Abstract
Background
The repair of rotator cuff tears is often complicated by fatty degeneration, which is the combination of lipid accumulation, fibrosis, inflammation and muscle weakness. p38 MAPK is a signaling molecule that plays a central role in these processes. The purpose of this study was to evaluate a small molecule inhibitor of p38 MAPK, SB203580, in reducing fatty degeneration in a preclinical model of rotator cuff injury and repair.
Methods
Adult rats underwent a bilateral supraspinatus tenotomy that was repaired 30 days later. Rats were treated with SB203580 or vehicle every 2 days with injections beginning 3 days prior to surgery, and continued until 7 days after surgery. Two weeks after surgical repair muscles were analyzed using histology, lipid profiling, gene expression and permeabilized muscle fiber contractility.
Results
Inhibition of p38 MAPK resulted in a nearly 49% reduction in fat accumulation and a 29% reduction in collagen content, along with changes in corresponding genes regulating adipogenesis and matrix accumulation. There was also a marked 40–80% decrease in the expression of several proinflammatory genes, including IL1β, IL6 and COX2, and a 360% increase in the antiinflammatory gene IL10. No differences were observed for muscle fiber force production.
Conclusion
Inhibition of p38 MAPK was found to have a significant decrease in intramuscular lipid accumulation and fibrosis that is usually seen in the degenerative cascade of rotator cuff tears, without having negative effects on the contractile properties of the rotator cuff muscle tissue.
Keywords: fatty degeneration, myosteatosis, rotator cuff, fibrosis, atrophy, inflammation, p38 MAPK
Introduction
Rotator cuff disease is one of the most frequently occurring musculoskeletal conditions treated by orthopaedic surgeons, with nearly 250,000 surgical repairs performed in the US on an annual basis.5 Currently, the ability to repair the cuff and allow for normal strength and function is often complicated by atrophy, retraction and fatty infiltration of the diseased muscle.2, 15, 17 The degree of these changes, termed “fatty degeneration”, increases with time and is a limiting factor for adequate repair as well as post-operative functioning and recovery.13, 23 Restoring muscle size and strength is essential in the post-operative period, however for unclear reasons the failure of repair and overall failure remain high. Fatty degeneration is a common pathological change that occurs in torn rotator cuff muscles, however little is known behind the pathophysiologic pathway of this phenomenon.22 It has been shown that even following surgical repair, fatty degeneration does not improve, and in most cases continues to worsen over time.14 Fat accumulation and atrophy are correlated with poor functional outcomes and increased risk of re-tear,14, 15 and identifying new treatments that can reverse or prevent fatty degeneration will likely improve clinical outcomes for patients with chronic rotator cuff tears.
The protein p38 mitogen associated protein kinase (MAPK) is a signaling molecule within cells that is involved in many different cellular signaling processes associated with inflammation, muscle atrophy, fibrosis and adipogenesis.4, 19, 31, 32 p38 MAPK is activated in response to the treatment of cells with TGFβ and TNFα signaling pathways, among others.18, 19 The central role of p38 MAPK in inducing inflammatory responses in many different tissues has led to the development of specific inhibitors of this molecule, and several clinical trials are underway to test the ability of small molecule inhibitors of p38 MAPK to block inflammation and promote regeneration in different disease states.10, 11 For skeletal muscle, p38 MAPK signaling has been associated with an accumulation of extracellular matrix (ECM), inflammation, atrophy and an overall general loss of muscle function with aging, and the targeted inhibition of p38 MAPK can markedly reverse degenerative changes and restore muscle function.3, 6 As there are no known pharmacological treatments available to effectively prevent degeneration or cause regeneration of torn rotator cuff muscles following repair, using a preclinical rat model of rotator cuff injuries, we sought to determine the ability of a small molecule inhibitor of p38 MAPK, SB2035807, to reduce muscle tissue damage from inflammation, fatty degeneration, and muscle atrophy following rotator cuff repair. We hypothesized that the post-operative treatment of torn rotator cuff muscles with SB203580 would reduce tissue inflammation and muscle fatty degeneration while enhancing the formation of a stable enthesis following rotator cuff repair.
Materials and Methods
Overview
This study was approved by the University of Michigan IACUC. Six-month old male Sprague-Dawley rats (N=14 rats in the vehicle control group, N=14 rats in the drug group, with N=4 rats used in each group for pilot studies and N=10 rats in each group for the main tear and repair experiments), maintained in specific pathogen-free conditions, were used for this study. Based on anatomical similarities with humans and previous studies, a rat model was selected to study rotator cuff healing.35 While some rat models of rotator cuff tear do not fully reproduce fatty degenerative changes because the free end of the torn tendon will randomly reattach to surrounding tissue,36 substantial fatty degeneration will occur in the rat rotator cuff by ensuring the tendon cannot spontaneously reattach.16, 25 The general techniques and methodologies in this study have been previously published and are provided in brevity in the current manuscript.16, 17, 27, 30
Animal Surgery
A bilateral tear and repair approach was used in this study. To induce a supraspinatus tear, rats were anesthetized with 2% isoflurane, placed in a lateral recumbent position, and the surgical area was shaved and scrubbed with ChloraPrep (CareFusion, El Paso, TX). The supraspinatus tendon was visualized using a deltoid splitting incision and transacromial approach, and once clearly visualized and isolated the tendon was sharply detached from the greater tuberosity using a #15 blade and secured using a modified Mason-Allen stitch within sterile nonpyrogenic surgical tubing (Pharmed BPT/Saint Gobain, Valley Forge, PA, USA) to prevent the tendon from forming adhesions to the surrounding tissue, allowing the supraspinatus to freely retract. Animals were allowed to recover for 30 days. This time point was selected as many of the pathological changes in rats one month after a tear mimic the early chronic changes seen in patients with rotator cuff tears.8, 16, 28 To repair the tear, rats were anesthetized and prepared as described above. The tendon was identified and silicone tube removed. A modified Mason-Allen stitch was placed using 5-0 two-arm Ethibond sutures (Johnson & Johnson, New Brunswick, NJ, USA) was placed in the tendon stump. After removal of small amounts of scar tissue and complete debridement of the native enthesis on greater tuberosity, crossed bone tunnels were drilled at the anterior and posterior margins of the cuff footprint and 2 mm lateral to the articular surface using 0.7mm K-wire (gSource, Emerson, NJ, USA). Suture ends were passed through the bone tunnels and tied over the humeral metaphyseal cortex to anatomically repair the supraspinatus to the native footprint. In all surgical procedures, a splash block of 1% lidocaine was administered, the deltoid was closed using 4-0 chromic gut sutures (Johnson & Johnson), and the skin was closed using a subcutaneous running suture of 4-0 Vicryl (Johnson & Johnson) with GLUture (Abbott Laboratories, Abbott Park, IL, USA) applied over the incision. Subcutaneous buprenorphine at a dose of 0.05mg/kg was administered for analgesia during post-operative recovery. Weightbearing and cage activity was allowed postoperatively, and rats were monitored for signs of distress or infection. All rats were grossly ambulatory to the same extent, and demonstrated signs of adequate food and water intake.
For the pilot study to determine the efficacy of SB203580 treatment in the inhibition of p38 MAPK, rats were anesthetized one day after the repair and supraspinatus muscles were removed for analysis. The muscles were finely homogenized and prepared for the p38 MAPK phosphorylation assay. In the main study, rats were allowed to recover for 14 days, at which time after repair, rats were anesthetized, and the supraspinatus muscle was removed for analysis. The proximal two-thirds of the right supraspinatus was finely minced and used for biochemistry and molecular biology, while the distal one-third of the muscle was used for histology. The proximal one-third of the left supraspinatus was used for muscle fiber contractility testing. Following removal of tissue, rats were euthanized by overdose of sodium pentobarbital followed by induction of a bilateral pneumothorax.
Administration of Drug
SB203580 (Alfa Aesar, Ward Hill, MA, USA) was dissolved in 1:100 DMSO in phosphate buffered saline (PBS) and administered via intraperitoneal injection at a dose of 1mg/kg every 2 days. SB203580 is not directly soluble in water or PBS, and must first be dissolved in an organic solvent prior to dilution in a physiologic buffer. To mimic a potential clinical scenario where patients who undergo rotator cuff repair would begin treatment prior to repair surgery and continue treatment in the acute post-surgical recovery phase, injections began 3 days prior to surgery, and continued until 7 days after surgery. Rats in the control group received IP injections of vehicle only (1:100 DMSO in PBS). This dose and timing regime was selected based on pilot experiments, the interest in minimizing the stress animals experience with IP injections, and previous studies evaluating p38 MAPK inhibition in muscle and connective tissue.31, 32. An IP route was selected to precisely control the delivered dose, and the total volume of solution injected IP was less than 1mL per dose.
p38 Activation
A MAGPix Luminex-based system (Luminex, Austin, TX, USA) was used to measure p38 phosphorylation as described.27, 33 Briefly, 100 mg of muscle was homogenized in Tissue Protein Extraction Reagent (Thermo Scientific, Rockford, IL, USA) supplemented with a protease and phosphatase inhibitor cocktail (Thermo Scientific), homogenized, spun at 12,000 × g for 10 min, and the supernatant was collected and stored at −80°C until use. A BCA protein assay (Thermo Scientific) was used to determine protein content, and the relative abundance of phosphorylated p38 MAPK (phosphorylation at Thr180 and Tyr182 residues) from 50 μg of total protein was analyzed using a Milliplex-p38 phospho protein magnetic bead assay (EMD Millipore Corporation, Billerica, MA, USA).
Histology
The distal third of supraspinatus muscles were snap frozen in TissueTek (Sakura Finetek, Torrance, CA, USA) using isopentane-cooled liquid nitrogen and stored at − 80°C until use. Muscles were cryosectioned and stained with hematoxylin and Oil red O to measure lipid content, and also hematoxylin and eosin to measure fiber cross-sectional area (CSA) using ImageJ software (NIH, Bethesda, MD, USA).
Gene Expression
Gene expression analysis was performed as previously described.16 RNA was isolated from 100 mg portions of the distal third of supraspinatus muscles using a Qiagen miRNeasy kit (Qiagen, Venlo, The Netherlands) and treated with DNase I (Qiagen) to eliminate genomic DNA. RNA integrity was verified using a Bioanalyzer RNA system. After reverse transcription of RNA with an RT2 First Strand kit (Qiagen), quantitative PCR (qPCR) was conducted using RT2 SYBR Green reagents (Qiagen) and commercial primers (Qiagen). Target gene expression was normalized to the stable housekeeping gene β-actin and then to the vehicle control group using the 2−ΔCt technique.
Hydroxyproline assay
Hydroxyproline measurements of muscle tissue were performed from 25mg portions of muscle using a colorimetric assay as previously described.27, 30 The hydroxyproline content was normalized to the dry mass of the muscle tissue.
Muscle Fiber Contractility Measurements
Bundles from the proximal third of the supraspinatus muscle were prepared for muscle fiber contractility measurements as previously described.16, 17, 27, 30 Briefly, fibers were placed in a chamber filled with relaxing solution, and secured at one end to a servomotor (model 322C, Aurora Scientific, Aurora, ON, Canada) and at the other end to a force transducer (model 403A, Aurora Scientific). The length of the fiber was adjusted until an average sarcomere length of 2.5μm was achieved. The cross sectional area (CSA) of fibers was then measured from top and side view images, and the fiber was exposed to a high Ca2+ solution to develop maximum isometric force (Fo). Fo was then normalized by the CSA to determine specific force (sFo). Ten fast fibers from each muscle were tested.
Lipid Analysis
Lipids were extracted from fifty milligrams of muscle tissue and analyzed with thin layer chromatography (TLC) as described.8, 30 TLC plates were stained with a rhodamine 6G solution (Sigma, St. Louis, MO, USA) and imaged in a ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA). Densitometry of triglyceride bands was performed using ImageJ.
Statistical analysis
Differences between control and SB203580 groups were tested using unpaired t-tests with alpha=0.05 in in GraphPad Prism 6.0. We powered the current study based on contractility values from a previous study.16 To detect a 30% difference in specific force, using a power of 0.80 and α=0.05, requires N=8 for each group. We included an additional two rats per group to account for any rats that were lost to infection or other complications.
Results
All rats survived to analysis. All rotator cuff repairs in both groups were intact postoperatively at the time of sacrifice, with no signs of humeral fractures, damage to transosseous tunnels, or failed repairs. Values for control rats for many of these values have been previously published.16 In pilot studies, treatment of SB203580 resulted in a 68% reduction in p38 phosphorylation one day after repairing a chronically torn rotator cuff (Figure 1, P<0.01).
Figure 1. p38 activation.
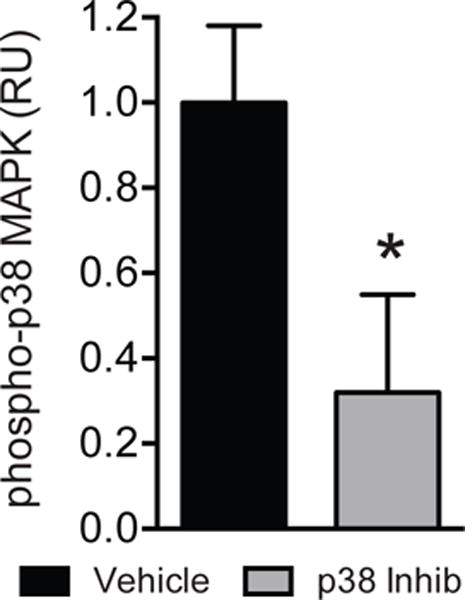
Relative units of p38 MAPK phosphorylation of vehicle and p38 MAPK inhibitor treated muscles. Values are mean±SD. N=4 for each group. *Significantly different from vehicle group (P<0.05).
After establishing that the dosing was effective at reducing p38 MAPK activation, we then conducted the main study. No differences in body mass (636±69g for the vehicle group, 612±40g for the p38 MAPK inhibitor group, P=0.17) were detected. Treatment with the p38 MAPK inhibitor SB203580 resulted in no change in muscle mass (Figure 2A, P=0.17) or muscle fiber cross-sectional area measured using histology (Figure 2B, P=0.33). p38 MAPK inhibitor treatment also resulted in a 29% decrease in the collagen biomarker hydroxyproline (Figure 2C, P=0.01) and a 48% decrease in triglyceride (Figure 3A–B, P<0.01), which is the major lipid species present in skeletal muscle. This reduction in triglyceride levels was also observed grossly in Oil red O stained muscle cross sections (Figure 3C).
Figure 2. Muscle mass, size and hydroxyproline values.
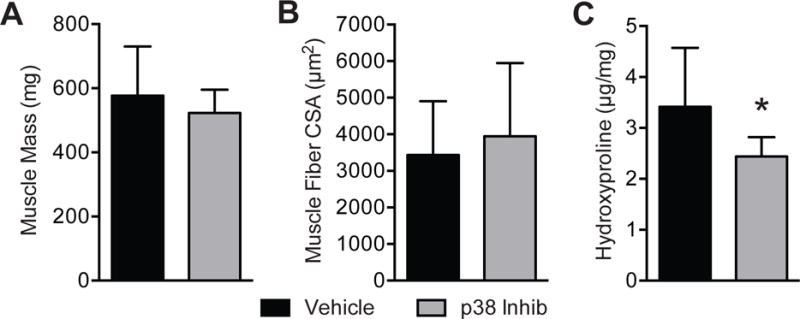
(A) muscle mass, (B) muscle fiber cross-sectional area and (C) hydroxyproline content (micrograms of hydroxyproline per milligram of dry muscle mass) of vehicle and p38 MAPK inhibitor treated muscles. Values are mean±SD. N=10 for each group. *Significantly different from vehicle group (P<0.05).
Figure 3. Lipid content.
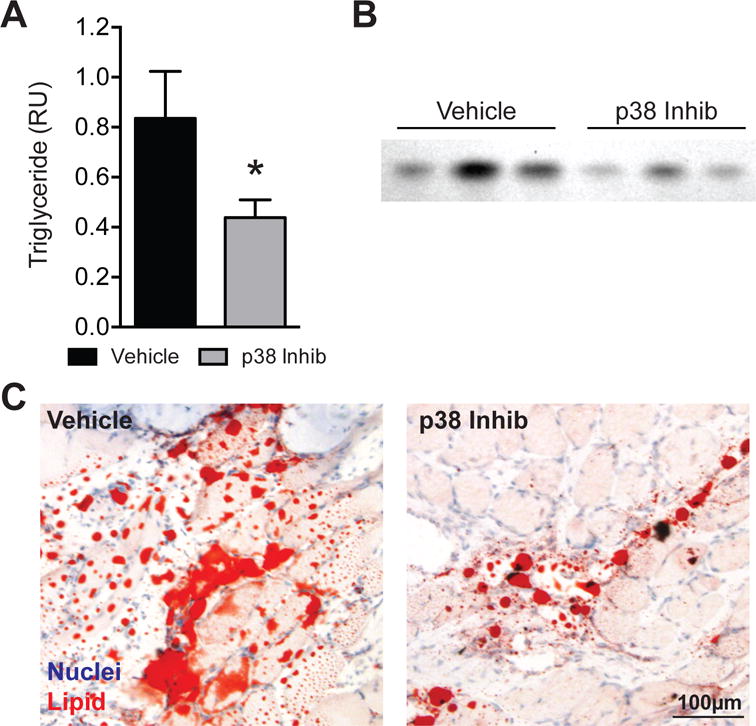
(A) Levels of triglyceride measured in relative units, (B) representative rhodamine 6G-stained thin layer chromatography triglyceride bands and (C) representative Oil red O histology of vehicle and p38 MAPK inhibitor treated muscles. Values are mean±SD. N=10 for each group. *Significantly different from vehicle group (P<0.05).
To assess muscle function, we measured the contractility of permeabilized muscle fiber cells. The permeabilization process does result in an increase in cross-sectional area compared to measurements performed using histological techniques, but simlar to histology no difference in permeabilized fiber cross-sectional area was observed between the two groups (Figure 4A, P=0.19). No differences were observed for maximum isometric force (Figure 4B, P=0.48) or specific force (Figure 4C, P=0.08), which is maximum isometric force normalized to cross-sectional area.
Figure 4. Permeabilized muscle fiber contractility.
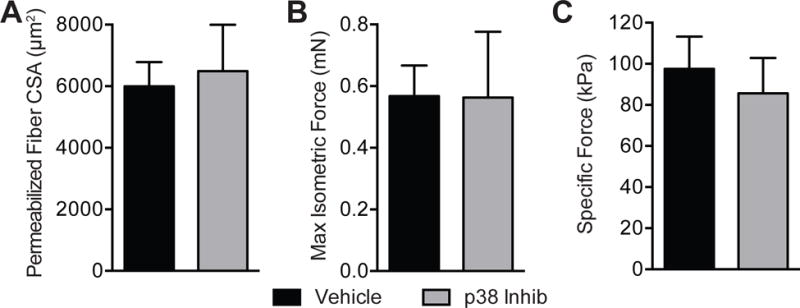
(A) Permeabilized muscle fiber cross-sectional area (CSA), (B) maximum isometric force and (C) specific force (maximum isometric force normalized to CSA) of vehicle and p38 MAPK inhibitor treated muscles. Values are mean±SD. N=10 for each group. No significant differences were found from vehicle group (P<0.05).
Finally, we measured the expression of several genes that are involved in the response of muscle to injury and regeneration (Figure 5). For genes associated with lipid accumulation and adipogenesis, the p38 MAPK inhibitor group had a 38% reduction in PPARγ (P=0.01), a 27% reduction in cEBPα (P<0.01) and a 24% reduction in CD36 (P=0.04). p38 MAPK inhibition also had an effect on several genes associated with extracellular matrix production and fibrosis, resuling in a 27% reduction in type I collagen (Col1α2, P=0.02), a 44% reduction in type III collagen (Col3α1, P=0.04), a 170% increase in matrix metalloproteinase 8 (MMP8, P<0.01), a 134% increase in tissue inhibitor of matrix metalloproteinases 2 (TIMP2, P<0.01) and a 27% reduction in the expression of fibroblast specific protein 1 (FSP1, P=0.01). No differences in MMP3 (P=0.06) or TIMP1 (P=0.18) were observed. p38 MAPK inhibition altered the expression of several genes associated with inflammation as well, including a 64% reduction in cyclooxygenase 2 (COX2, P<0.01), a 79% reduction in interleukin 1β (IL1β, P<0.01), a 44% reduction in IL6 (P<0.01) and a 360% increase in the expression of IL10 (P<0.01), although no differences were observed for COX1 (P=0.30).
Figure 5. Gene expression.
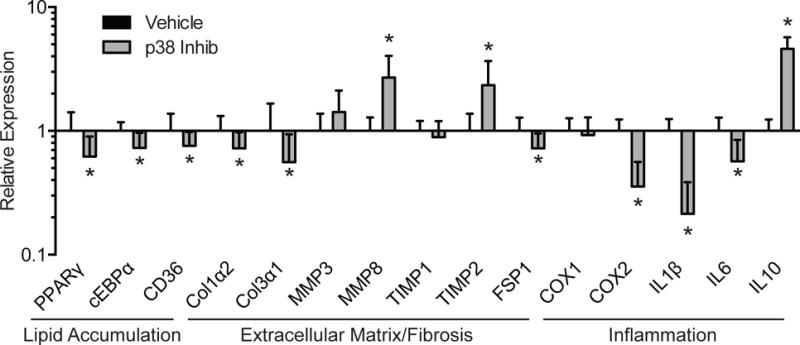
Expression of genes associated with lipid accumulation, extracellular matrix synthesis and fibrosis, and inflammation. The expression of each gene was normalized to β-actin and further normalized to the vehicle group. Values are mean±SD. N=10 for each group. *Significantly different from vehicle group (P<0.05). PPARγ, peroxisome proliferator-activated receptor γ; cEBPα, CCAAT/enhancer binding protein α; CD36, cluster of differentiation 36/fatty acid transporter; Col1α2, type I collagen α2; Col3α1, type III collagen α1; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase; FSP1, fibroblast specific protein 1; COX, cyclooxygenase; IL, interleukin.
Discussion
Fibrosis and fat infiltration into the muscle tissue are common pathological changes that occur in rotator cuff tears, however our current understanding of pathophysiology of this phenomenon is limited.22 Unfortunately, even following surgical repair, this fatty degeneration does not improve, and in most cases continues to worsen over time.14 As the muscle of patients with chronic rotator cuff tears is shortened by 30% or more, repairing a chronically torn rotator cuff by affixing the free tendon end back to the native footprint results in a secondary, stretch induced muscle injury that likely further exacerbates the inflammatory state of the muscle.8 Since fat accumulation and atrophy have been correlated with poor functional outcomes and increased risk of re-tear,14, 15 reversing or preventing the fatty degeneration cascade has the potential to improve clinical outcomes for patients with chronic rotator cuff tears. The p38 MAPK pathway appears to play an important role in promoting fibrosis, adipogenesis, inflammation and atrophy in multiple tissue types.4, 19, 32 In the current study we evaluated the efficacy of a small molecule inhibitor of p38 MAPK, SB203580, in preventing the accumulation of extracellular matrix and fat, and in improving the contractile function of muscle tissue, after repairing a chronic rotator cuff tear. The combined results of this preclinical study indicate that blocking p38 MAPK is able to effectively reduce collagen and fat accumulation without impacting muscle fiber contractility.
p38 MAPK is widely regarded as one of the key regulators of adipogenesis,4, 21 and the critical role of this kinase in regulating adipocyte activity and lipid storage was the primary rationale for conducting the current study. P38 MAPK functions in adipogenesis by activating various transcription factors like ATF2 that bind to DNA and induce the expression of the transcription factors PPARγ and cEBPα, which then direct the expression of several gene required for preadipocyte proliferation, adipocyte differentiation and lipid uptake and storage.4, 21 Consistent with this, in the current study we observed that inhibiting p38 MAPK resulted in a marked reduction in PPARγ and cEBPα, as well as CD36, which functions to transport fatty acids into adipocytes and muscle fibers. The reduction in PPARγ, cEBPα and CD36 corresponded with a decrease in triglyceride content, which is the major lipid species found in muscle fibers and adipocytes. These results indicate that blocking p38 MAPK activity is effective at reducing functional lipid accumulation in this preclinical model of rotator cuff disease.
Elevated inflammation is frequently observed in patients with chronic rotator cuff tears.2, 28 Since p38 MAPK plays a critical role in inducing inflammation in multiple tissue types,19, 20, 32 we sought to evaluate whether inhibition of p38 MAPK could regulate the expression of several genes known to be regulated by p38 MAPK that are involved in inflammation in skeletal muscle tissue. IL1β and IL6 are potent proinflammatory cytokines known to direct atrophy and collagen production,1, 12 and IL10 is an antiinflammatory gene that can protect against atrophy and fibrosis in skeletal muscle tissue.9 In the current study inhibition of p38 MAPK was effective at reducing the expression of IL1β and IL6, and inducing IL10. The generally favorable effects of p38 MAPK inhibition is further supported by the observed reduction in COX2 expression, which is a gene that produces proinflammatory prostaglandins that can inhibit muscle regeneration.29, 34 Combined, these results indicate that the targeted inhibition of p38 MAPK is able to effectively reduce the expression of proinflammatory biomarkers in injured rotator cuff muscles.
Muscle fibrosis is commonly observed in torn rotator cuff muscles, and likely contributes to poor functional outcomes.14, 16 Activation of p38 MAPK by TNFα, TGFβ and myostatin, which are cytokines that are induced and activate after muscle injury, leads to the induction of muscle fibrosis, type I and III collagen expression and connective tissue accumulation.18, 24, 26 In the current study, for rats treated with the p38 MAPK inhibitor we observed a decrease in the expression of the major muscle ECM components, type I and type III collagen, as well as the biomarker of collagen content, hydroxyproline. The TNFα, TGFβ and myostatin pathways can also induce muscle atrophy and a loss in muscle fiber force production through p38 MAPK, as well as other downstream pathways.18, 19 While we anticipated that blocking p38 MAPK would improve muscle fiber size and force production, no effect not observed, and the specific force values of both groups are 30% lower than uninjured muscles.16 These collective results suggest that in the context of rotator cuff injuries, the p38 MAPK pathway plays an important role in regulating fibrosis but not muscle atrophy and force production.
While we provided important insights into the potential therapeutic role of p38 MAPK inhibition in the treatment of rotator cuff tears, there are several limitations to this study. Rats are a frequently utilized preclinical model in the study of rotator cuff healing, but compared to humans they demonstrate a much better rate of structural regeneration and do not develop the same extent of fatty infiltration and atrophy. There is a gradient of degenerative changes seen throughout injured rotator cuff muscles, with the more pronounced changes observed at the distal end and decreasing in intensity moving proximally. The quantitative measures of triglyceride in the proximal regions of the muscle may therefore underestimate the changes that occurred in the distal region of the muscle. We measured the expression of several genes, and while we do not anticipate substantial posttranslational regulation of these transcripts, it is possible that changes in RNA levels would not predict subsequent changes in protein abundance. Based on our previous work and the work of others, we selected a single time point for the tear duration and repair duration that we think were predictive of long-term outcomes after the repair of chronically torn rotator cuff muscles. Despite these limitations, this study provided important insight into the biology of rotator cuff regeneration and identified a promising pharmacological target worth further investigation.
Conclusion
Several studies have shown that p38 MAPK signaling plays an important role in regulating the adaptation of tissues to mechanical loading, and the regeneration of tissue from injury. In the current study, inhibition of p38 MAPK at the time of rotator cuff repair resulted in a clinically favorable decrease in lipid accumulation, which is a hallmark in the rotator cuff degenerative cascade and predictive of clinical outcomes. p38 MAPK inhibition was also effective at reducing collagen content and inflammatory biomarkers. We also found that it is possible to markedly reduce fat accumulation and fibrosis without impacting muscle fiber force production. There are no approved p38 MAPK inhibitors currently on the market, but several of these compounds are being evaluated in clinical trials for a wide variety of conditions. Targeting p38 MAPK may be particular helpful to improve outcomes at the time of surgical repair of chronic rotator cuff tears, since the process of reattaching the chronically shortened muscle-tendon unit leads to a secondary stretch-induced injury to the muscle, and in the current study we were able to demonstrate inhibition of p38 MAPK limits some of the fibrosis and fat accumulation that occur after the repair. While further studies are necessary, given the encouraging results from this preclinical model of rotator cuff injury, the targeted inhibition of p38 MAPK may be able to improve the outcomes of patients with chronic rotator cuff tears.
Acknowledgments
The authors would like to thank Mr. Michael D. Flood and Mr. Stuart M. Roche for technical assistance with this project.
Disclaimer
This work was supported by an OREF Resident Research grant and NIH/NIAMS grants R01-AR063649 and F31-AR065931. The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article. This study was approved by the University of Michigan IACUC (approval number PRO00005154).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Level of evidence: Basic Science Study, In-vivo Animal Model
References
- 1.Andersen M, Pingel J, Klaer M, Langberg H. Interleukin-6: a growth factor stimulating collagen synthesis in human tendon. J Appl Physiol. 2011;110(6):1549–54. doi: 10.1152/japplphysiol.00037.2010. [DOI] [PubMed] [Google Scholar]
- 2.Bedi A, Dines J, Warren RF, Dines DM. Massive Tears of the Rotator Cuff. J Bone Joint Surg Am. 2010;92(9):1894–908. doi: 10.2106/jbjs.i.01531. [DOI] [PubMed] [Google Scholar]
- 3.Bernet JD, Doles JD, Hall JK, Tanaka KK, Carter TA, Olwin BB. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20(3):265–71. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bost F, Aouadi M, Caron L, Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87(1):51–6. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National Trends in Rotator Cuff Repair. J Bone Joint Surg Am. 2012;94(3):227–33. doi: 10.2106/jbjs.j.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20(3):255–64. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364(2):229–33. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 8.Davis ME, Stafford PL, Jergenson MJ, Bedi A, Mendias CL. Muscle fibers are injured at the time of acute and chronic rotator cuff repair. Clin Orthop Relat Res. 2015;473(1):226–32. doi: 10.1007/s11999-014-3860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189(7):3669–80. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominguez CC, Powers DA, Tamayo N. p38 MAP kinase inhibitors: many are made, but few are chosen. Curr Opin Drug Discov Devel. 2005;8(4):421. No doi. [PubMed] [Google Scholar]
- 11.Fisk M, Gajendragadkar PR, Mäki-Petäjä KM, Wilkinson IB, Cheriyan J. Therapeutic potential of p38 MAP kinase inhibition in the management of cardiovascular disease. Am J Cardiovasc Drugs. 2014;14(3):155–65. doi: 10.1007/s40256-014-0063-6. [DOI] [PubMed] [Google Scholar]
- 12.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35:661–4. doi: 10.1042/bst0350661. [DOI] [PubMed] [Google Scholar]
- 13.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: A study in thirteen patients. J Shoulder Elbow Surg. 2007;16(6):691–6. doi: 10.1016/j.jse.2007.02.122. doi: http://dx.doi.org/10.1016/j.jse.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 14.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–28. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 15.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78–83. [PubMed] [Google Scholar]
- 16.Gumucio JP, Davis ME, Bradley JR, Stafford PL, Schiffman CJ, Lynch EB, et al. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012;30(12):1963–70. doi: 10.1002/jor.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumucio JP, Korn MA, Saripalli AL, Flood MD, Phan AC, Roche SM, et al. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg. 2014;23(1):99–108. doi: 10.1016/j.jse.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43(1):12–21. doi: 10.1007/s12020-012-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumucio JP, Sugg KB, Mendias CL. TGF-β superfamily signaling in muscle and tendon adaptation to resistance exercise. Exerc Sport Sci Rev. 2015;43(2):93–9. doi: 10.1249/jes.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta JJ, Nebrada AR. Roles of p38α mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J. 2015;282(10):1841–57. doi: 10.1111/febs.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harms MM, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 22.Kang JR, Gupta R. Mechanisms of fatty degeneration in massive rotator cuff tears. J Shoulder Elbow Surg. 2012;21(2):175–80. doi: 10.1016/j.jse.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Laron D, Samagh SP, Liu X, Kim HT, Feeley BT. Muscle degeneration in rotator cuff tears. J Shoulder Elbow Surg. 2012;21(2):164–74. doi: 10.1016/j.jse.2011.09.027. doi: http://dx.doi.org/10.1016/j.jse.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008;283(28):19371–8. doi: 10.1074/jbc.M802585200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Manzano G, Kim HT, Feeley BT. A rat model of massive rotator cuff tears. J Orthop Res. 2011;29(4):588–95. doi: 10.1002/jor.21266. [DOI] [PubMed] [Google Scholar]
- 26.Mendias CL, Bakhurin KL, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci U S A. 2008;105(1):388–93. doi: 10.1073/pnas.0707069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendias CL, Lynch EB, Gumucio JP, Flood MD, Rittman DS, Van Pelt DW, et al. Changes in skeletal muscle and tendon structure and function following genetic inactivation of myostatin in rats. J Physiol. 2015;593(8):2037–52. doi: 10.1113/jphysiol.2014.287144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendias CL, Roche SM, Harning JA, Davis ME, Lynch EB, Sibilsky Enselman ER, et al. Reduced muscle fiber force production and disrupted myofibril architecture in patients with chronic rotator cuff tears. J Shoulder Elbow Surg. 2015;24(1):111–9. doi: 10.1016/j.jse.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 29.Mendias CL, Tatsumi R, Allen RE. Role of cyclooxygenase-1 and -2 in satellite cell proliferation, differentiation, and fusion. Muscle Nerve. 2004;30(4):497–500. doi: 10.1002/mus.20102. [DOI] [PubMed] [Google Scholar]
- 30.Oak NR, Gumucio JP, Flood MD, Saripalli AL, Davis ME, Harning JA, et al. Inhibition of 5-LOX, COX-1, and COX-2 increases tendon healing and reduces muscle fibrosis and lipid accumulation after rotator cuff repair. Am J Sports Med. 2014;42(12):2860–8. doi: 10.1177/0363546514549943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardí M, Caelles C, Serrano AL, et al. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. 2011;195(2):307–22. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz AJ, Sarver DC, Sugg KB, Dzierzawski JT, Gumucio JP, Mendias CL. p38 MAPK signaling in postnatal tendon growth and remodeling. PLoS One. 2015;10(3):e0120044. doi: 10.1371/journal.pone.0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma NN, Castarena CM, Cartee GD. Tissue-specific responses of IGF-1/insulin and mTOR signaling in calorie restricted rats. PLoS One. 2012;7(6):e38835. doi: 10.1371/journal.pone.0038835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen WW, Li Y, Tang Y, Cummins J, Huard J. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol. 2005;167(4):1105–17. doi: 10.1016/s0002-9440(10)61199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5(5):383–92. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 36.Ward SR, Sarver JJ, Eng CM, Kwan A, Würgler-Hauri CC, Perry SM, et al. Plasticity of muscle architecture after supraspinatus tears. J Orthop Sports Phys Ther. 2010;40(11):729–35. doi: 10.2519/jospt.2010.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]


