Abstract
Crohn’s disease and ulcerative colitis are common and debilitating manifestations of inflammatory bowel disease (IBD). IBD is characterized by a radical imbalance in the activation of pro-inflammatory and anti-inflammatory signaling pathways in the gut. These pathways are controlled by NF-κB, which is a master regulator of gene transcription. In IBD patients, NF-κB signaling is often dysregulated resulting in overzealous inflammation. NF-κB activation occurs through two distinct pathways, defined as either canonical or non-canonical. Canonical NF-κB pathway activation is well studied in IBD and is associated with the rapid, acute production of diverse pro-inflammatory mediators, such as COX-2, IL-1β, and IL-6. In contrast to the canonical pathway, the non-canonical or “alternative” NF-κB signaling cascade is tightly regulated and is responsible for the production of highly specific chemokines that tend to be associated with less acute, chronic inflammation. There is a relative paucity of literature regarding all aspects of non-canonical NF-κB signaling. However, it is clear that this alternative signaling pathway plays a considerable role in maintaining immune system homeostasis and likely contributes significantly to the chronic inflammation underlying IBD. Non-canonical NF-κB signaling may represent a promising new direction in the search for therapeutic targets and biomarkers associated with IBD. However, significant mechanistic insight is still required to translate the current basic science findings into effective therapeutic strategies.
Keywords: p100, NIK, alternative pathway, ulcerative colitis, Crohn’s disease
INTRODUCTION
Ulcerative Colitis (UC) and Crohn’s Disease (CD) are jointly defined as Inflammatory Bowel Disease (IBD) and are both chronic and debilitating disorders. The specific cause of IBD remains unknown. However, it is clear that both UC and CD are associated with complex genetic, immunological, and environmental interactions. Overzealous inflammation is the most prominent feature associated with IBD pathobiology. As such, immunosuppressive and anti-inflammatory drugs remain the first line of treatment for patients. Recently, the use of biologics targeting inflammatory signaling pathways have proven to be a highly successful therapeutic strategy. Indeed, a significant amount of basic and translational IBD research over the last decade has been focused on identifying and characterizing specific immune signaling pathways that may serve as therapeutic targets.
The transcription factor nuclear factor kappa B (NF-κB) is a central regulator of inflammation and modulates a diverse spectrum of biological processes. The NF-κB signaling cascade has been well studied in IBD pathogenesis and is often dysregulated in patients, resulting in aberrant cytokine and chemokine production in the gut. NF-κB activation occurs through two distinct pathways, defined as the canonical pathway and the non-canonical or “alternative” pathway. The vast majority of studies have thus far focused on the canonical NF-κB signaling cascade. Signal processing in the canonical pathway is rapid and constitutive. In the canonical pathway, (RelA/p65)/p50 heterodimers are maintained in the cytoplasm in an inactive state by a family of inhibitors of NF-κB (IκBα). Activation is mediated through a large IκB kinase complex consisting of the regulatory subunit IκB kinase γ (IKKγ; NF-κB essential modulator; NEMO), and catalytic subunits IκB kinase α (IKKα) and IκB kinase β (IKKβ). Upon upstream kinase activity, the IKK complex phosphorylates IκBα leading to its degradation and the subsequent release of the RelA/p50 heterodimer. Newly liberated RelA/p50 heterodimers rapidly translocate to the nucleus to activate the transcription of a diverse range of inflammatory mediators, such as COX2, TNF, IL-1β, and IL-6 (Figure 1).
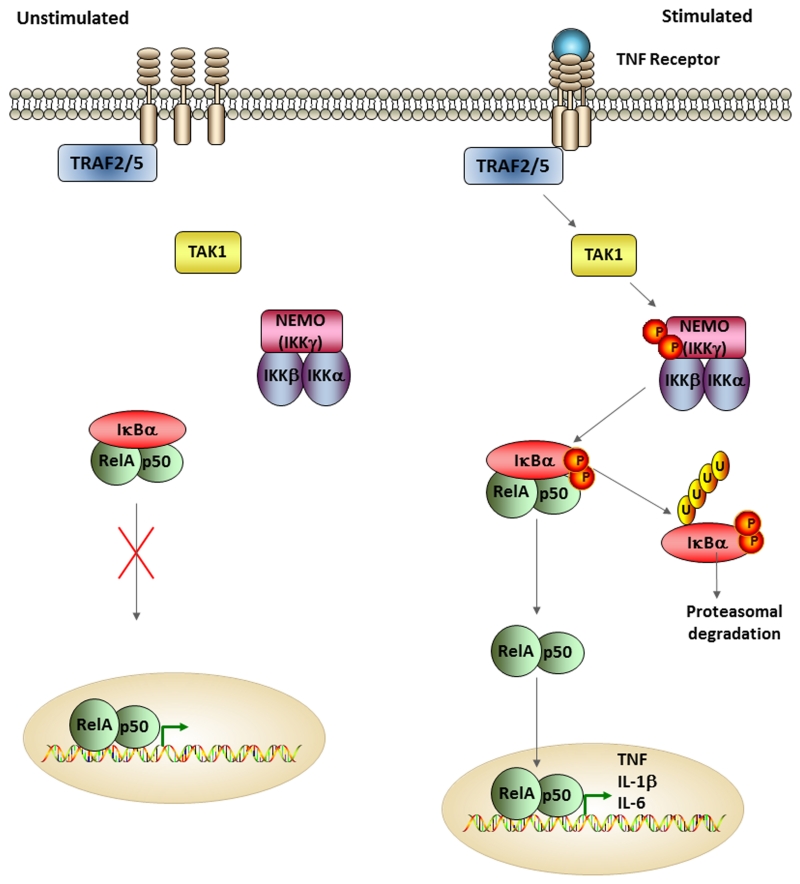
Figure 1. The Canonical NF-κB Signaling Pathway.
This schematic demonstrates some of the major steps associated with the canonical NF-κB signaling pathway under both unstimulated and stimulated conditions. The canonical pathway is triggered by a variety of stimuli that activate diverse receptors, such pattern recognition receptors, TNF receptors, and proinflammatory cytokine receptors. In this representative image, the TNF receptor is shown. When unstimulated, the IKK complex composed of NEMO (IKKγ), IKKβ, and IKKα, along with the heterodimer composed of NF-κB proteins RelA and p50 are inactive and located in the cytoplasm. The binding of a ligand to the cell surface receptor, such as TNF binding to TNF receptor, leads to the recruitment of adaptor proteins, such as TRAF2 or TRAF5 and TAK1. This upstream activity leads to the phosphorylation and activation of the regulatory subunit of the IKK complex, NEMO, which in turn leads to the phosphorylation of the catalytic subunit of the IKK complex, IKKβ. IKKβ then mediates the phosphorylation and induction of proteosomal degradation of IκBα, which then allows for nuclear localization of the heterodimer RelA/p50. Nuclear localization leads to the transcription of proinflammatory cytokines such as TNF, IL-1β, and IL-6.
In contrast to the canonical pathway, there is a relative paucity of data pertaining to non-canonical NF-κB signaling during IBD. Similar to the canonical cascade, the non-canonical NF-κB pathway involves an NF-κB heterodimer which comprises p52 and RelB, instead of p50 and RelA (1). However, before p52 is activated via the degradation of its C-terminal ankyrin repeat-containing domain, it is maintained in its precursor form, known as p100 where the DNA binding domain is not easily accessible (1). The p100 molecule acts as an IκB-like molecule, similar to p105 in the canonical pathway, and holds RelB in the cytoplasm (2). Upon receptor stimulation, p100 is targeted for proteasome degradation and subsequently processed to p52 (1, 3). This processing of p100 and degradation of the ankyrin repeat-containing domain unmasks the nuclear localization sequence (NLS), which facilitates the translocation of p52 to the nucleus (1).
Processing of p100 is tightly regulated by NF-κB-inducing kinase (NIK) and negatively regulated by a processing-inhibitory domain (PID) within p100. Under normal unstimulated conditions, NIK is constitutively degraded via the binding of a complex comprised of TNF-receptor associated factor 3 (TRAF3), TRAF2 and cellular inhibitor of apoptosis 1 and 2 (cIAP1/2), which together prevent basal activation (1, 2, 4). Upon recognition of a TNF family signaling molecule, such as CD40L, lymphotoxin β, or BAFFR, TRAF3 is degraded and NIK is stabilized. NIK is then available to phosphorylate IKKα (3, 5). The activation of IKKα leads to the phosphorylation of p100, causing its ubiquitination and targeting by proteasomes that cleave it into the active form of p52. The N-terminal portion of the p100 molecule is considered to be the active form, which translocates to the nucleus along with RelB. Meanwhile, the inhibitory C-terminal portion of p100 is degraded (2). Once in the nucleus, NF-κB initiates the transcription of a diverse, but limited repertoire of target genes, such as the chemokines CXCL12 and CXCL13 (3, 6) (Figure 2).
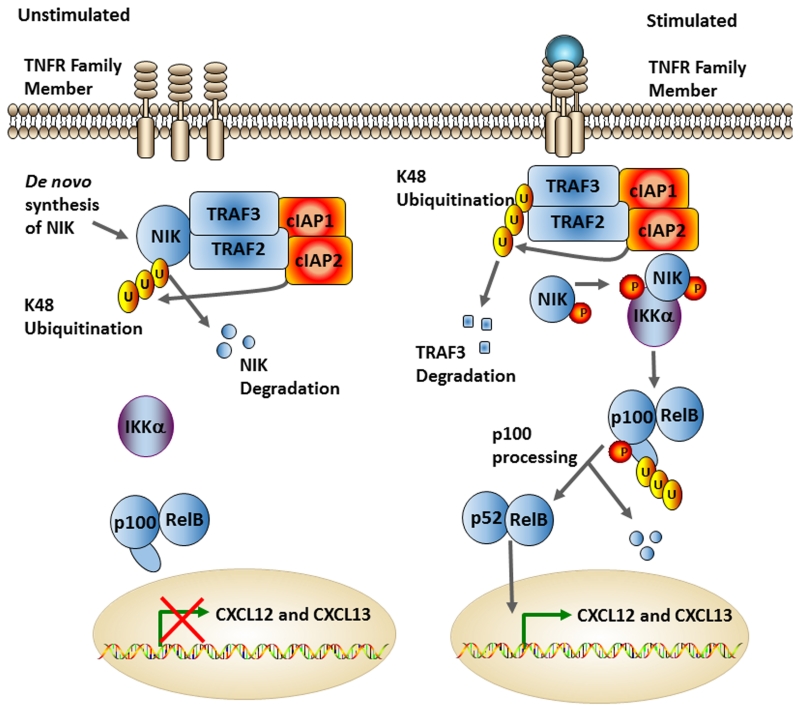
Figure 2. The Non-Canonical NF-κB Pathway.
NF-κB inducing kinase (NIK) is constantly being translated. However, under normal unstimulated conditions, NIK is ubiquitinated and degraded via the TRAF3/TRAF2/cIAP1/cIAP2 complex. Upon stimulation by TNF family ligands, this complex is degraded via K48 ubiquitination, which allows NIK to interact with and phosphorylate IKKα. IKKα then phosphorylates p100, leading to its cleavage to p52. The processing of p100 allows the RelB/p52 dimer to enter the nucleus and initiate transcription of non-canonical NF-κB associated genes, such as CXCL12 and CXCL13.
One of the major biological functions of the non-canonical NF-κB pathway is the development and organization of secondary lymphoid structures, such as Peyer’s patches in the gut (3). Mice lacking the lymphotoxin β receptor (LTβR) and mice with a mutation in NIK (Aly), which completely blocks non-canonical NF-κB signaling, lack lymph nodes and Peyer’s patches (7, 8). Peyer’s patches are critical for the maintenance of immune system homeostasis in the gastrointestinal tract. For example, dendritic cells isolated from Peyer’s patches are significantly more efficient in T cell activation compared with dendritic cells from other tissues. Indeed, murine dendritic cells isolated from Peyer’s patches have been shown to prime OVA TCR transgenic T cells to secrete fivefold higher levels of the anti-inflammatory cytokines IL-4 and IL-10 compared to dendritic cells from the spleen (9, 10). In addition to dendritic cells, gut macrophages from Peyer’s patches are also important for the regulation of the immune response to commensal components of the microflora. This was best illustrated using macrophages isolated from the jejunum and Peyer’s patches of normal patients undergoing tissue resection. These macrophages were challenged with LPS, which revealed that gut macrophages had much lower levels of the pro-inflammatory cytokines IL-1, IL-6, and TNF compared to monocytes (10, 11).
Beyond the generation of these secondary lymphoid structures in the gut, the non-canonical NF-κB signaling pathway is also associated with a myriad of inflammatory disorders. For example, it has been shown that loss of non-canonical NF-κB signaling through the disruption of NIK can lead to the development of a systemic inflammatory condition in mice known as hypereosinophilic syndrome-like disease (12). Hypereosinophilic syndrome (HES) is a family of inflammatory diseases characterized by an increase in eosinophils without a known cause. Hacker et al. (2012) showed that Nik−/− mice develop a progressive HES-like disorder characterized by eosinophilia, tissue destruction and premature death. Interestingly, they found that this disease progresses independent of IKKα phosphorylation because mice containing a point mutation in IKKα (IKKαAA/AA) did not show the classical signs of HES characteristic of the NIK deficient mice (12).
The non-canonical NF-κB signaling cascade is relatively understudied in the context of IBD. However, as new data emerges related to this alternative signaling cascade, the importance of this pathway in maintaining immune system homeostasis in the gut is becoming more evident. In addition to controlling the development of secondary lymphoid structures in mucosal tissues, recent studies have also found that non-canonical NF-κB signaling regulates T-cell differentiation and function (13, 14), IgA class switching (15, 16), cell migration (17), chemokine production (18), and interferon signaling (19) through mechanisms that are distinct from canonical NF-κB signaling. In essence, this signaling cascade is likely to influence IBD pathobiology through multiple mechanisms. This review focuses on our current knowledge of emerging concepts associated with the activation, regulation, and clinical relevance of non-canonical NF-κB signaling in maintaining immune system homeostasis in the gut. In addition to synthesizing recent findings related to the non-canonical NF-κB pathway and IBD, we also discuss potential therapeutic strategies and targets associated with this understudied signaling cascade.
STARTING THE ENGINE: NON-CANONICAL NF-κB STIMULATORY LIGANDS AND RECEPTORS
The first step of the non-canonical NF-κB pathway involves the recognition of a TNF family signaling molecule, such as TNF, CD40L, lymphotoxin β, or BAFFR. TNF is a potent pro-inflammatory cytokine produced by many leukocytes, including macrophages, lymphoid cells and mast cells. TNF is a type II transmembrane protein that signals through the TNF receptor (TNFR) family membrane receptors. Two receptors are known to bind TNF, TNFRI and TNFRII. TNFRI is constitutively expressed; whereas, TNFRII is highly regulated. TNF signaling is associated with diverse biological effects, including inflammation, cell differentiation and cell death. TNF signaling is typically associated with canonical NF-κB activation. However, TNF also activates the non-canonical pathway via TNFRI stimulation, which leads to TRAF2 degradation, NIK stabilization, IKKα phosphorylation and p100 processing (20). There is clearly functional interplay between the canonical and non-canonical NF-κB signaling pathways following TNFRI stimulation. This appears to be, at least in part, mediated by the receptor-interacting protein 1 (RIP1), which promotes TNF-mediated activation of the canonical pathway while functioning as a negative regulator of the non-canonical pathway (20). Mouse embryonic fibroblasts from Rip1−/− mice are highly sensitive to TNF induced cell death at early timepoints due to deficient canonical NF-κB signaling; whereas late activation of the non-canonical NF-κB cascade in these Rip1−/− cells appears to protect against cell death (20). Thus, it appears that RIP1 may function as a molecular switch between TNF induced canonical and non-canonical NF-κB signaling. It is clear that TNF and its receptors play a role in IBD pathogenesis. In both human patients and in IBD animal studies, increased colonic expression of TNF and both TNF receptors are routinely observed and heavily involved in IBD pathogenesis. In fact, compared to healthy controls, TNF levels are much higher in the stool and serum of patients with both UC and CD (21-23).
Traditionally, corticosteroids and immunomodulators were the preferred treatment strategies for patients with IBD (24). However, recently the use of anti-TNF monoclonal antibodies (mAbs) have shown significant promise (22, 24, 25). Infliximab was one of the first anti-TNF treatment to be used for IBD and was found to be very effective at inducing mucosal healing in patients with UC (22, 24-26). One of these early clinical trials revealed that 69% of patients who received 5 mg of infliximab and 61% who received 10 mg had a clinical response after 8 weeks of treatment. In contrast, only 37% of those who received placebo had a clinical response (26). Mucosal healing was significantly improved in patients who were given infliximab at 8, 30 and 54 weeks (26). In addition to Infliximab, adalimumab and certolizumab are also highly effective anti-TNF mAbs currently in use to treat IBD. The effects of these anti-TNF therapeutics on non-canonical NF-κB signaling has not been directly explored. However, the reduction in TNF signaling mediated by these therapeutics would almost certainly impact both canonical and non-canonical NF-κB signaling.
It is clear that TNF is a major contributing factor to IBD pathogenesis. However, this inflammatory mediator is only one piece of the puzzle that links the non-canonical NF-κB pathway with IBD. For example, in addition to TNF, CD40 is also a potent initiator of non-canonical NF-κB signaling and can dramatically impact IBD pathogenesis through multiple mechanisms. CD40 is a type I transmembrane protein and a member of the TNF-receptor superfamily that is primarily found on B cells and antigen-presenting immune cells, such as dendritic cells and macrophages. CD40 is activated through interactions with CD40 ligand (CD40L), which is a type II transmembrane protein expressed primarily by activated CD4+ T cells and platelets. The interaction between CD40 on B cells and CD40L on T cells is critical for the proliferation and differentiation of B cells and plays an important role in the regulation of inflammation. Meanwhile, ligation of CD40 on macrophages and dendritic cells increases the production of cytokines and acts as a survival signal (27). In the context of IBD, intestinal lamina propria T cell (LP-T cell) expression of CD40L plays an important role in pathogenesis (27). LP-T cells from inflamed mucosa do indeed express functional CD40L, which has been shown to induce production of IL-12 and TNF in monocytes, leading to increased inflammation in the gut (27). This LP-T cell mediated increase in monocyte cytokine expression can be attenuated using mAbs that block the CD40-CD40L interaction (27). For example, blockade of CD40 and CD40L in LP-T cell and monocyte co-cultures with the mAbs M90 and 5D12, respectively, resulted in a decrease in expression of IL-12 and TNF (27). The treatment with M90 led to a 39-100% inhibition of IL-12 and 34-100% inhibition of TNF. While the inclusion of 5D12 was associated with a 26-100% inhibition of IL-12 and a 33-100% inhibition of TNF production (27). This indicates a potential therapeutic strategy for IBD involving attenuation of the CD40-CD40L interactions. Indeed, it is clear that CD40 signaling underlies many diverse biological processes that are associated with IBD. Due to the potency of CD40 activation on non-canonical NF-κB signaling, it is highly likely that dysregulation of the non-canonical pathway underlies many of the pathobiological effects that have been attributed to CD40L-CD40 during disease.
The contribution of this CD40L/CD40/non-canonincal NF-κB axis in maintaining gastrointestinal health, immune system homeostasis in the gut, and as a mechanism associated with IBD has not been adequately explored. This is particularly true for cell populations beyond the immune system. While the best characterized functions for CD40/CD40L are associated with their roles in leukocyte function, expression of these proteins are not limited to immune cells. For example, there are many non-immune cell types, such as epithelial cells, that also express CD40 (28). One indication of this is seen in the increased expression of CD40 and CD40L in endoscopically obtained biopsies from patients with IBD (27, 29). In these studies, intestinal epithelial cells (IECs) of the colon lacked fluorescent staining of CD40 in healthy control patients. However, CD40 expression was significantly upregulated in IECs from patients with active IBD (29). In addition to IEC expression of CD40, platelets express CD40L and play a role in the inflammatory response and tissue injury in the gut (30). This is indicated by the fact that unstimulated platelets isolated from patients with active IBD have significantly increased levels of CD40L compared to platelets from normal healthy controls (30). In fact, unstimulated platelets from healthy subjects showed low expression of CD40L (30). Following thrombin stimulation, CD40L expression increased in both groups; however, a significant increase was observed between UC and CD groups compared to platelets isolated from healthy controls (30).
It has been suggested that this interaction between platelets and IBD is most likely via the activation of the mucosal microvasculature. Indeed, changes in the vasculature often accompany inflammation in response to various factors including vascular endothelial growth factor (VEGF). Angiogenesis and increased permeability are among the most common changes that occur during an inflammatory response. Typically, IBD pathogenesis is accompanied by these vasculature changes, mostly in the form of increased expression of cellular adhesion molecules (CAMs) and chemokine secretion via endothelial cells (31). This inflammatory-driven angiogenesis has been shown to occur in both CD and UC (29). As evidence of this, mucosal extracts and plasma of patients with active IBD have been reported to have higher levels of VEGF-A, as indicated by enzyme-linked immunosorbent assay (ELISA) (31). It is postulated that the CD40-CD40L interaction is crucial in mediating vascular changes in the inflamed mucosa. These changes are also accompanied by an increase in cellular adhesion molecules to allow leukocyte-endothelial cell interactions. Human intestinal microvascular endothelial cells (HIMEC) co-cultured with CD40L-positive platelets from normal donors increases the expression of both intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) (30). These adhesion molecules are expressed on endothelial and immune cells and are responsible for binding integrins, which allow the translocation of immune cells from the circulation to sites of inflammation. This increase in CAM expression is even stronger in co-cultures that contain platelets from IBD patients, indicating an important role for platelet-activated endothelium in IBD. Interestingly, blockade of CD40L with a neutralizing antibody resulted in a decrease in the upregulation of these adhesion molecules (30).
In addition to TNF and CD40, Lymphotoxin β-receptor (LTβR) is another TNF superfamily receptor involved in the activation of non-canonical NF-κB signaling (3). As mentioned earlier, one of the functions of LTβR is the development of Peyer’s patches in the gut. This was shown in prior studies utilizing mice deficient in LTβR (7). These mice also show a failure of normal B and T cell segregation, indicating a role of LTβR in the development of peripheral lymphoid tissue (7, 32). The role of LTβR in IBD has been previously characterized in the Citrobacter rodentium infection model of IBD mucosal inflammation. C. rodentium infection results in epithelial hyperplasia and mild to moderate inflammation of the colonic mucosa that can be used as a murine model for some aspects of IBD (33). C. rodentium infection causes RORγt+ cells to synthesize lymphotoxin (LT) which binds to LTβR on epithelial cells, resulting in the release of chemokines, such as CXCL-1 and CXCL-2, that recruit pro-inflammatory neutrophils and macrophages (34, 35). When mice conditionally deficient in LTβR in intestinal epithelial cells are infected orally with C. rodentium they display a deficiency in clearing the infection and increased pathology (34). This indicates a role for LTβR in the defense against mucosal pathogens in the gut. These data could be extended to IBD, suggesting that LTβR plays a protective role in the human condition.
CD40, LTβR, and TNFR are the most commonly studied initiators of non-canonical NF-κB signaling and most likely to directly influence IBD pathogenesis. However, another TNF superfamily receptor-ligand interaction is worth noting and has also been shown to be correlated with IBD, albeit through a more indirect mechanism. Receptor Activator of Nuclear factor κB (RANK) and its ligand RANKL also stimulate non-canonical NF-κB signaling (36). The stimulation of the non-canonical pathway via this route is most notable for promoting osteoclastogenesis and is associated with bone loss in humans particularly with relation to chronic gut inflammation (37, 38). Consistent with these prior findings, aly/aly mice that lack NIK and non-canonical signaling, show a baseline osteopetrosis, suggesting a defect in osteoclasts and bone remodeling (39). This defect was traced to the bone marrow in the mice, with RANK-L mediated generation of osteoclasts being impaired. Transfection of p100 or p52 coupled with RANKL stimulation abolished this impairment. Overexpression of RelB also abolished these changes in another aly/aly model (40). The RANKL-controlled osteoprotegerin (OPG) system has been shown to be dysregulated in UC and CD patients, and is correlated with increased bone loss in patient sub-groups (41). Indeed, bone loss is not uncommon in patients with chronic IBD, along with a variety of concurrent risk factors being identified including corticosteroid use, smoking, and vitamin D deficiency. Interestingly, treatment with infliximab is associated with improvements in bone metabolism (42). The exact mechanism linking RANKL/RANK/OPG, mucosal immunology, and bone remodeling has been investigated in several mouse models. Using IL-2 deficient mice, which develop a spontaneous autoimmune chronic colitis, bone loss was shown to be mediated via upregulation of RANKL, which resulted in promotion of intestinal dendritic cell survival and immune system activation (43). Recently, RANKL-RANK has also been shown to be critical in the development of regulatory T cells in models of chronic colitis (44). Immunodeficienct mice were administered adoptively transferred CD4+ effector T cells (CD4(+)CD45RB(high)) in order to induce T cell driven colitis, as well as, doses of CD4+CD25+CD45RBlo T regulatory cells, which function to suppress the effector-mediated colitis. The administration of anti-RANK mAbs negated the ability of T regulatory cells to suppress the effector cell driven colitis. Further underlining RANKL/RANKs effects in dendritic cells of the gut as suggested in IL-2 deficient mice (44). As with the other activating signals, there is a high likelihood that dysregulated non-canonical NF-κB signaling underlies many aspects of osteoclastogenesis associated with RANKL/RANK and may also be associated with increased bone loss in IBD patient sub-groups.
Finally, B cell Activating Factor (BAFF) and its partner BAFFR, are critical for non-canonical NF-κB signaling and are associated with B cell development and maturation (45). Although classically considered a stimulatory ligand for the canonical NF-κB pathway when it interacts with its normal partner BAFFR, BAFF can have additional regulatory effects on non-canonical signaling when it interacts with its other partner, the transmembrane activator and CAML interactor (TACI) (46). TACI signaling upon BAFF binding results in suppression of non-canonical signaling and upregulation of cIAP1, apparently by the formation of a multicomponent interaction with TANK, cIAP1, and TRAF2, which inhibits NIK (46). Studies focusing on BAFF and BAFFR specifically in the context of IBD are scarce, despite the involvement of B cells in IBD. However, suppression of BAFFR and TACI at the mRNA level, with concurrent hypo-methylation of their respective genes TNFRSF13B and TNFRSF13C, has been seen in the duodenal mucosa of dogs with IBD using methylation specific PCR (47). While BAFFR expression has been reported to be suppressed in canine IBD patients, BAFF itself has been observed to be upregulated at the mRNA level and is correlated with decreased in mucosal IgA and TGF-β (48). It would be highly interesting if the findings from these veterinary patients can be extended to human IBD.
Overall, the major ligands and receptors of non-canonical NF-κB signaling have a variety of effects ranging from adaptive immune cell development to skeletal remodeling; however, they all share a common link back to mucosal immunology, with several having been already implicated in IBD pathogenesis. The interaction between ligand and receptor is only the beginning of the signaling cascade. Non-canonical signaling also has an eclectic repertoire of positive and negative regulators that serve to fine-tune the pathway as needed, and are essential given the tightly-controlled nature of this alternative pathway. Thus, the failure of one or more molecules downstream may lead to significant loss of immune system homeostasis in the gut.
WORKING LIKE A WELL-OILED MACHINE: POSITIVE REGULATION OF NON-CANONICAL NF-κB SIGNALING
Non-canonical signaling is maintained by a variety of positive regulators that can affect various points of the pathway. These positive regulators typically target receptor-ligand interactions at the cell membrane, remove negative regulatory complexes, stabilize pathway components, and directly activate key signaling molecules. For example, a member of the tumor necrosis factor family, tumor necrosis factor-like weak inducer of apoptosis (TWEAK), activates NF-κB via interactions with the inducible cell-surface receptor Fn14 (Figure 3). TWEAK/Fn14 signaling can upregulate both canonical and non-canonical NF-κB signaling. However, under physiological conditions TWEAK/Fn14 signaling appears to primarily target the non-canonical pathway (49, 50). Specifically, TWEAK triggers p100 processing via Fn14 and has been associated with the production of non-canonical cytokines, such as CCL21 (50, 51). TWEAK signaling acts as a balance to cIAP1/2, inducing the degradation of the TRAF2, TRAF3, and cIAP1/2 complex to re-sensitize the cells to TNF (52) (Figure 3). Indeed, a TWEAK signaling complex consisting of FN14, TRAF2, and cIAP1 has been suggested (52).
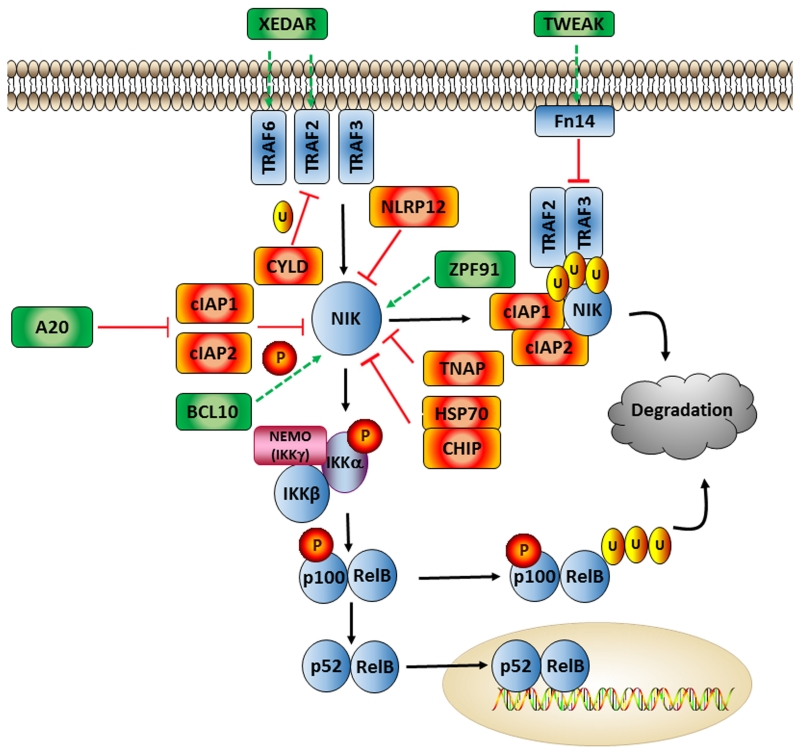
Figure 3. Major Positive and Negative Regulators of Non-canonical NF-κB Signaling.
Much of the regulation of the non-canonical NF-κB pathway revolves around stabilization or degradation of NIK. cIAP1/cIAP2 are the most well-known suppressors of NIK activity, forming a complex with TRAF2 and TRAF3, resulting in ubiquitination and proteosomal degradation. NLRP12 performs a similar function by binding to NIK and enhancing the formation of this complex. HSP70 also downregulates NIK via its partner CHIP, and TNAP interacts with NIK, TRAF2, and TRAF3 and inhibits NIK’s kinase activity. CYLD negatively regulates non-canonical NF-κB signaling further upstream, by ubiquinating TRAF2 and therefore inhibiting CD40 and XEDAR signaling. In terms of activators, XEDAR activates non-canonical NF-κB signaling and stimulates NIK by binding to TRAF6 and TRAF2. Also at the receptor level, TWEAK/Fn14 binding disassociates the ubiquitination complex itself and frees NIK. A20 binds to cIAP1/2 and also prevents proper NIK degradation. BCL10 controls the phosphorylation of NIK and promotes its activity, while ZPF91 binds directly to NIK to augment function.
TWEAK/Fn14 has been implicated in IBD (53, 54). In human UC patients, TWEAK levels are upregulated at the mRNA level in the intestinal mucosa along with Fn14 and IL-13 (55). Mechanistically, TWEAK appears to modulate damage to the intestinal epithelium associated with the production of IL-13 (55). In the gut, increased IL-13 has been shown to be associated with the loss of β-catenin in mouse primary intestinal explants and shown to disturb tight junction formation between epithelial cells (55). However, these responses were not seen in FN14 KO mice, suggesting that a loss of TWEAK/Fn14 signaling may actually be protective during IBD (55). Further support of this hypothesis was shown when TWEAK signaling was ablated, either through gene KO or mAbs. In both cases, TWEAK attenuation resulted in decreased progression of experimental colitis in a TNBS mouse model (56).
Similar to TWEAK, A20 represents another well studied regulatory protein in canonical and non-canonical NF-κB signaling. A20 is a ubiquitin-editing enzyme and can function to actually suppress signaling in the canonical NF-κB pathway, often along with a second subset of molecules termed A20 binding inhibitors of NF-κB or ABINS (57, 58). Although this mechanism isn’t fully understood, it is thought that A20 exerts its inhibitory effects via the disruption of TNFRI (59). However, in the non-canonincal NF-κB signaling cascade, A20 is associated with the activation of signaling. Under normal unstimulated conditions, the cIAP1/cIAP2/TRAF2/TRAF3 complex functions to continuously poly-ubiquitinate NIK in order to induce its degradation and repress the non-canonical pathway (Figure 2). However, A20 activation is induced by TNF family members and results in the suppression of the complex (60). Upon activation, A20 binding results in the interruption and disassociation of the cIAP1/cIAP2/TRAF2/TRAF3 complex and subsequent stabilization of NIK, which facilitates the activation of non-canonical signaling (60). Mice deficient in A20 develop severe hyper-inflammation and are extremely sensitive to both LPS and TNF (61). Mice that lack A20 in their enterocytes display increased susceptibility to experimental colitis and their enterocytes are particularly sensitive to TNF-driven apoptotic signals (62). In humans, the A20 locus contains several mutations that are associated with a diverse range of autoimmune and inflammatory conditions, including IBD (63, 64).
As with TWEAK and A20, B-cell CLL/lymphoma 10 (BCL10) is another molecule that significantly regulates non-canonical NF-κB signaling. BCL10 promotes NIK phosphorylation, rather than controlling the level of NIK, resulting in downstream p100/p52 processing (65). Mutations in BCL10, particularly those affecting the critical phosphorylation site at Ser(138), can directly affect downstream non-canonical NF-κB signaling (66, 67) (Figure 3). Studies in human intestinal epithelial cells have repeatedly shown BCL10’s necessity for inflammatory cytokine production and both canonical and non-canonical NF-κB activation. These effects are also seen in in vivo models using Bcl10−/− mice. When exposed to carrageenan, an inflammatory food additive that stimulates both the canonical and non-canonical NF-κB pathways, Bcl10−/− mice display decreased non-canonical activity, including decreased nuclear RelB and NIK levels (68). Additionally, the paracaspase MALT1, which acts as a partner to BCL10, also contributes to this non-canonical regulation. Lack of MALT1 in B cells results in decreased p100 phosphorylation and processing that is normally induced by BAFF, resulting in decreased survival of marginal zone B cells (69). Interestingly, in this same study, MALT activity was shown to be largely dispensable for canonical NF-κB activation.
Several non-canonical NF-κB regulatory proteins have roles in maintaining gastrointestinal homeostasis and cell growth, but have not been directly evaluated in the context of IBD. One of these is X-linked ectodermal dysplasia receptor (XEDAR, also known as EDAR2 or TNFRSF27), a lesser-known TNFR family member that has recently been shown to be an activator of the non-canonical NF-κB pathway (70). XEDAR requires the kinase activity of NIK and IKKα to bind TRAF2 and TRAF6 (70). This results in the processing of p100 and is negatively regulated by molecules such as cIAP1, A20, and TRAF3 (Figure 3). Although XEDAR has not been investigated in IBD, it has been shown to be greatly upregulated in the gastrointestinal mucosa of nonhuman primates and mice after radiation exposure (71, 72). This may have functional implications in IBD, where mucosal epithelial cell turnover and barrier integrity are critical events in the inflammatory process. In addition it serves as a link between constant renewal, proliferation and dysplastic potential. Another regulator of non-canoincal NF-κB signaling, but not directly evaluated in the context of IBD, is Zinc finger protein 91 (ZFP91) (73). ZFP91 is an E3 ubiquitin ligase that has the ability to bind directly to NIK, promoting its stabilization and downstream non-canonical signaling (Figure 3). ZFP91 is also essential in CD40-mediated NIK activation in vitro and plays a critical role in LIGHT (homologous to lymphotoxins, inducible expression, competes with herpesvirus glycoprotein D for herpesvirus entry mediator, a receptor expressed on T lymphocytes/LTβR interactions, leading to activation of non-canonical NF-κB signaling (74). In fact, knockdown of ZFP91 resulted in cessation of nuclear P52/RelB DNA binding, but has no effect on canonical p65 signaling. It remains unclear whether the mechanism of action is a direct promotion of NIK’s kinase activity, or rather a result of NIK stabilization. ZFP91 has not been investigated in mucosal immunology; however, due to its role in T-cell activation, it is highly likely that this pathway contributes to gastrointestinal immune responses and inflammation.
MAINTENANCE AND INSPECTION: NON-CANONICAL NF-κB PROCESSING AND EFFECTOR MOLECULES
The processing of p100 into p52 and subsequent translocalization of the p52/RelB heterodimer into the nucleus to activate gene transcription is the final step in the non-canonical NF-κB signaling cascade (75). This process is highly regulated compared to p105/p65 signaling, with only minimal constitutive action (76). In common mouse models of experimental colitis, wild type mice given DSS show a significant up-regulation of p100/p52 processing that is indicative of increased activation of the non-canonical NF-κB signaling cascade (77). Likewise, the generation of p100 is controlled by the gene Nfkb2, which has also been implicated in the pathobiology of IBD. The majority of data supporting a role for Nfkb2 in IBD was generated using mouse models of experimental colitis and colitis associated tumorigenesis. For example, Nfkb2−/− mice develop less severe colitis and dampened cytokine responses following DSS exposure, which results in fewer colonic tumors and attenuated tumorigenesis in the AOM/DSS model of colitis-associated cancer (78). Not surprisingly, aberrant p100/p52 expression has been seen in a variety of neoplasms and has been shown to interact with the critical proliferation protein cyclin D1, cooperating with p53 to express targeted proliferation and cell cycle genes (79). Together, these results are strong indicators that non-canonical signaling can have significant effects on both IBD and inflammation-driven tumorigenesis in the colon.
In addition to p100/p52, RelB is also an essential molecule in this final step of non-canonical NF-κB signaling. RelB dimerizes with p52 and functions to translocate p52 into the nucleus. All Rel proteins share a highly conserved 300 amino acid Rel homology domain (RHD), which includes domains involved in dimerization, nuclear localization, and DNA-binding. Significant inflammatory cell infiltration has been reported in several organs, including the GI tract, in Relb−/− mice (80). RelB deficiency is associated with significant defects in acquired and innate immunity, increased T-cell infiltration in the organs and severe skin inflammation (80-82). Together, these data illustrate the importance of RelB in maintaining immune system homeostasis and indicates that a lack of RelB function cannot be compensated for by any of the other Rel family members. Because of the specificity for RelB to non-canonical NF-κB signaling, it is commonly used as a cellular marker of activation of the alternative pathway.
RelB/p52 nuclear translocation results in the upregulation of a diverse group of pro-inflammatory cytokines and chemokines that are commonly associated with IBD pathobiology. The best characterized chemokines associated with IBD and attributed to non-canonical NF-κB activation are Chemokine (C-X-C Motif) Ligand 12 (CXCL12) and 13 (CXCL13) (6, 83). Indeed, both human and mouse studies have provided evidence supporting a strong role for the pleiotropic chemokine CXCL12 (Stromal-cell Derived Factor-alpha; SDF1-α) and its receptor CXCR4 in the pathogenesis of IBD. The genes encoding both CXCL12 and CXCR4 are ubiquitously expressed in a diverse range of cell types, including intestinal epithelial cells (84, 85). In the context of IBD, CXCL12 and CXCR4 are expressed in intestinal epithelial cells during homeostasis and differentially up-regulated in IBD patients, suggesting that CXCL12 is both constitutive and inflammatory during IBD (85). In addition to the intestinal epithelial cells, increased numbers of circulating immature plasma cells from IBD patients have also been found to have significantly higher expression of CXCR4 compared to healthy controls (86). Beyond gene expression studies, findings from genetic association studies have also identified polymorphisms in CXCL12 associated with disease progression in a Polish IBD patient population (87). This study reported a significant association between 3 mutations associated with the CXCL12/CXCR4 axis between UC patients and CD patients, compared to healthy subjects (87). Here, the authors concluded that having combinations of these 3 polymorphisms in the CXCL12/CXCR4 axis may significantly predispose individuals to the development of IBD.
Two notable studies have investigated the CXCL12 and CXCR4 interaction within the context of experimental murine colitis. The first employed a blockade of this pathway using a CXCR4 antagonist, which resulted in amelioration of disease in wild-type mice in the DSS experimental colitis model and in the IL-10 knockout mouse model (88). In both models, colitic mice not given the CXCR4 antagonist exhibited increased disease progression (88). However, the administration of the CXCR4 antagonist reduced TNF and IFN-γ production, independently of any effects on IL-10 generation and T cell differentiation (88). The second study also utilized pharmacological blockade of the CXCL12/CXCR4 axis. Here, the authors used a non-peptide antagonist AMD3100 (89). Administration of AMD3100 reduced colitis, prevented defects in intestinal permeability, and lowered cytokine production, including IL6 and TNF (89). Together, these mouse studies imply that increased CXCL12/CXCR4 signaling contributes to worsening of IBD pathogenesis. Conversely, a rat study that used lentiviral transduction to overexpress CXCR4 in mesenchymal stem cells, which were subsequently engrafted, showed a protective function for CXCR4 in a 2,4,6-trinitrobenzene sulfonic acid (TNBS) model (90). Engraftment of CXCR4 overexpressing bone marrow-derived mesenchymal stem cells (BMSCs) resulted in significant amelioration of both clinical and microanatomical severity of TNBS induced colitis (90). The mechanism associated with these findings indicated that CXCR4 overexpression resulted in more efficient migration of BMSCs to the inflamed colon and a suppression of pro-inflammatory cytokines in the injured colon associated with reduced STAT3 phosphorylation (90). The seemingly contradictory findings could simply reflect the systematic attenuation of the CXCL12/CXCR4 axis in the mouse models versus the immune cell specific overexpression of the axis in the rat model. Regardless, together with the human data, these findings indicate a complex and highly significant role for CXCL12/CXCR4 in IBD.
Similar to CXCL12, CXCL13 (BCA-1; BLC) and its receptor CXCR5 have also been shown up-regulated in IBD patients. A study evaluating CXCL13 (BCA-1) expression in frozen biopsy sections of normal and UC patients, and found that expression of CXCL13 was not only increased in inflamed tissue, but also highly expressed in both the peripheral elements of normal GALT patches and the abnormal lymphoid aggregates of UC patients (91). Consistent with these findings, mouse studies have shown that CXCL13 and CXCR5 are significantly involved in normal secondary lymphoid organ development (92). In mouse models, CXCL13 is significantly upregulated in various colitis models (93, 94). For example, increased transient pup consumption of n-6 fatty acid has recently been shown to reduce the severity of DSS induced experimental colitis in mice, likely through significantly altering the microbiome composition (94). In the transiently n-6-fed mice with attenuated disease progression, decreased numbers of CXCR5+ CD4+ T cells were detected in the mesenteric lymph nodes (94). Subsequent follow-up studies using this model revealed that antibody treatment with anti-CXCL-13 decreased the severity of DSS colitis and revealed critical roles for the CXCL13/CXCR5 axis in the progression of IBD (94). Indeed, elevated levels of CXCL13 were found in the serum of untreated pediatric IBD patients, linking the mouse findings to human subjects (94). Together, these data suggest that CXCL13/CXCR5 play an important role in the gastrointestinal tract, particularly in local mucosal lymphoid tissue, and in the pathogenesis of IBD.
In addition to CXCL12 and CXCL13, several C-C motif ligand chemokines are also regulated through the non-canonical NF-κB signaling pathway. For example, Chemokine (C-C motif) ligand 19 (CCL19; MIP-3-β; ELC) transcription is mediated through the non-canonical pathway and signals through the CCR7 receptor (95). The binding of CCL19 to CCR7 results in T cell and DC homing to T cell zones of lymphoid tissue. Little is known about CCL19’s importance in IBD, as the majority of research into this chemokine has focused on its function in the lymphoid system and various neoplastic conditions, including colorectal cancer. CCL19 expression is decreased in colorectal cancer, which is highly interesting, given the propensity of IBD patients to develop dysplasia (96). However, in CD patients, dendritic cells show increased CCL19 and CCR7 expression (97). Specifically, inflamed colonic tissue showed increased numbers of mature myeloid dendritic cells that expressed high levels of both CCR7 and CCL19 (97). Similar to CCL19, Chemokine (C-C motif) ligand 21 (CCL21) is another product of the non-canonical pathway. CCL21 also binds to CCR7 and functions as a potent lymphocyte chemoattractant. Interestingly, CCL21 has been shown to be upregulated in the serum of IBD patients, as well as, in reticular cells and lymphatic vessels in CD patients in concert with the CCR7/CCL19-expressing dendritic cells mentioned previously (97, 98). However, while the dysregulation of CCL19 and CCL21 appear to be associated with IBD, mechanistic insight associated with their exact roles in modulating disease pathogenesis is currently lacking.
PUTTING ON THE BRAKES: NEGATIVE REGULATION OF NON-CANONICAL NF-κB SIGNALING
Control of overzealous inflammation is critical to maintain immune system homeostasis in the gastrointestinal system. Attenuation of hyper-inflammation can be achieved through a variety of mechanisms, including the attenuation of NF-κB. Over the last decade, a variety of proteins have been characterized that function to negatively regulate both canonical and non-canonical NF-κB signaling. For example, a sub-group of the nucleotide-binding domain and leucine-rich-repeat containing (NBD-LRR; NLR) family of pattern recognition receptors have been shown to function as negative regulators of inflammation through attenuation of NF-κB signaling. NLRP12 is a member of this regulatory NLR sub-group that has been shown to reduce both canonical and non-canonical pathways. In the context of non-canonical NF-κB signaling, NLRP12 has been shown to bind to NIK and TRAF3 (6, 83). This binding is thought to result in the stabilization of TRAF3 and ultimately NIK degradation (6, 83). This mechanism is critical during IBD, where Nlrp12−/− mice have significantly increased non-canonical NF-κB signaling in models of experimental colitis and during colitis associated tumorigenesis (83). In acute and chronic models of experimental colitis utilizing DSS, Nlrp12−/− mice demonstrated increased morbidity and mortality, with significantly increased inflammation and damage to the epithelial barrier compared to wild type animals (83). While some changes were observed in the canonical NF-κB signaling cascade, a significant increase in non-canonical NF-κB signaling was observed in the Nlrp12−/− mice and strongly associated with disease pathogenesis (83, 99). Both NIK levels and p100 processing to p52 were found to be significantly increased in colonic explants from Nlrp12−/− mice (83). Furthermore, primary murine myeloid dendritic cells taken from Nlrp12−/− showed sustained and increased levels of NIK following activation with TNF compared to wild type cells. Downstream of NIK and p100 to p52 processing, a significant increase in non-canonical NF-κB associated chemokines were detected in the Nlrp12−/− mice, including CXCL12 and CXCL13 (83). Together, these data strongly support a role for NLRP12 in the attenuation of overzealous inflammation associated with IBD and further identify the critical nature of non-canonical NF-κB signaling in disease pathobiology.
The interaction between NLRP12, NIK, and TRAF3 support the hypothesis that NLRP12 negatively regulates NF-κB signaling through the formation of a multiprotein complex. Indeed, other NLRs in this functional subgroup, such as NLRC3, have also been suggested to form a multiprotein complex termed the “TRAFasome” (100). Multiprotein complexes are common players in major immunologic pathways, including the non-canonical NF-κB cascade. For example, a central inhibitory complex has been described that includes cIAP1/2, which act as negative regulatory molecules that suppress TNF-mediated NF-κB activation (101, 102). Mechanistically, a ubiquitin ligase complex consisting of TRAF2, TRAF3, and cIAP1/2 catalyzes the ubiquitination and degradation of NIK (103, 104). This is a potent negative regulatory mechanism, as stabilization of cIAP1 through this multi-protein complex limits downstream non-canonical activity, even during active TNF stimulation (105). cIAP1 and cIAP2 have become increasingly associated with IBD (106). For example, upregulation of cIAP2 has been seen in the colonic epithelium of ulcerative colitis patients (107, 108). Specifically, cIAP2 mRNA was detected at a significantly elevated rate in primary human cells isolated from colonocytes grown from biopsies of UC patients compared to control patients and UC patients in remission (108). In another study, biopsies from patients with active UC and control patients were evaluated and cIAP2 was upregulated in the regenerating colonocytes of the UC group (107, 108). Interestingly, the activity of cIAP1 and cIAP2 can be modulated by another sub-group of regulatory NLRs that function as positive regulators of inflammation. This NLR subgroup includes NOD1 and NOD2. Both of these NLRs are pro-inflammatory and function through the formation of a large macromolecular complex termed the “NODosome” (109). This complex modulates a variety of inflammatory signaling pathways, including activation of the NF-κB cascade. In human IBD patients, mutations in NOD2 have been highly associated with disease pathogenesis in specific sub-populations (110). These mutations in NOD2 are some of the best characterized in the context of disease and are routinely evaluated in IBD patients. In vitro work showed that macrophages from cIAP1 and cIAP2 deficient mice displayed defective NOD signaling, as did colonocytes deprived of cIAP1 or cIAP2 by iRNA (111). In mouse studies done by the same group, administration of the NOD2 agonist Muramyldipeptide (MDP) protected wild type mice, but not cIAP2-deficient mice, in models of experimental colitis (111), suggesting the protective role of NOD2 in IBD may be cIAP2 dependent.
TRAF3 is associated with the TRAF2-TRAF3-cIAP1-cIAP2 complex and has been shown to directly interact with NIK (103). In human IBD patients, TRAF3 was detected at significantly higher levels in plasma, peripheral blood mononuclear cells (PBMCs), and biopsies of inflamed and normal colon tissue from patients with IBD compared to healthy controls (112). In mouse models, depletion of TRAF3 results in constitutively activated non-canonical NF-κB signaling. For example, TRAF3 depleted B cells demonstrate constitutive p100 processing (113). Traf3−/− mice do not survive into adulthood. However, fetal liver transplants have been successfully utilized to study TRAF3 function and the postnatal lethality of Traf3−/− could be rescued by the additional loss of p100. Traf3−/−/p100−/− mice were reported to grow at normal rates and survive into adulthood (113). In addition to these conventional TRAF3 deficient mice, cell type specific Traf3−/− animals have also been generated. For example, myeloid cell-specific Traf3−/− mice have been characterized as developing spontaneous and chronic inflammation and increased tumorigenesis in multiple organs, including the colon (114). Similar to TRAF3, TRAF2 deletion has also been demonstrated to result in hyperactive non-canonical NF-κB signaling. Western blot analysis detected elevated p100 processing and RelB/p52 DNA binding in B cells isolated from mice lacking TRAF2 (115). Traf2−/− mice develop spontaneous, severe colitis as early as 10 days after birth, and succumb within 3 weeks of age (116). Interestingly, the administration of antibiotics to the drinking water of nursing mothers was able to partially rescue the Traf2−/− pups from the colitis symptoms (116). Together these data emphasize the importance of the interactions between the TRAF2/TRAF3/cIAP1/cIAP2 complexes with elements of the non-canonical NF-κB signaling cascade in IBD.
Positive and negative modulation of ubiquitination is an important post-translational regulatory mechanism for the control of non-canonical NF-κB signaling. While cIAP1/2, TRAF2, and TRAF3 all play important roles in ubiquination, deubiquitination is also highly relevant. For example, CYLD is a deubiquitinase that acts as a negative regulator of the NF-κB pathway (Figure 3). It plays a role in the regulation of canonical signaling via its interactions with NEMO (117, 118). Likewise, CYLD is also a significant negative regulator of the non-canonical signaling cascade via modulation of TRAF2 (119). A negative regulatory role of CYLD for NF-κB signaling was suggested after it was proven that expression of CYLD in HEK cells inhibited the activity of co-expressed NF-κB activators (119). Ubiquitination of TRAF2 by CYLD was found to be nearly abolished in the presence of a dominant negative ubiquitin-specific protease mutant deficient in CYLD (119). Indeed there appears to be a reciprocal autoregulation between NF-κB and CYLD, with induction of the latter also requiring the former (120). Upon stimulation with NF-κB activators TNF and nontypeable Haemophilus influenzae (NTHi), CYLD expression increased in the presence of functional IKKβ and NEMO (120). Because of the implications of TRAF2 as a negative regulator of CYLD, a TRAF2 dominant negative mutant was utilized and shown that when stimulated with TNF, the induction of CYLD was negated (120). CYLD’s role in mucosal immunity is also gaining traction. Its expression is reduced in human colorectal carcinomas (121). Likewise, genome wide association studies (GWAS) have detected mutations associated with CYLD as a risk factor for IBD (122). In animal studies, Cyld−/− mice showed prominent increases in the size and number of mucosal lymphoid tissue proliferation and hypertrophy, and eventually these animals develop spontaneous colitis with inflammatory cell infiltrate, crypt damage, and increases in pro-inflammatory cytokines (123). The spontaneous colitis observed in these Cyld−/− mice appeared consistent with features associated with human disease, including histological tissue damage, increased expression of pro-inflammatory cytokines IL-4 and IL-12, and weight loss and clinical symptoms. CYLD’s regulatory effects seem to be primarily via T cells, given that Cyld−/− T cells are hyper-responsive to TCR stimuli and Rag1−/− mice given Cyld−/− T cells develop a significant immune-mediated colitis compared to those given wild type T cells (123). Of note, the Rag1−/− mice adoptively transferred Cyld−/− T cells demonstrated increased lymphocyte infiltration into the periportal regions of the liver, infiltration of inflammatory cells in the colon, thickening of mucosa, and goblet cell depletion compared to mice transferred wild type T cells (123). Consistent with the expression data from human colorectal carcinoma patients, Cyld−/− mice also show increased sensitivity in AOM/DSS models of inflammation driven colon tumorigenesis (124). This was observed as an increase in leukocyte infiltration, histologic damage, mucosal ulcers, and dysplasia to colonic epithelium in the Cyld−/− mice compared to the wild type animals after one 21-day cycle exposure to AOM/DSS. Following a second 21-day cycle with AOM/DSS, the Cyld−/− mice presented with an increase in colonic tumors compared to wild type mice (124). Increased NF-κB signaling was associated with the mechanism underlying the increase in colitis and cancer in these CYLD deficient animals (124).
Heat shock 70 kDa (Hsc70) is another regulator of non-canonical NF-κB signaling that appears to function through the modulation of ubiquitination. Hsc70 is a positive regulator of cell cycle transition and carcinogenesis, regulating nuclear accumulation of cyclin D1 along with Connexin43 (Cx43) (125). It is a stress-induced molecule that is both constitutively expressed (Hsc70c) and inducible (Hsc70i), and interacts with an E3 ubiquitin ligase partner, called C-terminus of Hsc70 Interacting Protein (CHIP) (126). CHIP has been shown to a downregulate NIK and the non-canonical NF-κB pathway (127). Hsc70 has been linked to IBD both at the cellular and clinical level, particularly with ulcerative colitis (128). Both CD and UC patients show strong immunostaining of this protein in intestinal biopsies, especially in epithelial cells (128) and expression is decreased during treatment and remission (129, 130). This epithelial cell expression is mimicked in vitro, with induction of Hsc70 resulting in protection of intestinal epithelial cells from oxidative damage (131). In the mucosa of CD patients, but not UC, there is additional increased expression of Hsc70 in submucosal and mucosal mononuclear cells, potentially indicating a unique leukocyte-focused mechanism of Hsc70 in CD versus UC (128). Hsc70 expression is also associated with increasing colonic dysplasia (129), a significant lesion in IBD that can lead to colorectal cancer. Compared to tissue biopsies taken at initial diagnosis, six months after treatment there was a significant increase in Hsc70 expression in the colonic mucosa of patients who were unresponsive to treatment in both inflamed and dysplastic tissue (129). Overall, these findings suggest that during active damage and disease, Hsc70 may be activated in an attempt to curtail non-canonical signaling. In addition to Hsp70 inhibiting NIK signaling, other Heat shock proteins can actually stabilize NIK and are suggested to have a role in IBD. For example, Hsp90 binds to and stabilizes NIK in order to protect it from autophagy (132). Geldanamycin, an anti-tumor agent that inhibits Hsc90, was found to decrease p100 processing in a dose-dependent manner and was associated with decreased levels of NIK (132). Hsp90 is elevated in UC patients suggesting that it may prolong non-canonical NF-κB signaling and augment IBD pathogenesis (129).
Although there is no current literature directly detailing an association between TRAF and NIK-associated protein (TNAP) and F-Box and WD Repeat Domain Containing 7, E3 Ubiquitin Protein Ligase (FBXW7) in IBD, these two additional molecules also negatively regulate non-canonical NF-κB activity. TNAP has been shown to be down-regulated during both canonical and non-canonical signaling (133). In regards to non-canonical signaling, TNAP interacts with NIK, TRAF2, and TRAF3 to inhibit NIK’s kinase activity, resulting in suppression of downstream p100 processing through both knockdown and overexpression studies (133) (Figure 3). It does not, however, appear to affect NIK stability, only its activity. Although TNAP has not been characterized in immune-mediated disease, it is interesting to speculate that its ability to inhibit NIK plays a major role in the attenuation of disease pathology. Likewise, little is known regarding the role of FBXW7 in IBD. FBXW7 binds to NF-κB2 and has previously been characterized as a tumor suppressor (134). FBXW7 interacts with and binds to substrates via interaction between the degron motif on the substrate and its WD40 repeats (134). FBXW7 ubiquitylates p100 and promotes its degradation in a GSK3β-dependent manner (135). The inactivation of FBXW7 at the cellular level was shown to result in elevated p100 levels, and may therefore be interpreted as an increase in non-canonical NF-κB activity (135). Despite the fact that there is currently no evidence in the literature evaluating its involvement in IBD, some data have concluded that FBXW7 is downregulated in gastric cancer (136) and is inversely correlated with the tumor promoter ENO1 in colorectal cancer (137). In addition to TNAP and FBXW7, an assortment of other miscellaneous molecules have been shown to bind to NIK including epidermal growth factor (EGF) and its receptor (EGFR) (138), caspase death domains (139) and the protein of the proto-oncogene Cot/Tpl-2 (140), although only the effects on the canonical NF-κB pathway has been fully characterized for these additional proteins.
CONCLUSIONS
Given the powerful effects of TNF family members on inflammation, it is unsurprising that the majority of approved therapies for IBD target one or more of these molecules. Infliximab, adalimumab, and certolizumab are all anti-TNF mAbs currently approved for Crohn’s disease and are often used when more moderate medications, such as immunosuppressants, fail to result in a better quality of life for the patient. Under optimal conditions, these drugs have great efficacy in changing the clinical course of the disease. However, the effects are variable and often temporary. Indeed, the high risk of patients becoming nonresponsive to these treatments is of great concern amongst clinicians. Although the majority of patients initially respond well, only one-third of patients on biologics are still in remission after 1 year without steroids (141). Even in those patients who do not completely lose responsiveness, increasing dosages in order to maintain clinical remission is often required. Losing this efficacy is exceptionally problematic, as IBD is a chronic and progressive disease that can result in significant surgical intervention and complications over the course of the patient’s lifetime.
In addition, as TNF ligand-receptor interactions are important in non-canonical NF-κB signaling, the effects of these treatments on non-canonical signaling is a question worthy of investigation. For example, it is not currently clear if these current therapeutics target or suppress the non-canonical NF-κB cascade to the same extent as the canonincal pathway. Given that this signaling cascade is tightly regulated, as opposed to the rapid and constitutive expression of the canonical signaling pathway, it is not unreasonable to assume that the non-canonical NF-κB cascade significantly contributes to IBD at multiple stages of disease pathogenesis. It is our opinion that the contribution of this pathway to IBD pathobiology has been significantly overlooked, due to the high level of interest in canonical signaling. Because of the paucity of data regarding non-canonical signaling in IBD, it is our belief that a deeper investigation of this pathway and its components is warranted. Further research will certainly provide additional insight into disease pathogenesis. It is interesting to speculate that further mechanistic studies associated with the non-canonical NF-κB pathway will identify novel therapeutic targets, something that is greatly in demand for patients and clinicians alike.
Acknowledgments
SUPPORT:
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Numbers K01DK092355 and R03DK105975. Student work on this publication was supported by the National Institute of Allergy and Infectious Diseases Animal Model Research for Veterinarians (AMRV) training grant (T32-OD010430). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported through the VA-MD College of Veterinary Medicine.
REFERENCES
- 1.Betts JC, Nabel GJ. Differential regulation of NF-kappaB2(p100) processing and control by amino-terminal sequences. Mol Cell Biol. 1996;16:6363–6371. doi: 10.1128/mcb.16.11.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun SC. Controlling the fate of NIK: a central stage in noncanonical NF-kappaB signaling. Sci Signal. 2010;3:pe18. doi: 10.1126/scisignal.3123pe18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao G, Zhang M, Harhaj EW, et al. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 5.Ling L, Cao Z, Goeddel DV. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci U S A. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lich JD, Williams KL, Moore CB, et al. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178:1256–1260. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
- 7.Kanai T, Ilyama R, Ishikura T, et al. Role of the innate immune system in the development of chronic colitis. J Gastroenterol. 2002;37(Suppl 14):38–42. doi: 10.1007/BF03326411. [DOI] [PubMed] [Google Scholar]
- 8.Shinkura R, Kitada K, Matsuda F, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker H, Chi L, Rehg JE, et al. NIK prevents the development of hypereosinophilic syndrome-like disease in mice independent of IKKalpha activation. J Immunol. 2012;188:4602–4610. doi: 10.4049/jimmunol.1200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh NC, Waters LR, Fowler JA, et al. LKB1 inhibition of NF-kappaB in B cells prevents T follicular helper cell differentiation and germinal center formation. EMBO Rep. 2015;16:753–768. doi: 10.15252/embr.201439505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onder L, Nindl V, Scandella E, et al. Alternative NF-kappaB signaling regulates mTEC differentiation from podoplanin-expressing presursors in the cortico-medullary junction. Eur J Immunol. 2015;45:2218–2231. doi: 10.1002/eji.201545677. [DOI] [PubMed] [Google Scholar]
- 15.Jin J, Xiao Y, Chang JH, et al. The kinase TBK1 controls IgA class switching by negatively regulating noncanonical NF-kappaB signaling. Nat Immunol. 2012;13:1101–1109. doi: 10.1038/ni.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bram RJ. TBK1 suppression of IgA in the NIK of time. Nat Immunol. 2012;13:1027–1029. doi: 10.1038/ni.2451. [DOI] [PubMed] [Google Scholar]
- 17.Nottingham LK, Yan CH, Yang X, et al. Aberrant IKKalpha and IKKbeta cooperatively activate NF-kappaB and induce EGFR/AP1 signaling to promote survival and migration of head and neck cancer. Oncogene. 2014;33:1135–1147. doi: 10.1038/onc.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kew RR, Penzo M, Habiel DM, et al. The IKKalpha-dependent NF-kappaB p52/RelB noncanonical pathway is essential to sustain a CXCL12 autocrine loop in cells migrating in response to HMGB1. J Immunol. 2012;188:2380–2386. doi: 10.4049/jimmunol.1102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin J, Hu H, Li HS, et al. Noncanonical NF-kappaB pathway controls the production of type I interferons in antiviral innate immunity. Immunity. 2014;40:342–354. doi: 10.1016/j.immuni.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JY, Morgan M, Kim DG, et al. TNFalpha induced noncanonical NF-kappaB activation is attenuated by RIP1 through stabilization of TRAF2. J Cell Sci. 2011;124:647–656. doi: 10.1242/jcs.075770. [DOI] [PubMed] [Google Scholar]
- 21.Cartagene C, Flores I, Appleyard C. Role of tumor necrosis factor receptors in an animal model of acute colitis. Cytokine. 2005;32:9. doi: 10.1016/j.cyto.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Heel DAv, Udalova IA, Dilva APD, et al. Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF-kB transcription factors. Human Molecular Genetics. 2002;11:9. doi: 10.1093/hmg/11.11.1281. [DOI] [PubMed] [Google Scholar]
- 23.Braegger C, Nicholis S, Murch S, et al. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:3. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 24.Danese S, Colombel J, Peyrin-Biroulet L, et al. Review article: the role of anti-TNF in the management of ulcerative colitis -- past, present and future. Alimentary Pharmacology and Therapeutics. 2013;37:11. doi: 10.1111/apt.12284. [DOI] [PubMed] [Google Scholar]
- 25.Peyrin-Biroulet L. Anti-TNF therapy in inflammatory bowel diseases: a huge review. Minerva Gastroenteroligica E Dietologica. 2010;56:11. [PubMed] [Google Scholar]
- 26.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for Induction and Maintenance Therapy for Ulcerative Colitis. The New England Journal of Medicine. 2005;353 doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Colpaert S, D’Haens GR, et al. Hyperexpression of CD40 Ligand (CD154) in Inflammatory Bowel Disease and Its Contribution to Pathogenic Cytokine. Production Journal of Immunology. 1999;163:9. [PubMed] [Google Scholar]
- 28.Gelbmann CM, Leeb SN, Vogl D, et al. Inducible C40 expression mediates NFkB activation and cytokine secretion in human colonic fibroblasts. Gut. 2003;52:9. doi: 10.1136/gut.52.10.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danese S, Scaldaferri F, Vetrano S, et al. Critical role of the CD40-CD40-ligand pathway in regulating mucosal inflammation-driven angiogenesis in inflamamtory bowel disease. Gut. 2007;56:9. doi: 10.1136/gut.2006.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danese S, Motte CDL, Sturm A, et al. Platelets Trigger a CD40-Dependent Inflammatory Response in the Microvasculature of Inflammatory Bowel Disease Patients. Gastroenterology. 2003;124:16. doi: 10.1016/s0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 31.Scaldaferri F, Vetrano S, Sans M, et al. VEGF-A Links Angiogenesis and Inflammation in Inflammatory Bowel Disease Pathogenesis. Gastroenterology. 2009;136:16. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 32.Togni PD, Goellner J, Ruddle N, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:5. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 33.Lei L, Shi Q, Fang L, et al. Cytokine IL-6 is required for Citrobacter rodentium infection-induced intestinal Th17 and promotes IL-22 expression in inflamamtory bowel disease. Molecular Medicine Reports. 2014;9:6. doi: 10.3892/mmr.2014.1898. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Koroleva EP, Kruglov AA, et al. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity. 2010;32:23. doi: 10.1016/j.immuni.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends in Immunology. 2011;32:9. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Novack DV, Yin L, Hagen-Stapleton A, et al. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada T, Nakashima T, Hiroshi N, et al. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Tilg H, Moschen AR, Kaser A, et al. Gut, inflammation and osteoporosis: basic and clinical concepts. Gut. 2008;57:684–694. doi: 10.1136/gut.2006.117382. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama T, Fukushima H, Nakao K, et al. Processing of the NF-kappa B2 precursor p100 to p52 is critical for RANKL-induced osteoclast differentiation. J Bone Miner Res. 2010;25:1058–1067. doi: 10.1359/jbmr.091032. [DOI] [PubMed] [Google Scholar]
- 40.Cardoso MP, Pasternak J, Giglio AE, et al. Meningitis due to Haemophilus influenzae type f. Einstein (Sao Paulo) 2013;11:521–523. doi: 10.1590/S1679-45082013000400020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moschen AR, Kaser A, Enrich B, et al. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut. 2005;54:479–487. doi: 10.1136/gut.2004.044370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lima CA, Lyra AC, Rocha R, et al. Risk factors for osteoporosis in inflammatory bowel disease patients. World J Gastrointest Pathophysiol. 2015;6:210–218. doi: 10.4291/wjgp.v6.i4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashcroft AJ, Cruickshank SM, Croucher PI, et al. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity. 2003;19:849–861. doi: 10.1016/s1074-7613(03)00326-1. [DOI] [PubMed] [Google Scholar]
- 44.Totsuka T, Kanai T, Nemoto Y, et al. RANK-RANKL signaling pathway is critically involved in the function of CD4+CD25+ regulatory T cells in chronic colitis. J Immunol. 2009;182:6079–6087. doi: 10.4049/jimmunol.0711823. [DOI] [PubMed] [Google Scholar]
- 45.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanno Y, Sakurai D, Hase H, et al. TACI induces cIAP1-mediated ubiquitination of NIK by TRAF2 and TANK to limit non-canonical NF-kappaB signaling. J Recept Signal Transduct Res. 2010;30:121–132. doi: 10.3109/10799891003634509. [DOI] [PubMed] [Google Scholar]
- 47.Maeda S, Ohno K, Fujiwara-Igarashi A, et al. Methylation of TNFRSF13B and TNFRSF13C in duodenal mucosa in canine inflammatory bowel disease and its association with decreased mucosal IgA expression. Vet Immunol Immunopathol. 2014;160:97–106. doi: 10.1016/j.vetimm.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Maeda S, Ohno K, Uchida K, et al. Decreased immunoglobulin A concentrations in feces, duodenum, and peripheral blood mononuclear cells of dogs with inflammatory bowel disease. J Vet Intern Med. 2013;27:47–55. doi: 10.1111/jvim.12023. [DOI] [PubMed] [Google Scholar]
- 49.Enwere EK, Holbrook J, Lejmi-Mrad R, et al. TWEAK and cIAP1 regulate myoblast fusion through the noncanonical NF-kappaB signaling pathway. Sci Signal. 2012;5:ra75. doi: 10.1126/scisignal.2003086. [DOI] [PubMed] [Google Scholar]
- 50.Roos C, Wicovsky A, Muller N, et al. Soluble and transmembrane TNF-like weak inducer of apoptosis differentially activate the classical and noncanonical NF-kappa B pathway. J Immunol. 2010;185:1593–1605. doi: 10.4049/jimmunol.0903555. [DOI] [PubMed] [Google Scholar]
- 51.Sanz AB, Sanchez-Nino MD, Izquierdo MC, et al. TWEAK activates the non-canonical NFkappaB pathway in murine renal tubular cells: modulation of CCL21. PLoS One. 2010;5:e8955. doi: 10.1371/journal.pone.0008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vince JE, Chau D, Callus B, et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol. 2008;182:171–184. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slebioda TJ, Kmiec Z. Tumour necrosis factor superfamily members in the pathogenesis of inflammatory bowel disease. Mediators Inflamm. 2014;2014:325129. doi: 10.1155/2014/325129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dohi T, Burkly LC. The TWEAK/Fn14 pathway as an aggravating and perpetuating factor in inflammatory diseases: focus on inflammatory bowel diseases. J Leukoc Biol. 2012;92:265–279. doi: 10.1189/jlb.0112042. [DOI] [PubMed] [Google Scholar]
- 55.Kawashima R, Kawamura YI, Oshio T, et al. Interleukin-13 damages intestinal mucosa via TWEAK and Fn14 in mice-a pathway associated with ulcerative colitis. Gastroenterology. 2011;141:2119–2129. e2118. doi: 10.1053/j.gastro.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 56.Dohi T, Borodovsky A, Wu P, et al. TWEAK/Fn14 pathway: a nonredundant role in intestinal damage in mice through a TWEAK/intestinal epithelial cell axis. Gastroenterology. 2009;136:912–923. doi: 10.1053/j.gastro.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 57.Heyninck K, De Valck D, Vanden Berghe W, et al. The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN. J Cell Biol. 1999;145:1471–1482. doi: 10.1083/jcb.145.7.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verstrepen L, Carpentier I, Verhelst K, et al. ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling. Biochem Pharmacol. 2009;78:105–114. doi: 10.1016/j.bcp.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 59.He KL, Ting AT. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol Cell Biol. 2002;22:6034–6045. doi: 10.1128/MCB.22.17.6034-6045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamaguchi N, Oyama M, Kozuka-Hata H, et al. Involvement of A20 in the molecular switch that activates the non-canonical NF-small ka, CyrillicB pathway. Sci Rep. 2013;3:2568. doi: 10.1038/srep02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee EG, Boone DL, Chai S, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vereecke L, Sze M, Mc Guire C, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207:1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Wellcome Trust Case Control C Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhattacharyya S, Borthakur A, Tyagi S, et al. B-cell CLL/lymphoma 10 (BCL10) is required for NF-kappaB production by both canonical and noncanonical pathways and for NF-kappaB-inducing kinase (NIK) phosphorylation. J Biol Chem. 2010;285:522–530. doi: 10.1074/jbc.M109.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhattacharyya S, Borthakur A, Anbazhagan AN, et al. Specific effects of BCL10 Serine mutations on phosphorylations in canonical and noncanonical pathways of NF-kappaB activation following carrageenan. Am J Physiol Gastrointest Liver Physiol. 2011;301:G475–486. doi: 10.1152/ajpgi.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhattacharyya S, Borthakur A, Dudeja PK, et al. Lipopolysaccharide-induced activation of NF-kappaB non-canonical pathway requires BCL10 serine 138 and NIK phosphorylations. Exp Cell Res. 2010;316:3317–3327. doi: 10.1016/j.yexcr.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhattacharyya S, Xue L, Devkota S, et al. Carrageenan-induced colonic inflammation is reduced in Bcl10 null mice and increased in IL-10-deficient mice. Mediators Inflamm. 2013;2013:397642. doi: 10.1155/2013/397642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tusche MW, Ward LA, Vu F, et al. Differential requirement of MALT1 for BAFF-induced outcomes in B cell subsets. J Exp Med. 2009;206:2671–2683. doi: 10.1084/jem.20091802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verhelst K, Gardam S, Borghi A, et al. XEDAR activates the non-canonical NF-kappaB pathway. Biochem Biophys Res Commun. 2015;465:275–280. doi: 10.1016/j.bbrc.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 71.Zheng J, Wang J, Pouliot M, et al. Gene expression profiling in non-human primate jejunum, ileum and colon after total-body irradiation: a comparative study of segment-specific molecular and cellular responses. BMC Genomics. 2015;16:984. doi: 10.1186/s12864-015-2168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng J, Garg S, Wang J, et al. Laser capture microdissected mucosa versus whole tissue specimens for assessment of radiation-induced dynamic molecular and pathway changes in the small intestine. PLoS One. 2013;8:e53711. doi: 10.1371/journal.pone.0053711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin X, Jin HR, Jung HS, et al. An atypical E3 ligase zinc finger protein 91 stabilizes and activates NF-kappaB-inducing kinase via Lys63-linked ubiquitination. J Biol Chem. 2010;285:30539–30547. doi: 10.1074/jbc.M110.129551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin HR, Jin X, Lee JJ. Zinc-finger protein 91 plays a key role in LIGHT-induced activation of non-canonical NF-kappaB pathway. Biochem Biophys Res Commun. 2010;400:581–586. doi: 10.1016/j.bbrc.2010.08.107. [DOI] [PubMed] [Google Scholar]
- 75.Scheinman RI, Beg AA, Baldwin AS., Jr. kappa B p100 (Lyt-10) is a component of H2TF1 and can function as an I kappa B-like molecule. Mol Cell Biol. 1993;13:6089–6101. doi: 10.1128/mcb.13.10.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heusch M, Lin L, Geleziunas R, et al. The generation of nfkb2 p52: mechanism and efficiency. Oncogene. 1999;18:6201–6208. doi: 10.1038/sj.onc.1203022. [DOI] [PubMed] [Google Scholar]
- 77.Hollenbach E, Neumann M, Vieth M, et al. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 2004;18:1550–1552. doi: 10.1096/fj.04-1642fje. [DOI] [PubMed] [Google Scholar]
- 78.Burkitt MD, Hanedi AF, Duckworth CA, et al. NF-kappaB1, NF-kappaB2 and c-Rel differentially regulate susceptibility to colitis-associated adenoma development in C57BL/6 mice. J Pathol. 2015;236:326–336. doi: 10.1002/path.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schumm K, Rocha S, Caamano J, et al. Regulation of p53 tumour suppressor target gene expression by the p52 NF-kappaB subunit. EMBO J. 2006;25:4820–4832. doi: 10.1038/sj.emboj.7601343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weih F, Carrasco D, Durham SK, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 81.Burkly L, Hession C, Ogata L, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 82.Freyschmidt EJ, Mathias CB, MacArthur DH, et al. Skin inflammation in RelB(-/-) mice leads to defective immunity and impaired clearance of vaccinia virus. J Allergy Clin Immunol. 2007;119:671–679. doi: 10.1016/j.jaci.2006.12.645. [DOI] [PubMed] [Google Scholar]
- 83.Allen IC, Wilson JE, Schneider M, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Werner L, Guzner-Gur H, Dotan I. Involvement of CXCR4/CXCR7/CXCL12 Interactions in Inflammatory bowel disease. Theranostics. 2013;3:40–46. doi: 10.7150/thno.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dotan I, Werner L, Vigodman S, et al. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16:583–592. doi: 10.1002/ibd.21106. [DOI] [PubMed] [Google Scholar]
- 86.Hosomi S, Oshitani N, Kamata N, et al. Increased numbers of immature plasma cells in peripheral blood specifically overexpress chemokine receptor CXCR3 and CXCR4 in patients with ulcerative colitis. Clin Exp Immunol. 2011;163:215–224. doi: 10.1111/j.1365-2249.2010.04290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mrowicki J, Przybylowska-Sygut K, Dziki L, et al. The role of polymorphisms of genes CXCL12/CXCR4 and MIF in the risk development IBD the Polish population. Mol Biol Rep. 2014;41:4639–4652. doi: 10.1007/s11033-014-3335-y. [DOI] [PubMed] [Google Scholar]
- 88.Xia XM, Wang FY, Xu WA, et al. CXCR4 antagonist AMD3100 attenuates colonic damage in mice with experimental colitis. World J Gastroenterol. 2010;16:2873–2880. doi: 10.3748/wjg.v16.i23.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mikami S, Nakase H, Yamamoto S, et al. Blockade of CXCL12/CXCR4 axis ameliorates murine experimental colitis. J Pharmacol Exp Ther. 2008;327:383–392. doi: 10.1124/jpet.108.141085. [DOI] [PubMed] [Google Scholar]
- 90.Liu X, Zuo D, Fan H, et al. Over-expression of CXCR4 on mesenchymal stem cells protect against experimental colitis via immunomodulatory functions in impaired tissue. J Mol Histol. 2014;45:181–193. doi: 10.1007/s10735-013-9541-4. [DOI] [PubMed] [Google Scholar]
- 91.Carlsen HS, Baekkevold ES, Johansen FE, et al. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut. 2002;51:364–371. doi: 10.1136/gut.51.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 93.Abad C, Juarranz Y, Martinez C, et al. cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm Bowel Dis. 2005;11:674–684. doi: 10.1097/01.mib.0000171872.70738.58. [DOI] [PubMed] [Google Scholar]
- 94.Nagy-Szakal D, Mir SA, Harris RA, et al. Loss of n-6 fatty acid induced pediatric obesity protects against acute murine colitis. FASEB J. 2015;29:3151–3159. doi: 10.1096/fj.14-267690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoshida R, Imai T, Hieshima K, et al. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–13809. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- 96.Lu J, Zhao J, Feng H, et al. Antitumor efficacy of CC motif chemokine ligand 19 in colorectal cancer. Dig Dis Sci. 2014;59:2153–2162. doi: 10.1007/s10620-014-3138-y. [DOI] [PubMed] [Google Scholar]
- 97.Middel P, Raddatz D, Gunawan B, et al. Increased number of mature dendritic cells in Crohn’s disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55:220–227. doi: 10.1136/gut.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh UP, Singh NP, Murphy EA, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44–49. doi: 10.1016/j.cyto.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zaki MH, Vogel P, Malireddi RK, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schneider M, Zimmermann AG, Roberts RA, et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-kappaB. Nat Immunol. 2012;13:823–831. doi: 10.1038/ni.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahoney DJ, Cheung HH, Mrad RL, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Varfolomeev E, Goncharov T, Fedorova AV, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zarnegar BJ, Wang Y, Mahoney DJ, et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vallabhapurapu S, Matsuzawa A, Zhang W, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gentle IE, Wong WW, Evans JM, et al. In TNF-stimulated cells, RIPK1 promotes cell survival by stabilizing TRAF2 and cIAP1, which limits induction of non-canonical NF-kappaB and activation of caspase-8. J Biol Chem. 2016;291:2547. doi: 10.1074/jbc.A110.216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pedersen J, LaCasse EC, Seidelin JB, et al. Inhibitors of apoptosis (IAPs) regulate intestinal immunity and inflammatory bowel disease (IBD) inflammation. Trends Mol Med. 2014;20:652–665. doi: 10.1016/j.molmed.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 107.Seidelin JB, Vainer B, Andresen L, et al. Upregulation of cIAP2 in regenerating colonocytes in ulcerative colitis. Virchows Arch. 2007;451:1031–1038. doi: 10.1007/s00428-007-0517-1. [DOI] [PubMed] [Google Scholar]
- 108.Seidelin JB, Nielsen OH. Expression profiling of apoptosis-related genes in enterocytes isolated from patients with ulcerative colitis. APMIS. 2006;114:508–517. doi: 10.1111/j.1600-0463.2006.apm_116.x. [DOI] [PubMed] [Google Scholar]
- 109.Tattoli I, Travassos LH, Carneiro LA, et al. The Nodosome: Nod1 and Nod2 control bacterial infections and inflammation. Semin Immunopathol. 2007;29:289–301. doi: 10.1007/s00281-007-0083-2. [DOI] [PubMed] [Google Scholar]
- 110.Lin Z, Hegarty JP, John G, et al. NOD2 mutations affect muramyl dipeptide stimulation of human B lymphocytes and interact with other IBD-associated genes. Dig Dis Sci. 2013;58:2599–2607. doi: 10.1007/s10620-013-2696-8. [DOI] [PubMed] [Google Scholar]
- 111.Bertrand MJ, Doiron K, Labbe K, et al. Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity. 2009;30:789–801. doi: 10.1016/j.immuni.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 112.Shen J, Qiao YQ, Ran ZH, et al. Up-regulation and pre-activation of TRAF3 and TRAF5 in inflammatory bowel disease. Int J Med Sci. 2013;10:156–163. doi: 10.7150/ijms.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He JQ, Zarnegar B, Oganesyan G, et al. Rescue of TRAF3-null mice by p100 NF-kappa B deficiency. J Exp Med. 2006;203:2413–2418. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lalani AI, Moore CR, Luo C, et al. Myeloid cell TRAF3 regulates immune responses and inhibits inflammation and tumor development in mice. J Immunol. 2015;194:334–348. doi: 10.4049/jimmunol.1401548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grech AP, Amesbury M, Chan T, et al. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 116.Piao JH, Hasegawa M, Heissig B, et al. Tumor necrosis factor receptor-associated factor (TRAF) 2 controls homeostasis of the colon to prevent spontaneous development of murine inflammatory bowel disease. J Biol Chem. 2011;286:17879–17888. doi: 10.1074/jbc.M111.221853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kovalenko A, Chable-Bessia C, Cantarella G, et al. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 118.Brummelkamp TR, Nijman SM, Dirac AM, et al. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 119.Trompouki E, Hatzivassiliou E, Tsichritzis T, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 120.Jono H, Lim JH, Chen LF, et al. NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway. J Biol Chem. 2004;279:36171–36174. doi: 10.1074/jbc.M406638200. [DOI] [PubMed] [Google Scholar]
- 121.Hellerbrand C, Bumes E, Bataille F, et al. Reduced expression of CYLD in human colon and hepatocellular carcinomas. Carcinogenesis. 2007;28:21–27. doi: 10.1093/carcin/bgl081. [DOI] [PubMed] [Google Scholar]
- 122.Dinu I, Mahasirimongkol S, Liu Q, et al. SNP-SNP interactions discovered by logic regression explain Crohn’s disease genetics. PLoS One. 2012;7:e43035. doi: 10.1371/journal.pone.0043035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reiley WW, Jin W, Lee AJ, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J, Stirling B, Temmerman ST, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest. 2006;116:3042–3049. doi: 10.1172/JCI28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hatakeyama T, Dai P, Harada Y, et al. Connexin43 functions as a novel interacting partner of heat shock cognate protein 70. Sci Rep. 2013;3:2719. doi: 10.1038/srep02719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith MC, Scaglione KM, Assimon VA, et al. The E3 ubiquitin ligase CHIP and the molecular chaperone Hsc70 form a dynamic, tethered complex. Biochemistry. 2013;52:5354–5364. doi: 10.1021/bi4009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiang B, Shen H, Chen Z, et al. Carboxyl terminus of HSC70-interacting protein (CHIP) down-regulates NF-kappaB-inducing kinase (NIK) and suppresses NIK-induced liver injury. J Biol Chem. 2015;290:11704–11714. doi: 10.1074/jbc.M114.635086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ludwig D, Stahl M, Ibrahim ET, et al. Enhanced intestinal expression of heat shock protein 70 in patients with inflammatory bowel diseases. Dig Dis Sci. 1999;44:1440–1447. doi: 10.1023/a:1026616221950. [DOI] [PubMed] [Google Scholar]
- 129.Abou El Azm AR, Yousef M, Kobtan A, et al. Colonic mucosal expression of heat-shock proteins may have a potential prognostic value in ulcerative colitis. Arab J Gastroenterol. 2015;16:20–24. doi: 10.1016/j.ajg.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 130.Tomasello G, Sciume C, Rappa F, et al. Hsp10, Hsp70, and Hsp90 immunohistochemical levels change in ulcerative colitis after therapy. Eur J Histochem. 2011;55:e38. doi: 10.4081/ejh.2011.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Musch MW, Ciancio MJ, Sarge K, et al. Induction of heat shock protein 70 protects intestinal epithelial IEC-18 cells from oxidant and thermal injury. Am J Physiol. 1996;270:C429–436. doi: 10.1152/ajpcell.1996.270.2.C429. [DOI] [PubMed] [Google Scholar]
- 132.Qing G, Yan P, Qu Z, et al. Hsp90 regulates processing of NF-kappa B2 p100 involving protection of NF-kappa B-inducing kinase (NIK) from autophagy-mediated degradation. Cell Res. 2007;17:520–530. doi: 10.1038/cr.2007.47. [DOI] [PubMed] [Google Scholar]
- 133.Hu WH, Mo XM, Walters WM, et al. TNAP, a novel repressor of NF-kappaB-inducing kinase, suppresses NF-kappaB activation. J Biol Chem. 2004;279:35975–35983. doi: 10.1074/jbc.M405699200. [DOI] [PubMed] [Google Scholar]
- 134.Hao B, Oehlmann S, Sowa ME, et al. Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol Cell. 2007;26:131–143. doi: 10.1016/j.molcel.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 135.Arabi A, Ullah K, Branca RM, et al. Proteomic screen reveals Fbw7 as a modulator of the NF-kappaB pathway. Nat Commun. 2012;3:976. doi: 10.1038/ncomms1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li H, Wang Z, Zhang W, et al. Fbxw7 regulates tumor apoptosis, growth arrest and the epithelial-to-mesenchymal transition in part through the RhoA signaling pathway in gastric cancer. Cancer Lett. 2016;370:39–55. doi: 10.1016/j.canlet.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 137.Zhan P, Wang Y, Zhao S, et al. FBXW7 negatively regulates ENO1 expression and function in colorectal cancer. Lab Invest. 2015;95:995–1004. doi: 10.1038/labinvest.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen D, Xu LG, Chen L, et al. NIK is a component of the EGF/heregulin receptor signaling complexes. Oncogene. 2003;22:4348–4355. doi: 10.1038/sj.onc.1206532. [DOI] [PubMed] [Google Scholar]
- 139.Chaudhary PM, Eby MT, Jasmin A, et al. Activation of the NF-kappaB pathway by caspase 8 and its homologs. Oncogene. 2000;19:4451–4460. doi: 10.1038/sj.onc.1203812. [DOI] [PubMed] [Google Scholar]
- 140.Lin X, Cunningham ET, Jr., Mu Y, et al. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-kappaB acting through the NF-kappaB-inducing kinase and IkappaB kinases. Immunity. 1999;10:271–280. doi: 10.1016/s1074-7613(00)80027-8. [DOI] [PubMed] [Google Scholar]
- 141.Peyrin-Biroulet L, Deltenre P, de Suray N, et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–653. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]