Abstract
Vertical sleeve gastrectomy (VSG) is one of the most commonly performed clinical bariatric surgeries used for the remission of obesity and diabetes. However, the precise molecular mechanism by which VSG exerts its beneficial effects remains elusive. We report that the membrane‐bound G protein‐coupled bile acid receptor, GPBAR‐1 (also known as TGR5), is required to mediate the effects of anti‐obesity, anti‐hyperglycemia, and improvements of fatty liver of VSG in mice. In the absence of TGR5, the beneficial metabolic effects of VSG in mice are lost. Moreover, we found that the expression of TGR5 increased significantly after VSG, and VSG alters both BA levels and composition in mice, resulting in enhancement of TGR5 signaling in the ileum and brown adipose tissues, concomitant with improved glucose control and increased energy expenditure. Conclusion: Our study elucidates a novel underlying mechanism by which VSG achieves its postoperative therapeutic effects through enhanced TGR5 signaling. (Hepatology 2016;64:760‐773)
Abbreviations
- ANOVA
analysis of variance
- BA
bile acid
- BAT
brown adipose tissue
- cAMP
cyclic adenosine monophosphate
- D2
type 2 iodothyronine deiodinase
- DIO
diet‐induced obesity
- ELISA
enzyme‐linked immunosorbent assay
- eWAT
epididymal white adipose tissue
- FXR
farnesoid X receptor
- GPBAR‐1/TGR5
G protein‐coupled bile acid receptor 1
- GLP‐1
glucagon‐like peptide‐1
- H&E
hematoxylin and eosin
- HFD
high‐fat diet
- KO
knockout
- RYGB
Roux‐en‐Y gastric bypass
- T3
3‐5‐3′‐triiodothyronine
- VSG
vertical sleeve gastrectomy
- WT
wild‐type
Bariatric surgery has emerged as an attractive clinical intervention given its ability to achieve greater and more sustainable weight loss than those observed with lifestyle changes or pharmacological therapy.1, 2, 3 To date, Roux‐en‐Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) represent the two most commonly performed bariatric surgeries; both surgical interventions induce significant weight loss and improve glucose tolerance in humans and rodent models.1, 4, 5 Additionally, the RYGB and VSG procedures are no longer considered purely restrictive surgical procedures. Instead, both approaches result in profound metabolic impacts, whose benefits are just beginning to be elucidated.6 Over the past decade, VSG as a single‐stage surgical procedure has gradually become more popular owing to its relative simplicity and comparable clinical outcomes when compared with RYGB.7 Despite the absence of intestinal rearrangement, VSG produces physiological effects similar to those observed in RYGB, and with fewer complications and lower mortality. However, the molecular players governing the positive effects of VSG have remained largely elusive.
Several investigators have proposed mechanisms to explain the positive, metabolic effects of bariatric surgery.3, 8, 9, 10, 11 One important new mechanistic insight relates to signaling pathways regulated by bile acids (BAs). BAs are primarily known for their role as liver‐secreted molecules that aid in the emulsification and absorption of lipids in the small intestine. Additionally, BAs also serve as hormones that alter metabolism by targeting the nuclear farnesoid X receptor (FXR, NR1H4)12 and the cell membrane‐associated G protein‐coupled BA receptor 1 (GPBAR‐1, MBAR1, hereafter referred to as TGR5).13, 14 Many studies have described the clinical impact on BA circuitry following RYGB and VSG.2, 8 In general, surgical changes to the gastrointestinal anatomy increase BA absorption by both the ileum (the distal part of the small intestine) and the liver. Specifically, bariatric surgeries alter the profile of bile acids species in both humans and rodents.8, 9, 15 Such studies have hypothesized that bile acid action may underlie many of the metabolic improvements caused by VSG.
A growing body of evidence suggests that circulating BAs act as signaling molecules that regulate their own synthesis and multiple metabolic pathways by targeting the transcription factor FXR and the membrane protein TGR5.14 FXR signaling has been identified and implicated as necessary in some of the metabolic benefits of VSG as well as the changes to gut microbial communities10; however, the relationship between the increased circulating BAs with the beneficial metabolic effects of VSG remains unclear.10 Moreover, previous studies have reported that VSG produces weight loss in a manner independent of small heterodimer partner (SHP), a direct target gene of FXR that regulates important processes including BA, lipid, glucose homeostasis, and immune responses in the liver.15
In contrast to the nuclear receptor FXR, TGR5 represents a plasma membrane‐bound, G protein‐coupled receptor for BAs. TGR5 is expressed in multiple tissues, including the liver, intestine, adipose tissue, and muscle.16 As signaling molecules, BAs regulate various metabolic processes via TGR5.17 Stimulation of the TGR5 signaling by BAs confers the ability to modulate energy expenditure by regulating the activity of type 2 iodothyronine deiodinase (D2) and the subsequent activation of thyroid hormone in brown adipose tissue (BAT) and muscle.18 Furthermore, the activation of TGR5 by the selective agonist INT‐777 triggers an increase in glucagon‐like peptide‐1 (GLP‐1) secretion in enteroendocrine L cells of the intestinal epithelium.19 This incretin gut hormone modulates insulin secretion and sensitivity, glucagon secretion, and β cell mass,19, 20 thus improving glucose homeostasis.21 In light of these findings, we hypothesized that the activation of BA signaling, through its receptor TGR5, contributes to the insulin‐sensitizing effects of VSG surgery. We surmised that the subsequent induction of GLP‐1 secretion in L cells and activation of the TGR5‐D2 signaling in BAT contributes to the maintenance of weight loss and improvements in glucose control following VSG. To test this hypothesis, we performed VSG and examined its effects in Tgr5 knockout (Tgr5 −/−) and wild‐type mice.
Materials and Methods
ANIMALS
C57BL/6J wild‐type mice (WT) were purchased from Jackson Laboratory (Bar Harbor, ME). Tgr5 −/− (KO) mice, in the C57BL/6J background, were kindly provided by Vassileva Galya at Merck.22 All procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The animal study was approved by City of Hope Institutional Animal Care and Use Committee. Cohorts of mice used in the study included KO and WT male mice that were 6‐10 weeks of age. Mouse cohorts received a 60 kcal% saturated high‐fat diet (HFD) (D12492; Research Diets, New Brunswick, NJ) for 14 weeks and were then randomly subdivided into two body weight−matched groups (VSG and sham) before undergoing gastric surgery. The mice were maintained on the HFD after surgery except the recovery during the immediate postoperative period. Body weight and food intake were measured by weighing the mice and their food hoppers weekly.
SURGERY
VSG surgery was performed using isoflurane anesthesia. The lateral 80% of the stomach was resected, leaving a tubular gastric remnant in continuity with the esophagus superiorly and the pylorus and duodenum inferiorly as reported previously.10 The sham procedure involved analogous isolation of the stomach followed by manually applying pressure with blunt forceps along a vertical line between the esophageal sphincter and the pylorus.
GLUCOSE‐STIMULATED GLP‐1 AND INSULIN SECRETION IN VIVO
For insulin secretion test, mice were fasted overnight and gavaged with d‐glucose (1.5 g · kg−1 body weight). Blood was collected by retro‐orbital puncture at the indicated times. Next, serum insulin levels were tested using an enzyme‐linked immunosorbent assay (ELISA) kit (Crystal Chem, Downers Grove, IL). For in vivo plasma GLP‐1 measurements, mice were fasted and gavaged with the dipeptidyl‐peptidase‐IV inhibitor sitagliptin (3 mg · kg−1 body weight) 60 minutes before oral administration of d‐glucose (1 g · kg−1 body weight). Blood was collected by way of retro‐orbital puncture at the indicated times. Plasma was collected by way of centrifugation, and total GLP‐1 levels were assessed using an ELISA kit (Millipore, Billerica, MA).
EUGLYCEMIC‐HYPERINSULINEMIC CLAMPS
Hyperinsulinemic euglycemic clamp was performed on VSG and sham‐operated mice at 14 weeks after surgery at Baylor College of Medicine. As described previously,23 1 week before the clamp procedure, catheters were placed into the right internal jugular vein extending to the right atrium for sampling and infusions, respectively. Additional information can be found in the Supporting Information.
BODY FAT ANALYSIS
Body fat was measured at 14 weeks after surgery by way of 1H magnetic resonance spectroscopy (Bruker BioSpin, Billerica, MA).
INDIRECT CALORIMETRY AND LOCOMOTOR ACTIVITY ASSAYS
Mice from each group were put in a comprehensive animal metabolic monitoring system (Columbus Instruments, Columbus, OH). The volume of O2 consumption and CO2 production were recorded over a 24‐hour period. Energy expenditure was calculated as described previously.24 Locomotor activity was evaluated as described previously.25 Additional information can be found in the Supporting Information.
HISTOLOGICAL ANALYSIS OF LIVER AND ADIPOSE TISSUES
Liver and adipose tissue were fixed in 4% paraformaldehyde and then embedded in optimal cutting temperature compound (OCT) or paraffin. Oil red O staining and hematoxylin and eosin (H&E) staining were performed as described previously.26
BILE ACIDS PROFILE ANALYSIS
Bile acids composition was analyzed by ultra‐performance liquid chromatography‐mass spectrometry with a modification method.27 Detailed information on bile acid analysis can be found in the Supporting Information.
SAMPLING CECAL CONTENTS AND MICROBIOME ANALYSIS
WT and Tgr5 KO mice fed with HFD for 14 weeks after VSG and sham surgery were killed by way of CO2 asphyxiation and cecal contents were collected immediately. The microbiome genomic DNA was extracted using QIAamp DNA stool Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Sequencing library preparation, sequencing, and data analysis were performed by Integrative Genomics Core at City of Hope National Medical Center. Detailed information on microbiome analysis can be found in the Supporting Information.
STATISTICAL ANALYSIS
All data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was analyzed using an unpaired Student t test or one‐way analysis of variance (ANOVA) with Dunn's post‐test. The threshold of statistical significance was set at P < 0.05.
Results
TGR5 IS REQUIRED TO MAINTAIN WEIGHT LOSS AND IMPROVE HEPATIC STEATOSIS FOLLOWING VSG
In an effort to determine whether TGR5 mediates any positive, physiological role following bariatric surgery, VSG was performed on mice using a diet‐induced obesity (DIO) model.28 After 14 weeks of the feeding, the body weights of HFD‐fed WT and KO mice increased to 60% more than that of mice fed with a normal diet (Supporting Fig. 1A). Moreover, the blood glucose levels of the DIO mice also increased, and this increase correlated with diminished glucose tolerance relative to mice fed with a normal diet (Supporting Fig. 1B‐D). Next, we performed VSG or sham surgery in both WT and KO mice following a DIO model as described previously.10 In the first 3 weeks after surgery, WT and KO mice displayed a similar loss in body weight when compared with their respective sham‐operated controls (Fig. 1A). Over the course of the first 3 weeks, decreased food intake measurements were observed in mice receiving VSG surgery compared with sham‐operated controls (Fig. 1B). It should be noted, however, that food intake among all cohorts was similar by week 4. Additionally, the body weights of both WT‐VSG and KO‐VSG mice remained lower than those observed in sham‐operated control mice, despite no significant differences in their food intake after week 4. These data are consistent with findings that support VSG as a metabolic procedure rather than purely restrictive bariatric surgery.10 WT‐VSG mice maintained their weight loss throughout the post‐VSG period (Fig. 1A). Although the body weights of KO‐VSG mice remained lower than the weights of KO‐sham mice initially, by week 11, the body weights of both groups were similar (Fig. 1A).
Figure 1.
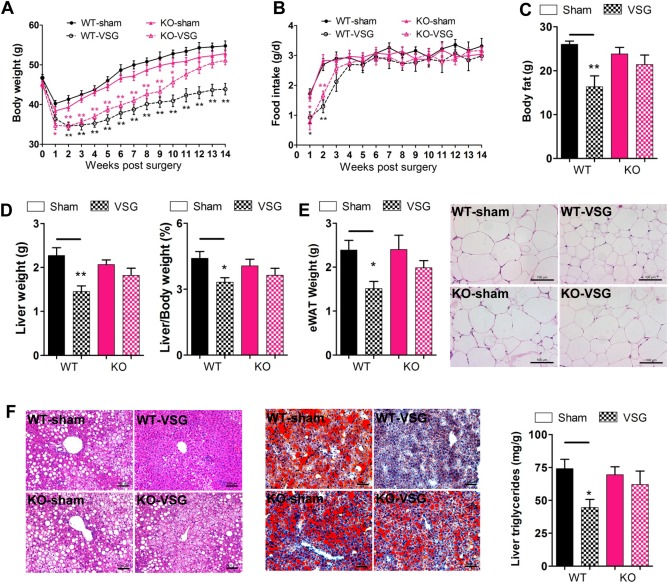
TGR5 is required for the maintenance of weight loss and improvement of hepatic steatosis following VSG. WT and KO mice were fed with HFD to induce DIO, and then subjected to VSG or sham surgical procedures. (A) Body weight of mice after surgery. (B) Food intake of mice after surgery. (C) Body fat weight at 14 weeks after surgery as measured by way of nuclear magnetic resonance. (D) Liver weight and liver/body weight ratio of mice at 14 weeks after surgery. (E) eWAT weight and H&E staining of eWAT sections. (F) H&E and Oil red O staining of liver sections, and liver triglycerides levels of mice at 14 weeks after surgery. *P < 0.05, **P < 0.01 versus sham‐operated mice by one‐way ANOVA with Dunn's post‐test. Values are presented as the mean ± SEM (n = 9‐12 per group, except for n = 5 per group in panel C). Scale bars, 100 μM.
Next, we examined the role of TGR5 in determining body composition after VSG. The results from nuclear magnetic resonance analysis showed that the body fat of WT‐VSG mice dropped dramatically compared with that of WT‐sham controls at 14 weeks after surgery, whereas there was no difference between KO‐VSG and KO‐sham mice (Fig. 1C). In addition, the liver and epididymal white adipose tissue (eWAT) weights were assessed. These data were consistent with our nuclear magnetic resonance findings and revealed a decrease in eWAT weights, liver weights, and liver/body weight ratio after surgery (Fig. 1D,E). Importantly, these changes were lost in Tgr5 KO mice (Fig. 1D,E). The critical role of TGR5 for maintaining the loss of body weight after VSG was further confirmed by way of histological analysis. As expected, VSG significantly reduced adipocyte hypertrophy as well as hepatic steatosis in WT but not Tgr5 KO mice (Fig. 1E,F). Together with the decreased hepatic triglycerides levels (Fig. 1F) and liver/body weight ratio (Fig. 1D), we observed a decrease in serum alanine aminotransferase and aspartate aminotransferase activities, as well as reduced inflammatory genes expression in liver (Supporting Fig. 2) after VSG. These results demonstrate that TGR5 is required to reduce hepatic steatosis and maintain weight loss and fat mass reduction after VSG.
TGR5 IS REQUIRED FOR THE GLUCOSE CONTROL AFTER VSG SURGERY
To investigate the role of TGR5 on glucose homeostasis after VSG surgery, glucose tolerance tests and the metabolites were analyzed in all surgical cohorts. No significant difference in fasting blood glucose levels and glucose tolerance was observed between WT and KO obese mice (Supporting Fig. 1B‐D) prior to VSG surgery. However, fasting blood glucose and insulin levels measured postoperatively at 12 weeks were lower in WT‐VSG mice compared with WT‐sham controls, whereas no significant difference was discerned between KO‐VSG and KO‐sham mice (Fig. 2A,C). In line with these findings, glucose tolerance was improved in WT‐VSG mice challenged 12 weeks after surgery compared with sham‐operated control mice in an intraperitoneal glucose tolerance test (Fig. 2B), whereas no differences were observed between KO‐VSG and sham‐operated mice (Fig. 2B). Notably, WT‐VSG mice exhibited a substantial improvement in the ability to clear glucose, an observation reflected as a 36% reduction in the area under the curve relative to sham‐operated controls (Fig. 2B). These demonstrate directly that TGR5 plays an important role in improving glucose tolerance following VSG.
Figure 2.
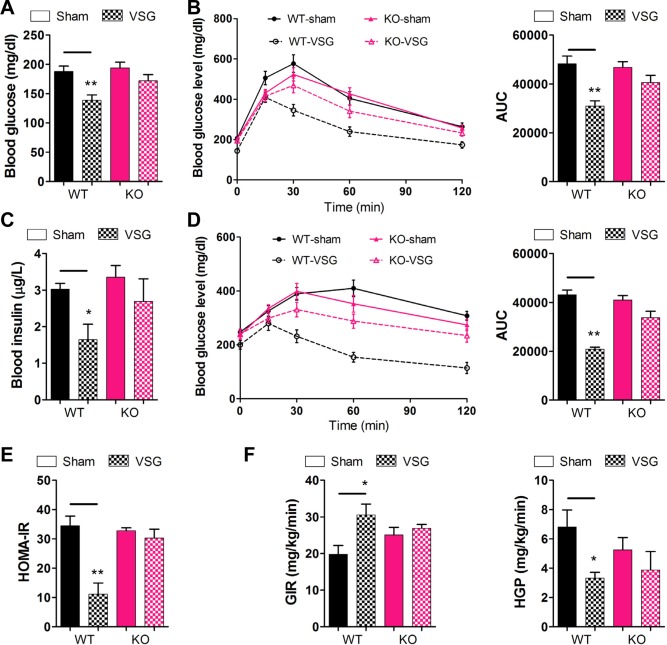
TGR5 contributes to improvement in glucose tolerance and insulin sensitivity after VSG. (A) Fasting blood glucose of mice. (B) Intraperitoneal glucose tolerance test and area under the curve from 0 to 120 minutes in WT and KO mice after intraperitoneal injection of a bolus of 1 g · kg−1 d‐glucose. (C) Fasting blood insulin level of mice. (D) Intraperitoneal pyruvate tolerance tests and area under the curve from 0 to 120 minutes in mice after intraperitoneal injection of a bolus of 1.5 g · kg−1 sodium pyruvate. (E) HOMA‐IR was measured in mice at 12 weeks after surgery. (F) Glucose infusion rates and hepatic glucose production were assessed using hyperinsulinemic‐euglycemic clamps in mice at 14 weeks after surgery. *P < 0.05, **P < 0.01 versus sham‐operated mice by way of one‐way ANOVA with Dunn's post‐test. Values are presented as the mean ± SEM (n = 9‐12 per group, except for n = 4‐5 per group in panel F).
The pyruvate bolus elicits a glycemic excursion that reflects hepatic gluconeogenesis and insulin resistance. The results from our intraperitoneal glucose tolerance test were further supported by intraperitoneal pyruvate tolerance tests, in which the WT‐VSG mice profoundly attenuated the obesity‐induced defect, with an approximately 52% decrease in blood glucose area under the curve (Fig. 2D) compared with the sham‐operated mice. However, there was no appreciable difference between the KO‐VSG mice and the sham‐operated mice (Fig. 2D). Consistent with the aforementioned data, glucose and insulin levels changes of WT‐VSG mice corresponded to a significantly decreased HOMA‐IR (homeostatic model assessment‐insulin resistance) compared with sham‐operated mice (Fig. 2E). In the same way, no difference was observed between KO‐VSG and KO‐sham mice. Thus, our data support a positive role for TGR5 in mediating improvements in insulin sensitivity after VSG.
To better define the potential alterations in insulin sensitivity after VSG, we subjected a cohort of WT and KO mice to hyperinsulinemic‐euglycemic clamps. Consistent with improvements in whole body insulin sensitivity, glucose infusion rates (Fig. 2F), glucose disposal rate, and whole body glucose flux (Supporting Fig. 3) were increased significantly and hepatic glucose production (Fig. 2F) was markedly suppressed in WT‐VSG, but not KO‐VSG mice, compared with corresponding sham‐operated mice. These data indicate that TGR5 could improve insulin sensitivity through the simultaneous suppression of de novo glucose production and elevation of peripheral glucose utilization.
ALTERED CIRCULATING BAS IN WT AND Tgr5 KO MICE AFTER VSG
Even though the bariatric surgery procedure was designed with the aim of restricting food intake and nutrient malabsorption, evidence suggests that these contributions to weight loss are minimal.9, 10, 15 Our results showed that fecal excretion of triglycerides, cholesterol, and free fatty acids in both WT and KO mice post‐VSG are similar to sham‐operated mice (Supporting Fig. 4), indicating that fat absorption post‐VSG may not contribute to the phenotype observed in this mouse model. Surgical intervention by VSG reduces body weight by variable physiological changes in food preferences, gut hormones, caloric malabsorption, gut microbiota, and bile acids.11 Studies have shown that bariatric surgery, including RYGB and VSG, increases circulating BA concentrations.29 To gain insight into the mechanisms underlying TGR5‐dependent metabolic improvements following VSG, we assessed serum and fecal BA compositions in each group 12 weeks after surgery. Using ultra‐performance liquid chromatography‐mass spectrometry, altered trends of BA compositions in wild‐type and Tgr5 KO mice after VSG were similar (Fig. 3). VSG surgery did not affect serum total bile acids levels (TBA) at 14 weeks post‐VSG after overnight fasting, but increased fecal TBA in both WT and KO mice (Supporting Fig. 5). Specifically, the serum concentrations of most individual unconjugated and taurine‐conjugated BAs, except glycine‐conjugated BAs, tended to be higher in WT‐VSG mice as well as KO‐VSG mice relative to their sham‐operated controls (Fig. 3A‐C). Serum bile acids proportion shifted similar in WT‐VSG and KO‐VSG compared with their respective sham‐operated controls (Supporting Fig. 6). In contrast, decreased levels of fecal taurine‐conjugated BAs were observed in WT and KO mice post‐VSG (Supporting Fig. 7), whereas increased levels of the unconjugated BAs were detected after surgery in both WT and KO mice (Supporting Fig. 7). No changes in fecal glycine‐conjugated BA content were observed among the analyzed groups (Supporting Fig. 7). These results are consistent with previous reports of human studies14, 29; the fact that BA composition was already altered as early as 4 weeks after the VSG surgery (Supporting Fig. 8) further suggests that the altered BAs might initiate metabolic changes at the beginning of the postsurgical period.
Figure 3.
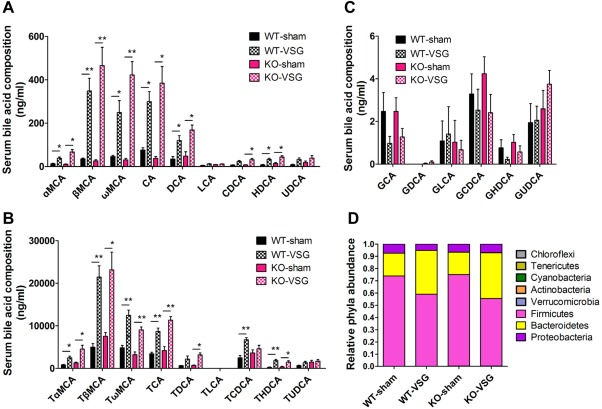
VSG alters BA composition and the gut microbial communities in both WT and KO mice. (A) Serum unconjugated BAs, (B) taurine‐conjugated BAs, and (C) glycine‐conjugated BAs in WT and KO mice at 12 weeks after surgery. (D) Gut microbiome composition of mice caecum samples at 14 weeks after surgery. *P < 0.05, **P < 0.01 versus sham‐operated mice by one‐way ANOVA with Dunn's post‐test. Values are presented as the mean ± SEM (n = 9‐12 per group, except for n = 4‐5 per group in panel D).
Next, in an attempt to better understand our BA analyses, we examined the gene expression profiles of several molecular players involved in mediating BA dynamics. BA synthesizing genes cholesterol 7 alpha‐hydroxylase (Cyp7a1) and 25‐hydroxycholesterol 7‐alpha‐hydroxylase (Cyp7b1) were highly induced in the livers of WT and KO mice that underwent VSG surgery versus sham mice, whereas the expression of sterol 12α‐hydroxylase (Cyb8b1) remained unchanged (Supporting Fig. 9); these data were also consistent with significantly increased muricholic acid levels (Fig. 3). In addition, we measured the expression of bile acids signaling components related to gene expression in the ileum of each mouse cohort. The expression of apical sodium‐dependent bile acid transporter (Asbt) was increased significantly in VSG mice (Supporting Fig. 9). These data suggest that the increase in serum taurine‐conjugated bile acids and the decrease in feces might be explained by the reabsorption of conjugated BAs via increased apical sodium‐dependent bile acid transporter in the ileum following VSG.
Intestinal microbiotas have been implicated in modulating bile acid metabolism and composition.30 Conversely, bile acids composition can also regulate the gut microbial communities.31 To investigate the changes of gut microbial communities, caecal contents from mice (14 weeks post‐VSG) were subjected to pyrosequencing, targeting the V3‐V5 region of the 16S ribosomal RNA gene32 (Fig. 3D). Indeed, phylum‐level shifts from Firmicutes to Bacteroidetes in the gut microbiome composition were observed in mouse caecum at 14 weeks post‐VSG both in WT and KO mice (Fig. 3D). The dominant phylum, Firmicutes, represented 72.8%‐75.5% of the sequences observed in the WT‐sham mice and 73.8%‐76.6% in the KO‐sham mice, while only representing 51.6%‐65.1% of the sequences for the WT‐VSG mice and 55.3%‐67.9% for the KO‐VSG mice. In contrast, the other small population tested did not display significant differences in VSG mice when compared with sham‐operated mice (Fig. 3D). It has been reported that obese humans, mice, and rats all display a reduction in the abundance of Bacteroidetes and an increase in Firmicutes.14, 33 Also, a reduced Firmicutes‐to‐Bacteroidetes ratio correlates with increased tauro‐muricholic acid levels and decreased obesity.18, 34 Thus, our results demonstrate that the therapeutic effects of VSG on both WT and Tgr5 KO mice might be due in part to the phylum‐level shifts that are beneficial for weight loss. Although our results suggest an alteration of BA composition and gut microbial communities following VSG surgery, these changes do not appear to be dependent on TGR5.
VSG ACTIVATES TGR5 IN ILEUM
It has been reported that TGR5 is critical in mediating the transcription of proglucagon, the precursor of GLP‐1.35 To test whether TGR5 is activated by VSG, the expression of proglucagon, as an indicator, was monitored along with Tgr5 using quantitative reverse‐transcription polymerase chain reaction from the ileums of the four mouse groups. As expected, Tgr5 mRNAs were virtually undetectable in Tgr5 −/− mice (Fig. 4A). Consequently, the expression of proglucagon was lower in Tgr5 −/− mice compared with WT mice (Fig. 4A). Notably, Tgr5 expression increased in WT‐VSG mice compared with the WT‐sham mice, and accordingly, the expression of proglucagon significantly increased in WT‐VSG mice as well (Fig. 4A). TGR5 also plays an essential role in mediating enhanced GLP‐1 release.35 To identify whether GLP‐1 and insulin secretion is induced by activated TGR5, glucose tolerance tests were performed while monitoring plasma GLP‐1 levels and insulin levels (Fig. 4B‐E). Following glucose challenge, we observed enhanced glucose clearance in WT‐VSG mice. Importantly, these improvements in glucose dynamics were associated with robust increases in GLP‐1 secretion and insulin release in response to an oral glucose load (Fig. 4B‐E). In Tgr5 KO mice, however, this response was severely blunted (Fig. 4B‐E), indicating that the TGR5 might be an essential component in mediating GLP‐1 and insulin secretion following VSG. Our data indicate that the enhanced expression of TGR5 and BA‐activated TGR5 may be essential for the improvement of insulin resistance and anti‐hyperglycemia effects of VSG.
Figure 4.
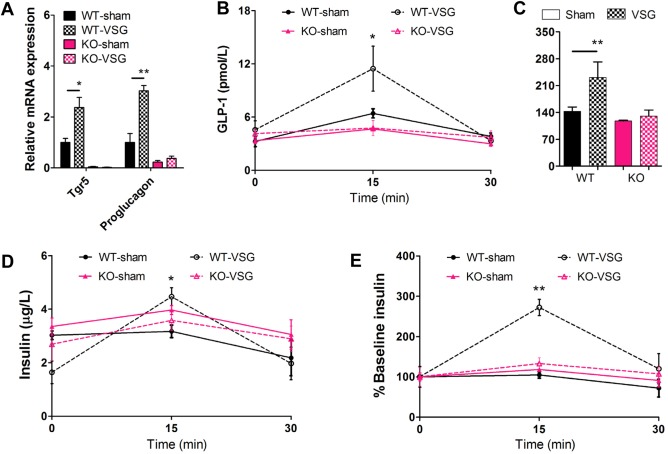
VSG activates TGR5 in the ileum to improve glucose control. (A) Messenger RNA levels of Tgr5 and proglucagon in ileum of mice at 14 weeks after surgery. (B) GLP‐1 secretion and (C) the area under the curve, and (D) insulin secretion and (E) the ratio of secreted insulin compared with the baseline (% baseline insulin) were measured in WT and KO mice at 13 weeks after surgery. *P < 0.05, **P < 0.01 versus sham‐operated mice by ANOVA with Dunn's post‐test. Values are presented as the mean ± SEM (n = 6‐12 per group).
VSG INCREASES ENERGY EXPENDITURE BY ACTIVATING TGR5 PATHWAY
BAT represents a major site for improving metabolic disorders, including obesity and diabetes.36 In BAT, BAs activate TGR5, resulting in increased concentrations of the secondary messenger cyclic adenosine monophosphate (cAMP). Elevated concentrations of cAMP subsequently activate D2, which in turn, convert the inactive thyroid hormone thyroxine to active 3‐5‐3′‐triiodothyronine (T3).37 Both peroxisome proliferator‐activated receptor γ coactivator (Pgc1α) and uncoupling protein 1 (Ucp1), master genes for thermogenesis in BAT, are T3‐response genes,38, 39 with Pgc1α also regulating Ucp1 expression.40 Thus, up‐regulated Pgc1α and Ucp1 triggered by increased levels of T3 resulted in increased BAT activity and enhanced energy expenditure in murine and human BAT.37 Moreover, direct administration of BAs to mice increases energy expenditure in BAT through prompting of the BA‐TGR5‐cAMP‐D2 signaling pathway, preventing obesity and insulin resistance.18 It has also been reported that overexpression of TGR5 or administration of the selective TGR5 agonist INT‐777 increases energy expenditure and reduces hepatic steatosis and obesity.19 To clarify the mechanism governing the TGR5‐dependent maintenance of weight loss phenotype observed following VSG, we examined the expression of genes involved in energy expenditure in the BAT of mice that underwent surgery. As shown in Fig. 5A, the expression of D2 was increased significantly in WT‐VSG compared with WT‐sham mice, whereas no difference was observed in KO mice. These data indicate that the regulation of D2 was dependent on both the elevated BAs levels and their target, TGR5. A similar profile of energy expenditure genes, including Pgc1α, Ucp1, Ucp3, straight‐chain acyl‐CoA oxidase1, and muscle‐type carnitine palmitoyltransferase I (mCPT‐I) were further indicative of a BA‐activated TGR5‐D2 signaling axis in the BAT of WT mice following VSG (Fig. 5A). This result was further supported by histological analysis of BAT in which Tgr5 KO mice exhibited adipocyte hypertrophy with many fat vacuoles even after VSG, whereas adipocyte hypertrophy in WT mice was strongly reduced post‐VSG (Fig. 5B).
Figure 5.
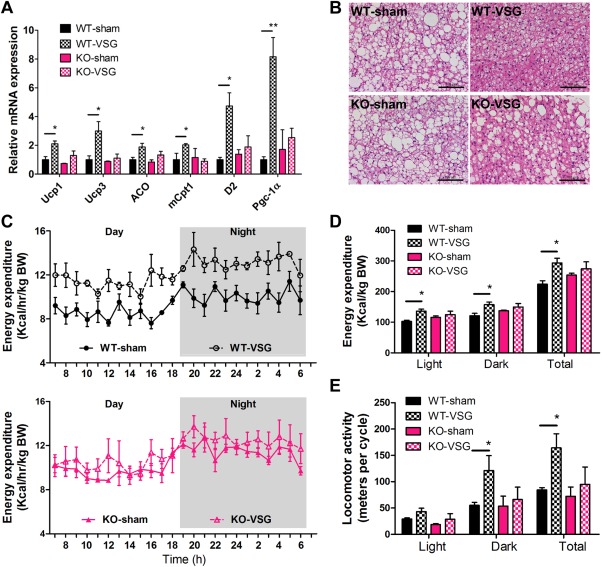
VSG increases energy expenditure by activating TGR5 in BAT. (A) Messenger RNA levels of thermogenic genes. (B) H&E staining of BAT. (C) Energy expenditure over a 24‐hour period and (D) total energy expenditure over light phase, dark phase, and a whole day. (E) Locomotor activity was measured in WT and KO mice at 14 weeks after surgery. *P < 0.05, **P < 0.01 versus sham‐operated mice by one‐way ANOVA with Dunn's post‐test. Values are presented as the mean ± SEM (n = 4‐6 per group). Scale bars, 100 μM.
To further explore whether TGR5 is involved in the regulation of physical activity and energy expenditure, which might contribute to the metabolic improvements in this VSG model, we subjected cohorts of VSG or sham‐operated mice to metabolic cages to measure indirect calorimetry and physical activity over a 24‐hour period under standard conditions 14 weeks after surgery. Our studies established that higher CO2 production and O2 consumption were evident in WT‐VSG mice compared with sham‐operated mice, indicating increased energy expenditure during light phase, dark phase, and the total 24‐hour period (Fig. 5C,D and Supporting Fig. 10). However, there was no significant difference in energy expenditure between KO‐VSG and sham‐operated mice (Fig. 5C,D). As expected, the total locomotor activity of WT‐VSG mice in a 24‐hour period was about two‐fold greater than that in sham‐operated controls, but there was no significant difference in KO‐VSG mice compared with sham‐operated Tgr5 KO mice (Fig. 5E). The frequency of locomotor activity revealed a similar differential trend with KO mice receiving sham surgery or VSG (Fig. 5E). Taken together, the aforementioned data demonstrate that the metabolic improvements of VSG surgery may be partially due to the TGR5‐activated BAT, increased physical activity, and energy expenditure.
Discussion
Over the past decade, the physiological function of BAs have progressed beyond digestive surfactants and have emerged as signaling molecules that regulate a myriad of biological functions, including glucose and lipid metabolism, energy homeostasis, liver regeneration, and liver repair.17, 19 The physiological functions of BAs are mainly the result of the activated BA receptors FXR12 and TGR5.13, 18, 19, 21 Activation of TGR5 by ligands and overexpression of the receptor itself confers strong beneficial effects on glucose and fat homeostasis.18, 19, 21 However, as endogenous BA ligands for TGR5, BAs are typically not present at high enough concentrations to activate TGR5 in a robust manner. Our studies demonstrate that this status, however, is changed following bariatric surgery. We propose that VSG elevates circulating concentrations of BAs to efficacious and safe levels, as well as increase the expression of TGR5, resulting in enhanced TGR5 signaling, and sustainable metabolic improvements, that include the maintenance of weight loss and remission of insulin resistance. In our study, we found that after VSG, concentrations of most unconjugated and taurine‐conjugated BAs in serum were significantly increased and activate TGR5 signaling. These increases subsequently induced intestinal GLP‐1 release and increased energy expenditure and physical activity, leading to sustained weight loss, and the improvement of glucose tolerance and insulin resistance in obese mice post‐VSG. Importantly, our data establish that the positive effects of VSG are dependent on TGR5.
Although it has been suggested that VSG is associated with increased circulating concentrations of BA and compositions,8, 9 their therapeutic value remains unclear. In contrast, several additional mouse models have described altered BA compositions, including sterol 12‐alpha‐hydroxylase (Cyp8b1−/−) mice,41 mice with short‐term calorie restriction,42 and antibiotic‐administered mice.43 While all of these mice display improved metabolic phenotypes similar to VSG, they do provide supporting evidence that BAs are critical factors capable of influencing whole body metabolism. Our study sought out to address this issue directly and determined that VSG confers metabolic improvements by activating TGR5 via elevated BAs and increased TGR5 expression. Our data show that although circulating concentrations of BAs following VSG are similar between Tgr5‐deficient mice and wild‐type mice, distinct differences exist. Postsurgical phenotyping revealed that metabolic improvements were only discernible in WT mice, but not Tgr5 deficient mice. Thus, the integrity of TGR5 signals was required for conferring the positive, therapeutic effects of the surgery.
GLP‐1 is a powerful hormone whose ability to stimulate insulin secretion and normalize glucose levels has been widely documented, and GLP‐1 levels increase after bariatric surgery.20, 44, 45 Surprisingly, it has been reported that the GLP‐1 receptor is not required for the therapeutic effects of bariatric surgeries.46 However, GLP‐1 can also regulate metabolism independent of the GLP‐1 receptor.47, 48 In our study, both the expression of proglucagon and GLP‐1 levels were increased following VSG surgery in WT mice but not in Tgr5 KO mice, results that are consistent with evidence that the GLP‐1 signal can be stimulated by TGR5 activity.19, 35 Our results demonstrate that GLP‐1 signal is regulated by TGR5, and GLP‐1 release might be related with the up‐regulated BA signals after VSG. However, the exact role of GLP‐1 in mediating the effects of bariatric surgery requires further study.
Recently, McGavigan et al.49 also reported that TGR5 contributes to VSG‐induced improvements in glucose regulation. These findings are consistent with the results of the present study; however, their data implicate the independent role of TGR5 in mediating the positive effects of VSG on weight loss, energy expenditure, and insulin secretion.49 The discrepancy in conclusions could be explained by several factors. First, the source of the Tgr5 −/− model mice used in our study versus the study by McGavigan et al. is different. Specifically, the Tgr5 −/− mice used in the latter report was obtained from Taconic Laboratories,50 whereas our Tgr5 −/− mouse line was provided by Vassileva Galya at Merck.22 Second, the age of the mouse cohorts also varied; in their study, mice were initiated on HFD from 1 month of age until 3 months of age, when VSG was performed. Our study design initiated HFD feeding at 2 months of age until mice were 5 months old before being subjected to VSG. Third, the two studies employed different types of diet feed. Whereas the previous study used a HFD feed deriving 45% energy from fat, the HFD used in our study contained 60% kcal% fat. Collectively, these differences contribute to different baseline body weights for mice used in two studies (average of 33 g and 45 g body weight per mouse in the McGavigan et al. study and the present study, respectively). The data provided in Supporting Fig. 1 together with our histological analyses of liver, WAT, and BAT indicated that the HFD‐induced obese model used in our study represented an ideal disease model that recapitulates the excessive adiposity, hyperglycemia, and insulin resistance often observed in human subjects.1 The evidence that BAs or a synthetic TGR5 agonist increases energy expenditure and ameliorates hepatic steatosis and obesity upon HFD by activating BA‐TGR5‐D2 signaling axis in mice18, 19 suggests that TGR5 plays a critical role in the control of body weight. Moreover, these results may have broad implications for examining the role TGR5 plays in mediating the positive effects of bariatric surgery in human subjects. To this end, Broeders et al.37 reported that administration of chenodeoxycholic acid increases energy expenditure in a TGR5‐mediated manner in human BAT. Our data further clarify that the energy expenditure regulated by BAs is dependent on TGR5 after VSG.
Taken together, our studies identify a relationship between the increased circulating concentrations of BAs observed after bariatric surgery and their therapeutic value on obesity, diabetes, and nonalcoholic fatty liver disease. Additionally, our study supplies evidence that VSG generates a new, steady level of BAs that confer the metabolic benefits of VSG through activation of TGR5.
Author names in bold designate co‐first authorship.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.28689/suppinfo.
Supplementary Table 1. The primers for RT‐PCR.
Acknowledgment
We thank Art Riggs, Barry Forman, Rama Natarajan, Debbie Thurmond, and Shiva Andrali for discussion and support, as well as William Davis and other members of the Huang laboratory (Xiaoxiao Ma, Jinyan Tian, Hongli Zhang) for helpful discussion. We also thank Ian Talisman for help editing the manuscript.
Potential conflict of interest: Nothing to report.
This study was supported in part by the Schaeffer Foundation and the National Cancer Institute (grant R01‐139158 to W.H. and P30CA33572), the National Natural Science Foundation of China (grants 81303186 and 81573581), the International Postdoctoral Exchange Fellowship Program of China (to L.D.), and the Open Research Fund of State Key Laboratory of Cellular Stress Biology, Xiamen University (grant SKLCSB2016KF002).
REFERENCES
- 1. Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel‐Courtin C, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 2009;250:234–241. [DOI] [PubMed] [Google Scholar]
- 2. Chakravartty S, Tassinari D, Salerno A, Giorgakis E, Rubino F. What is the mechanism behind weight loss maintenance with gastric bypass? Curr Obes Rep 2015;4:262–268. [DOI] [PubMed] [Google Scholar]
- 3. Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 2009;5:469–475. [DOI] [PubMed] [Google Scholar]
- 4. Pereferrer FS, Gonzàlez MH, Rovira AF, Blasco SB, Rivas AM, del Castillo Déjardin D. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg 2008;18:97–108. [DOI] [PubMed] [Google Scholar]
- 5. Stefater MA, Wilson‐Pérez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev 2012;33:595–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, Krause KR, et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg 2013;257:791–797. [DOI] [PubMed] [Google Scholar]
- 7. Reames BN, Finks JF, Bacal D, Carlin AM, Dimick JB. Changes in bariatric surgery procedure use in Michigan, 2006‐2013. JAMA 2014;312:959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steinert RE, Peterli R, Keller S, Meyer‐Gerspach AC, Drewe J, Peters T, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1‐year prospective randomized pilot trial. Obesity (Silver Spring) 2013;21:E660–E668. [DOI] [PubMed] [Google Scholar]
- 9. Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight‐loss‐independent manner. Obesity (Silver Spring) 2014;22:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva‐Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014;509:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 2013;10:575–584. [DOI] [PubMed] [Google Scholar]
- 12. Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 1999;3:543–553. [DOI] [PubMed] [Google Scholar]
- 13. Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, et al. Identification of membrane‐type receptor for bile acids (M‐BAR). Biochem Biophys Res Commun 2002;298:714–719. [DOI] [PubMed] [Google Scholar]
- 14. Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 2014;66:948–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myronovych A, Salazar‐Gonzalez RM, Ryan KK, Miles L, Zhang W, Jha P, et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity (Silver Spring) 2014;22:2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis 2014;46:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap‐bile acids in metabolic control. Nat Rev Endocrinol 2014;10:488–498. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006;439:484–489. [DOI] [PubMed] [Google Scholar]
- 19. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5‐mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009; 10:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuhre RE, Holst JJ, Kappe C. The regulation of function, growth and survival of GLP‐1‐producing L‐cells. Clin Sci (Lond) 2016;130:79–91. [DOI] [PubMed] [Google Scholar]
- 21. Brighton CA, Rievaj J, Kuhre RE, Glass LL, Schoonjans K, Holst JJ, et al. Bile acids trigger GLP‐1 release predominantly by accessing basolaterally located G‐protein coupled bile acid receptors. Endocrinology 2015;156:3961–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J 2006;398:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi CS, Savage DB, Abu‐Elheiga L, Liu ZX, Kim S, Kulkarni A, et al. Continuous fat oxidation in acetyl CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A 2007;104:16480–16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flynn CR, Albaugh VL, Cai S, Cheung‐Flynn J, Williams PE, Brucker RM, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun 2015;6:7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lugo JN, Brewster AL, Spencer CM, Anderson AE. Kv4.2 knockout mice have hippocampal‐ dependent learning and memory deficits. Learn Mem 2012;19:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang JJ, Li JG, Qi W, Qiu WW, Li PS, Li BL, et al. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab 2011;13:44–56. [DOI] [PubMed] [Google Scholar]
- 27. Yang L, Xiong A, He Y, Wang Z, Wang C, Wang Z, et al. Bile acids metabonomics study on the CCl4‐ and α‐naphthylisothiocyanate‐induced animal models: quantitative analysis of 22 bile acids by ultraperformance lipid chromatography‐mass spectrometry. Chem Res Toxicol 2008;21:2280–2288. [DOI] [PubMed] [Google Scholar]
- 28. Wang CY, Liao JK. A mouse model of diet‐induced obesity and insulin resistance. Methods Mol Biol 2012;821:421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klaassen CD, Cui JY. Review: Mechanisms of how the intestinal microbiota alters the effects of drugs and bile acids. Drug Metab Dispos 2015;43:1505–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 2014;30:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stearns JC, Lynch MD, Senadheera DB, Tenenbaum HC, Goldberg MB, Cvitkovitch DG, et al. Bacterial biogeography of the human digestive tract. Sci Rep 2011;1:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242–249. [DOI] [PubMed] [Google Scholar]
- 34. Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 2013;4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harach T, Pols TW, Nomura M, Maida A, Watanabe M, Auwerx J, et al. TGR5 potentiates GLP‐1 secretion in response to anionic exchange resins. Sci Rep 2012;2:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 2011;12:722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Broeders EP, Nascimento EB, Havekes B, Brans B, Roumans KH, Tailleux A, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 2015;22:418–426. [DOI] [PubMed] [Google Scholar]
- 38. Hall JA, Ribich S, Christoffolete MA, Simovic G, Correa‐Medina M, Patti ME, et al. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology 2010;151:4573–4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC‐1. Cell 1999;98:115–124. [DOI] [PubMed] [Google Scholar]
- 40. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold‐inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92:829–839. [DOI] [PubMed] [Google Scholar]
- 41. Hu X, Bonde Y, Eggertsen G, Rudling M. Muricholic bile acids are potent regulators of bile acid synthesis via a positive feedback mechanism. J Intern Med 2014;275:27–38. [DOI] [PubMed] [Google Scholar]
- 42. Fu ZD, Klaassen CD. Increased bile acids in enterohepatic circulation by short‐term calorie restriction in male mice. Toxicol Appl Pharmacol 2013;273:680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 2015;125:386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP‐1 and energy balance: an integrated model of short‐term and long‐term control. Nat Rev Endocrinol 2011;7:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux‐en‐Y gastric bypass. Ann Surg 2007;246;780–785. [DOI] [PubMed] [Google Scholar]
- 46. Wilson‐Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon‐like peptide 1 receptor deficiency. Diabetes 2013; 62:2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomas E, Habener JF. Insulin‐like actions of glucagon‐like peptide‐1: a dual receptor hypothesis. Trends Endocrinol Metab 2010;21:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nuche‐Berenguer B, Portal‐Núñez S, Moreno P, González N, Acitores A, López‐Herradón A, et al. Presence of a functional receptor for GLP‐1 in osteoblastic cells, independent of the cAMP‐linked GLP‐1 receptor. J Cell Physiol 2010;225:585–592. [DOI] [PubMed] [Google Scholar]
- 49. McGavigan AK, Garibay D, Henseler ZM, Chen J, Bettaieb A, Haj FG, et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 2015; doi: 10.1136/gutjnl-2015-309871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, et al. Targeted disruption of G protein‐coupled bile acid receptor 1 (Gpbar1/M‐Bar) in mice. J Endocrinol 2006;191:197–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.28689/suppinfo.
Supplementary Table 1. The primers for RT‐PCR.


