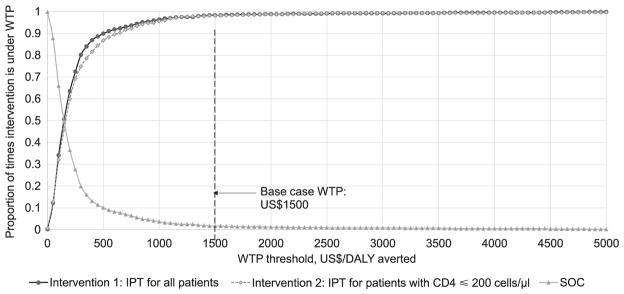
Results of PSA are shown in Figure 3, with mean ICERs of $126.00/DALY averted (95% UR US$13–609.00) for Intervention 1 and US$71/DALY averted for Intervention 2 (95% UR US$2–315.00). At a WTP threshold of US$1500.00/DALY averted, there was a 98.1% chance that Intervention 1 and a 98.3% chance that Intervention 2 was cost-effective.
Figure 3.
Cost-effectiveness acceptability curve from probabilistic sensitivity analysis showing the percentage of simulations in which Intervention 1 or 2 would be considered cost-effective compared to current SOC at varying WTP thresholds. We also show the percentage of simulations in which SOC would be considered the preferred option compared to Intervention 1 and Intervention 2 at varying WTP thresholds. Arrow = WTP threshold of Indian gross per capita at US$1500.00/DALY averted. WTP = willingness to pay; DALY = disability-adjusted life-years; IPT = isoniazid preventive therapy; SOC = Standard of Care.
In the base case, we did not include ART costs as part of intervention costs. Given the potential for increased life expectancy through averted TB death as a result of IPT, individuals in the intervention arms may incur additional health care costs associated with ART compared to SOC. In secondary analysis, accounting for ART costs, the net cost of Intervention 1 was US$1149.74/individual, with an ICER of US$246.00/DALY averted compared to SOC. The cost of Intervention 2 was US$1124.56/individual with an ICER of US$285.00/DALY averted compared to SOC.
Impact of TST on cost-effectiveness of IPT
In the base case, we assumed implementation of IPT irrespective of TST status, given the logistical challenges of TST. In secondary analysis, we explored the cost-effectiveness of TST-driven strategies for antepartum IPT. Overall, we found that providing IPT irrespective of TST status was cost-effective when compared to TST-driven strategies. If Intervention 1 was implemented exclusively for TST-positive individuals, the intervention costs were reduced to US$5.80, but it was less effective (20.304 DALY/individual) than implementation, irrespective of TST status (US$1028.00/DALY averted comparing IPT for all TST-positive vs. IPT for all irrespective of TST). This TST-driven strategy was also considered cost-effective compared to SOC (US$36.00/DALY averted). If Intervention 2 was implemented based on TST positivity, the intervention cost was US$2.58, with an ICER of US$1155.00/DALY averted compared to strategies without TST implementation, and US$31.00/DALY averted compared to SOC. When comparing TST-driven Intervention 1 to TST-driven Intervention 2, the ICER was US$37.00/DALY averted.
DISCUSSION
HIV-TB co-infection during pregnancy remains an important public health concern. Co-infection is associated with increased maternal and infant morbidity and mortality and HIV MTCT. To prevent progression of LTBI to active disease, the WHO recommends 6 months of IPT for TST-positive PLHIV, and consideration of IPT irrespective of TST status, particularly in settings where TST implementation may be impractical.1 Despite the efficacy of IPT, many countries have not adopted this strategy. Our results suggest that it is highly cost-effective to provide 6 months of IPT—irrespective of CD4 count stratification or TST status—for HIV-infected pregnant women in India, substantiate evidence that IPT is cost-effective for PLHIV, and provide insights into its use in a population with increased susceptibility to adverse TB outcomes.22
Although individuals with low CD4 counts have the highest risk of progression to active TB, the greatest population-level health benefits are obtained by implementing IPT for all HIV-infected pregnant women, irrespective of CD4 count and at more favorable cost-effectiveness thresholds. Nonetheless, while it is less effective than universal IPT for HIV-infected pregnant women, our data show that IPT for pregnant women with CD4 count ≤ 200 cells/μl is cost-effective compared to SOC. It is also a less expensive option that may be considered for resource-limited settings.
The diagnosis of LTBI in endemic settings in PLHIV can be challenging due to the impaired sensitivity of the TST with immunosuppression as well as the logistical barriers. We show that IPT for all HIV-infected pregnant women, irrespective of TST status, is highly cost-effective, and likely to result in greater health benefits (i.e., fewer DALYS/person) than TST-driven strategies. These results are reassuring given the worldwide shortages of tuberculin for TST and difficulties in test implementation, such as operator dependency, required follow-up, and tuberculin refrigeration.6 Furthermore, our results reinforce results of a recent South African clinical trial in non-pregnant PLHIV showing that combination IPT and ART was efficacious in preventing active TB irrespective of TST status.13
These results have important implications for TB and HIV programs. TB incidence peaks in women of reproductive age, and pregnancy is an important entry point for women into the health care system.6 The National Health and Family Survey in India found that 74% of all pregnant women in both rural and urban settings received antenatal care in a health care facility.35 Combining TB prevention efforts with peripartum care allows IPT to be more effective by taking advantage of this time frame. Furthermore, as 75% of TB cases in developing countries occur between the ages of 15 and 50 years, public health efforts should be increased for women of childbearing age, as they represent a highly economically productive age group.1
Our study has some limitations. First, our model did not consider downstream secondary TB transmissions that may be averted through IPT. Nonetheless, we show that antepartum IPT for HIV-infected pregnant women was cost-effective and projected to avert TB cases; consideration of additional TB transmission averted would lead to further improvements in the cost-effectiveness ratios. Our model also assumed ideal availability and usage of ART. Suboptimal linkage and retention in HIV care may compromise efforts to reduce HIV-TB morbidity and mortality. Finally, our model incorporated current literature estimates of IPT efficacy; in the future, as the proportion of cases of TB in India is increasingly attributed to multidrug-resistant strains, IPT efficacy may be reduced.
Despite these limitations, our study has several important findings. Despite current recommendations for IPT among PLHIV, uptake in India has been low. Pregnant women represent a target population where IPT may have particular benefits, but for whom there may be concerns about toxicity. Our results suggest that IPT for all HIV-infected pregnant women would be expected to lead to overall improved health outcomes at relatively low cost, even when considering the potential for severe INH-induced hepatotoxicity in pregnancy. Our study also has implications for other settings. By performing extensive sensitivity analyses, we show that IPT is a cost-effective strategy even in populations with lower LTBI prevalence or higher IPT costs. In PSA, both interventions of IPT were cost-effective nearly 100% of the time within a range of WTP thresholds of US$500–5000.00/DALY averted.
Moreover, the benefits of treating HIV-infected pregnant women with IPT extend beyond those modeled in our study. As active TB in HIV-infected pregnant women has been shown to increase the risk of MTCT4 and infant mortality,6,7 increased IPT could potentially reduce HIV-related morbidity and mortality.
CONCLUSION
The WHO-recommended regimen for IPT for HIV-infected pregnant women is highly cost-effective for TB prevention in India, with or without CD4 stratification and irrespective of TST usage. As endemic countries implement TB prevention strategies, consideration should be given to treating all HIV-infected pregnant women with 6 months of IPT.
Acknowledgments
This project was funded with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under grant # K23AI089259, UM1AI069465, USB1-31147-XX-13 and National Institute of Child Health and Human Development, National Institutes of Health (Bethesda, MD, USA) under grant R01HD081929. Additional funding was received from the Ujala Foundation (New Delhi, India), Wyncote Foundation (Philadelphia, PA, USA) and Gilead Foundation (Foster City, CA, USA).
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. TB/HIV: a clinical manual. Geneva, Switzerland: WHO; 2004. [Accessed October 2015]. WHO/HTM/TB/2004.329. http://www.who.int/maternal_child_adolescent/documents/9241546344/en/ [Google Scholar]
- 2.World Health Organization. Global tuberculosis report, 2014. Geneva, Switzerland: WHO; 2014. [Accessed October 2015]. pp. 13–14. WHO/HTM/TB/2014.08. http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 3.Getahun H, Sculier D, Sismanidis C, Grzemska M, Raviglione M. Prevention, diagnosis, and treatment of tuberculosis in children and mothers: evidence for action for maternal, neonatal, and child health services. J Infect Dis. 2012;205(Suppl 2):S216–S227. doi: 10.1093/infdis/jis009. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Bhosale R, Kinikar A, et al. Maternal tuberculosis: a risk factor for mother-to-child transmission of human immunodeficiency virus. J Infect Dis. 2011;203:358–363. doi: 10.1093/jinfdis/jiq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45:1192–1199. doi: 10.1086/522182. [DOI] [PubMed] [Google Scholar]
- 6.Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis. 2012;55:1532–1549. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis. 2007;45:241–249. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health and Family Welfare of India. TB India 2012: Revised National TB Control Program (RNTCP) Annual Status Report 2012. New Delhi, India: Ministry of Health and Family Welfare of India; 2012. pp. 1–10.pp. 47–49. [Google Scholar]
- 9.United Nations Children’s Fund. HIV/AIDS Statistics for India 2012. New York, NY, USA: UNICEF; 2012. [Accessed October 2015]. http://www.unicef.org/infobycountry/india_statistics.html. [Google Scholar]
- 10.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 11.Fox-Rushby JA, Hanson K. Calculating and presenting disability-adjusted life-years (DALYs) in cost-effectiveness analysis. Health Policy Plan. 2001;16:326–331. doi: 10.1093/heapol/16.3.326. [DOI] [PubMed] [Google Scholar]
- 12.National AIDS Control Organization (NACO), Government of India. Updated guidelines for prevention of parent to child transmission (PPTCT) of HIV using multi drug anti-retroviral regimen in India 2013. New Delhi, India: NACO; 2013. [Accessed October 2015]. pp. 10–13. http://www.naco.gov.in/upload/NACP%20-%20IV/18022014%20BSD/National_Guidelines_for_PPTCT.pdf. [Google Scholar]
- 13.Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–690. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23:1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma SK, Mohanan S, Sharma A. Relevance of latent TB infection in areas of high TB prevalence. Chest. 2012;142:761–773. doi: 10.1378/chest.12-0142. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49(RR-6):1–51. [PubMed] [Google Scholar]
- 17.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franks AL, Binkin NJ, Snider DE, Jr, Rokaw WM, Becker S. Isoniazid hepatitis among pregnant and postpartum Hispanic patients. Public Health Rep. 1989;104:151–155. [PMC free article] [PubMed] [Google Scholar]
- 19.Tedla Z, Nyirenda S, Peeler C, et al. Isoniazid-associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in HIV-infected adults in Botswana. Am J Respir Crit Care Med. 2010;182:278–285. doi: 10.1164/rccm.200911-1783OC. [DOI] [PubMed] [Google Scholar]
- 20.Durovni B, Cavalcante SC, Saraceni V, et al. The implementation of isoniazid preventive therapy in HIV clinics: the experience from the TB/HIV in Rio (THRio) study. AIDS. 2010;24(Suppl 5):S49–S56. doi: 10.1097/01.aids.0000391022.95412.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO-CHOICE unit cost estimates for service delivery 2011. Geneva, Switzerland: WHO; 2011. [Accessed October 2015]. http://www.who.int/choice/cost-effectiveness/inputs/health_service/en/ [Google Scholar]
- 22.Pho MT, Swaminathan S, Kumarasamy N, et al. The cost-effectiveness of tuberculosis preventive therapy for HIV-infected individuals in southern India: a trial-based analysis. PLOS ONE. 2012;7:e36001. doi: 10.1371/journal.pone.0036001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Management Services for Health. International Drug Price Indicator Guide 2014. Medford, MA, USA: MSH; 2014. [Accessed October 2015]. http://erc.msh.org/mainpage.cfm?file=1.0.htm&module=DMP&language=english. [Google Scholar]
- 24.Lu C, Liu Q, Sarma A, Fitzpatrick C, Falzon D, Mitnick CD. A systematic review of reported cost for smear and culture tests during multidrug-resistant tuberculosis treatment. PLOS ONE. 2013;8:e56074. doi: 10.1371/journal.pone.0056074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah M, Miele K, Choi H, et al. QuantiFERON-TB Gold InTube implementation for latent tuberculosis diagnosis in a public health clinic: a cost-effectiveness analysis. BMC Infect Dis. 2012;12:360. doi: 10.1186/1471-2334-12-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stouthard M, Essink-Bot M. Disability weights for diseases in the Netherlands. Rotterdam, The Netherlands: Department of Public Health, Erasmus University; 1997. [Google Scholar]
- 28.Holland DP, Sanders GD, Hamilton CD, Stout JE. Costs and cost-effectiveness of four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med. 2009;179:1055–1060. doi: 10.1164/rccm.200901-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Life tables for WHO Member States 2012. Geneva, Switzerland: WHO; 2012. [Accessed October 2015]. http://www.who.int/gho/mortality_burden_disease/life_tables/life_tables/en/ [Google Scholar]
- 30.Nuzzo JB, Golub JE, Chaulk P, Shah M. Analysis of latent tuberculosis infection treatment adherence among refugees and other patient groups referred to the Baltimore City Health Department TB Clinic, February 2009–March 2011. J Immigr Minor Health. 2015;17:56–65. doi: 10.1007/s10903-013-9882-9. [DOI] [PubMed] [Google Scholar]
- 31.Swaminathan S, Subbaraman R, Venkatesan P, et al. Tuberculin skin test results in HIV-infected patients in India: implications for latent tuberculosis treatment. Int J Tuberc Lung Dis. 2008;12:168–173. [PubMed] [Google Scholar]
- 32.Churchyard GJ, Fielding KL, Grant AD. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:1662–1663. doi: 10.1056/NEJMc1402073. [DOI] [PubMed] [Google Scholar]
- 33.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 34.World Bank. GDP per capita by country 2014. Washington, DC, USA: World Bank; 2014. [Accessed October 2015]. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. [Google Scholar]
- 35.Ministry of Health and Family Welfare, Government of India. Maternal health data 2005. New Delhi, India: MOHFW; 2005. [Accessed October 2015]. http://www.rchiips.org/nfhs/nfhs3.shtml. [Google Scholar]