Abstract
There are limitations in the current classification of danger associated molecular patterns (DAMP) receptors. To overcome these limitations, we propose a new paradigm by using endogenous metabolites lysophospholipids (LPLs) as a prototype. By utilizing a data mining method we pioneered, we made the following findings: 1) Endogenous metabolites such as LPLs at basal level have physiological functions; 2) under sterile inflammation, expression of some LPLs are elevated. These LPLs act as conditional DAMPs or anti-inflammatory homeostasis-associated molecular pattern molecules (HAMPs) for regulating the progression of inflammation or inhibition of inflammation, respectively; 3) receptors for conditional DAMPs and HAMPs are differentially expressed in human and mouse tissues; and 4) complex signaling mechanism exists between pro-inflammatory mediators and classical DAMPs that regulate the expression of conditional DAMPs and HAMPs. This novel insight will facilitate identification of novel conditional DAMPs and HAMPs, thus promote development of new therapeutic targets to treat inflammatory disorders.
Keywords: Lysophospholipid receptor, Rheumatoid arthritis, Atherosclerosis, Danger-associated molecular pattern molecules receptor, Homeostasis-associated molecular pattern molecules receptor
1. Introduction
Pathogen-associated molecular patterns (PAMPs) and danger associated molecular patterns (DAMPs) generated during a tissue injury act as sensors and activate the immune system to respond to infection or injury [1]. One of the key cellular receptors that recognize the “threat” signals initiated by PAMPs and DAMPs are Toll-like receptors (TLRs). These receptors are mainly located on the plasma membrane and activate inflammatory genes to counteract tissue injury and mediate repair. Moreover, TLRs work in synergy with cytosolic sensing receptor families like NLRs [NOD (nucleotide binding and oligomerization domain)-like receptors] to recognize DAMPs, particularly in inflammation privileged tissues where inflammasome component genes that activate pyrotopsis are not constitutively expressed [2,3]. In addition to TLRs and NLRs, four additional DAMP receptors including transmembrane C-type lectin receptors, retinoid acid inducible gene I (RIG-I), AIM2 (absent in melanoma 2), receptor for advanced glycation end products (RAGE, also a receptor for high mobility group box 1(HMGB1)) have been characterized [4]. Herein, we refer the above mentioned 6 categories of DAMP receptors as classical DAMP receptors.
Dr. Matzinger’s proposal of danger model has significantly improved our understanding on the role of endogenous metabolites in the pathogenesis of inflammatory and immunological diseases [5]. Based on the current understanding, we can identify following principles that classify DAMPs and their receptors: (1) endogenous metabolites released under cellular stress could serve as the DAMPs; (2) DAMPs has to bind to classical DAMP receptors to initiate inflammation; (3) classical DAMP receptors initiates pro-inflammatory transcription factor-mediated signaling pathways [6]; and (4) DAMP receptor genes are evolutionally conserved from mice to humans. However, the current classification and paradigm of DAMP receptors raise the following concerns: (1) Do all the endogenous metabolites that can act as DAMPs initiate inflammation via binding only to the classical DAMP receptors? (2) Do all the pro-inflammatory endogenous metabolites have high affinity and signal-generating interactions to classical DAMP receptors to facilitate the downstream signaling cascades? Is it feasible in the biochemical point of view? (3) Does the endogenous metabolites that regulate normal cellular functions at physiological concentrations initiate inflammatory response under elevated conditions? (4) The current DAMP receptor model emphasizes only the danger signals generated from endogenous metabolic processes. It fails to recognize the roles of potential endogenous metabolites in anti-inflammatory responses, inflammation resolution and maintenance of homeostasis; and (5) the current DAMP receptor model emphasizes only the danger signals released from damaged/dying cells [7] but fails to identify the roles of pathological elevated endogenous metabolites released from activated viable cells.
The significance of addressing these limitations and shifting the paradigm to form a new model is that it will encourage investigators [8] to recognize novel conditional DAMP receptors and anti-inflammatory and homeostatic signals derived from endogenous metabolites. Recent advances in immunology has clearly demonstrated the well-published “two arms model”. This model states that in addition to the pro-inflammatory immunoeffector and T cell co-stimulatory mechanisms, there are several immunotolerance and anti-inflammatory mechanisms mediated by the immune system. These anti-inflammatory mechanisms include T cell co-inhibition/co-suppression pathways [9], T cell anergy [10], regulatory T cells [11], and secretion of anti-inflammatory/ immunosuppressive cytokines such as transforming growth factor-β (TGF-β), interleukin-10 (IL-10), IL-35, and IL-37 as we and others reported [12–15], etc.
In order to shift the current paradigm on DAMP receptors and address the limitations mentioned above, we utilized an extensive literature survey and database mining method, which we have pioneered and published previously [16]. For this study we used the endogenous metabolite lysophospholipids (LPLs) and its receptors as the prototype. LPLs are bioactive, lipid-derived metabolites that act through G-protein coupled receptors (GPCRs) to either serve as signaling molecules at physiological concentrations that control cellular homeostasis or as inflammatory regulators at elevated concentrations [17]. LPLs are generated by regiospecific phospholipases on substrates such as membrane phospholipids and sphingolipids.
Some of the LPLs that have been identified and studied so far are lysophosphatidic acid (LPA), lysoyphosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), lipophosphoglycan (LPG), lysophosphatidylinositol (LPI), and lysophosphatidylserine (LysoPS) [17]. The role of LPLs in chronic inflammatory disorders such as coronary artery disease (CAD), hypertension [18,19], atherosclerosis and severe vascular diseases is well established [20,21]. Furthermore, LPLs contribute to pathophysiology of autoimmune disorders such as rheumatoid arthritis, and is involved in exacerbating the progression of the disease [22,23].
Our comprehensive literature survey revealed that LPLs can either act as pro- or anti-inflammatory agents that follow the logic of aforementioned “two arms” model in immunity. For an example, LysoPS [24] and LPE [25] were identified as endogenous metabolites that have anti-inflammatory and homeostatic functions, while elevated LPA, LPI and LPC levels were shown to induce inflammation. Therefore, we propose a conceptually innovative paradigm that identifies endogenous metabolites such as LPLs that act as pro-inflammatory mediators at elevated concentrations but mediate normal cellular functions at physiological concentrations as conditional DAMPs. Moreover, we designate the receptors that exert the effects of conditional DAMPs as conditional DAMP receptors and suggest that activation of inflammation by conditional DAMPs may not necessarily always involve or “converge to” TLRs, NLRs and other classical DAMP receptors. Herein, we further advance the current paradigm by suggesting that endogenous metabolites such as LysoPS and LPE that maintain homeostasis at physiological levels, but act as anti-inflammatory mediators to inhibit inflammation and promote inflammation resolution as homeostasis-associated molecular patterns (HAMPs). Furthermore, we propose that these HAMPS bind to their receptors (HAMP receptors) to initiate anti-inflammatory/homeostatic signaling and promote inflammation resolution. Moreover, we suggest that LPL-GPCRs can be classified as novel conditional DAMP receptors for the following reasons: (1) LPLs, which are endogenous pro-inflammatory lipid metabolites, are elevated during cellular stress and bind to their LPL-GPCRs; (2) Elevated pro-inflammatory LPLs initiate signaling cascade to activate inflammatory genes via LPL-GPCRs.
In this study, we have used LPLs as a group of prototypic molecules to demonstrate the proof of principle and do not exclude the possibilities of other bio-lipids such as n-6 polyunsaturated fatty acids to function as DAMPs or HAMPs to regulate inflammation [26]. Our newly proposed HAMPs include lipid-based specialized pro-resolving mediators (SPMs) [27] and other anti-inflammatory endogenous metabolites. Our new classification of LPL-GPCRs as conditional DAMP receptors and our novel concept of anti-inflammatory endogenous metabolite receptors as HAMP receptors will lead to the future development of novel therapeutics for autoimmune diseases, inflammatory diseases and inflammatory cancers.
2. Methods
2.1 Tissue expression profile of genes encoding G protein-coupled LPLs receptors (LPL-GPCRs)
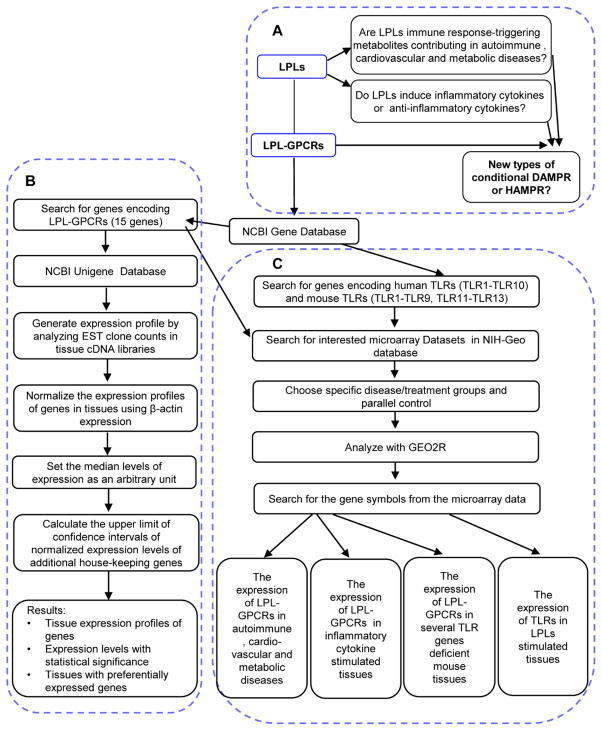
We used our previously reported experimental data mining strategy to analyze the expression profile of LPL-GPCRs (Part A, B in Figure 1) [28,2]. We analyzed LPL-GPCR gene expression in cardiovascular and other tissues by mining experimentally verified human and mouse mRNA expressions in the expressed sequence tag (EST) databases of the National Institutes of Health (NIH)/ National Center of Biotechnology Information (NCBI) UniGene (http://www.ncbi.nlm.nih.gov/sites/entrez?db=unigene) (see the justification for this database mining approach in the Discussion).
Figure 1. Flow chart of database mining strategy and three parts of data organization.
A) We propose a new paradigm for the first time that LPLs are pro-inflammatory danger-associated molecular pattern molecules (DAMPs) or anti-inflammatory homeostasis-associated molecular pattern molecules (our newly proposed HAMPs). B) The strategy in generating tissue expression profile of LPL-GPCRs. C) Result analysis and presentation.
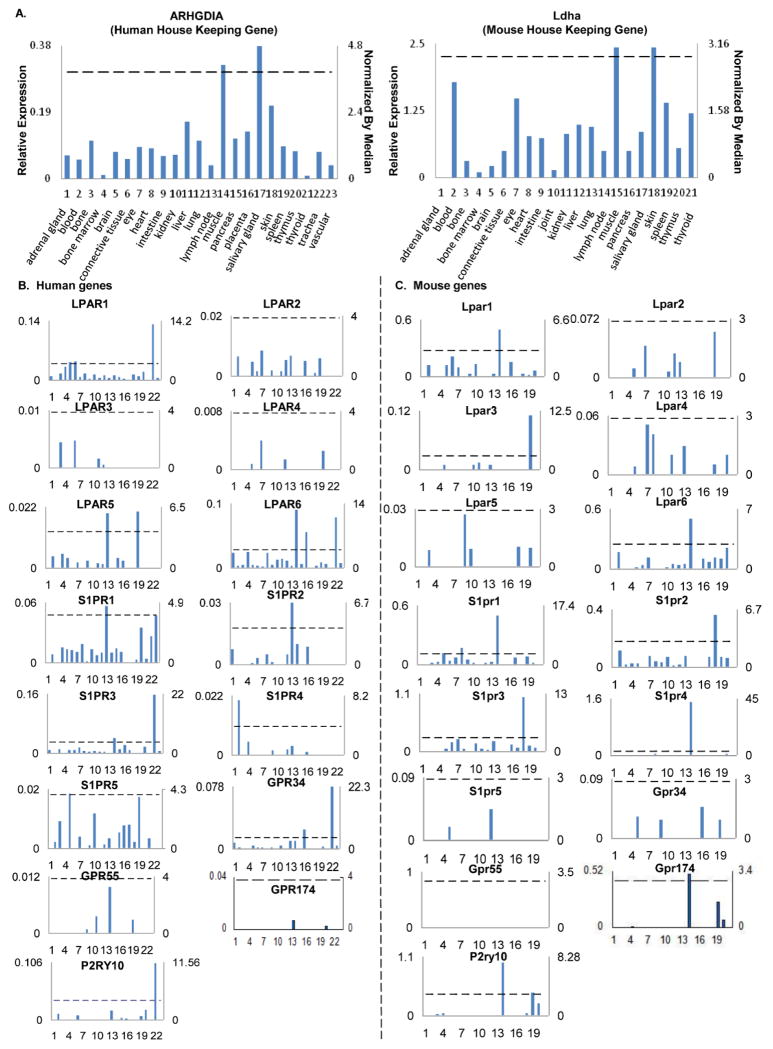
Transcripts per million of genes of interest were normalized with that of housekeeping β-actin in each given tissue to calculate the arbitrary units of gene expression. A confidence interval of the expression variation of housekeeping genes was generated by calculating the mean plus two times that of the standard deviation of the arbitrary units of three randomly selected housekeeping genes (ARHGDIA, GADPH and PRS27A in human; Ldha, Nono and Rpl32 in mouse) normalized by β-actin in the given tissues (Figure 4). If the expression variation of a given gene in the tissues was larger than the upper limit of the confidence interval (the mean plus two times the standard deviation) of the housekeeping genes, the high expression levels of genes in the tissues were considered statistically significant. Gene transcripts lower than one per million were technically presented as no expression.
Figure 4. LPL-GPCRs genes are differently expressed in human and mouse tissues.
A. Data presentation format (The data presented in X-, Y-axis, and tissue order of ARHGDIA and Ldha were applied to all the human and mouse genes examined respectively). As an example, the gene expression profiles of human housekeeping gene Rho GDP dissociation inhibitor (GDI) alpha (ARHGDIA) in the twenty-three tissues were presented, with the tissue names and position numbers shown on the X-axis. The gene expression data were normalized by the β-actin (Hs. 520640) expression data from the same tissue, which were presented on the left Y-axis. The expression ratios among tissues were generated by normalizing the arbitrary units of the gene in the tissues with the median level of the arbitrary units of the gene in all the tissues which are presented on the right Y-axis. In order to define confidence intervals for statistically higher expression levels of given genes, we calculated the confidence intervals of tissue expression for three housekeeping genes [the mean X+ 2 x standard deviations (SD) = 3.84] including ARHGDIA (Hs. 159161), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Hs. 544577), and ribosomal protein S27a (RPS27A, Hs. 311640). The expression variations of given genes in tissues, when they were larger than 3.84-fold, were defined as the high expression levels with statistical significance (the right Y-axis). To define confidence intervals for statistically higher expression levels of given genes in 21 mouse tissues, we calculated the confidence intervals of tissue expression [the mean X + 2 x standard deviations (SD) = 2.86] for three mouse housekeeping genes including Lactate dehydrogenase A (Ldha, Mm. 29324), non-POU-domain-containing octamer binding protein (Nono, Mm. 280069), and ribosomal protein L32 (Rpl32, Mm. 104368). The expression variations of given genes in tissues, when they were larger than 2.86-fold, were defined as the high expression levels with statistical significance (the right Y-axis). B. The expression profiles of LPL-GPCRs genes in human tissues (Left 2 columns, with LPL-GPCRs genes designated with capital letters). C. The expression profiles of LPL-GPCRs genes in mouse tissues (right 2 columns, with LPL-GPCRs genes designated with lowercase letters).
2.2 Expression profiles of LPL-GPCRs in human disease, mouse disease models and several TLR genes deficient mouse tissues, and expression profiles of TLRs in LPLs stimulated tissues
Gene expression profiles were collected from 15 microarray datasets in NIH-Geo database (Part C in Figure 1). Specific samples were chosen as disease or treatment groups and parallel control. The number of samples was always greater than 3 except for the pooled samples. By searching for the gene symbols from the microarray data, we selected the genes with significant changes (p<0.05). The genes of which the expression changes were more than 1 fold were defined as the upregulated genes while genes with their expression changes less than 1 fold were defined as downregulated genes.
3. Results
3.1. LPLs bind to distinct LPL-GPCRs to exert its pro-/ anti-inflammatory effects
The biological effects of LPLs are exerted via GPCRs. By an extensive literature survey, we found 15 GPCRs that are recognized as LPL receptors and tabulated this data in Table 1 [29–34]. Of note, previously claimed LPL-GPCRs including GPR3, GPR4, GPR6, GPR12, GPR35, GPR45, GPR65 (TDAG8), GPR68 (ORG1), GPR87, GPR119, GPR132 (G2A) and PAFR are now not recognized as LPL receptors by the LPL community (unidentified leading LPL expert, personal communication).
Table 1.
Three lysophospholipids (LPLs) including LPI and PAF bind to one specific G protein-coupled LPLs receptor (LPL-GPCRs), respectively, while LPA, S1P, SPC and LysoPS bind to more than two LPL-GPCRs.
| Receptors | Ligands | Gene Symbols of receptors | Alias of receptor | G-proteins |
|---|---|---|---|---|
| LPA1 | LPA | LPAR1 | EDG2, vzg-1, GRCP26 | Gi/oGqG12/13 |
| LPA2 | LPA | LPAR2 | EDG4 | Gi/oGqG12/13 |
| LPA3 | LPA | LPAR3 | EDG7 | Gi/oGqGs |
| LPA4 | LPA | LPAR4 | P2Y9, GPR23 | GsGi/oGqG12/13 |
| LPA5 | LPA | LPAR5 | GPR92, GPR93 | GsGi/oGqG12/13 |
| LPA6 | LPA | LPAR6 | P2Y5 | G12/13 |
| S1P1 | S1P, SPC | S1PR1 | EDG1, LPB1 | Gi/o |
| S1P2 | S1P, SPC | S1PR2 | EDG5, GPCR13, LPB2 | GsGi/oGqG12/13 |
| S1P3 | S1P, SPC | S1PR3 | EDG3, LPB3 | GsGi/oGqG12/13 |
| S1P4 | S1P, SPC | S1PR4 | EDG6, LPC1 | GsGi/oG12/13 |
| S1P5 | S1P, SPC | S1PR5 | EDG8, LPB4 | Gi/oGqG12/13 |
| GPR34 | LysoPS | GPR34 | Gi/o | |
| GPR55 | LPI | GPR55 | GqG12/13 | |
| GPR174 | LysoPS | GPR174 | FKSG79, GPCR17, LYPSR3 | |
| P2RY10 | LysoPS | P2RY10 | P2Y10 |
Our comprehensive literature survey revealed that LPLs bind to distinct LPL-GPCRs. For example, so far only GPR55 is recognized as the receptor for LPI [35] while LPA, S1P, SPC (sphingosylphosphorylcholine) and LysoPS bind to more than one type of LPL-GPCRs. As shown in Table 1, LPA binds to six LPL-GPCRs, S1P and SPC bind to five LPL-GPCRs.
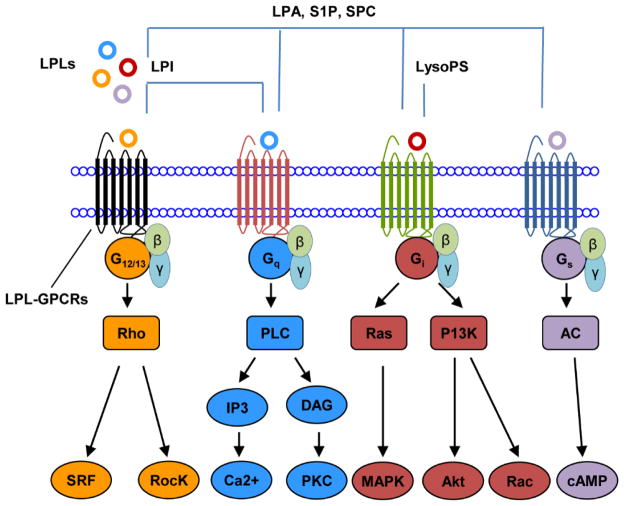
So far, GPR 34, which is the receptor for anti-inflammatory LysoPS [36,37] was identified to bind to the subunit Gi/o explicitly. Similarly, the LPI receptor GPR55 specifically binds to Gq and G12/13 subunits. Nevertheless, the other LPL-GPCRs which we have tabulated in Table 1 seem to bind to a variety of G protein subunits (the information of G protein subunits for GPR174 and P2RY10 was not available). This data suggested that certain LPLs such as LPI and LysoPS exerts distinct signaling mechanisms via GPR34 and GPR55, respectively, while some LPLs trigger broad spectrum of signaling by binding to variety of LPL-GPCRs. In order to have a comprehensive understanding about the signaling mechanisms of LPLs, we further examined the down-stream targets of currently identified LPL-GPCRs and illustrated our findings in Figure 2.
Figure 2. LPLs act as signaling mediators by binding to GPCRs.
GPR34, the receptor of LysoPS, binds to Gi to trigger specific signaling, GPR55, the receptors of LPI, bind to G protein (Gq and G12/13) to trigger signaling while the receptors of LPA, S1P and SPC are associated with more than one G protein subunits (Gq, G12/13, Gi and Gs) for signaling.
As shown in Figure 2, the LPLs act as signaling mediators by binding to seven-transmembrane domain GPCRs and either activate or inhibit downstream signaling pathways such as Rho-associated kinase (Rock), diacylglycerol (DAG), inositol 1,4,5-trisphosphate (IP3), mitogen-activated protein kinase (MAPK), Ca2+, adenylate cyclase (AC), phosphoinositide-3-kinase (PI3K) [29,38,39] etc. The specificities of LPL signaling via LPL-GPCRs may depend on the number of available receptors, as well as differentiation status, co-signaling pathways, and pathophysiological status in the cells.
3.2. For the first time, we propose a new paradigm that LPLs are either pro-inflammatory conditional DAMPs or anti-inflammatory HAMPs for regulating the balance between inflammation and inhibition/ resolution of inflammation
The Table 1 and Figure 2 clearly depicts that; (1) pro-inflammatory LPLs specifically bind to distinct LPL-GPCRs; (2) binding of pro-inflammatory LPLs to LPL-GPCR induce downstream inflammatory signaling cascades such as PKC (Protein Kinase C) [40–42], MAPK [43,44], ROCK [45] and SRF (Serum Response Factor) [46]. Therefore, it is evident that endogenous metabolites such as LPLs exert its inflammatory responses via LPL-GPCRs, thus it can be justified to designate LPL-GPCRs as conditional DAMP receptors. Furthermore, anti-inflammatory LysoPS has the capability of activating the survival PI3K and AKT pathway suggesting its receptors can be classified as HAMP receptors. To further consolidate this new hypothesis on conditional DAMPs and HAMPs, an extensive literature search was conducted.
Further supporting evidence for classifying LPLs as conditional DAMPs or HAMPs are presented in Table 2. As illustrated in Table 2A, endogenous LPLs were upregulated in the presence of metabolic and inflammatory disorders such as type 2 diabetes, diabetic retinopathy, acute coronary syndrome and autoimmune disorders. High serum levels of S1P, LPA and LPI was observed in patients with juvenile-onset systemic lupus erythematosus [47], proliferative diabetic retinopathy [48] and obese type 2 diabetes [49] respectively. Interestingly, acute coronary syndrome increased a bunch of pro-inflammatory LPLs (LPA, LPG, LPI and LPC) and anti-inflammatory LPLs (LysoPS and LPE). This indicates the presence of a complex signaling cascade that takes place to retain the balance of inflammation via conditional DAMPs and its resolution via HAMPs [21].
Table 2A.
The supporting evidence 1 for classifying LPLs as conditional DAMPs or HAMPs: LPLs are upregulated during the progression of some autoimmune, cardiovascular and metabolic diseases.
| Disease | Tissue | Upregulated Lysophospholipid | Downregulated Lysophospholipid | PMID |
|---|---|---|---|---|
| Juvenile-onset systemic lupus erythematosus | Human serum | S1P | - | 22648459 |
| Acute Coronary Syndrome | Human plasma | LPA, LPE, LPG, LPI, LysoPS, LPC | - | 25425621 |
| Proliferative diabetic retinopathy | Human vitreous fluid | LPA | - | 25496321 |
| Obese type 2 diabetes | Human plasma | LPI | - | 22179809 |
More supporting evidence for LPLs working as conditional DAMPs or HAMPs are presented Table 2B. Table 2B indicates that; (1) LPA, S1P, LPC, LPI and SPC induced the secretion of pro-inflammatory cytokines [50–56]; (2) in contrast, LysoPS and LPE suppressed the production of pro-inflammatory cytokines [57,25]; (3) additionally LPE also induced the secretion of anti-inflammatory cytokine IL-10; and (4) LPL mediated secretion of pro-inflammatory/ anti-inflammatory cytokines was dose dependent.
Table 2B.
The supporting evidence 2 for classifying LPLs as conditional DAMPs or HAMPs: The bindings of LPLs to their receptors regulate inflammation cytokine generation as the inflammation readouts.
| Treatment | Dose | Cell type/tissue | Induced cytokines/signaling | Suppressed cytokines/signaling | PMID |
|---|---|---|---|---|---|
| LPA | 1μM | Human Synoviocytes | IL-8 | - | 18006645 |
| 2.5μM | IL-6, IL-8 | - | |||
| 5μM | IL-6, IL-8 | - | |||
|
| |||||
| S1P lyase knock-out | - | Mouse liver | TNF, IFN-γ, MCP-1, IL-6 | - | 20097939 |
|
| |||||
| S1P | 0.5μM | Human CD4+ T cells | NF-κB ligand (RANKL) | - | 22326262 |
|
| |||||
| S1P | 0.01–0.5μM | MH7A synovial cells | NF-κB ligand (RANKL) | - | 22326262 |
|
| |||||
| LPC | 10μM | Mouse THP-1-derived macrophages | GM-CSF, TNF-α, MCP-1, IL-1β, IL-6, IL-8 | - | 21957201 |
|
| |||||
| LPI | - | hGPR55-HEK293 Cells | MAPK Signaling (ERK1/2 Phosphorylation) | - | 22027819 |
|
| |||||
| LPI | 1μM | hGPR55-HEK293 Cells | RhoA-dependent Ca2+ signaling and NFAT activation | - | 18757503 |
|
| |||||
| SPC | 10μM | Human keratinocytes | TNF-α, IL -6, ICAM-1 | - | 9886270 |
|
| |||||
| LysoPS | - | Mouse peritoneal macrophages | - | TNF-α, MMP-9, L-6, MCP-1 | 25445889 |
|
| |||||
| LysoPS | - | RAW 264.7 cells | - | TNF-α, MMP-9 | 25445889 |
|
| |||||
| LPE (2-ARA-LPE) | 50μg/kg | Mouse peritoneal lavage fluids of Zymosan A-Induced Peritonitis | - | TNF-α, IL-1β, IL-6 | 21744277 |
| 150μg/kg | IL-10 | TNF-α, IL-1β, IL-6 | |||
|
| |||||
| LPE (2-DHA-LPE) | 15μg/kg | Mouse peritoneal lavage fluids of Zymosan A-Induced Peritonitis | IL-10 | IL-6 | 21744277 |
We have previously demonstrated that under low concentrations, LPLs induce physiological signaling without exerting pro/ anti-inflammatory effects at a threshold that is detectable [58]. The data provided in the Table 2B indicates that LPLs have distinct functions in a dose dependent manner indicating LPLs can indeed be classified as conditional DAMPs or HAMPs. We have presented our novel working model in Figure 3.
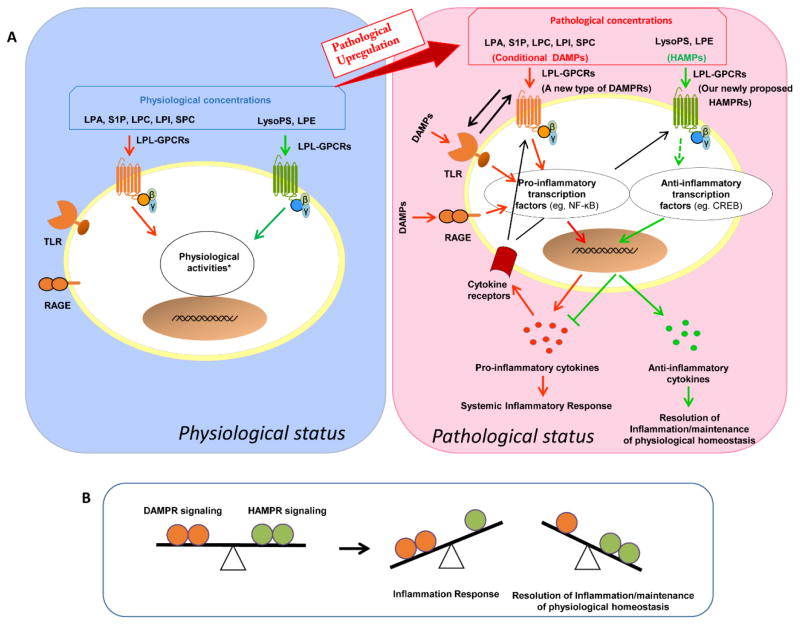
Figure 3. Our new working model: LPL-GPCRs are a new type of conditional DAMPRs or HAMPRs (receptors for DAMPs or HAMPs).
A) Upregulated LPA, S1P, LPC, LPI and SPC act as new types of conditional DAMPs to induce systemic /local inflammatory responses while LysoPS and LPE act as newly proposed HAMPs to suppress systemic/local inflammatory responses and induce the resolution of inflammation. B) LPLs regulate the balance between the progression of inflammation and resolution of inflammation via newly designed conditional DAMPR signaling and HAMPR signaling. Our new report showed that LPC stimulates mitochondrial reactive oxygen species generation without compromising ATP production in human aortic endothelial cells [66].
As illustrated in Figure 3A, pro-inflammatory LPLs such as LPA, S1P, LPC, LPI and SPC act as conditional DAMPs to induce systemic inflammatory responses. However, LysoPS and LPE act as HAMPs to suppress systemic inflammatory response and induce the resolution of inflammation. Thus, LPLs regulate the balance between initiation and resolution of inflammation via newly designated conditional DAMP receptors and HAMP receptors (Figure 3B). This new paradigm significantly improve our understanding of endogenous metabolites’ biological functions at pathophysiological conditions and their ability to modulate inflammation, systemic homeostasis and immune tolerance.
3.3. Some conditional DAMPs such as LPA, S1P and LPC regulate physiological signaling ubiquitously while others may have tissue specific signaling functions
We hypothesized that conditional DAMPs fulfill differential physiological roles at basal level. We further postulated that physiological functions of conditional DAMPs are regulated by differential expression of conditional DAMP receptors and also by tightly controlling the receptor level in the tissues. To validate this hypothesis, a database mining method we pioneered (Part 2 in Figure 1) was used to examine experimentally-verified expression profiles of mRNA transcripts of LPL-GPCRs in NCBI-UniGene database. The expression of 15 LPL-GPCR genes in 23 human tissues and 21 mouse tissues that were available in the NCBI-Unigene database was examined (Figure 4A). The analyzed data are summarized in Figures 4B and 4C.
The analysis implicated that the expression level of human LPL-GPCRs were relatively higher compared to mouse counterparts. Human brain, eye, intestine, lung and placenta expressed a variety of LPL-GPCRs, suggesting that LPLs mediate vital physiological functions in these tissues. Nevertheless, human adrenal gland, bone, bone marrow, salivary gland, thyroid, trachea and vascular tissues expressed least variety (less than eight) of LPL-GPCRs. Moreover, human heart and vascular tissues had less LPL-GPCRs compared to connective tissue and lymph nodes, suggesting that LPL-GPCRs signaling is stronger in connective tissue and lymph nodes than in the heart and vascular tissues. LPL-GPCRs were not expressed in mouse adrenal gland, muscle and thyroid.
Table 3 is a summary of figure 4 that shows the tissue distribution of LPL-GPCRs based on the ligand. Our data clearly demonstrates that GPCRs for LPA, S1P and SPC are widely expressed in most of the human and mouse tissues examined, suggesting that these metabolites may have more ubiquitous physiological roles. In contrast, LPI-GPCR expression is limited in humans and are only seen in intestine, muscle, liver and spleen, while it was not detected in the mouse tissues. Furthermore, GPCRs for anti-inflammatory LysoPS was expressed in 15 out of 23 human tissues and 10 out of 21 mouse tissues respectively, implicating that LysoPS is a potential HAMP as its presence may regulate homeostatic functions in tissues at basal level. Moreover, LysoPS, as a potential HAMP may play role in inflammation resolution if its levels are elevated due to cellular stress or pathological conditions.
Table 3A.
LPA, S1P and SPC have ubiquitous physiological signaling functions in human tissues whereas other LPLs, especially LPI, may have tissue-specific signaling functions.
| tissue | LPA-GPCRs | S1P-GPCRs | SPC-GPCRs | LPI-GPCRs | LysoPS-GPCRs |
|---|---|---|---|---|---|
| intestine | + | + | + | + | + |
| blood | + | + | + | − | + |
| brain | + | + | + | − | + |
| kidney | + | + | + | − | − |
| connective tissue | + | + | + | − | + |
| lung | + | + | + | − | + |
| thymus | + | + | + | − | + |
| pancreas | + | + | + | − | + |
| eye | + | + | + | − | + |
| lymph node | + | + | + | − | + |
| placenta | + | + | + | − | + |
| muscle | + | + | + | + | + |
| liver | + | + | + | + | + |
| spleen | + | + | + | + | − |
| vascular | + | + | + | − | + |
| thyroid | − | + | + | − | − |
| bone | + | + | + | − | − |
| bone marrow | + | + | + | − | − |
| heart | + | + | + | − | − |
| skin | + | + | + | − | − |
| trachea | + | + | + | − | + |
| adrenal gland | + | + | + | − | + |
| salivary gland | − | + | + | − | − |
3.4. The expressions of conditional DAMP receptors are significantly upregulated in rheumatoid arthritis, coronary artery disease, and other metabolic inflammatory diseases of human and mouse
Table 2A and 2B implicate that LPLs may regulate physiological signaling at basal levels. In addition, we hypothesized that the expressions of LPL-GPCRs, the newly designated conditional DAMP receptors are significantly upregulated in human and mouse inflammatory diseases. To examine this hypothesis, we extensively analyzed microarray experimental datasets deposited in NIH-Geo database by other laboratories. These depositors have utilized these microarray data for various other non-LPL studies. We analyzed the expression of LPL-GPCRs in five metabolic inflammatory diseases including coronary artery disease, metabolic syndrome, type 2 and type 1 diabetes as well as autoimmune rheumatoid arthritis and have tabulated the data in Table 4.
Table 4A.
The expression of certain LPL-GPCRs are significantly upregulated in rheumatoid arthritis, coronary artery disease, and other metabolic inflammatory diseases in human.
| Disease | Tissue or cell type | Upregulated genes | Downregulated genes | GEO Dataset ID |
|---|---|---|---|---|
| Rheumatoid Arthritis | synovial tissue | P2RY10**(LysoP) LPAR2* (LPA) LPAR6* (LPA) S1PR5 (S1P, SPC) |
LPAR1 (LPA) | GSE55235 |
|
| ||||
| Coronary Artery Disease | peripheral blood | GPR34* (LysoPS) GPR174*(LysoPS) P2RY10* LysoPS) |
- | GSE23561 |
|
| ||||
| Metabolic Syndrome | peripheral blood | - | - | GSE23561 |
|
| ||||
| Type 2 Diabetes | peripheral blood | - | P2RY10 (LysoPS) | GSE23561 |
|
| ||||
| Type 1 Diabetes | peripheral blood mononuclear cell | LPAR1 (LPA) LPAR2 (LPA) LPAR3 (LPA) S1PR3(S1P, SP) S1PR4(S1P, SP) |
S1PR5 (S1P, SPC), GPR174 (LysoPS) | GSE55100 |
These microarray experiments were not designed for LPL-GPCRs studies; FC, fold change;
1.5≤FC<3,
3≤FC<10,
10≤FC<100,
FC≥100
Interestingly, our analysis indicated that 4 LPL-GPCRs in synovial tissue of patients with rheumatoid disease, and 3 LPL-GPCRs in peripheral blood cells in patients with coronary artery disease were significantly upregulated with the scales ranging from ≥1.5 to ≥3.0. In addition, 5 LPL-GPCRs in peripheral blood mononuclear cells in patients with type 1 diabetes were upregulated in a low scale <1.5. This suggested that specific LPL-GPCR signaling contributes to the pathogenesis of these diseases. The downregulation of LPL-GPCRs was found in patients with rheumatoid arthritis, type 2 and type 1 diabetes but not in the patients with coronary artery disease. P2RY10 GPCR upregulation was common in both rheumatoid arthritis and coronary artery disease; and the majority of upregulated LPL-GPCRs were somewhat disease-specific.
These results suggest that, (1) pro-inflammatory LPLs including SPC, LPA, and S1P may contribute to the pathogenesis and progression of rheumatoid arthritis, coronary artery disease and type 1 diabetes; (2) expression of GPCRs for anti-inflammatory/ homeostatic LysoPS was attenuated in type 1 and type 2 diabetes; and (3) however, LysoPS-GPCR is induced in rheumatoid arthritis and coronary artery disease, indicating LysoPS may act as a potential HAMP under these pathologies. This is similar to our recently reported responsive anti-inflammatory cytokine IL-35 [13,14]. Similarly, LysoPS-GPCR expression was elevated in the aortas of ApoE−/− mice fed with high fat diet further validating the role of anti-inflammatory LysoPS as responsive HAMPs (Table 4B).
Table 4B.
The expression of certain LPL-GPCRs are significantly upregulated in collage induced arthritis, high fat diet mediated atherosclerosis, and diabetes in mouse.
| Model | Tissue or cell type | Upregulated genes | Downregulated genes | GEO Dataset ID |
|---|---|---|---|---|
| CIA mice, without joint swelling | knee joint synovium | - | S1pr1 (S1P, SPC)# | GSE13071 |
|
| ||||
| CIA mice, with joint swelling | knee joint synovium | Lpar4** (LPA) P2ry10**(LysoPS) Lpar1* (LPA)# Lpar6* (LPA) S1pr2*(S1P,SPC)# S1pr3* (S1P, SPC) Gpr34* (LysoPS)# Gpr174*(LysoPS)# |
- | GSE13071 |
|
| ||||
| APOE−/− mice, with 8/16/24 weeks high fat diet | aortic arch | P2ry10***(LysoPS Gpr34** LysoPS)# |
- | GSE18443 |
|
| ||||
| diabetic mice | glomerular endothelial cell | Lpar4* (LPA) Lpar6* (LPA) S1pr1 (S1P, SPC) |
- | GSE21324 |
As shown in Table 4B, the expressions of GPCRs for pro-inflammatory LPLs and anti-inflammatory LysoPS were elevated in collagen-induced arthritis model in mice. Out of these receptors most significant upregulation was seen for LPA-GPCRs, which was also the case observed in diabetes (db/db mice). Taken together, our results suggest that differential expression of LPL-GPCRs promote the pathogenesis of human and mouse rheumatoid arthritis, coronary artery disease, and other metabolic inflammatory diseases.
3.5. Pro-inflammatory cytokine signaling regulates the expression of LPL receptors
In order to determine how inflammatory and metabolic diseases mediate the upregulation of conditional DAMPs, we hypothesized that pro-inflammatory cytokines play an important role in regulating the expression of LPL-GPCRs, which is the prototype used for this study. To test this hypothesis, we examined the LPL-GPCRs expression in several GEO microarray datasets with inflammatory cytokine stimulated endothelial cells and monocytes. It is well established that the pro-inflammatory cytokines that were examined in this study are involved in development and progression of rheumatoid arthritis [22], coronary heart disease [59] and other metabolic diseases [60], suggesting that our hypothesis is pathophysiologically relevant.
Table 5 implicate that tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) induces Lpar2 (LPA-GPCR) in mouse endothelial cells and LPAR6 in human endothelial cells, respectively. Further, interleukin-1β (IL-1β) induces the expressions of GPR55 (LPI-GPCR). Since the GRP55 expression is not found in human vascular tissue in unstimulated conditions as shown in Figure 2, the upregulation of GPR55 induced by IL-1β in human endothelial cells is significant. Interestingly, IL-1β increased the expression of S1PR1 (receptors for SPC and SP1) while decreasing the other receptors (S1PR5, S1PR3) where the functions are mediated by the same ligands in human endothelial cells. This indicates that cytokines can differentially modulate the functions of conditional DAMPs in response to inflammation. Additionally, the data shown in Table 5 implicate that TNF-α and IL-1β can significantly upregulate the expression of GPR34, which is receptor for anti-inflammatory LysoPS. This indicates the presence of complex signaling mechanism where cytokines can directly or indirectly regulate the expression of conditional DAMPs and HAMPs in inflammatory diseases.
Table 5.
Pro-inflammatory cytokine signaling regulates the expression of LPL receptors.
| Treatment | Cell type | Upregulated gene | Downregulated gene | GEO Datasets ID |
|---|---|---|---|---|
| TNF-a vs Control | Mouse endothelial cells | Lpar2** | - | GSE4518 |
|
| ||||
| IL-1b vs Control | Human endothelial cells | GPR55** | S1PR5** | GSE19240 |
| S1PR1* | S1PR3* | |||
| - | LPAR2** | |||
|
| ||||
| IFN-g vs Control | Human endothelial cells | LPAR6* | - | GSE3920 |
|
| ||||
| TNF-a vs Control (2 hours) | Human Peripheral Blood Mononuclear Cells (PBMCs) | GPR34*** | - | GSE40838 |
|
| ||||
| IL-1b vs Control (6 hours) | Human Peripheral Blood Mononuclear Cells (PBMCs) | GPR34* | - | GSE40838 |
Of note, due to the limitations of microarray datasets in the current GEO database, the extent we could analyze the LPL-GPCRs in different tissues/ cells in response to different pro-inflammatory mediators were limited. Further, we also acknowledge that variety of factors other than pro-inflammatory cytokines may contribute to the disease mediated upregulation of LPL-GPCRs. However, our results demonstrated the proof of principle that pro-inflammatory cytokine signaling regulates the expression of conditional DAMPs and its receptors.
3.6. TLR signaling regulates the expression of LPL receptors
By utilizing GEO microarray data sets, we further studied the interrelation between classical DAMP receptors such as TLRs and LPL-GPCRs, which we have newly designated as conditional DAMP/ HAMP receptors. We hypothesized that in response to endogenous DAMPs, TLRs act as a set of prototypic DAMP receptors and play an important role in regulating the expressions of LPL-GPCRs. To test this hypothesis, we examined LPL-GPCR expression in several GEO microarray datasets in TLR gene deficient mouse tissues. This data is shown in Table 6. So far, four TLRs have been identified to bind to DAMPs such as HMGB1, RNA and DNA. These four receptors are TLR2 (HMGB1), TLR3 (RNAs), TLR4 (HMGB1) and TLR9 (HMGB1 and DNAs) [61].
Table 6.
DAMPRs/HAMPRs signaling interactions (1): TLR signaling regulates the expression of LPL receptors
Table 6 showed that the expressions of P2ry10 (LysoPS-GPCR) and Gpr55 (LPI-GPCR) are downregulated in TLR2 gene knock-out (KO) mouse in proximal jejunum. In addition, we found that the expressions of S1pr2 (S1P/ SPC-GPCR), P2ry10 (LysoPS-GPCR), and Lpar1 (LPA-GPCR) are downregulated in TLR4 KO mouse kidney. Moreover, the expression of S1pr2 (S1P/ SPC-GPCR) is decreased in TLR3 KO mouse liver.
This further validates that TLRs and pro-inflammatory mediators (Table 5) together with other classical DAMPs are involved in regulating the expression of conditional DAMPs and HAMPs to mediate the progression and resolution of inflammation in tissues.
Of note, the GEO datasets for TLR9 KO mice were not available for the analysis. Although the GEO datasets are limited, our results demonstrated the proof of principle that TLR signaling regulates the expressions of LPL receptors; and that classical DAMP receptor signaling regulates the expression of LPL receptors.
3.7. LPL receptor signaling regulates the expression of TLRs
We further studied whether newly designated conditional DAMPs regulate expression of classical DAMP receptors such as TLRs. Therefore, we hypothesized that in response to endogenous LPL metabolite danger signals, LPL-GPCRs regulate the expressions of TLR receptors. To test this hypothesis, we examined the TLR receptor expressions in several GEO microarray datasets that was conducted on LPA-stimulated human PBMCs and one S1P-stimulated mouse liver (Table 7).
Table 7.
DAMPRs/HAMPRs signaling interactions (2): LPL receptor signaling regulates the expression of TLRs
Of note, ten human TLRs have been identified including TLR1-TLR10 whereas twelve mouse TLRs have been found including TLR1-TLR9, and TLR11-TLR13 [62]. Table 7 illustrates that the expression several TLRs/ Tlrs are modulated in the presence of pro-inflammatory LPLs in human and mouse tissues. This suggests that, when elevated, endogenous metabolites such as LPLs can regulate the expression of TLRs and can modulate the inflammatory signals in response to classical DAMPs and other pro-inflammatory cytokines.
Again, although the GEO datasets are limited, our results demonstrated the proof of principle that LPLs regulate the expressions of TLR receptors. Of note, the reason for LPA to downregulate TLR7 and TLR3, and for S1P to downregulate Tlr5 may be due to signal complexity of these LPL receptors with stimulatory GPCRs and inhibitory GPCRs shown in Figure 3. Once again, our results suggest that: (1) the interplay between classical DAMP receptors and newly designated conditional DAMP receptors collaborate in endogenous danger signal-initiated sterile inflammation; (2) LPL-GPCR (as conditional DAMP receptors and HAMP receptors) signaling not only regulates various inflammatory cytokine signaling but also modulates other DAMP receptor-initiated signaling.
3. Discussion
Recently DAMPs and its six types of receptors had gained widespread attention as major players in regulating sterile inflammation. This was further validated by the recent reports from our lab, which demonstrated that NLR/ caspase-1 signaling pathway is responsible for inducing endothelial activation and subsequent atherogenesis during early hyperlipidemia [58] and hyperhomocysteinemia [63], for decreasing vascular endothelial growth factor receptor 2 (VEGFR2) mediated angiogenesis in ischemia [64], and for weakening of the progenitor cell mediated vessel repair [3,64].
However, despite the well-established roles of currently identified DAMPs and their receptors, there are limitations in the classification of DAMP receptors. To overcome the limitations we have elaborated above, we utilized a family of endogenous lipid metabolites (LPLs) and their receptors as a prototype. We performed a panoramic analysis on the tissue expression of all currently identified 15 LPL receptors in 23 human tissues and 21 mouse tissues. In addition, we also examined the expression changes of the 15 LPL receptors in five prototypic chronic sterile inflammations including autoimmune rheumatoid disease, coronary artery disease, metabolic syndrome, type 2 diabetes and type 1 diabetes. Based on our analysis we propose the following paradigm: (1) endogenous metabolites play a role in regulating normal cellular functions at basal level. However, the expression of these metabolites can be elevated in sterile inflammatory conditions and can induce inflammation in tissues. One potential mechanism that pro-inflammatory LPLs induce inflammation is by activating pro-inflammatory transcription factors (NF-kB, C/EBPδ and RUNX1) [6]. Thus, we named these endogenous metabolites as conditional DAMPs; (2) anti-inflammatory metabolites expression can get elevated in response to inflammation, and may play a role in inhibition or resolution of inflammation. We designated such metabolites as HAMPs; (3) conditional DAMPs and HAMPs exert their pathophysiological signaling via their receptors, and we designated these receptors as conditional DAMP receptors or HAMP receptors respectively; (4) conditional DAMPs and HAMPs are differentially expressed in tissues at normal physiological conditions (healthy state). Some conditional DAMPs are ubiquitously expressed while some may have distinct tissue specific functions; (5) under inflammatory and metabolic diseases, expression of conditional DAMP receptors and HAMP receptors are modulated, which will be responsible for either progression or inhibition/ resolution of inflammation; (6) complex signaling mechanism exists between various pro-inflammatory mediators and classical DAMPs and their receptors, that regulate the expression of conditional DAMPs and HAMPs and their receptors. We have illustrated our working model in Figure 3.
As we have emphasized, LPLs and LPL-GPCRs were used as a prototype to formulate this novel paradigm and we acknowledge that there may be myriad of other endogenous metabolites that can behave similarly as LPLs, thus they can either act as conditional DAMPs or HAMPs. At present, various types of LPLs and LPL-GPCRs were identified to play a role in inflammatory disorders including cardiovascular diseases [65]. Most recently, our lab further validated that the pro-inflammatory LPC is induced at early stages of atherogenesis and it induces mitochondrial reactive oxygen species production in viable endothelial cells [66].
We previously reported that although LPLs at low levels cannot initiate pro-inflammatory/anti-inflammatory cytokine secretions above the lowest threshold of current technique-detectable levels, still they can initiate a physiological signaling [58]. This finding is important since it emphasizes that LPLs and other pro-inflammatory metabolites are not pathologically generated only for the purpose to be the danger signals and HAMPs for regulating inflammation. Of note, recent reports attributed that LPA receptors may also initiate immune-suppressive signals [67–69] in addition to pro-inflammatory signals [70]. Similar conflicting results have been reported to pro-inflammatory mediators such as caspase-1 [71], anti-inflammatory estrogen [72] and pro-inflammatory cytokine interleukin-17 [73]. The contextual dependence of these biologicals systems that were used to study these inflammatory mediators may contribute to these dissimilarities observed [74]. Future work is needed to solve these discrepancies.
As we pointed out previously, our sets of experimental data on LPL receptor tissue expression are collected from cDNA cloning and DNA sequencing experiments deposited in NIH-UniGene database, rather than theoretical data derived from computer modeling. Therefore, it is our belief that this data require no further experimental verification. Further, these gene expression databases have been established based on precise DNA sequencing data, thus the data we obtained by NIH-UniGene database mining are more precise in providing the tissue expression profiles of genes than traditional hybridization- and primer annealing-based approaches like Northern blots and RT-PCRs [2].
In addition, the gene expression data we gathered by analyzing NIH-GEO databases have been generated by high throughput microarray experiments. Of note, the expression of LPL receptor genes among tissues and disease-related microarrays are significantly higher relative to housekeeping genes. Since a huge amount of “omics” experimental data are deposited in various NIH and other databases, it had become a well-accepted norm to analyze those large sets of experimental data to validate hypothesizes and formulate new paradigms without having additional experimental verification. Furthermore, the laboratories that have deposited the microarray data that we used for this study were conducted on other non-LPL-related projects. Therefore, to the best of our knowledge, the findings that we reported here have not been published elsewhere. This well-accepted practice allows us to claim that our analysis of existing data are novel and unpublished research findings, as such, do not represent a traditional literature review.
Our proposal of the new paradigm and new concept of conditional DAMPs, DAMP receptors, HAMPs and their receptors would significantly improve our understanding on the roles of endogenous metabolites in regulating the pathogenesis of inflammations. This will also eventually lead to future development of novel therapeutics for rheumatoid diseases, metabolic inflammatory diseases, cardiovascular diseases, inflammatory cancers and other diseases.
Table 3B.
LPA, S1P and SPC have ubiquitous physiological signaling functions in mouse tissues.
| tissue | LPA-GPCRs | S1P-GPCRs | SPC-GPCRs | LPI-GPCRs | LysoPS-GPCRs |
|---|---|---|---|---|---|
| lymph node | + | + | + | − | + |
| skin | + | + | + | − | + |
| spleen | + | + | + | − | + |
| thymus | + | + | + | − | + |
| brain | + | + | + | − | + |
| bone | + | + | + | − | + |
| bone marrow | − | + | + | − | + |
| eye | + | + | + | − | − |
| heart | + | + | + | − | − |
| intestine | + | + | + | − | + |
| joint | + | + | + | − | − |
| lung | + | + | + | − | − |
| salivary gland | + | + | + | − | + |
| pancreas | + | + | + | − | + |
| kidney | + | + | + | − | − |
| blood | + | + | + | − | − |
| liver | + | + | + | − | − |
| connective tissue | + | + | + | − | − |
| muscle | − | − | − | − | − |
| adrenal gland | − | − | − | − | − |
| thyroid | − | − | − | − | − |
Acknowledgments
Funding This work was partially supported by the National Institutes of Health Grants to XFY, HW and JY, and the American Heart Association Postdoctoral Fellowship to Dr. YFL.
Abbreviations
- PAMP
Pathogen associated molecular patterns
- DAMP
Danger associated molecular patterns
- TLR
Toll like receptors
- NLR
NOD like receptors
- NOD
Nucleotide binding and oligomerization domain
- RIG-I
Retinoid acid inducible gene I
- AIM2
Absent in melanoma 2
- RAGE
Receptor for advanced glycation end product
- HMGB1
High mobility group box 1
- IL
Interleukin
- TGF-β
Transforming growth factor-β
- LPLs
Lysophospholipids
- GPCRs
G-protein coupled receptors
- LPA
Lysophosphatidic acid
- LPC
Lysophophatidylcholine
- LPE
Lysophophatidylenthaolamine
- LPG
Lysophosphoglycan
- LPI
Lysophosphatidylinositol
- LysoPS
Lysophosphatidylserine
- CAD
Coronary artery disease
- SPM
Specialized pro-resolving mediators
- EST
Expressed sequence tags
- NIH
National institute of health
- NCBI
National center of biotechnology information
- ROCK
Rho-associated kinase
- DAG
Diacylglycerol
- IP3
Inositol 1,4,5-triphosphate
- MAPK
Mitogen activated protein kinase
- AC
Adenylate cyclase
- PI3K
Phosphoinositide-3-kinase
- PKC
Protein kinase C
- SRF
Serum response factor
- SPC
Sphingosylphosphorylcholine
- IFN-γ
Interferon-γ
Footnotes
Disclosures None
Ethics statement N/A. This study only employed a data mining strategy and did not involve human participants or experimental animal models.
References
- 1.Yang XF, Yin Y, Wang H. VASCULAR INFLAMMATION AND ATHEROGENESIS ARE ACTIVATED VIA RECEPTORS FOR PAMPs AND SUPPRESSED BY REGULATORY T CELLS. Drug Discov Today Ther Strateg. 2008;5(2):125–142. doi: 10.1016/j.ddstr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, et al. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22(2):311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, et al. Inflammasomes: sensors of metabolic stresses for vascular inflammation. [Research Support, N.I.H., Extramural] Front Biosci. 2013;18:638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venereau E, Ceriotti C, Bianchi ME. DAMPs from Cell Death to New Life. [Review] Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. [Research Support, Non-U.S. Gov’tReview] Nat Rev Immunol. 2009;9(10):692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 7.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] J Am Soc Nephrol. 2011;22(3):416–425. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn TS. The structure of scientific revolutions. 3. Chicago, IL: University of Chicago Press; 1996. [Google Scholar]
- 9.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. [Research Support, N.I.H., Extramural Review] Nat Rev Immunol. 2007;7(8):599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 11.Yang WY, Ying Shao Jahaira Lopez-Pastrana, Jietang Mai Hong Wang, Xiao-feng Yang. Pathological conditions re-shape physiological Tregs into pathological Tregs. Burns & Trauma. 2015;3:1–11. doi: 10.1186/s41038-015-0001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Nat Immunol. 2012;13(8):722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, et al. IL-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. [Research Support, N.I.H., Extramural] PLoS One. 2012;7(3):e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sha X, Meng S, Li X, Xi H, Maddaloni M, Pascual DW, et al. Interleukin-35 Inhibits Endothelial Cell Activation by Suppressing MAPK-AP-1 Pathway. [Research Support, N.I.H., Extramural] J Biol Chem. 2015;290(31):19307–19318. doi: 10.1074/jbc.M115.663286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng B, Yang F, Huston DP, Yan Y, Yang Y, Xiong Z, et al. Increased noncanonical splicing of autoantigen transcripts provides the structural basis for expression of untolerized epitopes. J Allergy Clin Immunol. 2004;114(6):1463–1470. doi: 10.1016/j.jaci.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YF, Li RS, Samuel SB, Cueto R, Li XY, Wang H, et al. Lysophospholipids and their G protein-coupled receptors in atherosclerosis. Front Biosci (Landmark Ed) 2016;21:70–88. doi: 10.2741/4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyth SS, Mueller P, Yang F, Brandon JA, Morris AJ. Arguing the case for the autotaxin-lysophosphatidic acid-lipid phosphate phosphatase 3-signaling nexus in the development and complications of atherosclerosis. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Arterioscler Thromb Vasc Biol. 2014;34(3):479–486. doi: 10.1161/ATVBAHA.113.302737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Latif A, Heron PM, Morris AJ, Smyth SS. Lysophospholipids in coronary artery and chronic ischemic heart disease. Curr Opin Lipidol. 2015 doi: 10.1097/MOL.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan M, Hao F, Xu X, Chisolm GM, Cui MZ. Lysophosphatidylcholine activates a novel PKD2-mediated signaling pathway that controls monocyte migration. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Arterioscler Thromb Vasc Biol. 2009;29(9):1376–1382. doi: 10.1161/ATVBAHA.109.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurano M, Suzuki A, Inoue A, Tokuhara Y, Kano K, Matsumoto H, et al. Possible involvement of minor lysophospholipids in the increase in plasma lysophosphatidic acid in acute coronary syndrome. [Research Support, Non-U.S. Gov’t] Arterioscler Thromb Vasc Biol. 2015;35(2):463–470. doi: 10.1161/ATVBAHA.114.304748. [DOI] [PubMed] [Google Scholar]
- 22.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. [Review] N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra100496510.7748/phc2011.11.21.9.29.c8797. [DOI] [PubMed] [Google Scholar]
- 23.Bourgoin SG, Zhao C. Autotaxin and lysophospholipids in rheumatoid arthritis. [Research Support, Non-U.S. Gov’t Review] Curr Opin Investig Drugs. 2010;11(5):515–526. [PubMed] [Google Scholar]
- 24.Frasch SC, Bratton DL. Emerging roles for lysophosphatidylserine in resolution of inflammation. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Prog Lipid Res. 2012;51(3):199–207. doi: 10.1016/j.plipres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung ND, Kim MR, Sok DE. 2-Polyunsaturated acyl lysophosphatidylethanolamine attenuates inflammatory response in zymosan A-induced peritonitis in mice. [Research Support, Non-U.S. Gov’t] Lipids. 2011;46(10):893–906. doi: 10.1007/s11745-011-3589-2. [DOI] [PubMed] [Google Scholar]
- 26.Marion-Letellier R, Savoye G, Ghosh S. Polyunsaturated fatty acids and inflammation. [Review] IUBMB Life. 2015;67(9):659–667. doi: 10.1002/iub.1428. [DOI] [PubMed] [Google Scholar]
- 27.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16(1):51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen NC, Yang F, Capecci LM, Gu Z, Schafer AI, Durante W, et al. Regulation of homocysteine metabolism and methylation in human and mouse tissues. [Research Support, N.I.H., Extramural] FASEB J. 2010;24(8):2804–2817. doi: 10.1096/fj.09-143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Review] J Biol Chem. 2004;279(20):20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 30.Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, et al. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. [Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t Review] Pharmacol Rev. 2013;65(3):967–986. doi: 10.1124/pr.112.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. [Review] Pharmacol Rev. 2005;57(2):279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- 32.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. [Review] Pharmacol Rev. 2002;54(2):265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. [Research Support, Non-U.S. Gov’t Review] Atherosclerosis. 2010;208(1):10–18. doi: 10.1016/j.atherosclerosis.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Pharmacol Rev. 2010;62(4):579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guy AT, Nagatsuka Y, Ooashi N, Inoue M, Nakata A, Greimel P, et al. NEURONAL DEVELOPMENT. Glycerophospholipid regulation of modality-specific sensory axon guidance in the spinal cord. [Research Support, Non-U.S. Gov’t] Science. 2015;349(6251):974–977. doi: 10.1126/science.aab3516. [DOI] [PubMed] [Google Scholar]
- 36.Ikubo M, Inoue A, Nakamura S, Jung S, Sayama M, Otani Y, et al. Structure-activity relationships of lysophosphatidylserine analogs as agonists of G-protein-coupled receptors GPR34, P2Y10, and GPR174. [Research Support, Non-U.S. Gov’t] J Med Chem. 2015;58(10):4204–4219. doi: 10.1021/jm5020082. [DOI] [PubMed] [Google Scholar]
- 37.Makide K, Uwamizu A, Shinjo Y, Ishiguro J, Okutani M, Inoue A, et al. Novel lysophosphoplipid receptors: their structure and function. [Research Support, Non-U.S. Gov’t Review] J Lipid Res. 2014;55(10):1986–1995. doi: 10.1194/jlr.R046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torkhovskaya TI, Ipatova OM, Zakharova TS, Kochetova MM, Khalilov EM. Lysophospholipid receptors in cell signaling. [Review] Biochemistry (Mosc) 2007;72(2):125–131. doi: 10.1134/s0006297907020010. [DOI] [PubMed] [Google Scholar]
- 39.Xiang SY, Dusaban SS, Brown JH. Lysophospholipid receptor activation of RhoA and lipid signaling pathways. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Biochim Biophys Acta. 2013;1831(1):213–222. doi: 10.1016/j.bbalip.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang SY, Wei CC, Shang TT, Lian Q, Wu CX, Deng JY. High glucose induces inflammatory cytokine through protein kinase C-induced toll-like receptor 2 pathway in gingival fibroblasts. [Research Support, Non-U.S. Gov’t] Biochem Biophys Res Commun. 2012;427(3):666–670. doi: 10.1016/j.bbrc.2012.09.118. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Zamel R, Bai XH, Liu M. PKC activation induces inflammatory response and cell death in human bronchial epithelial cells. [Research Support, Non-U.S. Gov’t] PLoS One. 2013;8(5):e64182. doi: 10.1371/journal.pone.0064182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cataisson C, Joseloff E, Murillas R, Wang A, Atwell C, Torgerson S, et al. Activation of cutaneous protein kinase C alpha induces keratinocyte apoptosis and intraepidermal inflammation by independent signaling pathways. J Immunol. 2003;171(5):2703–2713. doi: 10.4049/jimmunol.171.5.2703. [DOI] [PubMed] [Google Scholar]
- 43.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. [Research Support, Non-U.S. Gov’t Review] Biochim Biophys Acta. 2005;1754(1–2):253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 44.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. [Review] Physiol Rev. 2012;92(2):689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 45.Ma Z, Zhang J, Du R, Ji E, Chu L. Rho kinase inhibition by fasudil has anti-inflammatory effects in hypercholesterolemic rats. [Research Support, Non-U.S. Gov’t] Biol Pharm Bull. 2011;34(11):1684–1689. doi: 10.1248/bpb.34.1684. [DOI] [PubMed] [Google Scholar]
- 46.Taylor A, Tang W, Bruscia EM, Zhang PX, Lin A, Gaines P, et al. SRF is required for neutrophil migration in response to inflammation. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Blood. 2014;123(19):3027–3036. doi: 10.1182/blood-2013-06-507582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson L, Tullus K, Marks SD, Holt RC, Pilkington C, Beresford MW. Increased serum concentration of sphingosine-1-phosphate in juvenile-onset systemic lupus erythematosus. [Research Support, Non-U.S. Gov’t] J Clin Immunol. 2012;32(5):1019–1025. doi: 10.1007/s10875-012-9710-3. [DOI] [PubMed] [Google Scholar]
- 48.Abu El-Asrar AM, Nawaz MI, Mohammad G, Siddiquei MM, Alam K, Mousa A, et al. Expression of bioactive lysophospholipids and processing enzymes in the vitreous from patients with proliferative diabetic retinopathy. [Research Support, Non-U.S. Gov’t] Lipids Health Dis. 2014;13:187. doi: 10.1186/1476-511X-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreno-Navarrete JM, Catalan V, Whyte L, Diaz-Arteaga A, Vazquez-Martinez R, Rotellar F, et al. The L-alpha-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. [Research Support, Non-U.S. Gov’t] Diabetes. 2012;61(2):281–291. doi: 10.2337/db11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao C, Fernandes MJ, Prestwich GD, Turgeon M, Di Battista J, Clair T, et al. Regulation of lysophosphatidic acid receptor expression and function in human synoviocytes: implications for rheumatoid arthritis? [Comparative Study Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] Mol Pharmacol. 2008;73(2):587–600. doi: 10.1124/mol.107.038216. [DOI] [PubMed] [Google Scholar]
- 51.Bektas M, Allende ML, Lee BG, Chen W, Amar MJ, Remaley AT, et al. Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural] J Biol Chem. 2010;285(14):10880–10889. doi: 10.1074/jbc.M109.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeshita H, Kitano M, Iwasaki T, Kitano S, Tsunemi S, Sato C, et al. Sphingosine 1-phosphate (S1P)/S1P receptor 1 signaling regulates receptor activator of NF-kappaB ligand (RANKL) expression in rheumatoid arthritis. [Research Support, Non-U.S. Gov’t] Biochem Biophys Res Commun. 2012;419(2):154–159. doi: 10.1016/j.bbrc.2012.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vladykovskaya E, Ozhegov E, Hoetker JD, Xie Z, Ahmed Y, Suttles J, et al. Reductive metabolism increases the proinflammatory activity of aldehyde phospholipids. [Research Support, N.I.H., Extramural] J Lipid Res. 2011;52(12):2209–2225. doi: 10.1194/jlr.M013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anavi-Goffer S, Baillie G, Irving AJ, Gertsch J, Greig IR, Pertwee RG, et al. Modulation of L-alpha-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] J Biol Chem. 2012;287(1):91–104. doi: 10.1074/jbc.M111.296020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henstridge CM, Balenga NA, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. [Research Support, Non-U.S. Gov’t] FASEB J. 2009;23(1):183–193. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- 56.Imokawa G, TY, Higuchi K, Kondo H, Yada Y. Sphingosylphosphorylcholine is a potent inducer of intercellular adhesion molecule-1 expression in human keratinocytes. Journal of Investigative Dermatology. 1999;112:91–96. doi: 10.1046/j.1523-1747.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- 57.Nishikawa MKM, Ikeda H, Aoki J, Yatomi Y. Lysophosphatidylserine has Bilateral Effects on Macrophages in the Pathogenesis of Atherosclerosis. J Atheroscler Thromb. 2015;22:518–526. doi: 10.5551/jat.25650. [DOI] [PubMed] [Google Scholar]
- 58.Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y, et al. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. [Research Support, N.I.H., Extramural] Arterioscler Thromb Vasc Biol. 2015;35(4):804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Review] Atherosclerosis. 2000;148(2):209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 60.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. [Research Support, N.I.H., Extramural Review] Cell Metab. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. [Research Support, N.I.H., Extramural Review] Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 63.Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, et al. Caspase-1 Inflammasome Activation Mediates Homocysteine-Induced Pyrop-Apoptosis in Endothelial Cells. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.308501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez-Pastrana J, Ferrer LM, Li YF, Xiong X, Xi H, Cueto R, et al. Inhibition of Caspase-1 Activation in Endothelial Cells Improves Angiogenesis: A NOVEL THERAPEUTIC POTENTIAL FOR ISCHEMIA. [Research Support, N.I.H., Extramural] J Biol Chem. 2015;290(28):17485–17494. doi: 10.1074/jbc.M115.641191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y-f, Li Rong-shan, Samuel Sonia B, Cueto Ramon, Li Xinyuan, Wang Hong, Yang Xiao-feng. Lysophospholipids and their G protein-coupled receptors in atherosclerosis. Frontiers in Bioscience (Landmark Edition) 2016;21(January 1):70–88. doi: 10.2741/4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X, Fang P, Li Y, Kuo Y-M, Andrews AJ, Nanayakkara G, et al. Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-induced Endothelial Cell Activation. Atherosclerosis, Thrombosis and Vascular Biology. 2016 doi: 10.1161/ATVBAHA.115.306964. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oda SK, Strauch P, Fujiwara Y, Al-Shami A, Oravecz T, Tigyi G, et al. Lysophosphatidic acid inhibits CD8 T cell activation and control of tumor progression. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Cancer Immunol Res. 2013;1(4):245–255. doi: 10.1158/2326-6066.CIR-13-0043-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu J, Oda SK, Shotts K, Donovan EE, Strauch P, Pujanauski LM, et al. Lysophosphatidic acid receptor 5 inhibits B cell antigen receptor signaling and antibody response. [Research Support, N.I.H., Extramural] J Immunol. 2014;193(1):85–95. doi: 10.4049/jimmunol.1300429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emo J, Meednu N, Chapman TJ, Rezaee F, Balys M, Randall T, et al. Lpa2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] J Immunol. 2012;188(8):3784–3790. doi: 10.4049/jimmunol.1102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, et al. Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells. [Research Support, N.I.H., Extramural] Biochem J. 2006;393(Pt 3):657–668. doi: 10.1042/BJ20050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun Q, Gao W, Loughran P, Shapiro R, Fan J, Billiar TR, et al. Caspase 1 activation is protective against hepatocyte cell death by up-regulating beclin 1 protein and mitochondrial autophagy in the setting of redox stress. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] J Biol Chem. 2013;288(22):15947–15958. doi: 10.1074/jbc.M112.426791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khan D, Ansar Ahmed S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. [Review] Front Immunol. 2015;6:635. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mai J, Nanayakkara G, Lopez-Pastrana J, Li X, Li YF, Wang X, et al. Interleukin-17A Promotes Aortic Endothelial Cell Activation via Transcriptionally and Post-translationally Activating p38 MAPK Pathway. J Biol Chem. 2016 doi: 10.1074/jbc.M115.690081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taleb S, Tedgui A, Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. [Research Support, Non-U.S. Gov’t Review] Arterioscler Thromb Vasc Biol. 2015;35(2):258–264. doi: 10.1161/ATVBAHA.114.303567. [DOI] [PubMed] [Google Scholar]