Abstract
Objective
Hepatic encephalopathy (HE) is challenging to identify in children with acute liver failure (ALF), and was not a requirement for enrollment into the Pediatric ALF Study Group (PALFSG). The outcomes of PALFSG participants presenting with and without HE are presented.
Methods
PALFSG participants were classified based on daily assessment of HE during the first 7 days following study enrollment: Group1 - never developed HE; Group2 - no HE at enrollment with subsequent HE development; and Group 3 - HE at study enrollment. Clinical and biochemical parameters and outcomes of death, spontaneous recovery (SR), or liver transplantation (LT) were compared between groups.
Results
Data from 769PALFSG (54% male; median age 4.2 years; range 0–17.9 years) participants were analyzed, with 277 in Group 1 (36%), 83 in Group 2 (11%) and 409 in Group 3 (53%). Mortality occurred in 11% of all participants and was highest among Group 3 participants who demonstrated persistent grade III–IV HE (55%) or showed progression of HE (26%). Eleven (4%) Group 1 participants died within 21 days of enrollment. SR was highest in Group 1 (79%) and lowest in Group 2 (25%; p<.001).
Conclusion
Mortality21 days following enrollment was highest in participants enrolled with severe HE (grades III or IV) or demonstrating HE progression. However, 4% of participants without recorded clinical HE in the 7 days following enrollment died within 21 days. Improved assessment of neurological injury and PALF prognostication schema are needed.
Keywords: Fulminant, natural history, death, liver transplant, spontaneous recovery
Introduction
Hepatic encephalopathy (HE) is a sine qua non criterion for diagnosing fulminant liver failure in adults (1). Both initial and peak HE grades are associated with the likelihood of death during subsequent hospitalization in both adults and children (2–7).However, HE in children might not always be clinically apparent or reliably identified (3, 8–12). PALF is relatively rare so published studies have generally been limited to single center experiences with small patient numbers typically recruited over long periods of time (8–10, 13, 14). In particular, clinical outcomes have not been examined with respect to patterns of HE in PALF. The multicenter, international, NIH UO1-funded Pediatric Acute Liver Failure Study Group (PALFSG) was established in December 1999 with an overarching goal to develop a database to facilitate studies of pathogenesis, treatment and outcomes of children with acute liver failure. Participants enrolled in the PALFSG include children with severe uncorrectable liver-based coagulopathy (INR ≥2) even if HE was determined by an experienced pediatric hepatologist to be clinically absent at presentation. We hypothesized that children enrolled into the PALFSG who never developed HE would exhibit distinct demographic, clinical and biochemical characteristics at presentation and have better 21 day outcomes compared to participants with HE at presentation or who subsequently develop HE.
Methods
Participants
Inclusion criteria for enrollment into the PALFSG are: i) age from birth to 18 years with no known chronic liver disease; (ii) biochemical evidence of acute liver injury; (iii) coagulopathy not corrected by parenteral administration of vitamin K; and (iv) informed consent obtained from the parent or legal guardian. The degree of coagulopathy determined whether HE is required for PALF study enrollment, with all subjects having either (a) INR between 1.5 and 2.0 (or prothrombin time [PT] ≥15 and <20 seconds) in the presence of HE or (b) INR≥2 (PT≥20 seconds) with or without HE. The Whitington scale (Supplementary Table 1) was used for HE determinations in participants aged less than 3 years of age, whereas the standard clinical scale was used for participants aged 3 to 18 years of age (Supplementary Table 2) (15). A detailed description of the PALF study cohort has been previously published (16).For the present study, exclusion criteria included PALF attributed to acetaminophen toxicity (n=122), given that the vast majority of participants with acetaminophen-induced PALF did not develop HE and recovered spontaneously (7, 17, 18).
To understand the differing outcomes in PALF subjects who were enrolled with and without HE, participants enrolled in this prospective observational cohort study were classified as: Group 1 if they never had an episode of clinical HE reported within the first 7 days following study enrollment; Group 2 if they had no clinical HE at study enrollment but subsequently developed HE during the 7 days following enrollment; or Group 3 if they had HE at enrollment. Group 3 participants were further stratified by severity of coagulopathy at enrollment and HE persistence, progression or regression over the subsequent 7 days. HE progression was defined as an increase by at least 1 coma grade score, and HE regression as a decrease of at least 1 coma grade score within the 7 days following study enrollment (19) (See Figure 1).
Figure 1.
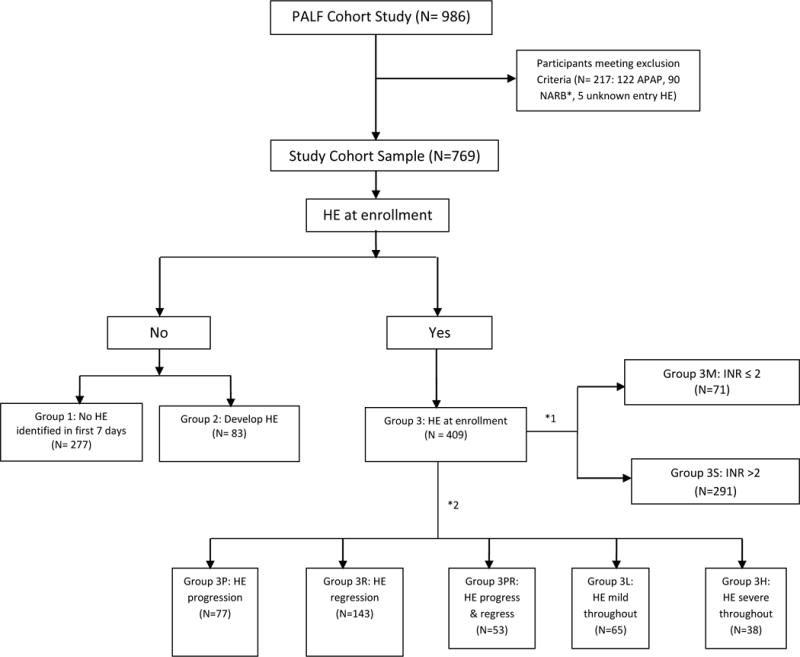
Classification of PALF Study Participants.
Study Design
This was a prospective observational cohort study previously published (16). Data available about participant demographic, clinical and laboratory information as recorded daily on data collection forms for up to 7 days following enrollment. Diagnostic evaluation and medical management of participants were performed as per local standard of care. Mutually exclusive outcomes examined were survival with native liver, i.e., spontaneous recovery (SR), death without transplant, and liver transplantation (LT) at 21 days after study entry.
Statistical Analysis
Baseline participant characteristics are summarized by medians and 25th–75th percentiles for continuous variables and by frequencies and percentages for categorical variables. To examine whether participants in Group 2 and Group 3 differed from those in Group 1, the Wilcoxon rank-sum test was used for continuous variables and the Pearson’s chi-squared test (or its exact version) for categorical variables.
For the subgroup analysis by severity of coagulopathy at enrollment, participants with milder hepatic-based coagulopathy (INR <2) were categorized as 3M, whereasparticipants with more severe hepatic-based coagulopathy (INR ≥ 2) were categorized as 3S.
For the subgroup analysis by patterns of HE progression, we included Group 3 participants with at least 2recordedHE measurements. HE change was categorized into 5 groups – these included HE progression (3P), HE regression (3R), both HE progression and regression (3PR), HE persisting as low/mild throughout the first 7 days (3L) and HE persisting as high/severe throughout the first 7 days (3H). The Pearson’s chi-squared test (or its exact version) was used to test whether the change groups differed with respect to categorical variables, and the Kruskal–Wallis test was used for continuous variables. The cumulative incidence probability curves, accounting for the competing risks of LT and death without transplantation, were computed using R (Version 3.0.1; R Foundation for Statistical Computing, Vienna, Austria) package cmprsk. We used the non-parametric methods and median (IQR) because they work well for both skewed and non-skewed outcomes, whereas parametric methods and the mean (SD) often requires the normality assumption and the symmetry of the distribution. INR was log-transformed due to its skewness. All other statistical analyses were conducted in SAS 9.4 (SAS Institute, Cary NC, version 9.4). For all statistical tests, statistical significance was defined as p<0.05. No adjustment for multiple testing was made.
RESULTS
Study Cohort
Between December 1999 and September 2010, 986 participants were enrolled in the PALFSG. Excluded from this analysis were those with a final diagnosis of acetaminophen-induced PALF (n=122), those with non-assessable HE status due to intubation or sedation (n=90), and those with a missing HE grade at PALF enrollment (n=5). The study cohort was comprised of 769 participants (Figure 1). We excluded from the main analysis those participants whose HE status could not be assessed due to being on a respirator or barbiturates (n=90) for the duration of the PALF study enrollment because we could not determine HE progression with certainty. Our PALF database did not record whether the sedation and/or intubation was or was not due to HE.
Patient Demographic and Clinical Characteristics
Demographics and clinical status of the study cohort on the day of enrollment into the PALF consortium are summarized in Table 1. Participants in Group 1 did not differ significantly from those in Groups 2 or 3 with respect to race and sex. A larger percentage of participants were reported as having the PALF etiologies of metabolic liver disease and autoimmune hepatitis in Group 1 (11% and 12% respectively) compared to Group 3 subjects (6% and 7%, p=0.03). Group 1 participants were significantly younger (median 3.0 years; 25th, 75th percentiles 0.1, 11.7) than Group 3 (median 4.2 years; 25th, 75th percentiles, 1.2, 11.0, p=0.01) and Group 2 (median 6.9 years; 25th, 75thpercentiles, 1.5, 10.3, p=0.03).Group 1 participants were less likely to have seizures (p=0.01), fever (p<0.001), or ICU admission (p<0.001) at presentation compared to Group 3 participants, and had less severe hyperbilirubinemia (p<0.001) compared to Group 2. A total of 492 (64%) patients had HE or developed HE with 7 days of PALF study entry.
Table 1.
Baseline Demographics, Clinical Status, and Outcomes of Study Cohort
| CharacteristicsA | All (N=769) | Group 1 (N=277) | Group 2 (N=83) | Group 3 (N=409) |
|
| ||||
| Sex (%) | ||||
| -Male | 54% | 55% | 60% | 52% |
|
| ||||
| Age (years) (median, q1–q3) | 4.2(0.7,11.1) | 3.0(0.1,11.7) | 6.9(1.5,10.3)* | 4.2(1.2,11.0)* |
|
| ||||
| Race (%) | ||||
| -White | 70% | 68% | 75% | 70% |
| -African American | 14% | 12% | 12% | 15% |
| -Asian | 9% | 12% | 8% | 7% |
| -American Indian | 2% | 1% | 1% | 2% |
| -Mixed | 3% | 4% | 1% | 3% |
| -Unknown | 2% | 3% | 2% | 2% |
|
| ||||
| Final Diagnosis(%) | * | |||
| -Indeterminate etiology | 51% | 45% | 63% | 54% |
| -Autoimmune Hepatitis | 9% | 12% | 10% | 7% |
| -Metabolic disorder | 8% | 11% | 5% | 6% |
| -Viral Hepatitis | 8% | 8% | 5% | 9% |
| -Other Diagnosis | 25% | 25% | 18% | 24% |
|
| ||||
| Entry Coma (%) | ||||
| 0 | 47% | 100% | 100% | 0% |
| I | 27% | 0% | 0% | 51% |
| II | 13% | 0% | 0% | 24% |
| III | 8% | 0% | 0% | 16% |
| IV | 5% | 0% | 0% | 9% |
|
| ||||
| Peak Coma (%) | ||||
| 0 | 36% | 100% | 0% | 0% |
| I | 22% | 0% | 48% | 31% |
| II | 18% | 0% | 24% | 28% |
| III | 14% | 0% | 18% | 22% |
| IV | 11% | 0% | 10% | 19% |
|
| ||||
| Seizure (N=759) (%) | 6% | 4% | 0% | 9%* |
| Jaundice(N=736) | 77% | 76% | 89%* | 76% |
| Fever (N=759) | 38% | 27% | 37% | 45%*** |
|
| ||||
| Require PICU (N=731) (%) | 58% | 40% | 31% | 76%*** |
| Requires Hemodialysis (N=728) | 1% | 0.4% | 0% | 2% |
| Requires Intracranial monitoring (N=726) | 2% | 0% | 0% | 4%*** |
|
| ||||
| INR (N=685)(median, q1–q3) | 2.6(2.1,3.8) | 2.4(2,3.2) | 2.6(2.2,3.5)* | 2.8(2.1,4.2)*** |
| Total Bilirubin (mg/dL) (N=707) | 12.1(4.2,19) | 9.6(3.4,16.4) | 16.5(11.2,20.4)*** | 12.2(4.0,19.9)* |
| Arterial ammonia (μmol/L)(N=139) | 80(44,126) | 60(41,99) | 45(6,92) | 86(55,142)* |
| Venous ammonia (μmol/L) (N=457) | 58(36,94) | 54(32,74) | 50(34,76) | 67(41,108)*** |
| Lactate (mmol/L) (N=406) | 2.8(1.9,4.8) | 2.5(1.6,4.4) | 3.1(2.1,5.2) | 2.8(2.0,5.1)* |
|
| ||||
| 21-day Outcome (N=768)(%) | *** | *** | ||
| Death by day 7 | 7% | 2% | 6% | 10% |
| LTx by day 7 | 26% | 9% | 46% | 34% |
| Death between day 8–21 | 5% | 2% | 6% | 6% |
| LTx between day 8–21 | 8% | 8% | 17% | 5% |
| Alive at day 21 | 55% | 79% | 25% | 45% |
|
| ||||
| Cause of death, (N=88) (%) | ||||
| Brain herniation/edema | 18% | 0% | 30% | 19% |
| Brain hemorrhage | 1% | 0% | 0% | 1% |
| Multi-system failure | 51% | 55% | 20% | 55% |
| Pulmonary hemorrhage | 6% | 9% | 0% | 6% |
| Pulmonary edema | 5% | 9% | 10% | 3% |
| Sepsis | 8% | 18% | 0% | 7% |
| Cardiac | 9% | 0% | 30% | 7% |
| Other | 2% | 9% | 10% | 0% |
p-value between 0.01 and 0.05,
0.001≤p<0.01, and
p<.001.
Group 1 is the reference group. The distributions of entry coma and peak coma were not compared between groups.
Sample size equals 769 unless otherwise specified.
Overall and Group-Specific Patient Outcomes
By 21 days after study enrollment, 11%of PALF participants died without having undergone LT,55% had spontaneous recovery of their native liver and34% underwent LT. Most (58%) of the 88 deaths occurred in the first 7 days after PALF study enrollment, with multi-system failure (51%) and brain herniation or edema (18%) being the most commonly cited causes of death (Table 1). Amongst Group 1 participants, 11 (4%) died. In comparison to those Group 1 participants with spontaneous recovery of native liver at day 21, these 11 subjects were younger (median age 0.05 years, range 0.01–11.9 years) when compared to median 2.3 years), and had evidence of a more severe illness at PALF enrollment as indicated by higher INR at study enrollment (median 3.1 (range 2.1–13.1) compared to median 2.3), and more often required PICU level care (64% compared to 37%). Their total bilirubin levels were not significantly higher, nor did their etiology for PALF differ statistically.
Figure 2 illustrates outcomes in each subgroup. Despite the absence of recorded HE, death occurred in 11 (4%) Group 1 participants, including 2 deaths occurring on day 1, and one each on day 2, day 4, and day 6 after PALF enrollment respectively. Group 1 causes of death were most commonly multi-organ failure (55%) (Table 1).Mortality was highest in Group 3 participants (16%), with more than half of these deaths occurring in the first 7 days following PALF enrollment. Causes of death were not significantly different between Group 1 and Group 3 participants. At 21 days following PALF study enrollment, LT was the most common outcome in Group 2 (63%) though less than half of participants in the other groups underwent LT (Group 1: 17%, Group 3: 39%)(Table 1).The majority of LT occurred within the first 7 days following PALF study enrollment (200/258, 78%) with the highest frequency of LT within the first 7 days occurring in group 3 (137/158; 87%) (Table 1).
Figure 2.
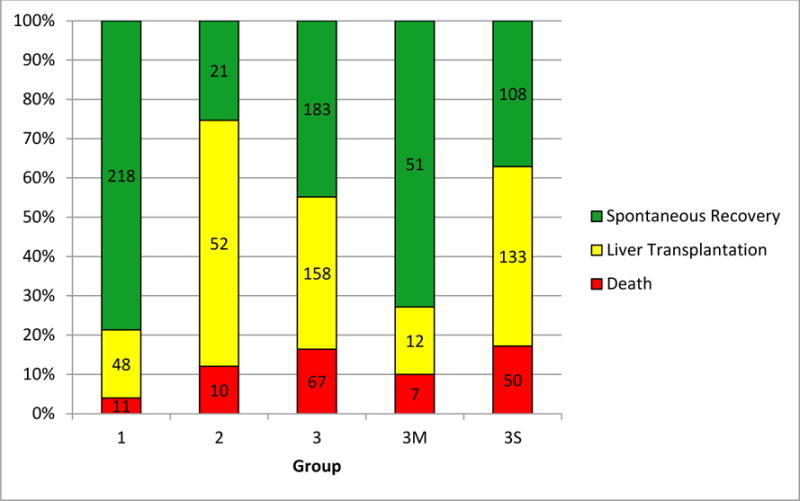
21 day outcomes for Participants in Groups 1, 2, 3, 3M and 3S
Using a Fine and Gray competing risks model to study the association between entry HE and the outcomes (LT or death without LT), adjusting for age, total bilirubin and log (INR), death without LT occurred more frequently in participants with entry HE of III or IV when compared to participants with entry HE score of 0. Specifically, the sub-distribution hazard ratio for HE grade III relative to grade 0 was 2.53 (95% CI 1.27–5.03, p=0.008), and the sub-distribution hazard ratio for HE grade IV relative to grade 0 was 4.48 (95% CI 2.27–8.85, p<.001).
Participants with entry HE grade of I were1.35 (95% CI 1.00–1.81, p=0.049) times more likely to undergo LT when compared to those with HE 0, whereas the estimated sub distribution hazard ratio HE grade II vs. grade 0 was 1.77 (95% CI 1.26–2.48, p=0.001). Participants with entry HE grade of III or IV were not significantly more likely to undergo LT than those with HE grade of 0. The sub distribution hazard ratio equals 0.98 (95% CI 0.53–1.82, p=0.95) for HE grade III vs. 0 and 0.95 (95% CI 0.43–2.09, p=0.89) for HE grade IV vs. 0.
Outcomes of Group 3 participants
Amongst the 409 Group 3 participants (with HE at the time of enrollment), the likelihood of death was highest in those with admission or peak HE grades III–IV, while LT occurred most often in participants with lower admission HE grade II or peak HE grades II–III (Table 2). Group 3 outcomes were further examined by degree of coagulopathy at presentation and by the progression or regression of HE over the 7 days following enrollment (Supplementary Table 3).
Table 2.
7 day and 21 day outcomes based on admission coma and peak coma respectively
| #$ | 7-day Outcomeˆ | 21-day Outcomeˆ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Died | Transplanted | Alive | p | Died | Transplanted | Alive | p | |||
| Admission coma (%) | 0 | 360 | 3% | 18% | 80% | *** | 6% | 28% | 66% | *** |
| I | 206 | 5% | 31% | 64% | 10% | 38% | 52% | |||
| II | 100 | 7% | 43% | 50% | 14% | 48% | 38% | |||
| III | 64 | 16% | 30% | 55% | 28% | 31% | 41% | |||
| IV | 38 | 34% | 29% | 37% | 39% | 29% | 32% | |||
|
| ||||||||||
| Peak Coma (%) | 0 | 277 | 2% | 9% | 89% | *** | 4% | 17% | 79% | *** |
| I | 166 | 2% | 27% | 72% | 5% | 34% | 61% | |||
| II | 135 | 3% | 36% | 61% | 10% | 50% | 41% | |||
| III | 104 | 10% | 46% | 44% | 18% | 50% | 32% | |||
| IV | 86 | 34% | 40% | 27% | 42% | 41% | 17% | |||
|
| ||||||||||
| Participants with INR<2 and HE at enrollment (3M) | ||||||||||
|
| ||||||||||
| Admission coma (%) | I | 34 | 0% | 12% | 88% | 0% | 21% | 79% | ||
| II | 16 | 6% | 13% | 81% | 13% | 25% | 63% | |||
| III | 10 | 10% | 0% | 90% | 20% | 0% | 80% | |||
| IV | 10 | 30% | 10% | 60% | 30% | 10% | 60% | |||
Outcome was missing for 1 of the 769 participants. The person had grade I admission coma and grade I peak coma. His INR was less than 2 at enrollment.
In each row, the cells represent the percentages of the outcome at a specific HE level.
p<.001
There were 71 participants with HE and milder (INR < 2) coagulopathy (Group 3M) and 291 with more severe (INR ≥ 2) coagulopathy (Group 3S). Demographic and clinical characteristics of these participants are provided in Supplementary Table 3. Etiologies of PALF were not significantly different between these groups (p=0.2). Jaundice at enrollment was less commonly seen in Group 3M (62%) versus Group 3S (79%) participants (p=0.005). While HE coma grades at enrollment did not differ significantly between those in Groups 3M and 3S, respectively (p=0.3), the percentage of participants undergoing LT was larger in 3S (46%) than in 3M (17%, p<0.001) participants (Figure 2). Twenty 3M subjects had an entry HE coma grade III or IV, all of whom required intensive care unit level of care despite mild coagulopathy, with 14 (70%)recovering spontaneously and death occurring in 5 (25%) participants. Despite an admission HE stage of IV, 6 Group 3M participants recovered spontaneously (Table 2).
Amongst Group 3S participants, 74 had entry HE grade of III–IV, and 97% of them were in ICU at baseline. There were 24 (32%) death and 30 (41%) LT by day 21. Among the Group 3 participants with severe entry HE of III–IV, 3S participants had worse outcomes than the 3M participants (p<.001).
Amongst 376 Group 3 participants with at least two HE measures following enrollment, 77 (20%) had HE progression (Group 3P), 143 (38%) had regression (Group 3R), 53 (14%) experienced both HE progression and regression (Group 3PR), 65 (17%) displayed persistently low HE (grade I–II; Group 3L), and 38 (10%) had persistently high HE (grade of III–IV; Group 3H) (Figure 1). Participants with clinically more significant HE (Groups 3P and 3H)were more likely to require ICU level care, including hemodialysis support and ICP monitoring (Supplementary Table 3). Mortality was highest in Group 3H (55%) and lowest in Group 3R (1%) subjects. Just under half of Group 3PR participants remained alive at 21 days, demonstrating that HE progression is not universally associated with a bad outcome. Spontaneous recovery was highest in Group 3R (88%) and lowest in Group 3P (6%). The cumulative incidence probability of death without LT was highest in Group 3H and lowest in Group 3R (p<0.001; See Figure 3).
Figure 3.
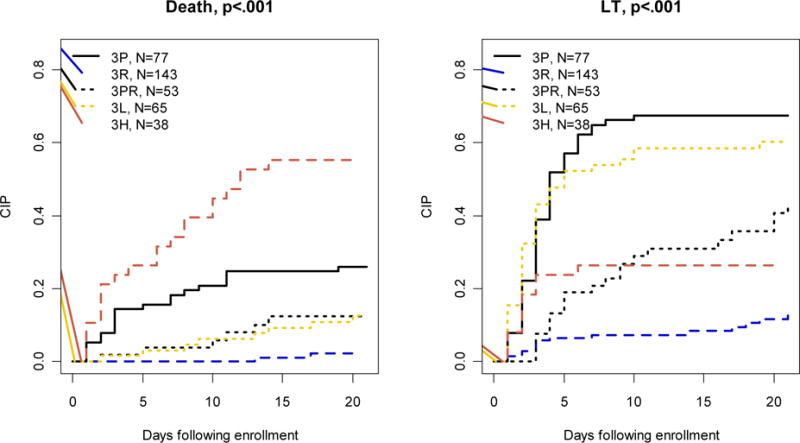
Cumulative incidence probability (CIP) curves of death and transplantation by patterns of HE evolution among participants in subgroups 3P–3H.
DISCUSSION
This study represents the most comprehensive description of HE in a contemporaneous cohort of children with acute liver failure followed in North America and in the United Kingdom. We not only incorporated HE evaluation at study entry, but also daily assessment of HE during a critical time in the clinical trajectory of PALF. We have made a number of important observations: (1) death or LT occurred within the first 7 days in 11% of children who never developed clinical HE, (2) HE is dynamic and associated with outcome, (3) 59% of patients who receive a LT in the first 7 days had mild HE with a peak HE score between 0–II, (4) 25% of children with a peak HE score of III–IV in the first 7 days were alive with their native liver at 21 days; and (5)almost two-thirds of children diagnosed with PALF developed or had HE within 7 days of study enrollment. We have confirmed that children who meet PALF study entry criteria without HE remain at risk for death or LT.
The classic definition of fulminant liver failure by Trey and Davidson (20) required HE as a sine qua non criterion. HE in children can be challenging to recognize and may be absent, late or apparent in children only at the terminal stages of acute liver failure (3, 8–12). Acknowledging the added challenges of i) HE being difficult to assess and not standardized across age groups and ii) reliance on HE detection might exclude potential participants with unrecognized HE, the PALFSG specified a coagulopathy-based definition of acute liver failure in children (16). In this present study, outcomes of 769 children with and without HE at PALF enrollment were evaluated. The findings reveal that PALFSG participants without HE were younger, and less likely to have fever, presented with seizures or require PICU admission at presentation. While spontaneous recovery with native liver was highest in PALFSG participants without HE, death also occurred in these participants. This suggests that the Trey and Davidson definition should not be relied upon to define acute liver failure in children. Serial neurological assessment routinely and throughout initial hospitalization care is necessary to capture the dynamic nature of PALF.
Since the inaugural publication describing the first 369 PALFSG participants (16), the PALFSG study entry criteria have been utilized in several retrospective single-center reports on PALF outcomes (9, 13, 21). Srivastava reported death in 56% of 79 patients with both HE plus INR>1.5 compared to no deaths in 18 patients without HE (10). In another study, death was reported in 81% of 27 PALF patients with HE versus 32% of 34 children without HE (11). Neither of these single-centered studies examined outcomes in participants as a function of changes in HE over time.
The results presented here demonstrate that outcomes were most favorable in PALFSG participants without HE. Mortality was lowest and LT was utilized much less frequently compared to subjects with clinically evident HE in the 21 days following enrollment. We acknowledge that data on daily HE assessments were available only for 7 days following PALF study enrollment and as such, HE may have developed beyond 7 days. It is also true that the cause of death in participants with multi-system organ failure is likely multi-factorial with the relative contribution of liver failure difficult to determine. Nonetheless, these data do reveal a real risk of death in children with acute liver failure who are assessed by physicians not to have HE, and meticulous attention to their clinical course by appropriate medical teams is paramount.
Rivera-Penera previously reported that spontaneous survivors of ALF who present with HE early in their clinical course had lower stage HE than non-survivors, and that non-survivors had delayed hospital admission and transfer to a tertiary care center in comparison to survivors (3). Time of progression to grade II HE was also longer in the non-survivors (18 days) versus the survivors (5 days) (3). Among the PALFSG participants, those who developed HE, or whose HE progressed from presentation through the first 7 days had the highest rates of LT by 3 weeks following study enrollment. Interestingly, LT occurred more frequently in participants with persistently low grade I and II HE compared to those with HE regression. These data suggest that observed HE progression after study enrollment informed consideration of the decision to transplant PALFSG patients.
Over ¼ of PALF participants who were enrolled with HE and mild hepatic-based coagulopathy (INR under 2) had Grade III or IV HE. All of these subjects were in the intensive care unit at time of PALFSG enrollment, with intracranial pressure monitoring reported in five children. Outcomes included spontaneous recovery in 70% and death in 25%. These data serve as a reminder that despite the presence of milder coagulopathy at time of PALFSG enrollment, the presence of HE in pediatric patients mandates meticulous attention similar to the scrutiny and rigorous surveillance of adult patients with fulminant liver failure (22).
Severity of coma grade was related to outcome in this study. Among those who had HE at PALF study enrollment, mortality was highest in those with persistent Grade III and IV HE highlighting the severity of a high coma grade with respect to outcome. LT occurred most frequently in participants who developed HE, demonstrated persistent mild HE, or whose HE progressed during the first 7 days following study enrollment. These findings highlight the potential influence of progressive HE on consideration of LT in PALF patients (14).Indeed, participants with worsening HE had the highest rate of LT and the lowest rate of spontaneous recovery, while participants with improving HE grade had the lowest LT rate and the highest likelihood of spontaneous recovery, despite those with HE regression having a higher proportion of children with severe admission HE stage III or IV. Within 3 weeks of study enrollment, LT was more common, and death less common in patients with mild HE in the first 7 days compared to those with persistently Grade III–IV HE. One plausible explanation for the discrepancy in LT rates between these 2 Groups (3L and 3H) may be that those with persistently mild HE (Group 3L) are considered “well enough” to benefit from LT in the setting of a timely and available organ, whereas those with persistently severe HE (Group 3H) may be considered “too sick to transplant” or may have not survived long enough prior to identification of an organ. It is important to note that some of the PALFSG participants with progressive HE who underwent LT might have been able to recover spontaneously. Some participants whose HE worsened were seen to have regression of HE but it is not yet possible to distinguish those who would go on to survive without LT from those for whom LT is a life-saving therapy. Future analyses on the influence of care-provider decision-making and time-to-organ-availability on PALF outcomes may well investigate these possibilities.
Finally, published data about causes of death in children with ALF are limited and sparse (12, 21). While a mortality rate of 17% among PALF patients presenting with HE has been previously published (16), mortality in PALF patients without HE has not. Over half of the reported causes of death in the PALFSG are attributed to multi-system failure, considerably higher than the 10% previously reported in single-center studies in the UK and Australia (12, 21). Importantly, our finding of death in 4% of PALF study participants without recorded clinical HE underscores the importance for further work on delineating the final pathway of death in this complex patient population.
As with all clinical studies, we acknowledge important limitations. Study participants presented to the PALF sites at various time points after the onset of illness. Data regarding potentially relevant clinical events between days 8 and 21 following PALF study enrollment were not available. Despite rigorous data quality controls, missing and incomplete data were common for variables due to institutional differences in standard of care. Another limitation is that 90 PALFSG participants were reported to have HE at some time during the study, but were excluded from subsequent analyses due to missing HE assessment data, thereby limiting the generalizability of this report to those whose HE could be assessed at enrollment. These excluded participants may well represent a sicker group of children compared to those who were included in this study, given that the majority of those who were excluded were either on a respirator or had received barbiturates rendering assessment and recording of HE grade impossible. Finally, we acknowledge that assessment tools for HE determinations and to assess brain injury in PALF are limited, and that there remains a pressing need to develop a reliable biomarker of brain impairment to use across pediatric age groups.
In conclusion, this study presents data collected from the largest clinical database of patients with PALF diagnosed in the United States, Europe and Canada. Results reported here serve to reiterate to all clinicians that children presenting with PALF without HE remain at risk for death. This finding underscores the importance of early transfer to an experienced pediatric liver transplant center for optimal care, even in the absence of severe encephalopathy or significant coagulopathy, and emphasizes the need for additional research to develop improved prognostic tools and optimize management in this often devastating condition.
Supplementary Material
What is Known?
PALF is not a single diagnosis.
HE may not be clinically apparent in PALF until the terminal stages of the disease process.
Diagnostic approaches and individualized management strategies that may include the decision to pursue liver transplantation are challenging.
What is New?
Children presenting with PALF without hepatic encephalopathy remain at risk of death.
Mortality was highest and transplant-free survival was lowest in PALF patients with severe or worsening hepatic encephalopathy.
Even in PALF patients presenting without HE, early transfer to a pediatric liver transplant center is recommended to enhance optimal outcomes.
Acknowledgments
Grant Support: Supported by NIH-NIDDK: 2U01DK072146
Abbreviations
- ALF
Acute Liver Failure
- ALFSG
Adult Liver Failure Study Group
- HE
Hepatic encephalopathy
- NIDDK
National Institute of Diabetes and Digestive Kidney Diseases
- NIH
National Institute of Health
- LT
liver transplantation
- PALF
Pediatric Acute Liver Failure
- PALFSG
Pediatric Acute Liver Failure Study Group
- TB
total bilirubin
Appendix 1: PALFSG Acknowledgements
Funding for the project is provided by the National Institutes of Health (NIH-NIDDK U01 DK072146).
Key individuals who have actively participated in the PALF studies include (by site): Current Sites, Principal Investigators and Coordinators –Robert H. Squires, MD, Kathryn Bukauskas, RN, CCRC (Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, Pennsylvania); Michael R. Narkewicz, MD, Michelle Hite, MA, CCRC (Children’s Hospital Colorado, Aurora, Colorado); Kathleen M. Loomes, MD, Elizabeth B. Rand, MD, David Piccoli, MD, Deborah Kawchak, MS, RD (Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania); Rene Romero, MD, Saul Karpen, MD, PhD, Liezl de la Cruz-Tracy, CCRC (Emory University, Atlanta, Georgia); Vicky Ng, MD, Kelsey Hunt, Clinical Research Coordinator (Hospital for Sick Children, Toronto, Ontario, Canada); Girish C. Subbarao, MD, Ann Klipsch, RN (Indiana University Riley Hospital, Indianapolis, Indiana); Estella M. Alonso, MD, Lisa Sorenson, PhD, Susan Kelly, RN, BSN, Dhey Delute, RN, CCRC, Katie Neighbors, MPH, CCRC (Lurie Children’s Hospital of Chicago, Chicago, Illinois); Philip J. Rosenthal, MD, Shannon Fleck, Clinical Research Coordinator (University of California San Francisco, San Francisco, California); Mike A. Leonis, MD, PhD, John Bucuvalas, MD, Tracie Horning, Clinical Research Coordinator (University of Cincinnati, Cincinnati, Ohio); Norberto Rodriguez Baez, MD, Shirley Montanye, RN, Clinical Research Coordinator, Margaret Cowie, Clinical Research Coordinator (University of Texas Southwestern, Dallas, Texas); Simon P. Horslen, MD, Karen Murray, MD, Melissa Young, Clinical Research Coordinator, Heather Vendettuoli, Clinical Research Coordinator (University of Washington, Seattle, Washington); David A. Rudnick, MD, PhD, Ross W. Shepherd, MD, Kathy Harris, Clinical Research Coordinator (Washington University, St. Louis, Missouri).
Previous Sites, Principal Investigators and Coordinators –Saul J. Karpen, MD, PhD, Alejandro De La Torre, Clinical Research Coordinator (Baylor College of Medicine, Houston, Texas); Dominic Dell Olio, MD, Deirdre Kelly, MD, Carla Lloyd, Clinical Research Coordinator (Birmingham Children’s Hospital, Birmingham, United Kingdom); Steven J. Lobritto, MD, SumerahBakhsh, MPH, Clinical Research Coordinator (Columbia University, New York, New York); Maureen Jonas, MD, Scott A. Elifoson, MD, Roshan Raza, MBBS (Harvard Medical School, Boston, Massachusetts); Kathleen B. Schwarz, MD, Wikrom W. Karnsakul, MD, Mary Kay Alford, RN, MSN, CPNP (Johns Hopkins University, Baltimore, Maryland); Anil Dhawan, MD, Emer Fitzpatrick, MD (King’s College Hospital, London, United Kingdom); Nanda N. Kerkar, MD, Brandy Haydel, CCRC, Sreevidya Narayanappa, Clinical Research Coordinator (Mt. Sinai School of Medicine, New York, New York); M. James Lopez, MD, PhD, Victoria Shieck, RN, BSN (University of Michigan, Ann Arbor, Michigan).
The authors are also grateful for support from the National Institutes of Health (Edward Doo, MD, Director Liver Disease Research Program, and Averell H. Sherker, MD, Scientific Advisor, Viral Hepatitis and Liver Diseases, DDDN-NIDDK) and for assistance from members of the Data Coordinating Center at the University of Pittsburgh (directed by Steven H. Belle, PhD, MScHyg).
Footnotes
Disclosures
Vicky Lee Ng1- None
RuoshaLi2 - None
Kathleen M. Loomes3 - None
Mike Leonis4 - None
David A. Rudnick5 - None
Steve H. Belle2,6-None
Robert H. Squires7 – None
Author Contributions
Vicky Lee Ng was involved in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision
Ruosha Li, Steve Belle, Kathleen Loomes, Mike Leonis, David Rudnick were involved in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
Robert Squires was involved in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative, technical, or material support; study supervision
References
- 1.Bernal W, Wendon J. Acute liver failure. New England Journal of Medicine. 2013;369(26):2525–34. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 2.Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. The Lancet. 2010;376(9736):190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 3.Rivera-Penera T, Moreno J, Skaff C, et al. Delayed encephalopathy in fulminant hepatic failure in the pediatric population and the role of liver transplantation. Journal of pediatric gastroenterology and nutrition. 1997;24(2):128–34. doi: 10.1097/00005176-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Shakil AO, Kramer D, Mazariegos GV, et al. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transplantation. 2000;6(2):163–69. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 5.Sturm E, Lexmond WS, Verkade HJ. Pediatric acute liver failure: variations in referral timing are associated with disease subtypes. European journal of pediatrics. 2014:1–7. doi: 10.1007/s00431-014-2363-x. [DOI] [PubMed] [Google Scholar]
- 6.Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: recommendations of the US Acute Liver Failure Study Group. Critical care medicine. 2007;35(11):2498–508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 7.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Annals of internal medicine. 2002;137(12):947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kaur S, Kumar P, Kumar V, et al. Etiology and prognostic factors of acute liver failure in children. Indian pediatrics. 2013;50(7):677–79. doi: 10.1007/s13312-013-0189-7. [DOI] [PubMed] [Google Scholar]
- 9.ÖZen H, YÃœCe A. Acute liver failure in children: 20-year experience. Turk J Gastroenterol. 2012;23(2):127–34. doi: 10.4318/tjg.2012.0319. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava A, Yachha SK, Poddar U. Predictors of outcome in children with acute viral hepatitis and coagulopathy. Journal of viral hepatitis. 2012;19(2):e194–e201. doi: 10.1111/j.1365-2893.2011.01495.x. [DOI] [PubMed] [Google Scholar]
- 11.Poddar U, Thapa BR, Prasad A, et al. Natural history and risk factors in fulminant hepatic failure. Archives of disease in childhood. 2002;87(1):54–56. doi: 10.1136/adc.87.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WS, McKiernan P, Kelly DA. Etiology, outcome and prognostic indicators of childhood fulminant hepatic failure in the United kingdom. J Pediatr Gastroenterol Nutr. 2005;40(5):575–81. doi: 10.1097/01.mpg.0000158524.30294.e2. [DOI] [PubMed] [Google Scholar]
- 13.Oh SH, Kim KM, Kim DY, et al. Improved outcomes in liver transplantation in children with acute liver failure. Journal of pediatric gastroenterology and nutrition. 2014;58(1):68–73. doi: 10.1097/MPG.0b013e3182a80362. [DOI] [PubMed] [Google Scholar]
- 14.Devarbhavi H, Singh R, Adarsh CK, et al. Factors that predict mortality in children with Wilson disease associated acute liver failure and comparison of Wilson disease specific prognostic indices. Journal of gastroenterology and hepatology. 2014;29(2):380–86. doi: 10.1111/jgh.12356. [DOI] [PubMed] [Google Scholar]
- 15.Whitington PF, Alonso EM. Fulminant Hepatitis and Acute Liver Failure Diseases of the Liver and Biliary System in Children. Blackwell Publishing Ltd; 2007. pp. 107–26. [Google Scholar]
- 16.Squires RH, Jr, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5):652–58. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun LJ, Tong MJ, Busuttil RW, et al. Acetaminophen hepatotoxicity and acute liver failure. Journal of clinical gastroenterology. 2009;43(4):342–49. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- 18.Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. The American journal of gastroenterology. 2007;102(11):2459–63. doi: 10.1111/j.1572-0241.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 19.Conn HO, Lieberthal MM. The hepatic coma syndromes and lactulose. 1979 [Google Scholar]
- 20.Trey C, Davidson CS. The management of fulminant hepatic failure. Progress in liver diseases. 1970;3(282) [PubMed] [Google Scholar]
- 21.Rajanayagam J, Coman D, Cartwright D, et al. Pediatric acute liver failure: etiology, outcomes, and the role of serial pediatric end-stage liver disease scores. Pediatric Transplantation. 2013;17(4):362–68. doi: 10.1111/petr.12083. [DOI] [PubMed] [Google Scholar]
- 22.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41(5):1179–97. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


