Abstract
Inflammatory bowel disease (IBD) is an inflammatory disorder of the intestine that affects an estimated 329 per 100,000 people in the United States and is increasing in incidence within a number of cultures worldwide. Likely due to its incompletely understood pathophysiology and etiology, standard treatments for IBD are only efficacious in subsets of patients and often do not induce lasting remission. As a result, novel therapies are needed. The success of anti-tumor necrosis factor-α treatment in a subset of IBD patients demonstrated that therapy targeting a single cytokine could be efficacious in IBD, and clinical trials investigating the blockade of a variety of cytokines have commenced. IL-27 is a relatively recently discovered type I cytokine with established roles in infectious disease, autoimmunity, and cancer in a variety of organs. IL-27 was identified as a candidate gene for IBD, and a number of studies in mouse models of IBD have demonstrated that IL-27 therapy is protective. However, in contrast to these investigations, genetic deletion of the IL-27 receptor has been shown to be protective in some mouse models of IBD. The purpose of this review is to highlight recent literature investigating the role of IL-27 in IBD, and to discuss possible explanations for the sometimes conflicting results of these studies. Evidence supporting IL-27 therapy as a treatment for IBD will also be discussed.
Keywords: Crohn’s disease, IBD, IL-27, ulcerative colitis
Introduction
Inflammatory bowel disease (IBD) refers to a collection of idiopathic inflammatory disorders of the intestine, the most common of which are Crohn’s disease and ulcerative colitis. IBD is thought to result from an abnormal inflammatory response to commensal organisms and other antigens normally confined to the intestinal lumen due to compromise of the mucosal epithelial barrier and subsequent inappropriate exposure of resident intestinal immune cells to luminal antigens. A single cause for IBD has not been identified. Development of the disease is likely multifactorial, as a variety of etiological factors, including hygiene status, previous gastrointestinal infection, genetics, diet, and various other lifestyle factors, have been implicated in its pathogenesis.1 Geographically, the highest incidence of IBD has been reported in northern Europe and North America, where an estimated 329 per 100,000 people in the United States suffer from IBD.1,2
Treatment for IBD typically involves some form of immunosuppression, necessitating a balance between achieving remission and managing potential adverse effects. Additionally, likely due to the complex pathophysiology and multifactorial etiology of IBD, rates for remission induction and maintenance with many of the current standard treatments for IBD remain modest and are often variable.3,4 As a result, investigation into novel therapeutic targets is sorely needed. The success of anti-tumor necrosis factor-α treatment in a subset of IBD patients demonstrated that therapy targeting a single cytokine could be efficacious in IBD, and antibodies against a variety of cytokines, including interleukin (IL)-13, IL-18, and IL-21, have entered clinical trials.3
IL-27 is a relatively recently discovered type I cytokine with established roles in infectious disease, autoimmunity, and cancer in a variety of organs, including the central nervous system, lung, skin, and gastrointestinal tract.5,6 However, it has not been definitively determined whether IL-27 ameliorates or promotes intestinal inflammation, as seemingly contradictory roles for IL-27 have been reported in murine models of IBD.6 Several excellent reviews have recently discussed the biology of IL-27.6–9 The purpose of this review is to highlight recent literature investigating the role of IL-27 in IBD, and to discuss possible explanations for the conflicting results of these studies. Evidence supporting IL-27 therapy as a potential treatment for IBD will also be discussed.
IL-27 and its receptor
IL-27 is a heterodimeric type I cytokine in the IL-12 cytokine family. It is composed of two subunits: Epstein-Barr virus-induced gene 3 (EBI3) and p28, so named due to its predicted molar mass by SDS-PAGE.5,8 IL-27 is predominantly produced by myeloid cells, such as macrophages and dendritic cells, but can also be expressed by epithelial cells, plasma cells, and endothelial cells.6 A variety of signals can induce IL-27 expression, including CD40 ligation, type I and II interferon signaling, and Toll-like receptor signaling.6 IL-27 expression induced by Toll-like receptor signaling has been shown to require interferon-alpha in human macrophages and interferon regulatory factor 3 in murine dendritic cells.10,11
IL-27 signals through a heterodimeric receptor consisting of IL-27Rα, previously termed TCCR or WSX-1, and gp130.6,12 Coexpression of both receptor subunits has been detected in T and B lymphocytes, mast cells, natural killer cells, natural killer T cells, monocytes, neutrophils, dendritic cells, intestinal epithelial cells, and endothelial cells.12–15 Interestingly, expression of the receptor differs among T cell subsets. IL-27Rα is highly expressed by regulatory, effector, and memory T cells, and increases with T cell activation. In contrast, naïve T cells express low levels of IL-27Rα. Natural killer and natural killer T cells express high levels of IL-27Rα, but activation of these cells results in decreased expression of this receptor subunit.15 Ligation of the IL-27 receptor induces signaling through the Jak/STAT pathway, resulting in the phosphorylation of STAT1, 3, 5, or 6.7,16 In addition to the Jak/STAT pathway, the activated IL-27 receptor has been reported to induce p38MAPK, ERK, and Akt signaling. IL-27 signaling also induces SOCS3, a suppressor of IL-27 signaling that directly binds to and inhibits the gp130 receptor subunit and associated Janus kinases and later facilitates their degradation.7
IL-27 activities in the immune system
IL-27: the T cell police
IL-27 boasts a diverse repertoire of functions in both the adaptive and innate branches of the immune system; however, the most well-known and thoroughly investigated functions of IL-27 relate to its ability to regulate the adaptive immune system by modulating T cell function. When first discovered, IL-27 was reported to induce expansion of naïve murine and human CD4+ T cells and to act synergistically with IL-12 to enhance interferon-γ production by both naïve T cells and NK cells.5 IL-27 blocks Th2 cytokine expression and the differentiation of Th2 cells by simultaneously increasing expression of the transcription factor T-bet while suppressing GATA-3 expression.17 Additionally, IL-27 can prevent the expression of both IL-17A and IL-17F by suppressing RORγt, a transcription factor necessary for Th17 cell differentiation.18 IL-27 also inhibits the production of Th17-polarizing cytokines by human dendritic cells. To demonstrate the functional consequences of this inhibition, Murugaiyan et al. showed that human memory CD4+ T cells cocultured with dendritic cells stimulated with both toll-like receptor (TLR) agonists and IL-27 produced significantly less IL-17 than those exposed to dendritic cells activated by TLR ligands alone.19
Additional noteworthy effects of IL-27 on T cells include its ability to prevent Fas-mediated activation-induced cell death20 and to induce IL-10 production.6 The ability of IL-27 to stimulate the expression of IL-10 by T cells is perhaps one of its most extensively characterized immunoregulatory functions, having been demonstrated in models of parasitic, viral, and autoimmune disease.6 IL-27 induces IL-10 and T-cell immunoglobulin and mucin domain-3 (Tim-3) in both CD4+ and CD8+ T cells by expression of the transcription factor nuclear factor, interleukin-3 regulated (NFIL3),21 and also induces the production of IL-10 by Th1 and Th2 cells in a STAT1 and STAT3 dependent manner.22 Even under Th17-polarizing conditions, generally thought to drive primarily proinflammatory cytokine production, the addition of IL-27 elicits significant increases in IL-10 production by human CD4+ T cells.19 Multiple studies have also demonstrated a critical role for IL-27 in the development of IL-10-secreting T regulatory type 1 (Tr1) cells in both humans and mice.19,23–27 Interferon-γ and IL-27 have both been shown to promote T-bet+ CXCR3+ regulatory T cells specialized to modulate Th1 responses; however, the development of this regulatory response in the gut-associated lymphoid tissue required IL-27.16 While IL-27 could play a role in the regulation of T regulatory cells by suppressing IL-2, the significance of this potential negative regulatory function has not been characterized in wild type animals.28
IL-27’s role in innate immunity
In addition to its impressive repertoire of regulatory functions in T cell biology, IL-27 has been demonstrated to have a number of both modulatory and complementary roles in innate immunity. After identifying the receptor responsible for IL-27 signaling, Pflanz and colleagues showed that IL-27 induced proinflammatory gene expression in both human mast cells and monocytes, but that this increase in expression occurred in notably fewer genes and at a later time point in monocytes.12 In contrast, in murine Trichuris muris infection and a murine model of mast cell-dependent passive cutaneous anaphylaxis, mice with genetic deletion of IL-27Rα had enhanced mast cell responses, suggesting that IL-27 may also negatively regulate mast cell activities under certain circumstances.29 IL-27 also acts on dendritic cells. IL-27 can induce expression of the regulatory molecule CD39 on dendritic cells,30 and when applied to monocyte-derived dendritic cells during their differentiation, improved antigen processing and subsequent T cell stimulation.31
A critical role for IL-27 in the suppression of neutrophil and monocyte function has been identified that demonstrated strikingly different outcomes in models of infectious disease based on the timing of IL-27 signaling.32,33 Mice with genetic deletion of IL-27Rα had more severe pulmonary histopathology and greater mortality following influenza infection than wild type mice, suggesting that IL-27 signaling is protective in influenza infection. In support of this finding, IL-27 treatment of mice in the late stage of influenza infection decreased both clinical signs of disease and lung histopathology scores without reducing clearance of the virus.32 This improvement in pathology corresponded to significantly lower pulmonary infiltrates of both neutrophils and monocytes, which were proposed by the authors to be due to IL-27-mediated suppression of the chemokines CXCL1, CCL5, and CCL4. However, in sharp contrast to this beneficial function of IL-27, the reduction of neutrophil and monocyte infiltrates due to administration of IL-27 early in influenza infection prevented viral clearance and worsened the clinical signs of disease. Accordingly, the highest endogenous levels of the IL-27 p28 subunit in wild type mice were measured in the late phase of influenza infection as viral load was declining,32 suggesting that endogenous release of IL-27 can be precisely timed for limiting collateral damage from the inflammatory response while not hindering pathogen clearance.
However, data from a model of septic murine peritonitis provide more evidence for the sometimes deleterious effects of the direct suppression of innate responses by IL-27.33 Mice lacking the EBI3 subunit of IL-27 had reduced mortality associated with greater bacterial clearance during septic peritonitis induced by cecal ligation and puncture. The authors further demonstrated that these mice lacking functional IL-27 had more robust intraperitoneal granulocytic infiltrates following cecal puncture, and that IL-27 directly inhibited the bacterial lipopolysaccharide-induced production of reactive oxygen intermediates by both granulocytes and macrophages/monocytes from wild type mice.33 Wild type mice were shown to upregulate the expression of IL-27 within 6 hours of cecal puncture,33 further suggesting that, when applied early in infection, the immunosuppressive effects of IL-27 may result in reduced pathogen clearance and more severe disease. Complementary to these findings in mice, IL-27 has also been shown to suppress human neutrophil adhesion and bacterial lipopolysaccharide-induced reactive oxygen species production in vitro.14
Collectively, these data inform critical stipulations for the use of IL-27 or anti-IL-27 as a therapy. As suggested by Wirtz et al., the inhibition of IL-27 signaling may prove effective in conditions such as sepsis in which infection control is key.33 Alternatively, the administration of IL-27 could be a novel tool for limiting immunopathology in autoimmune diseases and later in the course of infections when dampening inflammation becomes a larger priority than controlling pathogens. However, for both of these potential applications, the timing of therapy and the presence of primary or secondary infections would be crucial factors in determining treatment regimens.
IL-27 as a therapy for IBD: evidence from human patients and mouse models
IL-27 polymorphisms and mutations in IBD and cancer
Multiple studies have implicated IL-27 as a candidate gene for IBD.34–36 A genome wide association study in early-onset IBD identified IL-27 within a susceptibility locus in a North American-European cohort. In support of this conclusion, the authors also demonstrated that healthy individuals with two copies of the risk allele expressed significantly less IL-27 relative to individuals with two copies of the nonrisk allele and that colonic expression of IL-27 was significantly lower in early-onset Crohn’s disease patients than in healthy controls.34 IL-27 polymorphisms have also been associated with risk for IBD in both Chinese and Korean populations.35,36
The IL-27 receptor, either wild type or mutated, can contribute to hematopoietic cell transformation.37,38 Although IBD predisposes to colorectal cancer and IL-27 polymorphisms affect risk for IBD, thus far the potential role of IL-27 polymorphisms in colorectal cancer is unclear, as studies evaluating their association with the risk for colorectal cancer produced conflicting results.39–42
IL-27 ameliorates colitis induced by T cell transfer or chemical-induction in mice
Multiple studies have demonstrated the ability of IL-27 to ameliorate colitis in mice, either through lessening of induced colonic inflammation by IL-27 administration or demonstration of more severe colitis in mice deficient in IL-27 due to either antibody-mediated neutralization or genetic knockout.43–47 Mucosal administration of IL-27 synthesized in situ by a food-grade bacterium improved survival and significantly decreased disease activity, colon and small intestine histopathology scores, and proinflammatory gene expression within the intestine in a mouse model of enterocolitis induced by T cell transfer.44 The treatment effects in this study were both T cell- and IL-10-dependent; however, mucosal delivery of IL-27 was found to be more efficacious than direct mucosal delivery of IL-10 by the bacteria. A possible explanation is that IL-27 induces higher levels of endogenous IL-10 in the intestine and mesenteric lymph nodes than could be achieved by the bacteria producing IL-10 in situ. Interestingly, mucosal delivery of IL-27 was also more effective than systemic administration of recombinant murine IL-27 in this study, which had no detectable therapeutic effect.44 Consistent with previous literature,18 IL-27 treatment significantly decreased the expression of RORγt in the colons of enterocolitic mice in this study, and likely as a result, decreased the expression of both IL-17A and IL-17F as well. This study went further to show that IL-27 treatment also reduced disease activity in dextran sulfate sodium (DSS)-induced colitis, a widely used chemically-induced model of colitis.44
Subcutaneous treatment with IL-27 in an acute chemically-induced model of colitis using 2,4,6-trinitrobenzenesulfonic acid (TNBS) was also reported to be protective,46 with improved colonic macroscopic and histopathology scores and reductions in several of the same proinflammatory cytokines previously reported,44 including IL-6, TNF-α, IL-17A, and IL-1β.46 The ability of subcutaneous IL-27 treatment to protect the mice in this study from TNBS-induced colitis contrasts with the previously discussed findings of Hanson et al., in which systemically administered IL-27 by intraperitoneal injection showed no therapeutic effect.44 However, in addition to a different route of injection, the most beneficial effects of subcutaneous IL-27 treatment were at a higher dose than that administered intraperitoneally in the other study, and these two studies evaluated IL-27 treatment in two mechanistically very different models of colitis (acute chemically-induced versus chronic T cell transfer), potentially explaining this discrepancy. The ability of IL-27 to reduce intestinal inflammation by multiple routes of administration could be an advantage clinically, making it a more versatile therapy.44,46
Ablation of IL-27Rα is proinflammatory in murine models of colitis
Additional studies have indirectly demonstrated the anti-inflammatory effects of IL-27 in intestinal inflammation by documenting the impact of its absence. Antibody neutralization of IL-27 in mice infected with Citrobacter rodentium precipitated more severe colitis and increased production of IL-6.43 In another study, a more proinflammatory phenotype was observed in regulatory T cells lacking IL-27Rα, which produced more IL-17 and less IL-10 than regulatory T cells from wild type mice.45 The consequences of this altered phenotype due to IL-27Rα deletion were reported by another laboratory using the T cell transfer model of enterocolitis. In this model naïve CD4+ T cells are transferred into an immunodeficient recipient mouse, resulting in enterocolitis. However, the cotransfer of regulatory T cells can prevent the development of enterocolitis in this model. Interestingly, Do et al demonstrated that Foxp3+ regulatory T cells lacking IL-27Rα cotransferred with naïve CD4+ T cells were, unlike their wild type counterparts, unable to prevent the development of colitis. This group further showed that IL-27 stimulation of both human and murine regulatory T cells enhanced their ability to suppress inflammation and induced the expression of Lag3, a surface receptor critical for the suppressive function of regulatory T cells.48
Complementary to these findings, the induction of regulatory T cells by IL-10-secreting B cells is dependent on IL-27 signaling in T cells. Genetic deletion of IL-27Rα on CD4+ T cells or neutralization of IL-27 blocked the induction of regulatory T cells by IL-10-secreting B cells. This loss of IL-27 signaling also limited the ability of these B cells to suppress proinflammatory cytokine production by both T cells lacking IL-27Rα and wild type T cells in the presence of antibody-neutralized IL-27. Remarkably, while wild type B cells cotransferred with wild type CD4+ T cells reduced intestinal pathology due to T cell transfer, this effect was absent when the transferred T cells lacked IL-27Rα, demonstrating that IL-27 signaling is critical for the suppression of T cell-driven intestinal inflammation by IL-10-secreting B cells.25
In the DSS chemically-induced model of colitis, mice lacking the IL-27 receptor had significantly increased ratios of Th17 to Th1 cells in the mesenteric lymph nodes and colon both before and after DSS treatment compared to wild type controls.47 These mice developed more severe colitis more quickly when administered DSS compared to wild type controls and were significantly less likely to survive DSS exposure.47
IL-27 suppresses the innate inflammatory response
Recent experiments have shown that mucosally-administered IL-27 improves histopathology scores in both the DSS (Andrews, unpublished data) and TNBS models of acute chemically-induced colitis.49 In the TNBS model, this IL-27 treatment significantly reduced neutrophil infiltrates in inflamed segments of colon.49 These data complement the findings of Li et al, in which IL-27 treatment of human neutrophils suppressed their adhesion capability in vitro. Furthermore, IL-27 treatment of neutrophils in this study reduced gene and protein expression of the integrin Mac-1, which the authors proposed as a possible mechanism for the suppression of neutrophil adhesion by IL-27.14 Additionally, a separate study found that IL-27 treatment of neutrophils stimulated with a TLR ligand in vitro significantly reduced the production of the proinflammatory cytokines IL-6 and IL-12/IL-23p40.47
Further supporting a suppressive role for IL-27 on the innate immune system, deletion of IL-27Rα in RAG−/− mice lacking T and B cells made them more sensitive to DSS-induced colitis.47 These findings suggest that while IL-27 treatment may depend on modification of T cell function under certain conditions, its effects on the innate immune system alone may be sufficient for protection against some types of intestinal inflammation, widening its potential therapeutic indications. Collectively, these data lend further support for an immunosuppressive role of IL-27 in both innate and adaptive immunity in the intestine.
Additional protective functions of IL-27 in the intestine
In addition to the studies described above, which directly investigated the role of IL-27 in mouse models of intestinal inflammation, a number of studies have uncovered immunomodulatory and protective roles for IL-27 in the intestine while examining other aspects of intestinal biology. IL-27 promotes oral tolerance50 and mediates the ability of Bifidobacterium infantis to suppress IL-17 expression.51 Additionally, IL-27 was elevated in Fat-1 mice in chronic DSS-induced colitis in association with improved histopathology scores and decreased expression of Th17 cell cytokines.52 IL-27 can also promote intestinal epithelial barrier integrity. Intestinal epithelial cells have been shown to upregulate the IL-27 receptor in inflammation and bacterial infection, and IL-27 signaling increased intestinal epithelial cell proliferation and antibacterial peptide production.13 IL-27 clearly demonstrates a variety of protective functions in the intestine, and therefore may prove to be an effective therapy for IBD.
The other side: proinflammatory effects of IL-27 in mouse models of intestinal disease
Ablation of IL-27Rα signaling can attenuate intestinal inflammation due to T cell transfer, DSS administration, and IL-10 deficiency
In contrast to the evidence presented above, a collection of studies have reported deleterious effects of IL-27 in intestinal inflammation based on inhibiting IL-27 receptor signaling.53–56 Genetic deletion of IL-27Rα on either transferred T cells or in recipient mice effectively prevented the intestinal inflammation typical of the T cell transfer model of enterocolitis.53,54 Increased numbers of transferred T cells lacking IL-27Rα became Foxp3+, suggesting that IL-27 may negatively regulate the development of Foxp3+ regulatory T cells.53 The second study also noted that inhibition of IL-27Rα in recipient mice prevented Th17 cell development by decreasing production of IL-1β and IL-6 by antigen presenting cells.54 Similarly, IL-27Rα knockout mice reportedly develop less severe DSS-induced colitis than wild type mice, characterized by reduced expression of IL-6, TNF-α, and IFN-γ in intestinal lamina propria mononuclear cells.55 IL-10 deficient mice, which spontaneously develop intestinal inflammation, also reportedly have both delayed pathology and a survival advantage when IL-27Rα is concurrently genetically deleted.56 Interestingly, a number of these findings attributed to IL-27Rα deletion, including reduced inflammation in both the T cell transfer and DSS models of colitis and reductions in IL-1β, IL-6, and TNF- α, have also been reported as beneficial effects of IL-27 treatment, leaving more questions than answers for the interpretation of these aspects of IL-27 function. A summary of the reported roles for IL-27 in the models of murine colitis detailed above is presented in Table 1.
Table 1.
Summary of studies investigating the role of IL-27 in murine models of colitis.
| Studies supporting a beneficial role for IL-27 in murine colitis | ||
|---|---|---|
| Model | Result | Reference |
| T cell transfer | Mucosal administration of IL-27 improves pathology and clinical disease | 44 |
| IL-27Rα−/− renders Foxp3+ Tregs incapable of preventing enterocolitis | 48 | |
| IL-27Rα−/− on transferred T cells prevents the reduction of intestinal inflammation by IL-10-secreting B cells |
25 | |
| DSS | Mucosal administration of IL-27 improves pathology and clinical disease | 44, Andrews unpublished |
| IL-27Rα−/− results in more severe colitis and reduced survival | 47 | |
| Concurrent IL-27Rα−/− in RAG−/− mice worsened colitis and decreased survival | 47 | |
| Elevated IL-27 in Fat-1 mice associated with decreased pathology | 52 | |
| TNBS | Subcutaneous administration of IL-27 improves pathology | 46 |
| Mucosal administration of IL-27 improves pathology and clinical disease | 49 | |
| Citrobacter rodentium | IL-27 neutralization worsened colitis | 43 |
| Studies suggesting deleterious effects of IL-27 in murine colitis | ||
| Model | Result | Reference |
| T cell transfer | IL-27Rα−/− on transferred T cells reduces inflammation and clinical disease | 53 |
| IL-27Rα−/− in recipient mice prevents enterocolitis | 54 | |
| DSS | IL-27Rα−/− mice develop less severe colitis | 55 |
| IL-10−/− | Concurrent IL-27Rα−/− delays colitis and improves survival | 56 |
IL-27 and inflammatory bowel disease: clarity amid the confusion
Conflict resolution
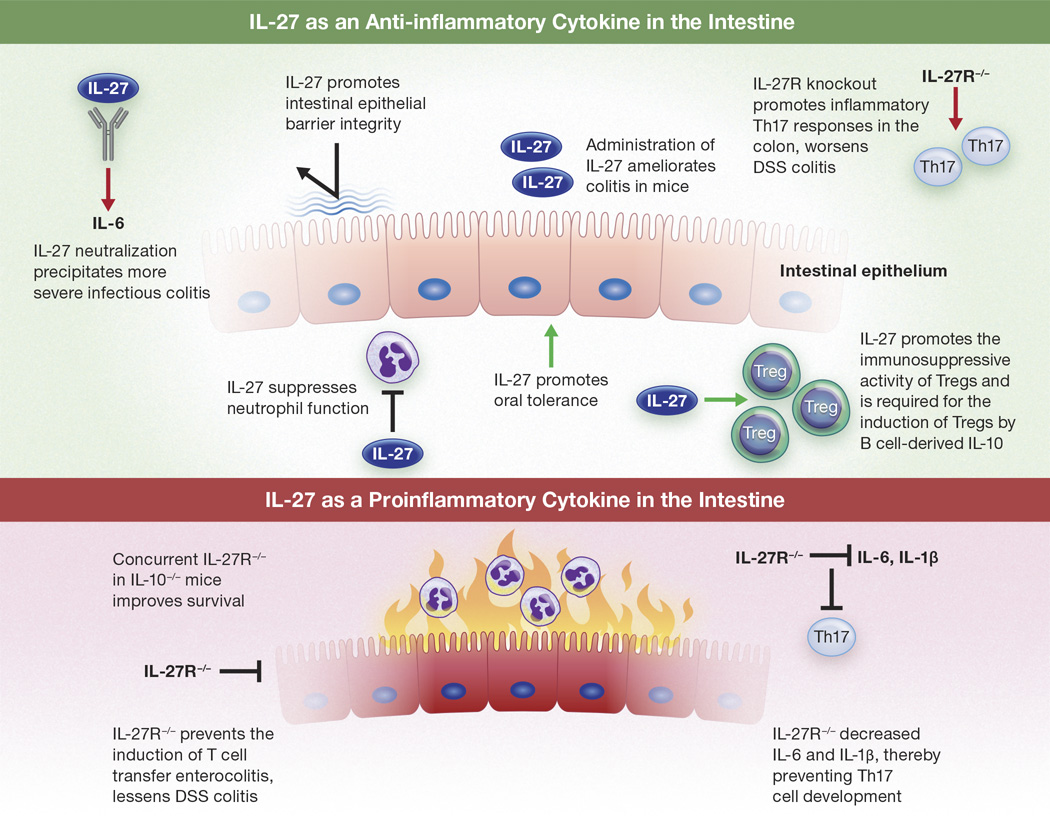
While a few studies suggest that inhibition of IL-27 receptor signaling can ameliorate intestinal inflammation, most of the literature supports IL-27 as a suppressor of intestinal inflammation caused by a variety of insults (Figure 1). It is important to note that all of the studies favoring a proinflammatory role for IL-27 in the intestine examined specific consequences of genetic deletion of the IL-27 receptor. In contrast, to our knowledge no report to date has demonstrated deleterious effects of exogenous IL-27 treatment on intestinal inflammation. Additionally, based on the complex biology of IL-27 discussed above, it is not hard to imagine how complete inhibition of IL-27 signaling could eliminate subtle checks and balances inherent to IL-27 function in wild type animals, as IL-27 signaling elicits distinct responses based on cell type and timing. For example, genetic deletion of IL-27Rα predicted that IL-27 would be protective in influenza infection. However, while the administration of IL-27 late in the course of influenza infection was beneficial, IL-27 therapy early in the infection resulted in more severe disease,32 an outcome impossible to predict by complete genetic inhibition of IL-27 signaling. Based on the ability of IL-27 to inhibit both Th2 and Th17 cell differentiation and promote Tr1 cell development,17–19,23–27 it is also reasonable to speculate that genetic ablation of IL-27 signaling could alter the differentiation and subsequent phenotype of multiple cell types, thereby creating an inflammatory microenvironment that is not representative of that of wild type animals.
Figure 1.
Evidence for IL-27 as an anti-inflammatory versus proinflammatory cytokine in the intestine. Results from conflicting studies are included.
Additionally, the timing of IL-27 signaling and the evaluation of its result are critical. In human mast cells, IL-27 rapidly elicits transcription of inflammatory cytokine genes, but elicits a slow response in human monocytes.12 As a result, conclusions taken at a single or limited time points may not fully illustrate the results of IL-27 therapy or elimination and may contribute to discrepancies between studies. Finally, different concentrations of IL-27 have been shown to elicit distinct patterns of cytokine expression.25 Different methods of IL-27 administration may produce different concentrations within the intestine, and may be an explanation for differing results between some studies. Furthermore, the different patterns of cytokine expression induced by different concentrations of IL-27 suggest that genetic deletion of IL-27 signaling in an entire animal or subsets of its cells may create a misleading “all or nothing” microenvironment unrepresentative of physiologic reality.
When interpreting literature describing the biology of IL-27, it is critical to acknowledge that each subunit of the heterodimeric IL-27 cytokine has unique biologic activities of its own.57,58 As a result, gene and/or protein expression of either of these subunits, even if coexpressed, is not necessarily indicative of the presence of the complete IL-27 dimer, and may in fact represent production of these subunits independently or some combination of complete IL-27 and free subunits.
Interestingly, the existence of a soluble form of IL-27Rα has been described that is able to antagonize IL-27 and is thought to originate by cleavage of the membrane-bound receptor.59 Dietrich et al. reported that varying levels of soluble IL-27Rα were produced by human CD4+ and CD8+ T cells, B cells, monocyte-derived dendritic cells, and monocytes.59 While only speculation, it is interesting to question whether genetic deletion of IL-27Rα in only specific cells, such as the transferred T cells or recipient cells in the studies detailed above, could also eliminate or reduce this negative regulator of IL-27, resulting in dysregulated IL-27 signaling in remaining cells with functional IL-27Rα. The discovery of this endogenous, soluble antagonist for IL-27 is intriguing, and further research into its function and regulation could impact concepts of IL-27 biology and potential therapeutic manipulation.
Lessons learned from targeting IL-17 to treat IBD
IL-23 promotes the development of Th17 cells,60,61 and a genome wide association study demonstrated a significant association between variants in the IL-23R gene and both Crohn’s disease and ulcerative colitis.62 However, while multiple studies have associated Th17 cells with the pathogenesis of IBD, the literature remains conflicted over whether their signature cytokines, IL-17A and IL-17F, drive intestinal inflammation or are protective (or both).63,64 In contrast to the efficacy of anti-tumor necrosis factor-α antibody treatment in a subset of IBD patients,3 antibody neutralization of IL-17A was surprisingly inefficacious, and in some cases, deleterious in patients with moderate to severe Crohn’s disease.65 Similarly, a clinical trial investigating antibody blockade of IL-17 receptor A in moderate to severe Crohn’s disease was terminated due to a lack of efficacy and worsening of disease in some patients.66 However, two other therapies known to inhibit Th17 responses have shown promise as treatments for Crohn’s disease and ulcerative colitis.67,68 Vidofludimus, an inhibitor of the enzyme dihydroorotate dehydrogenase known to also inhibit the production of both IL-17A and IL-17F, induced steroid free remission in at least 50% of both Crohn’s disease and ulcerative colitis patients in a clinical trial. Furthermore, of the remaining patients in the trial, partial remission was achieved in an additional 28.6% and 41.7% of patients with Crohn’s disease or ulcerative colitis, respectively.67 Two trials have investigated the use of tofacitinib in IBD, demonstrating that it may be an effective therapy for ulcerative colitis, but not Crohn’s disease.68,69 Tofacitinib inhibits Janus kinases 1, 2 and 3, and in doing so also blocks the differentiation of Th17 cells. Patients with moderately to severely active ulcerative colitis showed dose dependent clinical responses and clinical remission with tofacitinib therapy. Among patients receiving the highest dose, 78% showed a clinical response and 41% achieved clinical remission after 8 weeks of treatment.68 A shorter 4 week study found no significant reductions in Crohn’s disease activity index in patients with moderate to severe Crohn’s disease given tofacitinib;69 however, whether this lack of efficacy is due to differences between the two diseases or the shorter time of treatment (or both) is yet to be determined. Additionally, patients receiving placebo treatment in this study had a higher rate of response and remission than anticipated, leaving the authors to question whether this could have contributed to the nonsignificant difference in treatment results.69
Interestingly, a recent study reported that antibody neutralization of both IL-17A and IL-17F, but neither cytokine alone, reduced colon histopathology scores in the murine T cell transfer model of enterocolitis.70 This could explain why vidofludimus and tofacitinib were more successful in clinical trials for IBD than antibody blockade of IL-17A alone, but offers little insight as to why anti-IL-17 receptor A treatment, which should inhibit signaling of both IL-17A and IL-17F,71 was inefficacious. This is particularly relevant to the discussion herein, as IL-27 is able to block the expression of both IL-17A and IL-17F through inhibition of the transcription factor RORγt.18 This potential therapeutic effect has been demonstrated experimentally in murine enterocolitis, in which decreased expression of RORγt, IL-17A, and IL-17F was reported in the colons of mice following mucosal administration of IL-27.44 IL-27 also inhibits the development of Th17 cells by inducing expression of programmed death ligand 1 on naïve T cells, which when cultured with naïve CD4+ T cells, prevents their differentiation into Th17 cells.72 However, it is unclear what effect, if any, IL-27 may have on the secretion of IL-17 by immune cells other than T cells in the inflamed intestine, such as neutrophils and mast cells, which have been shown to be important sources of IL-17 in arthritis and psoriasis.73,74 In contrast to antibody neutralization of a cytokine or its receptor, it is interesting to question whether the administration of another cytokine, potentially more subject to endogenous regulation, could create a more physiologically relevant anti-inflammatory microenvironment that might be able to maintain balance in the pro- and anti-inflammatory effects of the immune mediators it regulates. For example, the elimination of cytokine signaling by antibody neutralization or genetic deletion could block both its beneficial and harmful effects, while modulating a cytokine with one of its physiologic regulators could potentially preserve its beneficial functions.
Potential roles for IL-27 as a therapy for both Crohn’s disease and ulcerative colitis
Although grouped together as IBD, Crohn’s disease and ulcerative colitis are distinct conditions that vary both clinically and immunopathologically.75 The lesions of Crohn’s disease may be located anywhere throughout the gastrointestinal tract and are characterized by transmural infiltrates of macrophages and lymphocytes that in many patients multifocally organize to form granulomas. In contrast, ulcerative colitis is limited to the colon and features histopathologic changes of the mucosa only, including infiltrates of granulocytes and lymphocytes. Mucosal ulceration may be present in both conditions. Based on characterizations of cytokine expression and signaling, Crohn’s disease is considered to be driven by Th1 responses, while ulcerative colitis is a Th2-mediated disease.75
Despite differences in their immunopathology, there are abundant mechanisms by which IL-27 administration could be an effective therapy for both Crohn’s disease and ulcerative colitis. Perhaps the most obvious mechanism by which IL-27 could reduce inflammation in IBD is through its stimulation of the immunoregulatory cytokine IL-10, which can be induced by IL-27 signaling in CD8+ and regulatory, Th1, Th2, and Th17 cells.6,19,21–27 IL-10 boasts an incredible number of anti-inflammatory functions, including blocking Th1 and Th2 responses; inhibition of inflammatory cytokine and chemokine production by monocytes and neutrophils; limiting the recruitment of dendritic cells, T cells, neutrophils, and monocytes; inducing anergy in activated T cells; stimulating the production of interleukin-1 receptor antagonist and soluble tumor necrosis factor receptor; and reducing monocyte activation of T cells.76,77 Complementing these functions of IL-10, IL-27 itself also limits tissue infiltration by neutrophils and monocytes, suppresses chemokine production, and inhibits the generation of reactive oxygen intermediates by both macrophages/monocytes and granulocytes.32,33 As demonstrated in a mouse model of experimental autoimmune encephalomyelitis, IL-27’s ability to induce CD39 on dendritic cells could contribute to the suppression of pathogenic T cell responses in IBD.30 As ulcerative colitis has been shown to be driven by Th2 immunopathology, IL-27’s capacity to block Th2 cell differentiation and cytokine expression makes it well suited to treat this condition.17,75 Additionally, while the treatment of Th1-driven Crohn’s disease with a cytokine known to promote Th1 responses sounds counterintuitive,6,75 IL-27 is also critical for the development of T-bet+ CXCR3+ regulatory T cells in the gut-associated lymphoid tissue that are specialized for regulating Th1 cells.16 IL-10 induced by IL-27 treatment could further contribute to the regulation of exuberant Th1 immunity in Crohn’s disease.6,19,21–27
How IL-27’s ability to curb Th17 responses may influence its efficacy as a treatment for IBD is uncertain. However, in contrast to the failed trials investigating direct targeting of IL-17 signaling alone as a treatment for Crohn’s disease, inhibition of Th17 responses is only one of many anti-inflammatory functions IL-27 exerts on adaptive and innate immunity. In this way IL-27 treatment would be more analogous to vidofludimus and tofacitinib therapy, which were effective in treating ulcerative colitis and/or Crohn’s disease,67,68 and, like IL-27, exert diverse anti-inflammatory functions in addition to the blockade of IL-17A and IL-17F.
IL-27 and IL-27R expression in IBD
Both subunits of the IL-27 receptor, IL-27Rα and gp130, are expressed at low levels in normal intestinal epithelial cells, but are upregulated in inflammation in both epithelial cells and infiltrating leukocytes.13 Increased expression of IL-27 in inflamed segments of the intestinal mucosa has been demonstrated in both Crohn’s disease and ulcerative colitis.13,78 Patients with active Crohn’s disease have also been shown to have both significantly increased serum IL-27 and soluble IL-27Rα relative to healthy controls; however, despite an overall positive correlation between these two values, the ratio of cytokine to soluble receptor varied widely among patients.59 Table 2 highlights the evidence for IL-27 as a factor in human IBD.
Table 2.
Summary of evidence linking IL-27 and IBD in human patients.
| Genetic associations | Reference |
|---|---|
| IL-27 identified within a susceptibility locus in a North American-European cohort | 34 |
| IL-27 polymorphisms are associated with risk for IBD in Chinese and Korean populations | 35, 36 |
| Individuals homozygous for an IBD risk allele containing IL-27 express less IL-27 than people homozygous for the nonrisk allele | 34 |
| IL-27 and IL-27 receptor expression in IBD patients | |
| Colonic IL-27 expression is reduced in early-onset Crohn’s disease patients relative to healthy individuals | 34 |
| Increased IL-27 expression has been documented in inflamed intestine from both Crohn’s disease and ulcerative colitis patients | 13, 78 |
| Crohn’s disease patients have increased serum IL-27 and soluble IL-27Rα | 59 |
| Intestinal epithelial cells in foci of inflammation upregulate the IL-27 receptor | 13 |
The question remains whether elevations of IL-27 in the inflamed intestinal mucosa and serum of IBD patients are contributing to inflammation in these patients or represent an anti-inflammatory response. However, based on the evidence presented herein, it seems most likely that these elevations in IL-27 represent an (inadequate) anti-inflammatory response in diseased segments of intestine. While IL-27 signaling in the intestine presumably acts on inflammatory cells, inflamed intestinal epithelial cells upregulate the IL-27 receptor and are therefore also capable of responding to IL-27.
Concluding remarks
While complex, the majority of the literature investigating the role of IL-27 in mouse models of IBD complements knowledge gained from human patients and supports an anti-inflammatory role for IL-27 in IBD. However, the studies suggesting a more pro-inflammatory role for IL-27 should not be ignored, but should rather inform future investigations into the complex physiology of this cytokine. While it’s unlikely that a single “silver bullet” treatment for all cases of IBD will ever be found, IL-27 is a promising potential therapy that warrants further investigation.
Acknowledgments
source of funding:
Drs Andrews and Durum are supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- 1.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 2.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012;61:918–932. doi: 10.1136/gutjnl-2011-300904. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Lemann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:870–879. doi: 10.1111/j.1365-2036.2011.04599.x. [DOI] [PubMed] [Google Scholar]
- 5.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naïve CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 7.Aparicio-Siegmund S, Garbers C. The biology of interleukin-27 reveals unique pro- and anti-inflammatory functions in immunity. Cytokine Growth Factor Rev. 2015;26(5):579–586. doi: 10.1016/j.cytogfr.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 9.Meka RR, Venkatesha SH, Dudics S, et al. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun Rev. 2015;14(12):1131–1141. doi: 10.1016/j.autrev.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molle C, Nguyen M, Flamand V, et al. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. The Journal of Immunology. 2007;178:7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 11.Pirhonen J, Siren J, Julkunen I, Matikainen S. IFN-α regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J Leukoc Biol. 2007;82:1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- 12.Pflanz S, Hibbert L, Mattson J, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. The Journal of Immunology. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 13.Diegelmann J, Olszak T, Göke B, et al. A novel role for interleukin-27 (IL-27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer and activator of transcription (STAT) protein signaling and induction of antibacterial and anti-inflammatory proteins. J Biol Chem. 2012;287(1):286–298. doi: 10.1074/jbc.M111.294355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JP, Wu H, Xing W, et al. Interleukin-27 as a negative regulator of human neutrophil function. Scandinavian Journal of Immunology. 2010;72:284–292. doi: 10.1111/j.1365-3083.2010.02422.x. [DOI] [PubMed] [Google Scholar]
- 15.Villarino AV, Larkin J, III, Saris CJM, et al. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 16.O-Hara Hall A, Beiting DP, Tato C, et al. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infections-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimoto T, Yoshimoto T, Yasuda K, et al. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. The Journal of Immunology. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 18.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. The Journal of Immunology. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 19.Murugaiyan G, Mittal A, Lopez-Diego R, et al. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;184:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim G, Shinnakasu R, Saris CJM, et al. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. The Journal of Immunology. 2013;190:1510–1518. doi: 10.4049/jimmunol.1201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu C, Sakuishi K, Xiao S, et al. An IL-27/NFIL3 signaling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun. 2015;23(6):6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 23.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8(12):1380–1390. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 24.Jeon SG, Kayama H, Ueda Y, et al. Probiotic Bifidobacterium breve induced IL-10-producing Tr1 cells in the colon. PLOS Pathogens. 2012;8(5):e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishima Y, Liu B, Hansen JJ, Sartor RB. Resident bacteria-stimulated interleukin-10-secreting B cells ameliorate T-cell-mediated colitis by inducing T-regulatory-1 cells that require interleukin-27 signaling. Cell Mol Gastroenterol Hepatol. 2015;1(3):295–310. doi: 10.1016/j.jcmgh.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pot C, Jin H, Awasthi A, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Meng R, Li Z, et al. IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunol Lett. 2011;136(1):21–28. doi: 10.1016/j.imlet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Tait Wojno ED, Hosken N, Stumhofer JS, et al. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artis D, Villarino A, Silverman M, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 30.Mascanfroni ID, Yeste A, Vieira SM, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nature Immunology. 2013;14(10):1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung JY, Roberts LL, Robinson CM. The presence of interleukin-27 during monocyte-derived dendritic cell differentiation promotes improved antigen processing and stimulation of T cells. Immunology. 2014;144:649–660. doi: 10.1111/imm.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu FDM, Kenngott EE, Schröter MF, et al. Timed action of IL-27 protects from immunopathology while preserving defense in influenza. PLoS Pathog. 2014;10(5):e1004110. doi: 10.1371/journal.ppat.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirtz S, Tubbe I, Galle PR, et al. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203(8):1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imielinski M, Baldassano RN, Griffiths A, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41(12):1335–1340. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li CS, Zhang Q, Lee KJ, et al. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J Gastroenterol Hepatol. 2009;24(10):1692–1696. doi: 10.1111/j.1440-1746.2009.05901.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Wang L, Fan R, et al. Association of IL-27 gene three polymorphisms with Crohn’s disease susceptibility in a Chinese Han population. Int J Clin Exp Pathol. 2014;7(12):8952–8957. [PMC free article] [PubMed] [Google Scholar]
- 37.Pradhan A, Lambert QT, Reuther GW. Transformation of hematopoietic cells and activation of JAK2-V617F by IL-27R, a component of a heterodimeric type I cytokine receptor. PNAS. 2007;104(47):18502–18507. doi: 10.1073/pnas.0702388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambert QT, Pradhan A, Roll JD, Reuther GW. Mutations in the transmembrane and juxtamembrane domains enhance IL-27R transforming activity. Biochem J. 2011;438(1):55–164. doi: 10.1042/BJ20110351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang ZQ, Wang JL, Pan GG, Wei YS. Association of single nucleotide polymorphisms in IL-12 and IL-27 genes with colorectal cancer risk. Clin Biochem. 2011;45(1–2):54–59. doi: 10.1016/j.clinbiochem.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Lyu S, Ye L, Wang O, et al. IL-27 rs153109 polymorphism increases the risk of colorectal cancer in Chinese Han population. Onco Targets Ther. 2015;8:1493–1497. doi: 10.2147/OTT.S80255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu XP, Hua LY, Chao HL, et al. Genetic association between IL-27 rs153109 polymorphism and cancer risk in Chinese population: a meta-analysis. J Recept Signal Transduct Res. 2014;26:1–6. doi: 10.3109/10799893.2014.986743. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Tan X, Huang J, et al. Association of 3 common polymorphisms of IL-27 gene with susceptibility to cancer in Chinese: evidence from an updated meta-analysis of 27 studies. Med Sci Monit. 2015;21:2505–2513. doi: 10.12659/MSM.895032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dann SM, Le C, Choudhury BK, et al. Attenuation of intestinal inflammation in interleukin-10 deficient mice infected with Citrobacter rodentium . Infect Immun. 2014;82(5):1949–1958. doi: 10.1128/IAI.00066-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanson ML, Hixon JA, Li W, et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology. 2014;146:210–221. doi: 10.1053/j.gastro.2013.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAleer JP, Saris CJM, Vella AT. The WSX-1 pathway restrains intestinal T cell immunity. Int Immunol. 2011;23(2):129–137. doi: 10.1093/intimm/dxq464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaoka T, Ito M, Yamashita J, et al. Treatment with IL-27 attentuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G568–G576. doi: 10.1152/ajpgi.00329.2010. [DOI] [PubMed] [Google Scholar]
- 47.Troy AE, Zaph C, Du Y, et al. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J Immunol. 2009;183:2037–2044. doi: 10.4049/jimmunol.0802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Do J, Visperas A, Sanogo YO, et al. An IL-27/Lag3 axis enhances Foxp3+ regulatory T cell-suppressive function and therapeutic efficacy. Mucosal Immunol. 2016;9(1):137–145. doi: 10.1038/mi.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLean MH, Hanson ML, Steidler L, et al. Intra-luminal interleukin (IL)-27 ameliorates acute murine TNBS colitis—a potential future therapeutic for inflammatory bowel disease? Gastroenterology. 2013;144(Supp 1):S-33. [Google Scholar]
- 50.Shiokawa A, Tanabe K, Tsuji NM, et al. IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunology Letters. 2009;125:7–14. doi: 10.1016/j.imlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Tanabe S, Kinuta Y, Saito Y. Bifidobacterium infantis suppresses proinflammatory interleukin-17 in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int J Mol Med. 2008;22(2):181–185. [PubMed] [Google Scholar]
- 52.Monk JM, Jia Q, Callaway E, et al. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J Nutr. 2012;142:117–124. doi: 10.3945/jn.111.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox JH, Kljavin NM, Ramamoorthi N, et al. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2010;208(1):115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visperas A, Do JS, Bulek K, et al. IL-27, targeting antigen- presenting cells, promotes Th17 differentiation and colitis in mice. Mucosal Immunol. 2013;7(3):625–633. doi: 10.1038/mi.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Honda K, Nakamura K, Matsui N, et al. T helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflamm Bowel Dis. 2005;11(12):1044–1052. doi: 10.1097/01.mib.0000191611.05466.1f. [DOI] [PubMed] [Google Scholar]
- 56.Villarino AV, Artis D, Bezbradica JS, et al. IL-27R deficiency delays the onset of colitis and protects from helminth-induced pathology in a model of chronic IBD. Int Immunol. 2008;20(6):739–752. doi: 10.1093/intimm/dxn032. [DOI] [PubMed] [Google Scholar]
- 57.Stumhofer JS, Tait ED, Quinn WJ, III, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wirtz S, Billmeier U, Mchedlidze T, et al. IL-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology. 2011;141:1875–1886. doi: 10.1053/j.gastro.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dietrich C, Candon S, Ruemmele FM, Devergne O. A soluble form of IL-27Rα is a natural IL-27 antagonist. J Immunol. 2014;192:5382–5389. doi: 10.4049/jimmunol.1303435. [DOI] [PubMed] [Google Scholar]
- 60.Aggarwal S, Ghilardi N, Xie MH, et al. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 61.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 62.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLean LP, Cross RK, Shea-Donohue T. Combined blockade of IL-17A and IL-17F may prevent the development of experimental colitis. Immunotherapy. 2013;5(9):923–925. doi: 10.2217/imt.13.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuss IJ. IL-17: intestinal effector or protector? Mucosal Immunol. 2011;4:366–367. [Google Scholar]
- 65.Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Targan SR, Feagan BG, Vermiere S, et al. A randomized, double blind, placebo controlled study to evaluate the safety, tolerability, and efficacy of AMG 827 in subjects with moderate to severe Crohn’s disease. Gastroenterology. 2012;143(3):e26. [Google Scholar]
- 67.Herrlinger KR, Diculescu M, Fellermann K, et al. Efficacy, safety and tolerability of vidofludimus in patients with inflammatory bowel disease: The ENTRANCE study. J Crohns Colitis. 2013;7(8):636–43. doi: 10.1016/j.crohns.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 68.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–24. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 69.Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12(9):1485–1493. doi: 10.1016/j.cgh.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 70.Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19(8):1567–1576. doi: 10.1097/MIB.0b013e318286fa1c. [DOI] [PubMed] [Google Scholar]
- 71.Russell CB, Kerkof K, Bigler J, et al. Blockade of the IL-17R with AMG 827 leads to rapid reversal of gene expression and histopathologic abnormalities in human psoriatic skin. J Invest Dermatol. 2010;130:S46. [Google Scholar]
- 72.Hirahara K, Ghoreschi K, Yang X, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katayama M, Ohmura K, Yukawa N, et al. Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS One. 2013;8(5):e62231. doi: 10.1371/journal.pone.0062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 76.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 77.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 78.Leon AJ, Gomez E, Garrote JA, et al. High levels of proinflammatory cytokines, but not markers of tissue injury, in unaffected intestinal areas from patients with IBD. Mediators Inflamm. 2009;2009:580450. doi: 10.1155/2009/580450. [DOI] [PMC free article] [PubMed] [Google Scholar]