Abstract
Cutaneous T-cell lymphomas (CTCL) are skin malignancies including mycosis fungoides (MF) and CD30+ lymphoproliferative disorders (LPD). In early disease, CTCL can be difficult to diagnose, especially in MF for which there is no reliable diagnostic marker. MF/CTCL have increased expression of thymocyte selection-associated HMG box protein (TOX). Although TOX has been proposed to be a diagnostic marker for MF, further validation studies are needed. Moreover, it is unclear what drives TOX expression or its role in MF/CTCL. We hypothesize evaluation of TOX levels across a spectrum of CTCL, including MF precursor (large plaque parapsoriasis, LPP), will help elucidate the implications of altered TOX expression. TOX staining was performed in MF, CD30+ LPD, LPP as well as benign inflammatory dermatoses (BID) and normal skin (NS). Positive TOX expression was identified in 73.6% of MF cases and in 31.6% of BID/NS. TOX had a positive predictive value (PPV) for MF of 86.7% and a negative predictive value (NPV) of 48.1%. TOX expression in MF was detected more commonly in Black patients (p=0.015) and less commonly in transformed MF (p=0.045). LPP had positive TOX staining in 70.0%. In CTCL cells, GATA3 knockdown decreased TOX mRNA and protein expression. TOX expression also decreased in the presence of CTCL therapeutics. Our data indicate that TOX is useful as a diagnostic marker in MF. Moreover, TOX expression was evident in LPP, indicating it may have a previously unappreciated role in the development of MF. Finally, our data suggest that GATA3 regulates TOX, revealing insight into TOX regulation.
Introduction
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma (CTCL) and is the most common form of all primary cutaneous lymphomas.1,2 Clinically, MF lesions progress from patches to plaques and tumors. In the patch stage, diagnosis can be difficult as the differential diagnosis is broad and includes chronic eczema, parapsoriasis, psoriasis, and pityriasis lichenoides chronica. Furthermore, histopathologic examination can be non-specific as the patches may not demonstrate the characteristic findings of epidermotropic, cerebriform lymphocytes, and Pautrier’s microabscesses.2,3 Instead MF patches may simply reveal an infiltrate of well-defined, small lymphocytes, and this can difficult to distinguish from an immunologic reactive process. Currently, there are no clinically utilized positive tumor markers that differentiate malignant T cells from the reactive T cells present in both early MF infiltrates and other benign conditions. Given the non-specific nature of these early MF lesions as well as empiric corticosteroid treatment blunting the histologic appearance, an accurate diagnosis is often delayed for years. While “negative” markers of MF exist, i.e. the loss of CD7, CD2, CD23, and CD26, determination of a positive tumor marker will be instrumental in the timely and appropriate diagnosis of MF, particularly in the early stages of disease.4
A study by Zhang et al5 demonstrated a potential positive MF tumor marker, thymocyte selection-associated HMG box protein (TOX), which showed significant upregulation in early MF as determined by real time quantitative-PCR. TOX expression, as evaluated by IHC and immunofluorescence, was strongly positive in MF lesions, but absent or minimally positive in the chronic dermatitis controls as well as a cohort of CD30+ lymphoproliferative disorders (LPD), another form of CTCL.5,6 TOX encodes a nuclear protein that is able to modify chromatin structure and therefore functions as a transcription factor.7,8 TOX is involved in lymphocyte maturation, specifically the transition from double positive CD4+CD8+ to mature CD4+ T cells. TOX expression is tightly regulated and normally dissipates after the lymphocytes exit the thymus.9 Cyclin-dependent kinase inhibitors p27 and p57 were recently determined to be increased in the setting of TOX knockdown, whereas overexpression of TOX led to AKT phosphorylation and CTCL proliferation.10,11 Another protein integral to T-cell development, GATA binding protein 3 (GATA3), which can be upregulated in lymphoma,12 has proposed binding sites on the TOX promoter.13 Additionally, a recent report demonstrated that both TOX and GATA3 were overexpressed in Sézary cells and identified that inhibition of TOX led to increased expression of the tumor suppressor runt-related transcription factor 3 (RUNX3).14 Further mechanisms of TOX expression and mechanism of action remain to be elucidated.
The purpose of this study was to determine patient or disease characteristics associated with TOX expression in CTCL and to further validate its use as a diagnostic marker in MF. The incidence of MF is higher among Black individuals, which are known to have a worse prognosis.15 Furthermore, young African American women with early onset MF have been reported to frequently have poor prognoses and more aggressive therapy should be considered for optimal outcomes.16 Given its potential utility as a tumor marker, we sought to determine if expression of TOX varied among patient cohorts and may be associated with these epidemiological observations. Although Zhang et al5 showed high specificity of TOX in differentiating MF from chronic dermatosis, it remains unclear whether TOX is a reliable marker in differentiating MF from other precursor lesions and CTCLs. Lastly we sought to further understand the role and regulation of TOX and GATA3 in MF.
Methods
Tissue
We identified patient samples with MF (n=53), CD30+ LPD [including lymphomatous papulosis (LyP; n=9)], cutaneous and systemic anapestic large cell lymphoma [c-ALCL (n=8) and s-ALCL (n=3)], and large plaque parapsoriasis (LPP; n=10) retrospectively through chart review and diagnosis based on clinical findings and histologic evaluation. All diagnoses were established by a single dermatologist and were based upon clinical and pathologic findings (J.A.Z). The negative control cohort was composed of normal discarded skin (NS; n=2), and skin biopsies from patients with benign inflammatory dermatoses (BID) (e.g. psoriasis, spongiotic dermatitis; n=17), also determined retrospectively. 4–6 mm biopsies were paraffin-embedded and sectioned. This study was approved by the Vanderbilt Institutional Review Board.
Immunohistochemistry
Slides were placed on the Leica Bond Max IHC stainer (Leica Microsystems, Inc., Buffalo Grove, IL). Slides were deparaffinized and heat induced antigen retrieval was performed using the Epitope Retrieval 2 solution for 20 minutes. Slides were incubated with anti-TOX (Sigma-Aldrich, Inc., St. Louis, MO. Catalog # HPA018322) at a 1:500 dilution for 1 hour. TOX staining intensity was scored 0–3 by a single dermatopathologist (J.P.Z).
Statistical analysis
Statistical analysis was performed with SPSS 20.0 (IBM, New York City, NY).
Cell Culture
The HH (CRL-2105) and Hut-78 (TIB-161) CTCL cell lines were cultured as described by the American Type Culture Collection (Manassas, VA).
RNA isolation and quantitative real-time PCR (qRT-PCR)
RNA was isolated from skin biopsies, PBMC, and cell lines using Trizol (Invitrogen, Grand Island, NY) according to the manufacturer’s protocol. Sequences for β-actin, GATA3, and TOX-specific qRT-PCR primer pairs were obtained from the Primer Bank (Harvard Medical School) and synthesized by Eurofins MWG Operon (Huntsville, AL). cDNA was generated and qRT-PCR was performed with SybrGreen (SABiosciences, Valencia, CA) in triplicate, as previously reported.17 The data are expressed in 2−ΔCt using β-actin as an internal reference.
Transfection
HH cells (2×106/sample) were prepared utilizing the Nucleofector Kit V (Lonza, Basel, Switzerland) and transfected with the X-005 program in the Nucleofector II instrument (Lonza). Hut-78 cells (2×106/sample) were prepared with the Nucleofector Kit R (Lonza) and transfected with the V-001 program. The TOX and GATA3 siRNA oligomer and control siRNA (200–600 nM) were utilized for knockdown (Origene).
Western Blotting
Cell pellets were lysed with RIPA buffer (50 mM Tris, 150 mM sodium chloride, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate) at 24 hours after transfection (see above) with GATA3 siRNA or control, and total cellular proteins western blotted, as previously described.17 Antibodies specific for GATA3 (#5852, Cell Signaling), TOX (HPA018322, Sigma-Aldrich), and α-tubulin (T6074, Sigma-Aldrich) were utilized.
Results
TOX is useful as a diagnostic marker for MF
We evaluated TOX expression in 53 skin biopsy samples of MF. Of these, 39 stained positively (73.6%) and 14 stained negatively (26.4%, Figure 1A and 1B). The positive predictive value (PPV) for any TOX expression (Grade 1–3) was 86.7% and the negative predictive value (NPV) was 48.1%. Strong TOX expression (Grade 2–3) was detected in 33 of 53 MF cases (62.3%, Figure 1B) and had a PPV of 97.1% and a NPV of 47.4%. Conversely, positive TOX expression was observed in 6 of 19 (31.6%) BID/NS samples, and strongly positive TOX expression was detected in only 1 of 19 (5.3%) BID samples.
Figure 1.
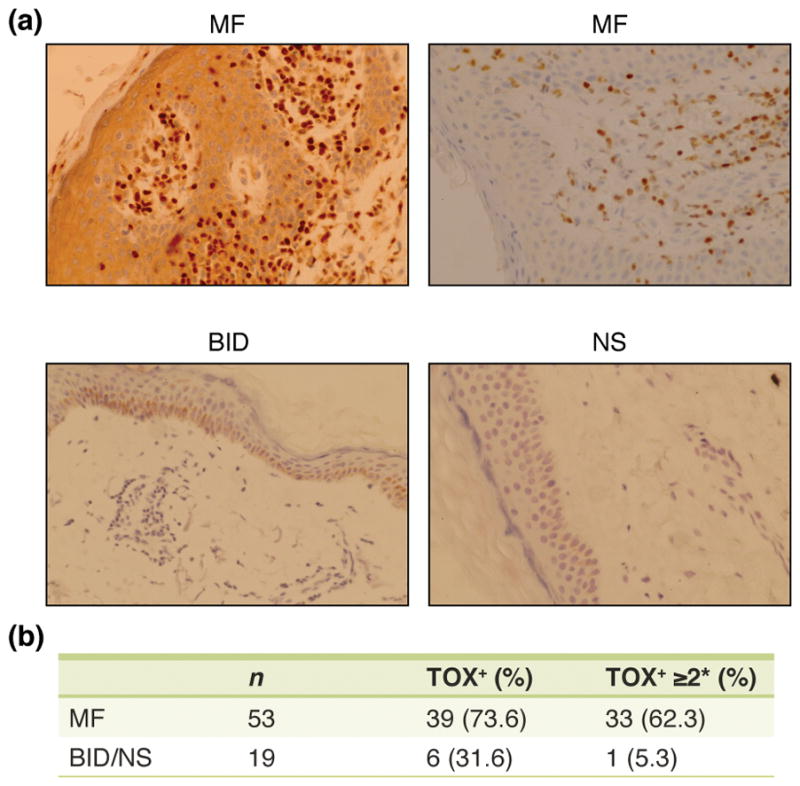
TOX staining was significantly increased in MF skin samples. A) Representative immunohistochemical staining with the TOX antibody on formalin-fixed, paraffin embedded tissues samples (MF = mycosis fungoides; BID = benign inflammatory dermatosis; NS = normal skin). B) Percentage of MF and BID/NS staining positively with TOX and staining strongly positive (* score ≥ 2) with TOX.
TOX expression across patient and disease characteristics
We determined that TOX expression was associated with ethnicity. 12 of 12 (100%) Black patients had positive TOX expression, whereas 27 of 41 (65.9%) Caucasian patients had TOX expression (p=0.015, Fisher’s Exact Test, Table 1). There was no difference in early (stage Ia-IIa) or advanced (stage IIb-IV) disease, or transformed disease between the Black and White patients, but there was a significant increase in Stage IIb disease in the White patients (29.2% versus 0%, p=0.047, Fisher’s Exact Test, Table 2).
Table 1.
| n | TOX+ (%) | TOX+ >2* (%) | |
|---|---|---|---|
| MF | 53 | 39 (73.6) | 33 (62.3) |
| Stage Ia | 19 | 16 (84.2) | 15 (78.9) |
| Stage Ib | 11 | 8 (72.7) | 4 (36.4) |
| Stage IIa | 2 | 1 (50) | 1 (50) |
| Stage IIb | 12 | 7 (58.3) | 6 (50) |
| Stage III | 1 | 1 (100) | 1 (100) |
| Stage IV | 7 | 5 (71.4) | 5 (71.4) |
| Female | 31 | 24 (77.4) | 19 (61.3) |
| Male | 22 | 15 (68.2) | 14 (63.6) |
| Black | 12 | 12 (100) | 10 (83.3) |
| White | 41 | 27 (65.9) | 23 (56.1) |
| Transformed | 12 | 6 (50) | 6 (50) |
Table 2.
| Black n (%) | White n (%) | |
|---|---|---|
| Stage Ia | 5 (42) | 14 (34) |
| Stage Ib | 4 (29) | 7 (17) |
| Stage IIa | 1 (8) | 1 (2) |
| Stage IIb | 0 (0) | 12 (29)* |
| Stage III | 0 (0) | 1 (2) |
| Stage IV | 2 (17) | 5 (12) |
| Transformed | 1 (8) | 11 (27) |
There was no statistically significant relationship between TOX expression and age (p=0.069, Mann Whitney Test) or sex (p=0.452, Table 1). Regarding stage of MF at time of biopsy versus TOX expression, there was decreased strong TOX expression in Stage Ib vs Stage Ia (p=0.027). Stage Ib had reduced strong TOX expression versus all MF (p=0.052, Table 1). There were no other significant differences between clinical stages of disease. We also determined that MF with large cell transformation was less commonly associated with TOX expression (p=0.045, Fisher’s Exact Test). There were 12 biopsy samples of transformed MF. Of these, 6 had positive TOX staining (50.0%) and 6 had negative TOX staining (50%, Table 1), whereas 80% of non-transformed MF cases had positive TOX expression (33 of 41).
TOX is variably expressed across subtypes of CTCL
We stained a variety of CD30+ lymphoproliferative CTCL samples with TOX antibody, including primary cutaneous anaplastic large cell lymphoma (c-ALCL) (n=8), systemic anaplastic large cell lymphoma (s-ALCL) (n=3), and lymphomatoid papulosis (LyP) (n=9). We determined that CD30+ LPD have reduced TOX expression as compared to MF. 50% of CD30+ LPD vs 73.6% of MF showed any TOX staining (p=0.053), and 5% of CD30+ LPD vs. 62.3% showed strongly positive TOX staining (p<0.001, Table 3). Additionally, LyP has less TOX expression compared to MF (22.2% vs 73.6% for any TOX staining and 0.0% vs 62.3% for strong TOX staining; both p<0.01, Table 3).
Table 3.
| n | TOX+ (%) | TOX+ >2* (%) | |
|---|---|---|---|
| MF | 53 | 39 (73.6) | 33 (62.3) |
| CD30+LPD | 20 | 10 (50) | 1 (5) |
| LyP | 9 | 2 (22.2) | 0 (0) |
| C-ALCL | 8 | 6 (75) | 1 (12.5) |
| S-ALCL | 3 | 2 (66.7) | 0 (0) |
| LPP | 10 | 7 (70.0) | 5 (50.0) |
TOX is expressed in Large Plaque Parapsoriasis
After staining 10 LPP biopsy samples with anti-TOX antibody, we determined that LPP, which is often considered a precursor to MF, showed similar staining patterns to MF. There was positive staining in 70.0% of biopsy samples and strongly positive expression in 50.0% of LPP. This compares to 73.6% of MF biopsies showing positive TOX staining and 62.3% with strongly positive TOX expression.
TOX and GATA3 mRNA inhibition are associated with an alteration in their protein expression
Both TOX and GATA3 are known to be important for T-cell development and there are proposed GATA3 binding sites on the TOX promoter.(9, 12, 13) TOX and GATA3 siRNA knocked down TOX and GATA expression, respectively 24 hours post-transfection in Hut-78 cells (Figure 2A). GATA3 siRNA reduced both GATA3 and TOX mRNA expression. TOX siRNA did not affect GATA3 mRNA expression (Figure 2B). Furthermore, Western blot of the CTCL Hut-78 cell line transfected with GATA3 siRNA demonstrated that TOX protein expression was decreased in the setting of GATA3 knockdown (Figure 2C).
Figure 2.
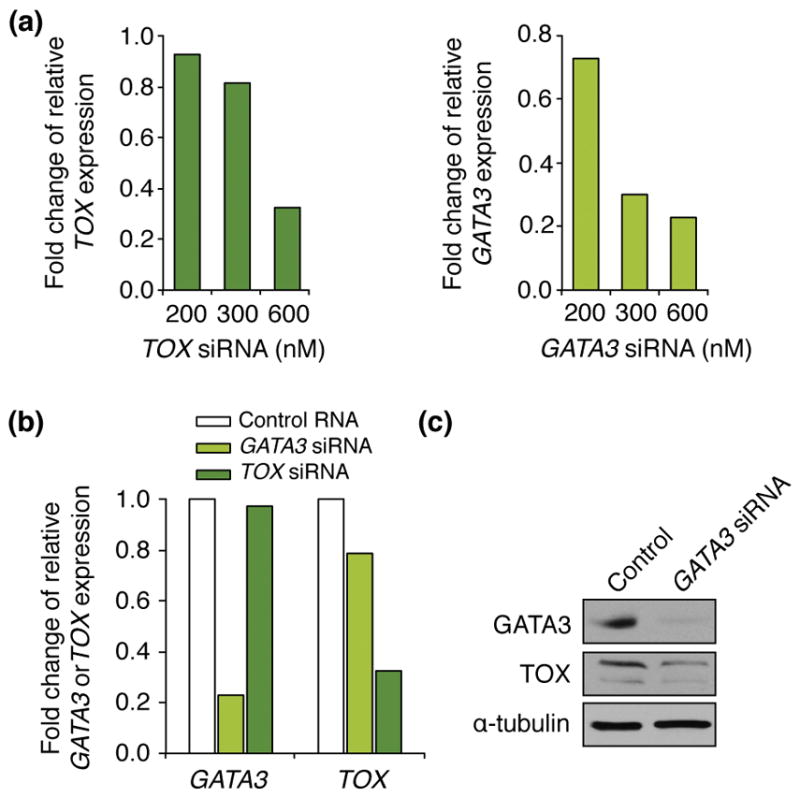
GATA3 reduced TOX expression. A) Hut-78 cells were transfected with the indicated concentrations of TOX or GATA3 siRNA, and 24 hours post-transfection TOX or GATA3 mRNA levels were evaluated by qRT-PCR. Values are relative to β-actin levels. B) Hut-78 cells were transfected with 600nM of TOX or GATA3 siRNA or control RNA and were harvested 24 hours later. TOX or GATA3 mRNA levels were evaluated by qRT-PCR and are relative to β-actin levels. C) Hut-78 cells transfected with GATA3 siRNA or control RNA (CTRL) were harvested 24 hours post-transfection. Western blots of whole cell protein lysates were performed for the proteins indicated.
TOX mRNA expression is reduced by CTCL therapies
The retinoid, bexarotene, and the histone deactylase inhibitor, depsipeptide, are commonly used to treat CTCL. It has been demonstrated that decreased TOX expression is associated with increased CTCL cell apoptosis.(10) To evaluate the role of TOX in CTCL response to therapeutics, Hut-78 cells were treated with bexarotene and demonstrated decreased levels of TOX mRNA expression at 24 and 48 hours (*p<0.001, Figure 3). The CTCL HH cells were treated with depsipeptide which also led to reduced TOX mRNA expression at 24 (**p=0.05) and 48 hours (#p=0.04, Figure 3). Notably, non-lethal doses of the medications were used. Thus, drugs that inhibit the proliferation of CTCL cells are associated with decreased TOX levels.
Figure 3.
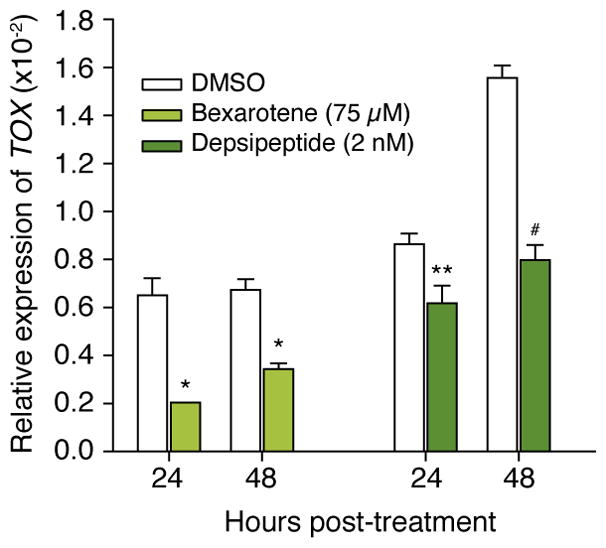
Hut-78 cells were treated with bexarotene and TOX mRNA expression was evaluated 24 and 48 hours (*p<0.001, t-test) later. HH cells were treated with depsipeptide and TOX mRNA expression was determined 24 hours (**p=0.05, t-test) and 48 hours (#p=0.04, t-test) later.
Discussion
The diagnosis of MF is often delayed due to the non-specific clinical presentation of early cutaneous involvement, sometimes requiring up to 10 years to properly diagnose the entity.18 Even the pathognomonic epidermotropism is not present in 4% of cases of MF and atypical lymphocytes are present in only 9% of cases.19 Despite previously described “negative” markers, determination of a positive tumor marker would be instrumental in the timely and appropriate diagnosis of MF, particularly in the early stages of disease. One such potential marker, thymocyte selection-associated HMG box protein (TOX), has been demonstrated to be significantly upregulated in early MF as determined by real time quantitative-PCR.5 Zhang et al also utilized IHC and immunofluorescence to demonstrate that early MF lesions were strongly positive for TOX expression, but chronic dermatitis controls showed no or minimally positive expression.
In this study we performed staining for TOX on biopsies of MF, control benign inflammatory dermatoses, normal skin, CD30+ LPD, and LPP. We determined that TOX is a useful marker for MF, especially with strongly positive expression (Grade 2–3), resulting in a PPV of 97.1%. Furthermore, the potential MF precursor LPP and MF have similar TOX staining profiles. These data indicate the TOX may play a role in MF pathogenesis. Indeed, the recent study by Yu, et al revealed that overexpression of TOX led to increased CTCL cell proliferation.11 Further research should address whether those cases of LPP with strong TOX expression progress at a higher rate to MF, which would allow clinicians to utilize TOX as a prognostic indicator. Interestingly, MF with large cell transformation was less commonly associated with TOX and only a small percentage of CD30+ LPD biopsies showed strong TOX expression, similar to the findings of Morimura, et al.6 It is interesting to note that LyP had less TOX staining as compared to c-ALCL and s-ALCL (22.2% vs 72.7%), but more work will be needed to definitively evaluate the role of TOX in distinguishing LyP from ALCL. Further studies are also needed to evaluate whether transition from non-transformed MF to large cell transformation leads to loss of TOX expression.
Given the tendency of MF to be more aggressive in the Black population, we sought to determine whether there was differential TOX expression among patient cohorts. Interestingly, 100% of the biopsies from Black patients had positive TOX expression, as compared to 65.9% of the biopsies of White patients. Although we did not have a significant difference in early stage, advanced stage, or transformed MF, we did have an increase in Stage IIb in the White as compared to the Black patients. It is possible that this discrepancy may be contributing to the differential TOX expression, but the smaller sample size within sub groups makes it difficult to draw appropriate conclusions. Additionally, a recent study demonstrated that TOX expression may be associated with worse outcome.20 TOX may play a role in the development and progression of MF in Black patients, but the molecular mechanism responsible for this relationship remains unclear. There was no association between TOX expression and patient age or sex. It will be important for larger studies to further assess the relationship between ethnicity and TOX expression.
Given its role in T cell development, overexpression in CTCL, and the presence of binding sites in the TOX promoter, GATA3 was studied to further elucidate the relationship between these two proteins and shed light its potential role in MF pathogenesis.12,13 Results from our experiments demonstrated that TOX mRNA and protein expression was decreased following GATA3 knockdown, indicating GATA3 regulates TOX expression. Thus, GATA3, commonly expressed in CTCL, may be able to drive the increased expression of TOX that is seen in MF/CTCL.
We present data that further demonstrates the utility of TOX as a tumor marker, especially in the setting of strongly positive staining patterns to differentiate MF from benign inflammatory disorders and CD30+ LPD. Furthermore, the similar staining patterns between LPP and MF support the theory that LPP is a related clinical entity to MF, and TOX expression may be responsible in the pathogenesis of MF. The significantly increased expression of TOX among Black MF patients compared to White MF patients, suggests that these disease processes may vary and therefore, targeted therapies may need to differ in the future. Furthermore, agents that inhibit CTCL proliferation led to decreased TOX levels, suggesting a link between TOX and cell growth. Finally, our data indicate that GATA3 has a role in the regulation of TOX expression providing insight, which may become important for targeted therapies as additional information is discovered about the role of TOX in MF pathogenesis.
Acknowledgments
Funding Sources: Dermatology Foundation Physician-Scientist Career Development Award (LYM), the NIH supported Vanderbilt Clinical Oncology Research Career Development Program (K12CA090625), NIH (UL1TR000445), Hematology Helping Hands Fund (CME and LYM), R01CA148950 (CME), and the Vanderbilt Department of Medicine/Dermatology, the McDaniel Gift Fund, the Vanderbilt Cutaneous Lymphoma Fund, and an anonymous donor.
We thank the members of the Eischen lab for helpful discussions. This work was funded in part by the Dermatology Foundation Physician-Scientist Career Development Award (LYM), the NIH supported Vanderbilt Clinical Oncology Research Career Development Program (K12CA090625), NIH (UL1TR000445), Hematology Helping Hands Fund (CME and LYM), R01CA148950 (CME), and the Vanderbilt Department of Medicine/Dermatology, the McDaniel Gift Fund, the Vanderbilt Cutaneous Lymphoma Fund, and an anonymous donor.
Footnotes
Conflict of interest: None to disclose
References
- 1.Weinstock MA, Horm JW. Mycosis fungoides in the United States. Increasing incidence and descriptive epidemiology. JAMA. 1988;260(1):42–6. [PubMed] [Google Scholar]
- 2.Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Critical reviews in oncology/hematology. 2008;65(2):172–82. doi: 10.1016/j.critrevonc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139(7):857–66. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 4.Lansigan F, Choi J, Foss FM. Cutaneous T-cell lymphoma. Hematology/oncology clinics of North America. 2008;22(5):979–96. x. doi: 10.1016/j.hoc.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang Y, Yu R, Huang Y, Su M, Xiao C, et al. Molecular markers of early-stage mycosis fungoides. The Journal of investigative dermatology. 2012;132(6):1698–706. doi: 10.1038/jid.2012.13. [DOI] [PubMed] [Google Scholar]
- 6.Morimura S, Sugaya M, Suga H, Miyagaki T, Ohmatsu H, Fujita H, et al. TOX expression in different subtypes of cutaneous lymphoma. Archives of dermatological research. 2014;306(9):843–9. doi: 10.1007/s00403-014-1501-7. [DOI] [PubMed] [Google Scholar]
- 7.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Molecular and cellular biology. 1999;19(8):5237–46. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas JO, Travers AA. HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends in biochemical sciences. 2001;26(3):167–74. doi: 10.1016/s0968-0004(01)01801-1. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson B, Chen JY, Han P, Rufner KM, Goularte OD, Kaye J. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nature immunology. 2002;3(3):272–80. doi: 10.1038/ni767. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Su MW, Jiang X, Zhou Y. Evidence of an oncogenic role of aberrant TOX activation in cutaneous T-cell lymphoma. Blood. 2015;125(9):1435–43. doi: 10.1182/blood-2014-05-571778. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Luo Y, Liu J, Liu Y, Sun Q. TOX acts an oncological role in mycosis fungoides. PloS one. 2015;10(3):e0117479. doi: 10.1371/journal.pone.0117479. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Gibson HM, Mishra A, Chan DV, Hake TS, Porcu P, Wong HK. Impaired proteasome function activates GATA3 in T cells and upregulates CTLA-4: relevance for Sezary syndrome. The Journal of investigative dermatology. 2013;133(1):249–57. doi: 10.1038/jid.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome research. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dulmage BO, Akilov O, Vu JR, Falo LD, Geskin LJ. Dysregulation of the TOX-RUNX3 pathway in cutaneous T-cell lymphoma. Oncotarget. 2015 Nov 6; doi: 10.18632/oncotarget.5742. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstock MA, Gardstein B. Twenty-year trends in the reported incidence of mycosis fungoides and associated mortality. American journal of public health. 1999;89(8):1240–4. doi: 10.2105/ajph.89.8.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun G, Berthelot C, Li Y, Glass DA, 2nd, George D, Pandya A, et al. Poor prognosis in non-Caucasian patients with early-onset mycosis fungoides. Journal of the American Academy of Dermatology. 2009;60(2):231–5. doi: 10.1016/j.jaad.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 17.McGirt LY, Adams CM, Baerenwald DA, Zwerner JP, Zic JA, Eischen CM. miR-223 regulates cell growth and targets proto-oncogenes in mycosis fungoides/cutaneous T-cell lymphoma. The Journal of investigative dermatology. 2014;134(4):1101–7. doi: 10.1038/jid.2013.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arai E, Katayama I, Ishihara K. Mycosis fungoides and Sezary syndrome in Japan. Clinicopathologic study of 107 autopsy cases. Pathology, research and practice. 1991;187(4):451–7. doi: 10.1016/S0344-0338(11)80006-3. [DOI] [PubMed] [Google Scholar]
- 19.Massone C, Kodama K, Kerl H, Cerroni L. Histopathologic features of early (patch) lesions of mycosis fungoides: a morphologic study on 745 biopsy specimens from 427 patients. The American journal of surgical pathology. 2005;29(4):550–60. doi: 10.1097/01.pas.0000153121.57515.c6. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Litvinov IV, Wang Y, Su MW, Tu P, Jiang X, et al. Thymocyte selection-associated high mobility group box gene (TOX) is aberrantly over-expressed in mycosis fungoides and correlates with poor prognosis. Oncotarget. 2014;5(12):4418–25. doi: 10.18632/oncotarget.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]


