Abstract
Progesterone (P4), a steroid produced during estrous cycles and gestation for maintenance of pregnancy, also plays key roles in breast development to allow lactation post-parturition. Progestins (P4 and related steroids) are also implicated in breast cancer etiology. Hormone replacement therapy containing both estrogen and progestins increases breast cancer incidence while estrogen hormone therapy lowers breast cancer risk. P4 signaling via nuclear P4 receptors (PRs) has been extensively studied in breast cancer, however, progestin signaling via non-classical membrane bound progestin receptors (MPRs and PGRMC1) remains unclear. Moreover, P4 metabolites and synthetic progestins may bind membrane progestin receptors. We hypothesized that PR-negative breast epithelial cells express non-classical progestin receptors, which activate intracellular signaling pathways differently depending on nature of progestin. Therefore, our objectives for the current study were to determine expression of MPRs and PGRMC1 in two PR-negative non-tumorigenic breast epithelial cell lines, assess progestin-mediated signaling and biological functions. We determined five MPR isoforms and PGRMC1 were present in MCF10A cells and all progestin receptors but MPRβ in MCF12A cells. MCF10A and MCF12A cells were treated with P4, select P4 metabolites (5αP and 3αHP), medroxyprogesterone acetate (MPA), or a specific MPRAgonist (MPR-Ag) and phosphorylation of ERK, p38, JNK, and AKT was characterized following treatment. To our knowledge this is the first report of ERK and JNK activation in MCF10A and MCF12A cells with P4, P4 metabolites, MPA, and MPR-Ag. Activation of ERK and JNK in cells treated with MPR-Ag implicates MPRs may serve as the receptors responsible for their activation. In contrast, p38 activation varied with cell type and with progestin treatment. P4 and MPA promoted AKT phosphorylation in the MCF12A cell line only whereas no activation was observed in MCF10A cells. Interestingly, cellular proliferation increased in MCF10A cells treated with MPA or 5αP, while MPR-Ag tended to slightly decrease proliferation. Collectively, our data highlights the importance of investigating the effects of synthetic progestins in breast cancer biology. Our results add to the understanding that various progestins have on breast epithelial cells and underscores the importance of considering both membrane bound receptors and progestin type in breast cancer development.
Keywords: Medroxyprogesterone acetate, membrane progesterone receptors, breast cancer, 5α-dihydroprogesterone, 3α-dihydroprogesterone
1. Introduction
Progesterone (P4) is a steroid produced predominantly by the corpus luteum during estrous cycles and pregnancy, as well as the placenta during gestation. P4 elicits a number of different biological responses in females principally associated with establishment and maintenance of pregnancy, influencing several target tissues. In breasts, P4 promotes formation of lobular-alveolar structures during pregnancy, regulating normal breast physiology (Topper and Freeman, 1980). Specifically, P4 stimulates cell proliferation through regulation of cell cycle genes, synthesis of growth factors, and growth factor receptors; during lactation, P4 stimulates differentiation. Progestins (P4 and related steroids) are also implicated in breast cancer etiology. The Women's Health Initiative randomized controlled trials of combined estrogen and progestin hormone therapy (CHT) and unopposed estrogen hormone therapy (EHT) clearly demonstrates CHT use increases breast cancer risk while EHT lowers risk (Rossouw, Anderson, Prentice et al., 2002, Anderson, Limacher, Assaf et al., 2004). Schairer and colleagues demonstrated breast cancer risk increased 8% per year in women taking CHT compared to 1% per year in women receiving estrogens alone in a study conducted with 46,355 women (Schairer, Lubin, Troisi et al., 2000). Many similar, large cohort studies have confirmed that combined estrogen-progestin treatment increases breast cancer risk compared to estrogen alone (Li, Weiss, Stanford et al., 2000,Persson, Weiderpass, Bergkvist et al., 1999, Ross, Paganini-Hill, Wan et al., 2000). One of the largest studies conducted, the “Million Women Study” involving 1,084,110 women (50-64 years old) in the United Kingdom revealed an increased risk of incidence and fatal breast cancer for women taking CHT compared to women using EHT (Bliss and Gray, 2003). These studies strongly indicate progestins play crucial roles in breast cancer etiology and progression.
Conflicting reports suggest progestins either induce or inhibit cell death in breast cancer cells (Formby and Wiley, 1998, Kandouz, Lombet, Perrot et al., 1999, Moore, Spence, Kiningham et al., 2006, Ory, Lebeau, Levalois et al., 2001). Fundamentally, progestins must bind and activate specific receptors to cause biological responses. Historically, most actions attributed to progestins involve binding to the nuclear progestin receptors (PRs), known as (PR-A) or (PR-B); research focused on nuclear receptors has yielded critical biological information. However, substantial evidence of progestin-initiated events in PR-negative cells exists. Progestins also bind and activate distinct membrane bound proteins such as the membrane P4 receptors (MPRs), which have 7-8 transmembrane domains and P4 receptor membrane component-1 (PGRMC1), which possesses a single transmembrane domain (Peluso, Romak and Liu, 2008,Thomas, 2008,Zhu, Bond and Thomas, 2003). The MPRs are members of the progestin and adipoQ receptor (PAQR) family and consist of 5 different subtypes (MPRα, −β, −γ, −δ, −ε) (Pang, Dong and Thomas, 2013, Tang, Hu, Arterburn et al., 2005, Thomas, Pang, Dong et al., 2007). The existence of non-classical P4 receptors (MPRs and PGRMC1), distinctive in protein sequence, function, and localization from PRs, increases the complexity of progestins signaling in breast cells and demands further investigation into progestins and breast cancer biology.
A notable attribute of mammary tissue and breast cancer cells is their ability to convert P4 to biologically active metabolites 5α-dihydroprogesterone (5αP) and 3α-dihydroprogesterone (3αHP) that cause opposing actions on cell proliferation and apoptosis (Wiebe, 2006, Wiebe, Beausoleil, Zhang et al., 2010, Wiebe, Muzia, Hu et al., 2000) in cells lacking PR (Weiler and Wiebe, 2000). Cell proliferation is increased by 5αP and decreased by 3αHP, (Wiebe, 2006,Wiebe et al., 2000); while apoptosis is stimulated by 3αHP with decreased Bcl2/Bax ratio while this ratio increases in 5αP treated breast cells (Wiebe et al., 2010). Notably, the progestin metabolites initiate cell signaling via receptors located in the plasma membrane and not through classical PRs (Weiler and Wiebe, 2000) yet identification of membrane receptors for progestin metabolites has not been revealed. Many conclusions based upon progestins signaling in breast cancer cells were made without the knowledge that MPRs and PGRMC1 existed or that P4 metabolites are biologically active. The lack of consistent findings with respect to progestins in breast cancer biology may in part be attributed to (1) the presence of different progestin receptors and (2) the potential biotransformation of P4 to bioactive metabolites, yielding varied cellular responses.
Limited studies suggest normal breast epithelial cells increase proliferation in response to synthetic but not natural progestins. In vitro experiments investigating the role of P4 regulating breast epithelial cell proliferation have produced inconsistent results, as reviewed by Clarke and Sutherland (Clarke and Sutherland, 1990). P4 either decreases or has no effect on proliferation of normal breast epithelium explanted into nude mice (Laidlaw, Clarke, Howell et al., 1995, McManus and Welsch, 1984). Treatment of MCF10A cells, PR-negative benign breast epithelial cells, with the CHT P4-derivative medroxyprogesterone acetate (MPA) with growth factors increased proliferation whereas P4 had no effect (Kramer, Seeger, Kramer et al., 2006, Kramer, Seeger, Kramer et al., 2006). These data suggest differences in the type of progestins may account for the discrepancies noted in cell proliferation of benign human breast epithelial cells. Indeed, the pharmacological properties of progestins vary depending on their parent compound in which they were synthesized from, leading to disparities in biological activities (Schindler, Campagnoli, Druckmann et al., 2003, Stanczyk, 2003). MPA is often used in contraception and CHT. Intriguingly, increased risk of breast cancer in women taking CHT depends on the type of progestin used, as women taking natural P4 had no statistically significant increase in breast cancer risk whereas an increased risk was noted in women taking synthetic progestins like MPA (Fournier, Berrino and Clavel-Chapelon, 2008, Fournier, Berrino, Riboli et al., 2005).
Overall, the functions of progestins on breast cells appear to depend on progestins present and receptors expressed. We hypothesize that PR-negative non-tumorigenic breast epithelial cells express non-classical progestin receptors, which function differently depending on nature of progestin. Therefore, the objectives of this study were to examine MPRs and PGRMC1 in two PR-negative non-tumorigenic breast epithelial cell lines and assess signaling, and biological functions using P4, MPA, P4 metabolites and a MPR agonist (MPR-Ag). In this study we used MCF10A and MCF12A cell lines to analyze the effects of P4, MPA, 5αP, 3αHP, and MPR-Ag on ERK, p38, JNK and AKT signaling and cell proliferation. Treatment with progestins resulted in varying levels of kinase activation depending on cell line and progestin. Cell proliferation was stimulated by MPA and 5αP in MCF10A cells, while MCF12A cells did not differ in cell proliferation with any progestin treatment. These results highlight the importance of elucidating progestin function in benign breast epithelial cells. Because natural P4, its metabolites, and synthetic progestins can initiate different biological functions, these data may have relevant clinical implications as well as advancing understanding of progestin functions in breast cancer biology.
2. Materials Methods
2.1. Cell Culture
MCF12A and MCF10A human epithelial breast cells were purchased from ATCC. Cells were maintained in a humidified incubator at 37°C with 5% CO2 in DMEM: F12 media (Life Technologies, Grand Island, NY) containing 100 units/mL penicillin/streptomycin (Life Technologies), 5% horse serum (Life Technologies), 20 ng/mL of epidermal growth factor (Life Technologies), 0.1 μg /mL cholera toxin (Sigma Aldrich, Saint Louis, MO), 10 μg /mL insulin (Sigma Aldrich, Saint Louis, MO), and 0.5 μg /mL hydrocortisone (Sigma Aldrich). Experiments were conducted in cells that were under passage 14 for all studies. For protein phosphorylation experiments, 500,000 cells were plated per 60 mm tissue culture dish in complete medium. Twenty-four hours after plating, cells were changed to serum-free medium and incubated for another 24 h prior to treatment. Treatments included 100 nM P4, 100 nM MPA, 1000 nM 3αHP, 1000 nM 5αP, 100 nM MPR-Ag (Org OD-02-0) or vehicle for 5, 10, 30, 60, or 120 min. To confirm progestin-induced phosphorylation of ERK and p38, cells were treated for 2h in the presence of ERK inhibitor (50 μM U0126; Sigma) or p38 inhibitor (50 μM SB203580; Sigma) prior to progestins treatment. Protein was harvested as previously described (Coleson, Sanchez, Ashley et al., 2015), transferred into a fresh tube and stored in −20°C. All experiments were repeated a minimum of three times.
2.2. Western Blot
Protein concentrations were determined using the Pierce BCA Protein Assay (Thermo Scientific) per manufacturer's instructions. Fifteen or twenty micrograms of protein lysate was loaded into 10% acrylamide gels. Gels were subjected to SDS-PAGE and transferred onto ImmunBlot PVDF membrane (BioRad) using the Semi-Dry transfer method. Membranes were blocked for an hour at room temperature in 5% non-fat milk or 5% BSA (Santa Cruz Biotechnologies, Santa Cruz, CA) in Tris buffered saline with Tween (TBS-T), the primary antibody was applied in blocking solution and incubated overnight at 4°C with agitation. Antibodies were purchased from Santa Cruz Biotechnologies, Aviva Systems Biology (San Diego, CA), or Cell Signaling Technology (Beverly, MA). Primary antibodies against MPRα (Santa Cruz, sc-134815), MPRβ (Santa Cruz, sc-134814), MPRγ (AbCam, 79517) MPRδ (Aviva, ARP49900_P050), MPRε (Santa Cruz, sc-99578), PGRMC1 (Santa Cruz, sc-98680), pERK (Santa Cruz, sc-7383), ERK (Santa Cruz, sc-93), pAKT (Cell Signaling 9271L), AKT (Cell Signaling 9272S), pJNK (Cell Signaling 9251L), JNK (Cell Signaling 9252L), p-p38 (Cell Signaling 4511L), p38 (Cell Signaling 9212S) and appropriate secondary antibodies goat anti-rabbit (Santa Cruz, sc-2004) or goat anti-mouse (Santa Cruz, sc-2005) were used according to manufacturer's recommendations. Blots were washed in TBS-T then incubated with secondary antibody at room temperature for an hour. Membranes were washed with TBS-T, and then exposed to Clarity Western ECL Substrate (BioRad) per manufacturer's instructions. Chemiluminescent images were collected on a ChemiDoc XRS+ using Image Lab 5.2.1 software (BioRad).
2.3. Cell viability
MCF10A and MCF12A cells (2,000 cells/well) were plated onto 96-well white clear-bottom plates (Costar, Washington DC) in complete media. The following day, media containing charcoal-stripped serum replaced complete media and cells were incubated for 24 h. Next, cells were treated with 100 nM of P4, MPA, 5αP, 3αHP, MPR-Ag, or vehicle, added in serum-free media and allowed to incubate for an additional 72 h. The CellTiter Glo assay (Promega, Madison, WI) was used per manufacturer's instructions to determine cell viability.
2.4. Statistical Analysis
All experiments were repeated a minimum of three times. Graph-Pad Prism was used to compare treatments to control using a student's t test.
3. Results
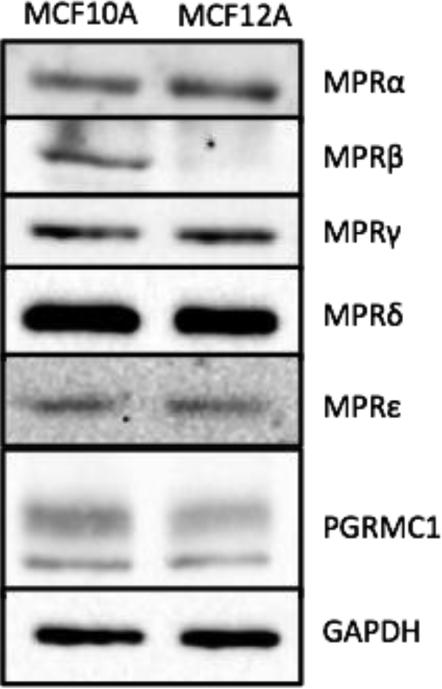
Protein levels of MPRα, MPRβ, MPRγ, MPRδ, MPRε, and PGRMC1 were determined in 15 μg total MCF10A and MCF12A cell lysate (Figure 1). Additional positive and negative controls were used to assure the fidelity of our immunoblotting (data not shown). MPRα, MPRγ, MPRδ, MPRε, and PGRMC1 were all similarly produced in MCF10A and MCF12A cells, whereas MPRβ was expressed only in MCF10A cell lines. Notably, MCF10A and MCF12A cell lines are triple negative and therefore do not express PR.
Figure 1. MPRβ is present in MCF10A but not MCF12A cell lines, and all other progestin receptors are similarly expressed in both.
Fifteen μg of protein was subject to western blot analysis, and probed for MPRα, MPRβ, MPRγ, MPRδ, MPRε, and PGRMC1 protein production.
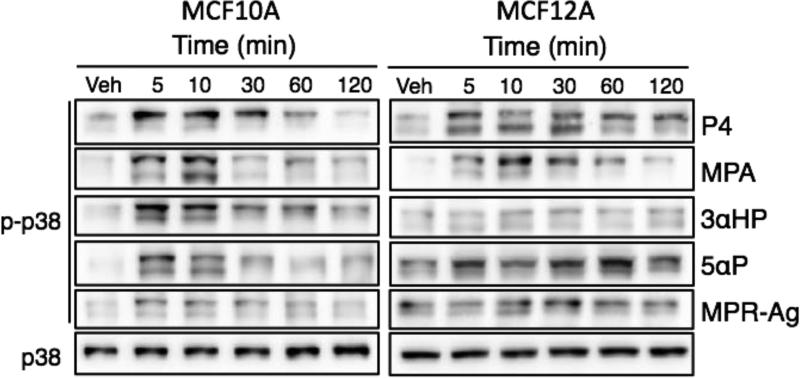
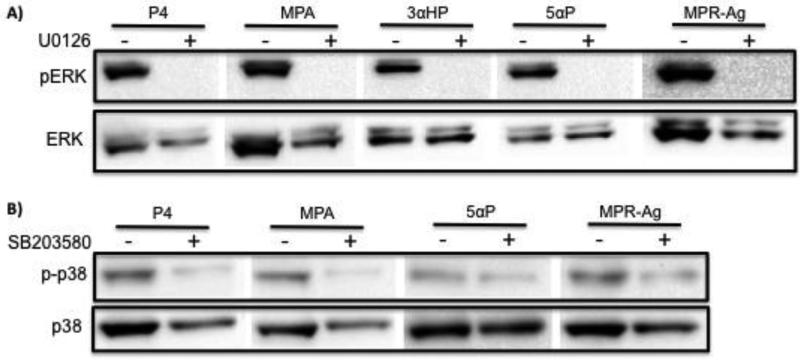
To determine if progestin compounds could activate key intracellular signaling pathways, we treated cells with 100 nM P4, 100 nM MPA, 1000 nM 3αHP, 1000 nM 5αP, 100 nM MPR-Agonist (MPR-Ag) or vehicle and protein was collected at 5, 10, 30, 60, or 120 min after treatment. For all experiments, images are representative of three separate experiments. Phosphorylation of ERK1/2 was assessed via western blot analysis and compared to total ERK1/2 (Figure 2). In both cell lines, all treatments promoted transient phosphorylation of ERK1/2. We next assessed phosphorylation of JNK using the treatments listed above. As with ERK1/2, phosphorylation of JNK was observed in all treatment conditions, with levels decreasing to baseline phosphorylation by 120 min following exposure (Figure 3). Phosphorylation of p38 was also evaluated. In contrast to ERK1/2 and JNK, p38 activation was observed following exposure to P4 and MPA in both cell lines, where as the metabolites 3αHP and 5αP induced phosphorylation only in MCF10A cell lines (Figure 4). Exposure to the agonist MPR-Ag elicited a very modest response in p38 phosphorylation in MCF10A but not MCF12A cell lines. Activation of AKT was ascertained, and P4 and MPA promoted AKT phosphorylation in the MCF12A cell lines only (Figure 5); no activation was observed in MCF10As with any treatment. To confirm activation of MAPK pathways were specific to treatment with progestins, inhibitors of ERK, JNK, or p38 were utilized prior to treatment. As shown in Figure 6, phosphorylation of ERK by P4, MPA, 3αHP, 5αP, or MPR-Ag was abrogated when MCF10A cells were pre-treated with the ERK inhibitor U0126. Phosphorylation of p38 by P4, MPA, 5αP, or MPR-Ag was also abolished when pre-treated with the p38 inhibitor SB203580 (Figure 6).
Figure 2. ERK1/2 is transiently phosphorylated in MCF10A and MCF12A cell lines following treatment with P4, MPA, 3αHP, 5αP, or MPR-Ag.
Cells were treated with 100 nM P4, 100 nM MPA, 1000 nM 3HαP, 1000 nM 5αP, 100 nM MPR-Agonist (MPR-Ag) or vehicle (Veh) and protein was collected at 5, 10, 30, 60, or 120 min after treatment. Phosphorylation of ERK1/2 was assessed via western blot analysis and compared to total ERK1/2. Images are representative of 3 experiments.
Figure 3. Phosphorylation of JNK in MCF10A and MCF12A cell lines after exposure to P4, MPA, 3αHP, 5αP, or MPR-Ag.
Cells were treated with 100 nM P4, 100 nM MPA, 1000 nM 3αHP, 1000 nM 5αP, 100 nM MPR-Agonist (MPR-Ag) or vehicle (Veh) and protein was collected at 5, 10, 30, 60, or 120 min after treatment. Phosphorylation of JNK was measured via western blot analysis and compared to total JNK. Images are representative of 3 experiments.
Figure 4. Transitory phosphorylation of p38 in MCF10A and MCF12A cell lines following treatment with P4, MPA, 3αHP, 5αP, or MPR-Ag.
Cells were treated with 100 nM P4, 100 nM MPA, 1000 nM 3αHP, 1000 nM 5αP, 100 nM MPR-Agonist (MPR-Ag) or vehicle (Veh) and protein was collected at 5, 10, 30, 60, or 120 min after treatment. Phospho-p38 was determined via western blot analysis and compared to total p38. Images are representative of 3 experiments.
Figure 5. AKT phosphorylation in MCF10A and MCF12A cell lines following treatment with P4, MPA, 3αHP, 5αP, or MPR-Ag.
Cells were treated with 100 nM P4, 100 nM MPA, 1000 nM 3αHP, 1000 nM 5αP, 100 nM MPR-Agonist (MPR-Ag) or vehicle (Veh) and protein was collected at 5, 10, 30, 60, or 120 min after treatment. AKT phosphorylation was established via western blot analysis and compared to total AKT. Images are representative of 3 experiments.
Figure 6. Activation of pERK and p-p38 by progestins is abrogated when pre-treated with inhibitors to pERK or p-p38.
Cells were pre-treated for 2h with ERK (U0126) or p38 (SB203580) inhibitors and then treated with 100 nM P4, 100 nM MPA, 1000 nM 3HαP, 1000 nM 5αP, or 100 nM MPR-Agonist (MPR-Ag) and protein was collected at 10 min after treatment. (A) Phosphorylation of ERK1/2 was assessed via western blot analysis and compared to total ERK1/2. (B) Phosphorylation of p38 was assessed via western blot analysis and compared to total p38.
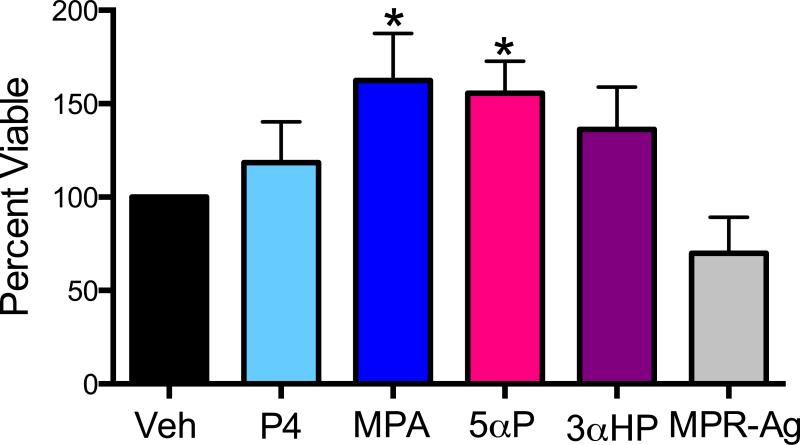
As we observed alterations in pathways known to modulate cell growth, we determined cell viability following treatment. No significant cell viability changes were observed in the MCF12A cells following 72 h exposure to any treatment. In contrast, proliferation of MCF10A cells increased significantly (P<0.05) following exposure to MPA and 5αP, but not P4 or 3αHP (Figure 7). Treatment with MPR-Ag tended (P=0.08) to decrease cell viability in the MCF10A cells.
Figure 7. Viability of MCF10A cells increased following treatment with either MPA or 5αP.
Cells were treated for 72 h with 100 nM of P4, MPA, 5αP, 3αHP, MPR-Ag or vehicle (Veh) control. Viability was determined as percent vehicle for each treatment. Treatment with MPA or 5αP stimulated cell growth (P<0.05), whereas MPR-Ag treatment tended (P=0.08) to impede proliferation. For all experiments n ≥ 3.
4. Discussion
Progestins are clearly involved in breast cancer biology, especially in light of the WHI and million women's studies demonstrating increased breast cancer risk in woman receiving CHT compared to EHT alone. In fact, women receiving EHT alone demonstrated a reduced risk (Rossouw et al., 2002, Anderson et al., 2004). Fundamentally, progestins induce biological response(s) by interacting with a receptor. Extensive study of the nuclear PR has provided a wealth of information. The recent discovery of other progestin receptors confounds interpretation of progestin intracellular signaling, as functions previously attributed to nuclear receptors may be partially or wholly due to non-nuclear receptor activation. Studying MPRs and PGRMC1 in breast cancer biology is needed to advance understanding of progestin functions. Progestins, including natural P4, biologically active metabolites, and synthetics such as MPA vary in their pharmacology and induce differing biological outcomes. Moreover, progestins are metabolized to bioactive metabolites that have distinctive biological responses further complicating the understanding of progestins in breast cancer biology. Several studies have reported progestin effects in breast cancer cells, but it is important to determine effects of progestins in benign breast epithelial cells. Presently, we lack clear understanding of how non-classical progestin receptors function in nontumorigenic breast cells as most research has been conducted in breast cancer cells. The objectives of the current study were to determine how natural P4, select progestin metabolites, MPA and the MPR-agonist alter signaling and cell proliferation in nontumorigenic breast cells and the involvement of non-classical progestin receptors.
While strong data exist implicating MPA in the etiology of breast cancer, much of the underlying biology remains poorly understood and the work completed largely focused on nuclear PR. Progestin-induced functions have been reported in the nontumorigenic breast cells, MCF10A and MCF12A, despite lacking PR (Kramer et al., 2006, Kramer et al., 2006, Behera, Dai, Garde et al., 2009). We determined that MCF10A and MCF12A cells express non-classical progestin receptors, which may account for progestin functions in breast epithelial cells (Figure 1). To our knowledge, this is the first report of MPRs and PGRMC1 production in MCF10A and MCF12A cells. MCF10A cells are positive for all membrane-bound progestin receptors assayed for, whereas the MCF12A express all but MPRβ (Figure 1). MPRα, the first cloned MPR (Zhu et al., 2003,Ashley, Clay, Farmerie et al., 2006,Zhu, Rice, Pang et al., 2003) is expressed in breast cancer cells such as MB468 and SKBR3 (Dressing, Alyea, Pang et al., 2012, Zuo, Li and You, 2010) and based on our results, the nontumorigenic MCF10A and MCF12A cell lines. We observed benign breast epithelial cells also express MPRβ and MPRγ, yet expression differs between the two lines with MCF12A being MPRβ null. More recently, two new isoforms MPRδ and MPRε, were discovered, and it was suggested only neural tissues produced these isoforms (Pang et al., 2013). We are the first to demonstrate MCF10A and MCF12A cells produce MPRδ and MPRε, and MPRδ appears to be the most highly expressed MPR in breast epithelial cells. The function of MPRδ and MPRε are unknown. PGRMC1 is produced in both breast epithelial cell lines, in agreement other reports indicating PGRMC1 and MPRs are often expressed in similar mammalian tissues with overlapping distributions (Thomas, 2008, Tang et al., 2005, Cahill, 2007). PGRMC1 may enhance cell surface expression and functions of MPRα in breast cancer cells (Thomas, Pang and Dong, 2014), but if PGRMC1 performs similar functions in breast epithelial cells as well as with other MPRs is not known. Collectively, these data demonstrate nontumorigenic breast cells express a number of different progestin receptors, which likely are responsible for progestin-induced functions in cells lacking PR.
Interestingly, phosphorylation of ERK occurred in both MCF10A and MCF12A nontumorigenic cells following treatment with all progestins tested (Figure 2). To our knowledge, this is the first report of progestins activating the ERK pathway in PR-negative benign breast epithelial cells. MCF10A cells are a spontaneously immortalized, non-malignant cell line obtained from a patient with fibrocystic breast disease (Dawson, Wolman, Tait et al., 1996) and are considered “normal” because they do not form tumors in immunodeficient mice. The MCF10A cells represent a unique model to study breast cancer progression and many groups have generated a series of cell lines originating from MCF10A cells that form tumors in immunodeficient mice as recently reviewed (So, Lee, Kramata et al., 2012). Using transformed MCF10A lines that caused tumors in immunodeficient mice, it was noted that pERK was highly phosphorylated in the lines that formed tumors quickly and it was suggested that ERK activation might be critical for developing malignant breast cancer (Lee, Liu, Goodman et al., 2006). We observed pERK activation with natural P4 and the synthetic progestin MPA, as well as the metabolites 3αHP and 5αP and the MPR-Ag. Thus, (1) the ERK pathway is stimulated by varying progestins and (2) MPRs are involved in pERK stimulation. It is important to note that MCF10A and MCF12A cells are PR null, yet we established MPRs and PGRMC1 are produced and may be responsible for ERK activation. The fact that MPR-Ag was able to induce pERK in both MCF10A and MCF12A cells indicate the MPRs may serve as the receptors for ERK activation. Comparable experiments using the MPR-Ag demonstrated activation of ERK in breast cancer, fish granulosa, and neuronal cells (Pang et al., 2013, Dressing et al., 2012, Dressing, Pang, Dong et al., 2010) similar to our results in nontumorigenic breast cells. Moreover, phosphorylation of ERK with all progestins tested was abolished when cells were pre-treated with the ERK inhibitor (Figure 6) further implicating MPRs and/or PGMRC1 as receptors responsible for ERK activation. Progestin-induced activation of ERK has not been previously reported in either MCF10A or MCF12A cell lines. Taken together, our findings provide a foundation to explore the potential involvement of progestins and ERK activation in breast cancer development.
Similar to ERK stimulation, all progestins induced JNK phosphorylation in both MCF10A and MCF12A cells (Figure 2). Progestin-induced JNK stimulation has not been reported in MCF10A or MCF12A cells to our knowledge. The effects of P4 or related progestins on JNK activation are not well understood. Expression of MPRα, −β, −γ was demonstrated in a variety of ovarian cancer cell lines lacking PR and in agreement with our studies, P4 treatment resulted in JNK activation (Charles, Thomas and Lange, 2010). As the ovarian cancer cells tested lack PR, the authors suggest P4 is functioning through MPRs. Treatment of human umbilical vein endothelial cells with supraphysiological levels of P4 was able to induce JNK phosphorylation, but JNK activation was not evident at physiological concentrations (Powazniak, Kempfer, de la Paz Dominguez et al., 2009). Studies evaluating P4 effects on epidermal growth factor (EGF) signaling in breast cancer cells revealed treatment of T47D-YB cells with R5020, a P4 agonist, for 48 h prior to EGF treatment resulted in JNK phosphorylation (Lange, Richer, Shen et al., 1998). However it should be noted that R5020 itself did not induce pJNK, but rather sensitized the breast cancer cells to the effects of EGF. Notably, unlike PR, MPRs do not bind R5020 (Kelder, Azevedo, Pang et al., 2010). Results from the current study indicate progestins induce pJNK activation directly in breast epithelial cells. As MCF10A and MCF12A are PR null and the MPR-Ag activates JNK, we suggest the progestin-induced effects are mediated through MPRs. The consequence of pJNK upon progestin stimulation requires further investigation, and we are currently exploring downstream functions.
Activation of p38 following progestin treatment, varied by cell type, with MCF10A cells demonstrating strong induction of phosphorylated p38 with P4, MPA, 3αHP and 5αP treatment and modest p38 activation with MPR-Ag administration (Figure 4). When cells were pre-treated with p38 inhibitor, activation by P4, MPA, 5αP, or MPR-Ag was diminished (Figure 6) suggesting membrane progestin receptors are responsible for the progestin-induced activation of p38. P4 and MPA only induced modest p38 activation in MCF12A cells and other progestins elicited no effect. It is intriguing that the various progestins tested would induce differences in intracellular signaling in two different breast epithelial cell lines. Currently, the reason is unclear, though only MPRβ was not detected in MCF12A cells, which may account for differences noted. Treatment of PR-negative ovarian cancer cells expressing MPRα, MPRβ, MPRγ with P4 results in p38 activation (Charles et al., 2010). Similar to our results, p38 activation occurs in human myometrial cells treated with P4 (Karteris, Zervou, Pang et al., 2006). The myometrial cells expressed MPRα and MPRβ. Though myometrial and breast cells differ, use of non-tumorigenic cells provides an advantageous model to study progestin actions. Our data provide novel information on how progestins can elicit differing p38 activation in benign breast epithelial cells.
Recent studies provide evidence progestins can regulate AKT activation, potentially through MPRα (Zuo et al., 2010, Tan and Thomas, 2014, Vares, Sai, Wang et al., 2015), yet most results are based on indirect evidence. In Atlantic Croaker sperm, stimulation with endogenous progestin results in AKT activation, a function believed to be induced via MPRs activation (Tan and Thomas, 2014). MPR-Ag treatment induced sperm hypermotility, though MPR-Ag induction of AKT activation was not reported. In our current study we did not observe AKT phosphorylation with any of the progestins tested, including MPR-Ag in MCF10A cells (Figure 5). This is contrary to a recent report by Vares and colleagues, in which the authors report AKT stimulation in MCF10A cells with P4 and MPR-Ag (Vares et al., 2015). However, AKT activity was monitored by inversely correlating to FOXO transcriptional activity whereas direct evidence of pAKT activation was not shown. We did not measure FOXO transcriptional activity in the current study. In MCF12A cells we observed AKT stimulation with P4 and MPA. In support, MPA induced pAKT activation in PR positive T47D cells (Saitoh, Ohmichi, Takahashi et al., 2005), but it is not known if T47D cells express MPRs. Similarly, MPA induces pAKT in C4HD cells, a progestin-dependent murine tumor line (Salatino, Beguelin, Peters et al., 2006,Salatino, Schillaci, Proietti et al., 2004), yet if MPRs or PGRMC1 are expressed in these cells is unknown.
Our results indicate MPA and 5αP increase cell proliferation in MCF10A cells, yet natural P4 does not (Figure 7), further highlighting the divergent effects progestins can elicit. In support, Kramer and colleagues reported MPA increased cell proliferation in MCF10A cells yet P4 did not alter proliferation, and authors suggested this was mediated through MAPK pathways (Kramer et al., 2006, Kramer et al., 2006). We observed ERK activation with all progestins tested and activation was abrogated upon pre-treating cells with ERK inhibitor (Figure 6), yet only MPA and 5αP increased cell proliferation. As natural P4 differs in chemical structure compared to its metabolites, synthetic MPA, and MPR-Ag, its possible the binding and subsequent activation of downstream pathways also differ. Whether other signaling cascades such as NFkB, Jak/Stat are activated by P4 metabolites, MPA or MPR-Ag is unclear, but an area we are investigating. Our data revealing 5αP increasing cell proliferation in MCF10A cells confirms several other reports in breast epithelial as well as cancer cells (Wiebe, 2006, Wiebe et al., 2010, Wiebe et al., 2000, Pawlak, Zhang and Wiebe, 2005). Treatment with MPR-Ag on the other hand tended (p=0.08) to decrease cell proliferation. Because MCF10A cells lack PR but express all membrane bound receptors assayed for, it is difficult to identify which receptor(s) is key in the MPA and 5αP induced effects. Studies to decipher these questions are currently underway in our laboratory.
5. Conclusions
As noted, MCF10A and MCF12A cells are triple negative suggesting progestins effects occur through other receptors and not PR. Our data indicate two benign breast cell lines express several MPRs and PGRMC1 and respond differently to various types of progestins. The fact that MPA increased cell proliferation in MCF10A cells yet P4 did not underscores the importance of investigating the roles synthetic progestins play in breast cancer biology compared to natural P4. Concurrently, our data demonstrate membrane bound progestin receptors are crucial to progestin signaling and activate MAPK and AKT pathways. Yet, because we also established MPRδ and MPRε are synthesized in both cell lines, this highlights the complexity of progestin signaling in breast epithelial cells and underscores the necessity for future research to delineate receptor specific pathway activation. The various family members of the MAPK family and AKT play several multifaceted roles in cell signaling such as cell growth, differentiation, and apoptosis, yet the cellular decision involves a balance among competing pathways (Denhardt, 1996). We suggest the overall biological results of progestin signaling is a summation of signaling induced by binding of various progestins to receptors and cross-talk between receptors. Our results add to the understanding that various progestins have on breast epithelial cells and emphasizes the importance of considering both membrane bound receptors and progestin type.
Highlights.
Five MPRs and PGRMC1 are synthesized in PR null breast epithelial cells.
Natural and synthetic progestins activate MAPK and AKT pathways in PR null cells.
The MPR agonist activated MAPK pathways in MCF10A and MCF12A cells.
Cellular proliferation increased in MCF10A cells treated with MPA or 5αP.
Membrane bound receptors and progestin types are central to breast cell functions.
Acknowledgements
The authors would like to thank Gail Silver, Nicole Sanchez, and Adriana Alire for assistance with conducting experiments. This research was funded by the Partnership for the Advancement of Cancer Research: NMSU/FHCRC, supported in part by NCI grants U54 CA132383 (NMSU) and U54 CA132381 (FHCRC); (awarded to A.K.A; awarded to R.L.A.), Cowboys for Cancer Research Grant (awarded to R.L.A.), the Howard Hughes Medical Institute Science Education Award #52006932 to NMSU, the Agriculture and Food Research Initiative Competitive Grants #2013-38422-20957 from the USDA National Institute of Food and Agriculture, and Interdisciplinary Research Grant provided by the New Mexico State University's Vice President for Research's Office (awarded to A.K.A. and R.L.A.)
List of abbreviations
- P4
progesterone
- MPR
membrane progesterone receptor
- MPR-Ag
membrane progesterone receptor agonist
- PR
nuclear progesterone receptor
- MPA
medroxyprogesterone acetate
- 5αP
5α-dihydroprogesterone
- 3αHP
3α-dihydroprogesterone
- PGRMC1
progesterone receptor membrane component-1
- CHT
Combined hormone therapy (estrogen + progestin)
- EHT
Unopposed estrogen hormone therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare no competing interests.
References
- 1.Topper YJ, Freeman CS. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980;60:1049–106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA : the journal of the American Medical Association. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA : the journal of the American Medical Association. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 4.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA : the journal of the American Medical Association. 2000;283:485–91. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 5.Li CI, Weiss NS, Stanford JL, Daling JR. Hormone replacement therapy in relation to risk of lobular and ductal breast carcinoma in middle-aged women. Cancer. 2000;88:2570–7. doi: 10.1002/1097-0142(20000601)88:11<2570::aid-cncr20>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Persson I, Weiderpass E, Bergkvist L, Bergstrom R, Schairer C. Risks of breast and endometrial cancer after estrogen and estrogen-progestin replacement. Cancer causes & control : CCC. 1999;10:253–60. doi: 10.1023/a:1008909128110. [DOI] [PubMed] [Google Scholar]
- 7.Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. Journal of the National Cancer Institute. 2000;92:328–32. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 8.Bliss JM, Gray R. Breast cancer and hormone-replacement therapy: the Million Women Study. Lancet. 2003;362:1328–9. doi: 10.1016/S0140-6736(03)14591-6. author reply 1330-1. [DOI] [PubMed] [Google Scholar]
- 9.Formby B, Wiley TS. Progesterone inhibits growth and induces apoptosis in breast cancer cells: inverse effects on Bcl-2 and p53. Annals of clinical and laboratory science. 1998;28:360–9. [PubMed] [Google Scholar]
- 10.Kandouz M, Lombet A, Perrot JY, Jacob D, Carvajal S, Kazem A, Rostene W, Therwath A, Gompel A. Proapoptotic effects of antiestrogens, progestins and androgen in breast cancer cells. The Journal of steroid biochemistry and molecular biology. 1999;69:463–71. doi: 10.1016/s0960-0760(99)00069-2. [DOI] [PubMed] [Google Scholar]
- 11.Moore MR, Spence JB, Kiningham KK, Dillon JL. Progestin inhibition of cell death in human breast cancer cell lines. The Journal of steroid biochemistry and molecular biology. 2006;98:218–27. doi: 10.1016/j.jsbmb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Ory K, Lebeau J, Levalois C, Bishay K, Fouchet P, Allemand I, Therwath A, Chevillard S. Apoptosis inhibition mediated by medroxyprogesterone acetate treatment of breast cancer cell lines. Breast cancer research and treatment. 2001;68:187–98. doi: 10.1023/a:1012288510743. [DOI] [PubMed] [Google Scholar]
- 13.Peluso JJ, Romak J, Liu X. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone's antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology. 2008;149:534–43. doi: 10.1210/en.2007-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Frontiers in neuroendocrinology. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2237–42. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang Y, Dong J, Thomas P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors delta and {epsilon} (mPRdelta and mPR{epsilon}) and mPRdelta involvement in neurosteroid inhibition of apoptosis. Endocrinology. 2013;154:283–95. doi: 10.1210/en.2012-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–80. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 18.Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148:705–18. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 19.Wiebe JP. Progesterone metabolites in breast cancer. Endocrine-related cancer. 2006;13:717–38. doi: 10.1677/erc.1.01010. [DOI] [PubMed] [Google Scholar]
- 20.Wiebe JP, Beausoleil M, Zhang G, Cialacu V. Opposing actions of the progesterone metabolites, 5alpha-dihydroprogesterone (5alphaP) and 3alpha-dihydroprogesterone (3alphaHP) on mitosis, apoptosis, and expression of Bcl-2, Bax and p21 in human breast cell lines. The Journal of steroid biochemistry and molecular biology. 2010;118:125–32. doi: 10.1016/j.jsbmb.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Wiebe JP, Muzia D, Hu J, Szwajcer D, Hill SA, Seachrist JL. The 4-pregnene and 5alpha-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer research. 2000;60:936–43. [PubMed] [Google Scholar]
- 22.Weiler PJ, Wiebe JP. Plasma membrane receptors for the cancer-regulating progesterone metabolites, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-4-pregnen-20-one in MCF-7 breast cancer cells. Biochemical and biophysical research communications. 2000;272:731–7. doi: 10.1006/bbrc.2000.2847. [DOI] [PubMed] [Google Scholar]
- 23.Clarke CL, Sutherland RL. Progestin regulation of cellular proliferation. Endocr Rev. 1990;11:266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- 24.Laidlaw IJ, Clarke RB, Howell A, Owen AW, Potten CS, Anderson E. The proliferation of normal human breast tissue implanted into athymic nude mice is stimulated by estrogen but not progesterone. Endocrinology. 1995;136:164–71. doi: 10.1210/endo.136.1.7828527. [DOI] [PubMed] [Google Scholar]
- 25.McManus MJ, Welsch CW. The effect of estrogen, progesterone, thyroxine, and human placental lactogen on DNA synthesis of human breast ductal epithelium maintained in athymic nude mice. Cancer. 1984;54:1920–7. doi: 10.1002/1097-0142(19841101)54:9<1920::aid-cncr2820540924>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Kramer EA, Seeger H, Kramer B, Wallwiener D, Mueck AO. The effect of progesterone, testosterone and synthetic progestogens on growth factor- and estradiol-treated human cancerous and benign breast cells. Eur J Obstet Gynecol Reprod Biol. 2006;129:77–83. doi: 10.1016/j.ejogrb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Kramer EA, Seeger H, Kramer B, Wallwiener D, Mueck AO. Characterization of the stimulatory effect of medroxyprogesterone acetate and chlormadinone acetate on growth factor treated normal human breast epithelial cells. J Steroid Biochem Mol Biol. 2006;98:174–8. doi: 10.1016/j.jsbmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 46 Suppl. 2003;1:S7–S16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–90. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast cancer research and treatment. 2008;107:103–11. doi: 10.1007/s10549-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. International journal of cancer. Journal international du cancer. 2005;114:448–54. doi: 10.1002/ijc.20710. [DOI] [PubMed] [Google Scholar]
- 32.Coleson MP, Sanchez NS, Ashley AK, Ross TT, Ashley RL. Human chorionic gonadotropin increases serum progesterone, number of corpora lutea and angiogenic factors in pregnant sheep. Reproduction. 2015;150:43–52. doi: 10.1530/REP-14-0632. [DOI] [PubMed] [Google Scholar]
- 33.Behera MA, Dai Q, Garde R, Saner C, Jungheim E, Price TM. Progesterone stimulates mitochondrial activity with subsequent inhibition of apoptosis in MCF-10A benign breast epithelial cells. Am J Physiol Endocrinol Metab. 2009;297:E1089–96. doi: 10.1152/ajpendo.00209.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology. 2006;147:4151–9. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2231–6. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dressing GE, Alyea R, Pang Y, Thomas P. Membrane progesterone receptors (mPRs) mediate progestin induced antimorbidity in breast cancer cells and are expressed in human breast tumors. Hormones & cancer. 2012;3:101–12. doi: 10.1007/s12672-012-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuo L, Li W, You S. Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells via a membrane progesterone receptor mediated pathway. Breast Cancer Res. 2010;12:R34. doi: 10.1186/bcr2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Thomas P, Pang Y, Dong J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor alpha (mPRalpha) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology. 2014;155:1107–19. doi: 10.1210/en.2013-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR. MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol. 1996;148:313–9. [PMC free article] [PubMed] [Google Scholar]
- 41.So JY, Lee HJ, Kramata P, Minden A, Suh N. Differential Expression of Key Signaling Proteins in MCF10 Cell Lines, a Human Breast Cancer Progression Model. Mol Cell Pharmacol. 2012;4:31–40. [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, Notterman D, Reiss M, Suh N. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol. 2006;72:332–43. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 43.Dressing GE, Pang Y, Dong J, Thomas P. Progestin signaling through mPRalpha in Atlantic croaker granulosa/theca cell cocultures and its involvement in progestin inhibition of apoptosis. Endocrinology. 2010;151:5916–26. doi: 10.1210/en.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charles NJ, Thomas P, Lange CA. Expression of membrane progesterone receptors (mPR/PAQR) in ovarian cancer cells: implications for progesterone-induced signaling events. Horm Cancer. 2010;1:167–76. doi: 10.1007/s12672-010-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powazniak Y, Kempfer AC, de la Paz Dominguez M, Farias C, Keller L, Calderazzo JC, Lazzari MA. Effect of estradiol, progesterone and testosterone on apoptosis- and proliferation-induced MAPK signaling in human umbilical vein endothelial cells. Mol Med Rep. 2009;2:441–7. doi: 10.3892/mmr_00000119. [DOI] [PubMed] [Google Scholar]
- 46.Lange CA, Richer JK, Shen T, Horwitz KB. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways, J Biol Chem. 1998;273:31308–16. doi: 10.1074/jbc.273.47.31308. [DOI] [PubMed] [Google Scholar]
- 47.Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P. Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists. Steroids. 2010;75:314–22. doi: 10.1016/j.steroids.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–34. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 49.Tan W, Thomas P. Activation of the Pi3k/Akt pathway and modulation of phosphodiesterase activity via membrane progestin receptor-alpha (mPRalpha) regulate progestin-initiated sperm hypermotility in Atlantic croaker. Biol Reprod. 2014;90:105. doi: 10.1095/biolreprod.113.112896. [DOI] [PubMed] [Google Scholar]
- 50.Vares G, Sai S, Wang B, Fujimori A, Nenoi M, Nakajima T. Progesterone generates cancer stem cells through membrane progesterone receptor-triggered signaling in basal-like human mammary cells. Cancer Lett. 2015;362:167–73. doi: 10.1016/j.canlet.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Saitoh M, Ohmichi M, Takahashi K, Kawagoe J, Ohta T, Doshida M, Takahashi T, Igarashi H, Mori-Abe A, Du B, Tsutsumi S, Kurachi H. Medroxyprogesterone acetate induces cell proliferation through up-regulation of cyclin D1 expression via phosphatidylinositol 3-kinase/Akt/nuclear factor-kappaB cascade in human breast cancer cells. Endocrinology. 2005;146:4917–25. doi: 10.1210/en.2004-1535. [DOI] [PubMed] [Google Scholar]
- 52.Salatino M, Beguelin W, Peters MG, Carnevale R, Proietti CJ, Galigniana MD, Vedoy CG, Schillaci R, Charreau EH, Sogayar MC, Elizalde PV. Progestin-induced caveolin-1 expression mediates breast cancer cell proliferation. Oncogene. 2006;25:7723–39. doi: 10.1038/sj.onc.1209757. [DOI] [PubMed] [Google Scholar]
- 53.Salatino M, Schillaci R, Proietti CJ, Carnevale R, Frahm I, Molinolo AA, Iribarren A, Charreau EH, Elizalde PV. Inhibition of in vivo breast cancer growth by antisense oligodeoxynucleotides to type I insulin-like growth factor receptor mRNA involves inactivation of ErbBs, PI-3K/Akt and p42/p44 MAPK signaling pathways but not modulation of progesterone receptor activity. Oncogene. 2004;23:5161–74. doi: 10.1038/sj.onc.1207659. [DOI] [PubMed] [Google Scholar]
- 54.Pawlak KJ, Zhang G, Wiebe JP. Membrane 5alpha-pregnane-3,20-dione (5alphaP) receptors in MCF-7 and MCF-10A breast cancer cells are up-regulated by estradiol and 5alphaP and down-regulated by the progesterone metabolites, 3alpha-dihydroprogesterone and 20alpha-dihydroprogesterone, with associated changes in cell proliferation and detachment. The Journal of steroid biochemistry and molecular biology. 2005;97:278–88. doi: 10.1016/j.jsbmb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Denhardt DT. Signal-transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochem J. 1996;318(Pt 3):729–47. doi: 10.1042/bj3180729. [DOI] [PMC free article] [PubMed] [Google Scholar]