Abstract
We previously showed that feeding a Western type diet (WTD) to Ldlr−/− mice lacking serum amyloid A (SAA) (Saa−/−Ldlr−/− mice), the level of total blood monocytes was higher than in Ldlr−/− mice. In this investigation we demonstrate that higher levels of bone marrow monocytes and macrophage-dendritic cell progenitor (MDP) cells were found in WTD fed Saa−/−Ldlr−/− mice compared to Ldlr−/− mice and lower levels of granulocyte-macrophage progenitor (GMP) cells and common myeloid progenitor (CMP) cells in Ldlr−/− mice. These data indicate that SAA regulates the level of bone marrow monocytes and their myeloid progenitors in hyperlipidemic Ldlr−/− mice.
Keywords: monocytes, monopoiesis, serum amyloid A, stem cells, Western-type diet
1. Introduction
SAA is an acute phase protein that is notably increased in both acute and chronic inflammatory states. It is a significant risk factor for cardiovascular disease, a chronic inflammatory disorder [1-3]. As previously shown, plasma SAA levels are very low in chow fed LDL receptor deficient (Ldlr−/−) and apolipoprotein E deficient (Apoe−/−) mice, but when the animals become hyperlipidemic upon feeding a WTD, there are moderately (~4 to 10 fold) increased plasma levels of SAA [4-6], significantly less than the ~1,000 fold increase observed in the acute phase response. Hepatocytes are the predominant source of plasma SAA, though macrophages do have the capacity to synthesize these proteins. While it has been suggested that SAA has properties that may promote atherogenesis [4, 7-12], recent studies in Apoe−/− mice [13] and in our laboratory in WTD fed Ldlr−/− mice [6] have shown that SAA promotes early lesion formation but does not influence advanced plaque development. Previous studies in patients and in hyperlipidemic mouse models have shown that the level of blood monocytes is a major risk factor for the development of cardiovascular disease [14-16]. Although the foam cell lesions in WTD fed Ldlr−/− mice were larger than in Saa−/−Ldlr−/− mice, the absence of SAA in the Saa−/−Ldlr−/− mice was associated with a higher level of total blood monocytes. However, in our study it was the Ly6clo and not the Ly6chi subset that was affected. Since the inflammatory Ly6chi subset has been reported to be associated with risk for cardiovascular disease [17, 18], this suggested that the difference in Ly6clo blood monocytes was not a major contributor to the development of the atherosclerosis phenotype in the absence of SAA. We showed both hepatic and bone marrow derived SAA appears to be capable of suppressing the increment in blood monocytes. Thus there appears to be a complex relationship between SAA and monocyte homeostasis in a hyperlipidemic state. The purpose of this investigation was to further investigate this relationship. We show that SAA influences bone marrow stem cell precursors of monocytes and regulates monopoiesis after WTD feeding in mice deficient in the LDL receptor. This is the first study to investigate the relationship between SAA and monopoiesis.
2. Materials and Methods
2.1 Murine studies
Globally deficient Saa−/−Ldlr−/− [6] and Ldlr−/− mice on the C57BL/6 background were bred in-house. Mice were maintained on chow diet #2918 (4% fat, 0% cholesterol) from Harlan Teklad (Indianapolis, IN) until 8 to 10 weeks of age, when they were switched to a high-fat/high-cholesterol WTD (21% milk fat, 0.2% cholesterol w/w) (TD.88137 from Harlan Teklad) for 6-7 weeks. For all experiments female mice were used. All mice were housed in a specific pathogen-free facility. The study was conducted in accordance with National Institute of Health guidelines. The protocol was approved by the Institutional Animal Care and Use Committee at the University of Chicago.
2.2 Flow cytometry for blood leukocytes
Mice were anesthetized with isoflurane and 80 μl of blood was collected. Blood was processed by lysing red blood cells with 1× Multi-species Red Blood Cell Lysis Buffer (eBioscience, San Diego, CA) and resuspended in 1% BSA/PBS. All antibodies were purchased from eBioscience (San Diego, CA) unless otherwise stated. Fc receptors were blocked with anti-FcγRII/III antibody 2.4G2.
Blood lymphocytes were stained with CD45.2 AF780 (104), CD3 APC (145-2C11), CD4 PE (GK1.5), CD8 FITC (53-6.7), CD19 PerCP (eBio1D3/1D3), and subsets were defined as total B cells, CD45+CD3−CD19+ and total T cells CD45+CD19−CD3+, which were further divided into CD4+ and CD8+ subsets. Neutrophils were stained with CD45.2 PerCP-Cy5.5 (104), CD11b FITC (M1/70), Ly6G APC (1A8, Biolegend, San Diego, CA) and defined as CD45+CD11bhiLy6G+. To exclude dead cells, all samples were stained with eFluor450 Fixable viability dye (eBioscience, San Diego, CA). All flow cytometry data was collected on LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software 10.0 (Tree Star, Ashland, OR).
2.3 Flow cytometry for bone marrow monocytes and stem cells
Bone marrow was flushed from femurs and tibias, filtered and red blood cells were lysed with ACK lysis buffer. All antibodies were purchased from eBioscience (San Diego, CA) unless otherwise noted. For monocyte analysis, Fc receptors were blocked with anti-FcγRII/III antibody 2.4G2 and cells were stained with CD45.2 PE (104), CD115 APC (AFS98), F4/80 FITC (Cl:A3-1, AbSerotec, Raleigh, NC), and Ly6c PE-Cy7 (HK1.4). Total monocytes were defined as CD45.2+CD115+F4/80+ and divided into Ly6clo and Ly6chi subsets [19].
For stem cell analysis, cells were stained with the following lineage markers to select undifferentiated cells (all in PE): Gr1 (RB6-8C5), B220 (RA3-6B2), CD19 (1D3, BD Biosciences, San Jose, CA), CD3 (145-2C11), CD4 (GK1.5), CD8 (53-6.7), NK1.1 (PK136), Ter-119 (TER-119), CD11c (N418), and CD11b (M1/70). MDP and common dendritic cell progenitor (CDP) cells were stained with Sca-1 PerCP-Cy5.5 (D7), c-Kit PE-Cy7 (2B8), CD115 APC (AFS98), fms-related tyrosine kinase 3 (Flt-3; CD135) Biotin (AF210) and Streptavidin FITC. MDP and CDP cells were differentiated based on their staining with c-Kit, the former being positive and the latter being intermediate. Both cells were otherwise lineage marker negative, Sca-1−c-Kit+CD115+Flt-3+.
Lineage marker negative, Sca-1+c-Kit+ (LSK) cells (also known as hematopoietic stem cells, HSCs) were stained with Sca-1 PerCP-Cy5.5 (D7), c-Kit PE-Cy7 (2B8) and defined as lineage marker negative, Sca-1+c-Kit+. GMP and CMP cells were stained with Sca-1 PerCP-Cy5.5 (D7), c-Kit PE-Cy7 (2B8), CD34 Biotin (RAM34), Streptavidin APC, FcγRII/III FITC (CD16/32; 2.4G2, BD Biosciences, San Jose, CA). GMP and CMP cells were distinguished by their staining for FcγRII/III, the former staining highly and the latter exhibiting intermediate staining. Otherwise they were both lineage marker negative, Sca-1−c-Kit+CD34+. For all staining, dead cells were excluded from analysis using eFluor450 Fixable viability dye.
2.4 Flow cytometry staining of spleen lymphocytes and dendritic cells
Spleens were processed into single cell suspensions using 70 μm filters and DMEM media (Fisher Scientific, Pittsburgh, PA) supplemented with 5% FCS, 1% Pen-Strep. Red blood cells were lysed with ACK lysis buffer. For lymphocyte and dendritic cell staining, cells were stained with CD45.2 AF780 (104), CD3 APC (145-2C11), and CD19 PerCP-Cy5.5 (eBio1D3/1D3), CD11c PE (N418), and MHC II (I-A/I-E) FITC (M5/114.15.2). T cells were defined as CD45+CD19−CD3+; B cells as CD45+CD3−CD19+ cells; and dendritic cells as CD45+CD11c+ MHC II+. Dead cells were excluded from analysis using eFluor450 Fixable viability dye.
2.5 Cell cycle analysis
Cell proliferation of stem cells was evaluated by quantifying the DNA content of stem cells ex vivo using flow cytometry and 4′, 6-diamidino-2-phenylindole (DAPI) staining. Bone marrow cells were stained with cell surface markers for MDP and CDP cell subsets as described above. Cells were fixed with 1% PFA for 1 hour and washed twice with 0.5% saponin, 0.1% NaN3, 0.1% BSA. Cells were stained with DAPI in 0.5% saponin, 0.1% NaN3, 0.1% BSA for 30 minutes at room temperature. Proliferating cells were defined as the percent of cells in the S/G2/M phases in the cell cycle in each cell subset, which was quantified using FlowJo software 10.0.
2.6 Statistical analysis
All statistical analysis was performed using GraphPad Prism version 6.00 for Macintosh X (GraphPad Software, Inc. La Jolla, California). For all comparisons, data was analyzed using Student’s unpaired t-test or One-way ANOVA, with Tukey’s multiple comparison test. Significance was determined at p values less than 0.05. All data is graphed as mean ± standard error of the mean.
3. Results
3.1 SAA does not influence peripheral hematopoietic cells except for Ly6clo monocytes in hyperlipidemic Ldlr−/− mice
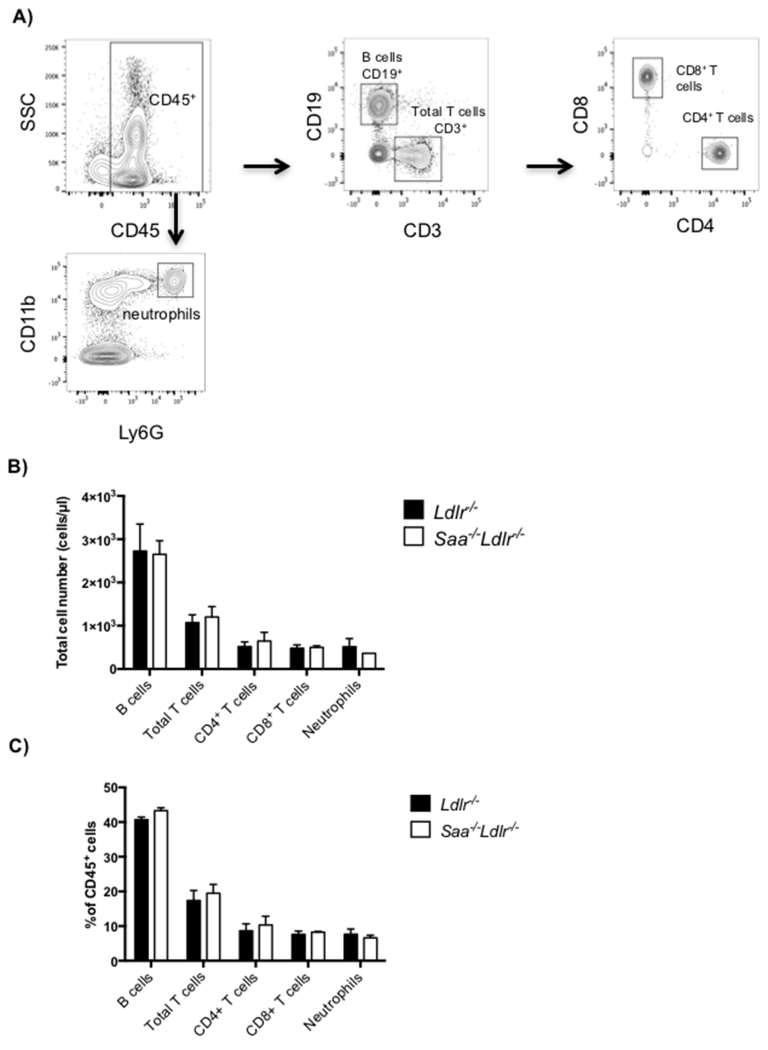
We have previously shown that blood monocytes were higher in Saa−/−Ldlr−/− mice compared to Ldlr−/− mice after 6 weeks of WTD feeding (8.24 ± 1.06% vs. 11.77 ± 0.93% expressed as % of CD45+ cells), specifically affecting the percent of Ly6clo and not the Ly6chi subset [6]. Higher total cell numbers of Ly6clo monocytes were found also in Saa−/−Ldlr−/− mice (186 cells/μl) compared to Ldlr−/− mice (90 cells/μl) after 6 weeks of WTD feeding, but not in chow fed mice. The chow fed Ldlr−/− mice had very low levels of plasma, with levels increasing approximately 4 fold after WTD feeding [4, 6]. Since no difference in monocytes was observed in chow fed mice and both strains of mice were equally hyperlipidemic after WTD feeding, this suggests a direct or indirect effect of SAA. To determine if the influence of SAA on blood monocyte levels was specific to the monocyte lineage, blood lymphocytes and neutrophils were quantified in Ldlr−/− and Saa−/−Ldlr−/− mice fed WTD for 6 weeks. No differences were found in T cell subsets (CD45+CD19−CD3+CD4+ and CD45+CD19−CD3+CD8+), B cells (CD45+CD3−CD19+), or neutrophils (CD45+CD11b+Ly6G+) (Fig. 1). Thus among blood leukocytes, only the levels of Ly6clo monocytes were influenced by SAA.
Fig 1. SAA does not influence blood lymphocyte or neutrophils in hyperlipidemic Ldlr−/− and Saa−/−Ldlr−/− mice fed WTD for 6 weeks.
A) Flow cytometry gating for blood lymphocytes. All cell subsets were defined as live CD45+ cells and further analyzed for T and B cells and neutrophils. Cell subsets were defined as: total T cells as CD19−CD3+, CD4+ T cells as CD19−CD3+CD4+, CD8+ T cells as CD19−CD3+CD8+, B cells as CD3−CD19+ cells, and neutrophils as CD11bhiLy6G+. Blood leukocytes as B) total cells number expressed as cells/μl and C) percent of live CD45+ cells in Ldlr−/− and Saa−/−Ldlr−/− mice fed 6 weeks WTD. The data is expressed as mean ± SEM with n= 4 Ldlr−/− mice and 3 Saa−/−Ldlr−/− mice.
To begin to understand how SAA influences blood monocyte levels, we examined if proliferation of blood monocytes was altered. No difference was found in the amount of proliferation in the blood Ly6chi monocytes in Ldlr−/− and Saa−/−Ldlr−/− mice fed WTD (S1 Fig.). The level of proliferation was not high enough to detect in Ly6clo monocytes.
3.2 SAA differentially regulates the level of myeloid progenitors in a hyperlipidemic state
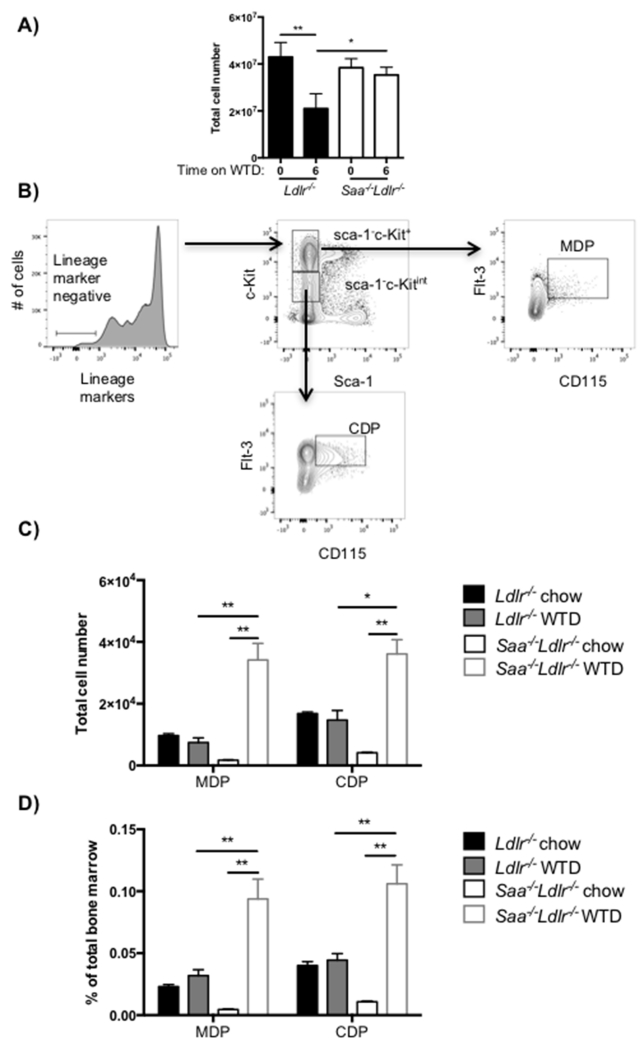
We next explored whether SAA influences monopoiesis in the bone marrow. Bone marrow monocytes (CD45+F4/80+ CD115+) were quantified in Ldlr−/− and Saa−/−Ldlr−/− mice fed chow and WTD. In chow fed mice, there was no difference in total bone marrow monocytes or Ly6chi or Ly6clo subsets between Ldlr−/− and Saa−/−Ldlr−/− mice (Fig. 2B and C). On the other hand, the feeding of WTD to Saa−/−Ldlr−/− mice, but not Ldlr−/− mice, led to a ~2-fold increase in total bone marrow monocytes. In contrast to the blood, both Ly6clo and Ly6chi subsets of bone marrow monocytes were increased in the WTD fed Saa−/−Ldlr−/− mice. The increment in bone marrow monocytes upon WTD feeding is only seen in the absence of SAA. Since the levels of SAA increase with WTD feeding, this suggests that SAA may be involved in suppression of an increment in bone marrow monocytes, either directly or indirectly, in hyperlipidemic Ldlr−/− mice.
Fig 2. SAA prevents an increase in bone marrow monocyte levels after 6 weeks of WTD.
A) Flow cytometry gating for bone marrow monocytes. Total monocytes were defined as live CD45+CD115+F4/80+ and further divided into Ly6chi/lo subsets. Bone marrow monocytes and Ly6chi/lo subsets expressed as B) total cell numbers or C) as a percent of live CD45+ cells in Ldlr−/− and Saa−/−Ldlr−/− mice fed chow or WTD for 6 weeks. The data is expressed as mean ± SEM. For chow-fed animals, total n = 8 Ldlr−/− and 8 Saa−/−Ldlr−/− mice pooled from two independent experiments. For WTD fed animals, total n = 7 Ldlr−/− and 8 Saa−/−Ldlr−/− mice pooled from two independent experiments. *p<0.05; **p<0.01; #p<0.0001
SAA may be influencing the level of myeloid stem cells in hyperlipidemic mice. Therefore, we quantified the level of these stem cell subsets in the bone marrow of Ldlr−/− and Saa−/−Ldlr−/− mice fed chow and WTD. We observed reduced recovery of bone marrow cells in WTD fed Ldlr−/− mice compared chow fed animals and WTD fed Saa−/−Ldlr−/− mice (Fig. 3A). This was reproducibly seen in three separate experiments. The decreased number of cells may be due to reduced recovery of bone marrow cells or lower number of bone marrow cells in the WTD fed Ldlr−/− mice. To take account of this difference in total bone marrow cell number, we have expressed the number of stem cells as the total number per mouse and as the percent of total bone marrow cells.
Fig 3. MDP and CDP cells are increased after 6 weeks of WTD in the absence of SAA.
Ldlr−/− and Saa−/−Ldlr−/− mice were fed a chow diet or WTD for 6 weeks. A) Total bone marrow cell numbers quantified after ACK lysis. B) Flow cytometry gating for MDP and CDP stem cells in bone marrow. Live lineage marker negative bone marrow cells were analyzed for the stem cell subsets MDP (Sca-1−c-Kit+CD115+ Flt-3+) and CDP (Sca-1−c-KitintCD115+Flt-3+) cell subsets. MDP and CDP cells as C) total number and D) percentage of MDP and CDP subsets per total bone marrow cells. The data is expressed as mean ± SEM. For chow fed animals, n = 4 Ldlr−/− and 5 Saa−/−Ldlr−/− mice in one experiment. For WTD fed animals, total n= 12 Ldlr−/− and 6 Saa−/−Ldlr−/− mice which were pooled from two independent experiments. *p<0.01, **p<0.0001
MDP cells are known to differentiate into CDP cells and monocytes [22, 23]. As expected based on the blood and bone marrow monocyte data, there was no significant difference in MDP and CDP cell levels in the bone marrow in the presence or absence of SAA in chow fed mice (Fig. 3C and D), although there was a trend towards lower levels in the Saa−/−Ldlr−/− mice. Regardless of how expressed, the level of MDP and CDP cells was not different with the feeding of WTD to the Ldlr−/− mice compared to chow fed mice, even in the face of a four-fold elevation of plasma SAA levels [13]. On the other hand, the percent and total cell number of MDP cells increased approximately 12 to 20-fold after feeding of WTD to Saa−/−Ldlr−/− mice. In WTD fed Saa−/−Ldlr−/− mice the level of MDP cells was 3-fold higher compared to Ldlr−/− mice. Similar to MDP cells, WTD induced higher levels of CDP cells in Saa−/−Ldlr−/− mice but not in Ldlr−/− mice. The increase in CDP cells may simply be a downstream effect of the increased level of MDP cells.
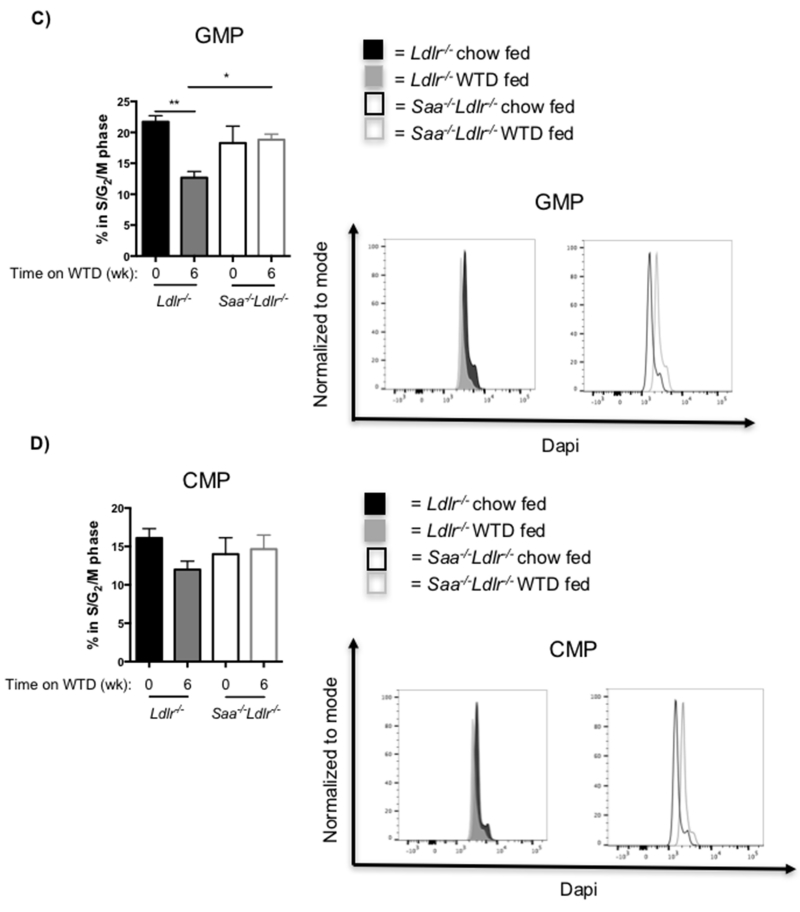
Studies have shown that diets high in cholesterol and fat result in the accumulation of cholesterol in stem cells, including LSK, CMP and GMP cells and that this regulates their proliferation [15, 16, 21, 24]. To determine if SAA was influencing proliferation of MDP and CDP cells in the WTD fed mice, cell cycle analysis was performed to quantify the percent of these cells in G2/M/S phase. Proliferating MDP and CDP cells were higher in Saa−/−Ldlr−/− compared to Ldlr−/− mice, but the difference was only significant for the CDP cells (Fig. 4A and B). Thus, SAA may be suppressing the proliferation of these myeloid stem cells, particularly CDP cells, in Ldlr−/− mice fed WTD.
Fig. 4. Proliferation of myeloid stem cells in Ldlr−/− and Saa−/−Ldlr−/− mice.
Cell cycle analysis was used to analyze the proliferation of A) MDP and B) CDP cells in mice fed WTD for 6 weeks and C) GMP and D) CMP cells in mice fed WTD or chow for 6 weeks. For each cell subset, the percent of the bone marrow progenitor cells in the S/G2/M phase was quantified. For MDP and CDP cells, n= 4 Ldlr−/− mice and 4 Saa−/−Ldlr−/− mice fed WTD for 6 weeks. For GMP and CMP cells, n=7 for each group of chow fed (0 wk) Ldlr−/− and Saa−/−Ldlr−/− mice; for 6 week WTD fed mice, n=11 for Ldlr−/− mice; and n=8 for Saa−/−Ldlr−/− mice. The data is expressed as mean ± SEM. *p<0.05; **p<0.01
The higher level of CDP cells in the bone marrow of WTD fed Saa−/−Ldlr−/− mice may indicate that SAA suppresses the development of dendritic cells. To explore this, dendritic cells and lymphocytes were quantified in the spleen of Saa−/−Ldlr−/− and Ldlr−/− after WTD feeding. As with the blood, there was no significant difference in splenic T and B cells levels (Fig. 5), but there is a trend towards slightly higher splenic dendritic cells in WTD fed Saa−/−Ldlr−/− mice.
Fig 5. SAA does not reduce dendritic cells or lymphocytes in the spleen after 6 weeks of WTD.
A) Flow cytometry gating for spleen T cells, B cells, and dendritic cells. All cell subsets were defined as live CD45+ and further analyzed for T cells, defined as CD19−CD3+, B cells, defined as CD3−CD19+, and dendritic cells, defined as CD11c+MHCII+. B) T cells, B cells, and dendritic cells in the spleen in Ldlr−/− and Saa−/−Ldlr−/− mice fed WTD for 6 weeks as a percent of live CD45+ cells. The data is expressed as mean ± SEM with n = 6 Ldlr−/− and 6 Saa−/−Ldlr−/− mice pooled from two independent experiments.
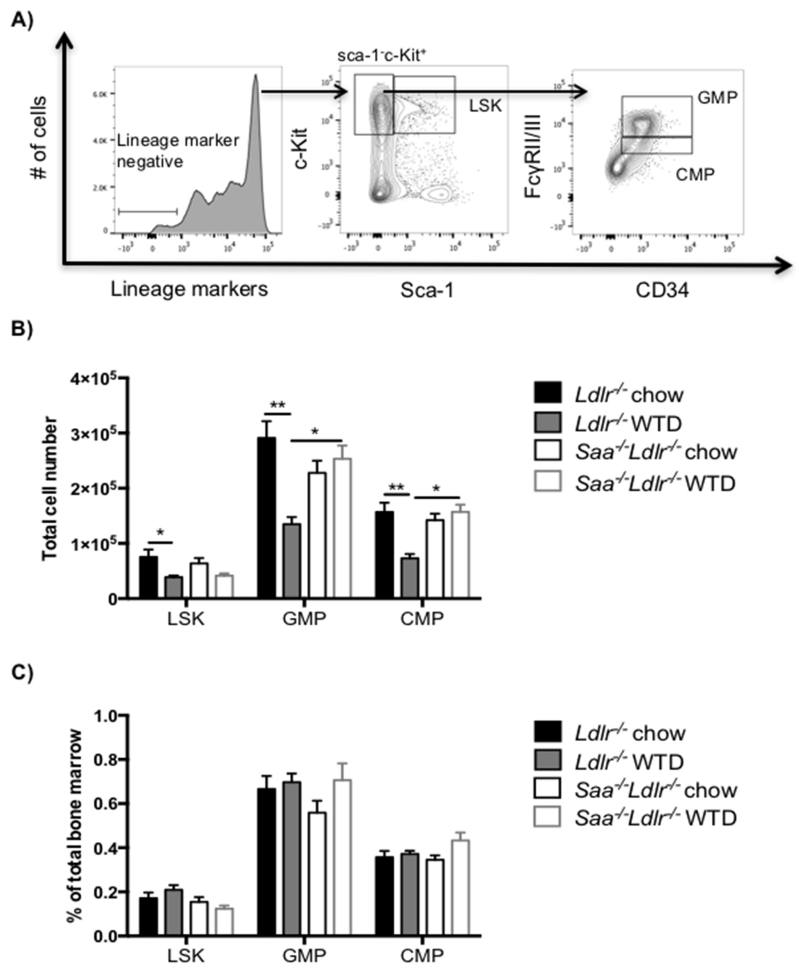
LSK cells are the most primitive stem cell subset and are known for their ability to differentiate into all hematopoietic populations [23, 25]. LSK cells were quantified to determine if the differences in MDP and CDP cells were simply a downstream influence of SAA on the level of their progenitor. Reduced absolute number, but not percent, of LSK cells was observed in Ldlr−/− mice after feeding WTD compared to chow-fed controls, while no diet effect was observed in Saa−/−Ldlr−/− mice (Fig. 6B and C). This could reflect the lower recovery or number of total bone marrow cells upon feeding WTD to the Ldlr−/− mice. Thus, the increase in the levels of MDP and CDP cells in Saa−/−Ldlr−/− mice upon WTD feeding does not appear to be a downstream effect of LSK changes as the LSK cell levels are comparable in chow and WTD fed Saa−/−Ldlr−/− mice. CMP and GMP cells are additional myeloid progenitors. On chow diet, there is no difference in the levels of these progenitor cells in the Ldlr−/− and Saa−/−Ldlr−/− mice. WTD feeding reduced the GMP and CMP number, but not percent, in Ldlr−/− mice but had no effect on the level of these progenitor cells in Saa−/−Ldlr−/− (Fig. 6B and C). Proliferation of GMP cells were also reduced in WTD fed Ldlr−/− mice (Fig. 4C and D). Differences in the level of these precursors may potentially contribute to the difference in blood monocytes levels observe between Ldlr−/− and Saa−/−Ldlr−/− mice fed WTD.
Fig 6. The absence of SAA does not reduce the proportion of LSK, GMP and CMP cells.
A) Flow cytometry gating for LSK, GMP and CMP stem cells in bone marrow. Live lineage marker negative bone marrow cells were analyzed for the stem cell subsets LSK (Sca-1+c-Kit+), GMP (Sca-1−c-Kit+CD34+ FcγRII/IIIhi), and CMP (Sca-1−c-Kit+CD34+ FcγRII/IIIint). Bone marrow progenitor cells in Ldlr−/− and Saa−/−Ldlr−/− mice fed chow or WTD for 6 weeks expressed as B) total cell number and C) percent of total bone marrow cells. The data is expressed as mean ± SEM. For chow fed mice, n=8 Ldlr−/− mice and 8 Saa−/−Ldlr−/− mice. For WTD fed mice, total n=12 Ldlr−/− and 7 Saa−/−Ldlr−/− mice pooled from two independent experiments. *p<0.01; **p<0.0001
4. Discussion
We have previously shown that a higher level of total blood monocytes were found in Saa−/−Ldlr−/− mice compared to Ldlr−/− mice after WTD feeding [6]. It was primarily the Ly6clo subset that was elevated. These differences in monocyte levels were not evident in animals fed chow, indicating that there is an interaction between SAA status and hyperlipidemia. The monocyte effect could be a function of the 4-fold increment in plasma SAA in Ldlr−/− animals fed the WTD [4, 6]. It could also be a result of the increment in plasma cholesterol interacting with the elevated SAA levels. We cannot positively distinguish between these two possibilities. In this paper we show that this increase is specific to the myeloid lineage, as no difference was found in blood or spleen lymphocytes after WTD feeding.
The purpose of this investigation was to further elucidate the relationship between SAA and blood monocytes after WTD feeding. We did this by analyzing the stem cell precursors of monocytes in the bone marrow. We made a number of novel observations. Our data suggests that in the absence of SAA, WTD results in an increment in bone marrow monocytes and their precursors MDP and CDP compared to the animals fed chow diets. This increase is not observed in the presence of SAA. Bone marrow Ly6chi and Ly6clo cells followed a trajectory similar to that observed with total bone marrow monocytes. This contrasts to our report of the monocyte subsets in the blood of these animals, where we observed only an increment of Ly6clo monocytes [6]. What is intriguing about our results is the observation that the regulation of monocytes and their precursors by exposure to the WTD is dependent upon whether SAA is present or not.
Blood monocytes are derived from bone marrow-derived stem cell populations, including the most primitive hematopoietic stem cells, HSPC (also known as LSK), which gives rise to all blood leukocytes. A number of intermediate precursors originate from these cells; MDP, CDP, GMP and CMP. These potential precursors, however, exhibited different responses to hyperlipidemia and SAA. After WTD feeding, MDP and CDP cells were elevated in the absence of SAA but not in its presence. Examination of cell cycling indicates that cell replication participates in achieving the steady state level of these stem cell precursors in these mice. The expanded steady state level of MDP may contribute to the elevated blood and bone marrow monocytes. Total number of bone marrow cells and the level of GMP and CMP cells were reduced in the WTD fed Ldlr−/− bone marrow compared to chow fed mice. However, the reduction in each of these cell numbers was not observed in the absence of SAA (Saa−/−Ldlr−/−). As a result, in WTD fed animals the steady state levels of GMP and CMP cells are much higher in the absence of SAA than in its presence. However, the lower number of bone marrow cells obtained from the WTD fed Ldlr−/− mice does not allow us to say whether the differences in cell numbers of GMP and CMP progenitors are contributing to the elevated blood monocytes in WTD fed Saa−/−Ldlr−/− animals compared to similarly fed Ldlr−/− animals. It is worth noting that the GMP and CMP precursors are about ten times as abundant in the bone marrow as are the MDP and CDP cells.
Studies have shown that high-cholesterol/high-fat diets lead to stem cell proliferation due to lower levels of cholesterol efflux [14, 26, 27]. This results in an increase in blood monocyte levels, specifically in the Ly6chi subset and neutrophils. In contrast we observed an increase in the blood Ly6clo monocytes in high fat diet fed Saa−/−Ldlr−/− mice compared to Ldlr−/− mice and chow fed Saa−/−Ldlr−/− mice and no difference in blood neutrophil levels suggesting that changes in cholesterol efflux did not contribute to the differences in monocyte and stem cell levels in these mice. Moreover, no difference was found in the level of cholesterol efflux in bone marrow GMP or CMP cells from Ldlr−/− and Saa−/−Ldlr−/− mice fed WTD (data not shown). In addition, plasma levels of apoE and apoA-I, which promote cholesterol efflux, were similar in Ldlr−/− and Saa−/−Ldlr−/− mice fed WTD (data not shown), indicating that SAA likely influenced myeloid stem cell and monocyte levels independent of cholesterol efflux.
We initiated the studies reported in this paper to explore the basis for the increment in total and Ly6clo blood monocytes without a change in Ly6chi monocytes seen in Saa−/−Ldlr−/− animals fed the WTD. We focused on monocytes and their precursors in the bone marrow of the two strains of mice, in part, because no other leukocytes differed in abundance in either the blood or the spleen as a function of SAA status. What we find is not a simple straightforward basis for the blood phenotype. We did find a significant increment in bone marrow monocytes in WTD fed Saa−/−Ldlr−/− mice, but, in contrast to the blood, this was largely accounted for by a large increase in the Ly6chi subset. This subset was a least three times as abundant in WTD fed Saa−/−Ldlr−/− mice as was the Ly6clo subset. The biogenetic relationship between these two subsets is not well defined. However, there is evidence that the Ly6clo subset may be derived from the Ly6chi subset [28-30]. Based upon this relationship, it is possible that SAA may directly or indirectly promote this conversion or potentially limit the migration of the Ly6chi cells from the bone marrow into the blood or limit migration of Ly6clo cells from the blood. For instance, SAA may influence expression of transcription factors such as Nr4a1, which has been shown to be required for Ly6clo monocyte survival and differentiation [31]. Alternatively, the conversion of Ly6chi to Ly6clo monocytes may be unusually efficient within the blood compartment in the absence of SAA or the expression of chemokine receptors on the Ly6clo cells is reduced so that they migrate from the blood at a lower rate. Clearly further work is required is dissect these possibilities.
These findings may have a more general applicability to inflammatory states. SAA, whose expression is induced during inflammation, may be part of a regulatory network that suppresses the expansion of the pro-inflammatory Ly6chi monocytes during active inflammation and allows for the participation of these cells in mediating a measured inflammation. As the level of SAA declines with the resolution of the inflammation, the reparative Ly6clo monocyte subset may be more active and contribute to the restoration of a non-inflammatory steady state. This hypothesis could be tested in other models of inflammation.
In conclusion, we have shown that SAA regulates monopoiesis in a hyperlipidemic state. This is the first study to show that a pro-inflammatory, acute phase protein, such as SAA, can suppress monocytosis and stem cell proliferation after WTD feeding. Further studies are needed to clarify the mechanistic relationship between the myeloid lineages and SAA.
Supplementary Material
Acknowledgements
We would like to thank Drs. Maria C. De Beer and Fredrick C. De Beer at the University of Kentucky Medical Center for their generous gift of Saa−/− mice. This work was supported by NIH grant P01 HL092969, the Leducq Foundation and the Cardiovascular Pathophysiology and Biochemistry Training grant HL007237.
Abbreviations
- Ldlr
Low-density lipoprotein receptor
- SAA
serum amyloid A
- WTD
Western-type diet
- MDP
macrophage-dendritic cell progenitor
- CDP
common dendritic cell progenitor
- CMP
common myeloid progenitor
- GMP
granulocyte-macrophage progenitor
- LSK
lineage marker negative, Sca-1+, c-Kit+
Footnotes
Contribution of Authors
PAK performed and designed experiments, analyzed data, and wrote the manuscript. TJS assisted in designing experiments and provided scientific input. CAR and GSG provided scientific guidance and assisted in writing the manuscript and designing experiments.
References
- 1.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 2.Arant CB, Wessel TR, Ridker PM, Olson MB, Reis SE, Delia Johnson B, Sharaf BL, Pauly DF, Handberg E, Zineh I, Sopko G, Kelsey SF, Noel Bairey Merz C, Pepine CJ. Multimarker approach predicts adverse cardiovascular events in women evaluated for suspected ischemia: results from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Clin Cardiol. 2009;32:244–250. doi: 10.1002/clc.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Bairey Merz CN, Sopko G, Olson MB, Reis SE, National Heart, L. a. B. I. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:726–732. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 4.Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O’Brien KD, Chait A. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–545. doi: 10.1161/01.CIR.0000136819.93989.E1. [DOI] [PubMed] [Google Scholar]
- 5.De Beer MC, Wroblewski JM, Noffsinger VP, Rateri DL, Howatt DA, Balakrishnan A, Ji A, Shridas P, Thompson JC, van der Westhuyzen DR, Tannock LR, Daugherty A, Webb NR, De Beer FC. Deficiency of endogenous acute phase serum amyloid A does not affect atherosclerotic lesions in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2014;34:255–261. doi: 10.1161/ATVBAHA.113.302247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishack PA, Bhanvadia CV, Lukens J, Sontag TJ, De Beer MC, Getz GS, Reardon CA. Serum Amyloid A Facilitates Early Lesion Development in Ldlr−/− Mice. J Am Heart Assoc. 2015;4:e001858. doi: 10.1161/JAHA.115.001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien KD, McDonald TO, Kunjathoor V, Eng K, Knopp EA, Lewis K, Lopez R, Kirk EA, Chait A, Wight TN, deBeer FC, LeBoeuf RC. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:785–790. doi: 10.1161/01.ATV.0000158383.65277.2b. [DOI] [PubMed] [Google Scholar]
- 8.Lee HY, Kim SD, Shim JW, Yun J, Kim K, Bae YS. Activation of formyl peptide receptor like-1 by serum amyloid A induces CCL2 production in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2009;380:313–317. doi: 10.1016/j.bbrc.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 9.Lee HY, Kim SD, Baek SH, Choi JH, Cho KH, Zabel BA, Bae YS. Serum amyloid A stimulates macrophage foam cell formation via lectin-like oxidized low-density lipoprotein receptor 1 upregulation. Biochem Biophys Res Commun. 2013;433:18–23. doi: 10.1016/j.bbrc.2013.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HY, Kim MK, Park KS, Bae YH, Yun J, Park JI, Kwak JY, Bae YS. Serum amyloid A stimulates matrix-metalloproteinase-9 upregulation via formyl peptide receptor like-1-mediated signaling in human monocytic cells. Biochem Biophys Res Commun. 2005;330:989–998. doi: 10.1016/j.bbrc.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 11.Lee HY, Kim SD, Baek SH, Choi JH, Bae YS. Role of formyl peptide receptor 2 on the serum amyloid A-induced macrophage foam cell formation. Biochem Biophys Res Commun. 2013;433:255–259. doi: 10.1016/j.bbrc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Lee HY, Kim MK, Park KS, Shin EH, Jo SH, Kim SD, Jo EJ, Lee YN, Lee C, Baek SH, Bae YS. Serum amyloid A induces contrary immune responses via formyl peptide receptor-like 1 in human monocytes. Mol Pharmacol. 2006;70:241–248. doi: 10.1124/mol.105.022103. [DOI] [PubMed] [Google Scholar]
- 13.Dong Z, Wu T, Qin W, An C, Wang Z, Zhang M, Zhang Y, Zhang C, An F. Serum amyloid A directly accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Mol Med. 2011;17:1357–1364. doi: 10.2119/molmed.2011.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy AJ, Dragoljevic D, Tall AR. Cholesterol efflux pathways regulate myelopoiesis: a potential link to altered macrophage function in atherosclerosis. Front Immunol. 2014;5:490–495. doi: 10.3389/fimmu.2014.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankaranarayanan S, Kellner-Weibel G, Llera-Moya M, Phillips MC, Asztalos BF, Bittman R, Rothblat GH. A sensitive assay for ABCA-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res. 2011;52:2332–2340. doi: 10.1194/jlr.D018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy AJ, Bijl N, Yvan-Charvet L, Welch C, Bhagwat N, Reheman A, Wang Y, Shaw JA, Levine RL, Ni H, Tall AR, Wang N. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med. 2013;19:586–594. doi: 10.1038/nm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 23.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 24.Seijkens T, Hoeksema MA, Beckers L, Smeets E, Meiler S, Levels J, Tjwa M, de Winther MP, Lutgens E. Hypercholesterolemia-induced priming of hematopoietic stem and progenitor cells aggravates atherosclerosis. FASEB J. 2014;28:2202–2213. doi: 10.1096/fj.13-243105. [DOI] [PubMed] [Google Scholar]
- 25.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 26.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolani S, Pagler TA, Murphy AJ, Bochem AE, Abramowicz S, Welch C, Nagareddy PR, Holleran S, Hovingh GK, Kuivenhoven JA, Tall AR. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis. 2013;229:79–85. doi: 10.1016/j.atherosclerosis.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114:1757–1771. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 30.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 31.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.