Abstract
BACKGROUND
More active high-dose chemotherapy (HDC) regimens are needed for refractory lymphomas. The authors previously combined infusional gemcitabine with busulfan and melphalan (Gem/Bu/Mel) pursuing DNA damage repair inhibition. Subsequently, they combined Gem/Bu/Mel with vorinostat, which facilitates chemotherapy access to DNA. The resulting regimen was safe and synergistic. However, vorinostat induced DNA methyltransferase up-regulation, which could be preclinically abrogated by azacitidine, increasing tumor-cell kill. Those observations led to a clinical combination of azacitidine with vorinostat/Gem/Bu/Mel.
METHODS
Patients ages 12 to 65 years with refractory or poor-risk relapsed lymphomas were eligible. They received intravenous azacitidine on days −11 through −3 at doses from 15 to 35 mg/m2 daily (dose levels 1–3), followed by oral vorinostat (1000 mg once daily on days −11 through −3), gemcitabine (2775 mg/m2 over 4.5 × 2), busulfan (at an area under the receiver operating characteristic curve of 4000 daily × 4), and melphalan (60 mg/m2 × 2). Patients who had tumors that were positive for CD20 (cluster of differentiation 20; B-lymphocyte antigen) received rituximab on day −9.
RESULTS
In total, 60 patients were enrolled, including 26 with diffuse large B-cell lymphoma (DLBCL) (10 double hit/double expressors), 21 with Hodgkin lymphoma, 8 with T-cell lymphoma, and 5 with other B-cell lymphomas. The median patient age was 41 years (range, 16–65 years), patients had received a median of 3 prior lines of chemotherapy (range, 2–7 lines of chemotherapy); and 32% of tumors were positive on positron emission tomography studies at the time of HDC. Two patients died from treatment complications (respiratory syncytial virus pneumonia and sepsis, respectively). The maximum tolerated dose of azacitidine was encountered at dose level 1 (15 mg/m2 daily). The toxicity profile (mainly mucositis and dermatitis) was manageable and was identical to that of vorinostat/Gem/Bu/Mel. Neutrophils and platelets engrafted promptly. At a median follow-up of 15 months (range, 8–27 months), the event-free and overall survival rates were 65% and 77%, respectively, among patients with DLBCL; 76% and 95%, respectively, among patients with Hodgkin lymphoma; and 88% for both among patients with T-cell lymphoma.
CONCLUSIONS
Double epigenetic modulation of Gem/Bu/Mel with azacitidine/vorinostat is feasible and highly active in patients with refractory/poor-risk relapsed lymphomas, warranting further evaluation.
Keywords: autologous stem-cell transplantation, azacitidine, high-dose chemotherapy, lymphoma, phase 1 trial, vorinostat
INTRODUCTION
High-dose chemotherapy (HDC) with regimens like combined carmustine, etoposide, cytarabine, and melphalan (BEAM) plus autologous stem-cell transplantation (ASCT) is standard treatment for chemosensitive, relapsed diffuse large B-cell lymphoma (DLBCL) and Hodgkin lymphoma (HL). Early randomized trials demonstrated a benefit from HDC in patients with chemosensitive, relapsed DLBCL during the era before rituximab and also in patients with HL.1–3 However, the more recent Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) study demonstrated worse outcomes after BEAM with or without rituximab in patients with DLBCL who relapsed after receiving rituximab plus combined cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) than the previously observed outcomes in patients who relapsed after CHOP alone.4 Furthermore, particularly poor outcomes were reported in patients who had primary refractory tumors or relapsed tumors with high-risk features, such as a first complete remission (CR) <12 months, a secondary International Prognostic Index (IPI) score >1 at relapse, exposure to multiple salvage regimens, or the presence of active tumor at HDC.5–8 Similar adverse prognostic factors have been described in HL.9,10 Therefore, there is a clear need to develop more effective HDC regimens for these patient populations.
We previously developed a high-dose combination of gemcitabine/busulfan/melphalan (Gem/Bu/Mel) that produced a synergistic interaction based on gemcitabine inhibition of DNA damage repair.11 Gemcitabine was infused at a fixed dose rate (FDR) of 10 mg/m2 per minute, optimizing the formation of its active intracellular triphosphate metabolite.12,13 Busulfan was administered intravenously with pharmacokinetic-guided dosing. Given its encouraging results,14 we then used Gem/Bu/Mel as a platform for epigenetic modulation of HDC. Changes in histone acetylation lead to changes in chromatin configuration. Inhibition of histone deacetylases (HDACs) weakens the histone-DNA bonds and de-condenses chromatin.15 In our prior preclinical experiments in resistant B-cell and T-cell lymphoma cell lines, a synergistic increase in cytotoxicity was demonstrated. Increased DNA damage and apoptosis were observed when the HDAC inhibitor vorinostat (suberoylanilide hydroxamic acid) was added to the Gem/Bu/Mel combination.16 Those preclinical observations led us to test vorinostat/Gem/Bu/Mel clinically, and we observed that vorinostat could be safely combined with full-dose Gem/Bu/Mel and produced marked activity in refractory lymphomas.17
However, in parallel preclinical work, we noted that the addition of vorinostat to Gem/Bu/Mel increased protein levels of DNA methyltransferases 3A (DNMT3A) and DNMT3B in lymphoma cells exposed to these agents, which peaked at approximately 48 hours after drug exposure.16 The addition of azacitidine to vorinostat/Gem/Bu/Mel abrogated the induction of DNMT3A and DNMT3B, causing a marked increase of cytotoxicity. There was important sequence specificity, and concurrent treatment was more active than sequential drug exposures. Those observations suggested that inhibition of DNA methyltransferases could further enhance the cytotoxicity of vorinostat/Gem/Bu/Mel. Our preclinical observations were consistent with previous reports indicating that synergism resulted from concurrent exposure of DLBCL lines to HDAC inhibitors and DNA methyltransferase inhibitors.18
Our intriguing preclinical observations motivated us to test azacitidine/vorinostat/Gem/Bu/Mel clinically. We hypothesized that azacitidine could be safely combined with Gem/Bu/Mel plus ASCT. Here, we report the results from our dose-finding study of azacitidine combined with vorinostat/Gem/Bu/Mel in patients with refractory/poor-risk relapsed HL and non-Hodgkin lymphoma (NHL).
MATERIALS AND METHODS
Patient Population
The study protocol was approved by the Clinical Research Committee and Institutional Review Board of The University of Texas MD Anderson Cancer Center. This trial was registered at ClinicalTrials.gov (NCI-2014-01025). Patients provided written informed consent before enrollment. Eligibility included ages 12 to 65 years and 1 of the following lymphomas: 1) DBLCL with primary refractory disease (less than a CR or induction failure after R-CHOP), relapse within 12 months of R-CHOP, a secondary IPI score >1, less than a PR to first salvage chemotherapy, or receipt of prior treatment with ≥3 chemotherapy lines; 2) HL with primary refractory disease (progression during or within 3 months of front-line chemotherapy), relapse within 12 months of front-line chemotherapy, relapse within a prior irradiation field, extranodal or bulky disease (defined as any lesion >5 cm), less than a metabolic CR to second-line chemotherapy, or second relapse or beyond; 3) refractory/relapsed T-cell lymphoma; and 4) all other refractory relapsed B-cell lymphomas. Additional eligibility criteria included adequate renal (creatinine clearance >50 mL/minute), hepatic (aspartate aminotransferase, alanine aminotransferase, and bilirubin levels <3 times the upper normal limit), pulmonary (forced expiratory volume in 1 second, forced vital capacity, and corrected single-breath carbon monoxide diffusing capacity values >50%) and cardiac function (left ventricular ejection fraction >40%), a performance status of 0 or 1, no prior whole-brain irradiation or radiation within 1 month of enrollment, no active hepatitis B, and no chronic hepatitis C causing cirrhosis/stage 3 or 4 fibrosis.
Tumors were restaged within 30 days pre-enrollment, at 1, 3, and 6 months after HDC; and every 6 months thereafter. Responses were assessed before planned post-HDC radiotherapy.19 Positron emission tomography (PET) scans were interpreted using the Deauville 5-point scale.20
HDC
Patients received an intravenous test dose of busulfan of 32 mg/m2 over 60 minutes during the preadmission week (Table 1). Vorinostat was administered orally on days −11 through −2 at a dose of 1000 mg daily within 1 hour before the start of chemotherapy. Azacitidine was administered intravenously at doses from 15 to 35 mg/m2 daily on days −11 through −2. On days −8 through −2, azacitidine was immediately followed by the other chemotherapy drugs. Gemcitabine was administered on days −8 and −3 as a loading bolus of 75 mg/m2 followed by a continuous infusion of 2700 mg/m2 over 4.5 hours (10 mg/m2 per minute) and was immediately followed by busulfan or melphalan. Busulfan was infused daily over 3 hours on days −8 through −5, targeting an average daily area under the receiver operating characteristic curve (AUC) of 4000 μM per minute, with the first 2 therapeutic doses calculated from the pharmacokinetics of the test dose. If necessary, the third and fourth doses were readjusted after the first therapeutic dose analysis, targeting an aggregate course AUC of 16,000 μM per minute. The sampling and analytical processes have been described previously.21,22 A fixed busulfan dose of 100 mg/m2 daily would be received by patients for whom pharmacokinetic dosing was not feasible. Melphalan was administered at a dose of 60 mg/m2 daily over 30 minutes on days −3 and −2.
TABLE 1.
Treatment Schedule
| Treatment | Day
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −11 | −10 | −9 | −8 | −7 | −6 | −5 | −4 | −3 | −2 | −1 | 0 | |
| Azacitidine | X | X | X | X | X | X | X | X | X | X | ||
| Vorinostat | X | X | X | X | X | X | X | X | X | X | ||
| Gemcitabine | X | X | ||||||||||
| Busulfana | X | X | X | X | ||||||||
| Melphalan | X | X | ||||||||||
| PBPCs | X | |||||||||||
| Rituximab (CD20+ tumors) | X | |||||||||||
Abbreviations: CD20+, cluster of differentiation 20 (B-lymphocyte antigen) positive; PBPCs, peripheral blood progenitor cells.
The busulfan test dose to be administered in the week prior to admission.
Supportive Care
Acetaminophen, azoles, and metronidazole were avoided on days −10 through −1. Patients received phenytoin 300 to 600 mg daily on days −9 through −4. Intravenous dexamethasone 8 mg was given twice daily on days −11 through −2. Intravenous hydration was started on admission and continued until day −1. Oral care with palifermin, glutamine, and supersaturated calcium/phosphate rinses and oral cryotherapy during melphalan were performed uniformly as previously described.11 Patients received an infusion of peripheral blood progenitor cells on day 0. Departmental guidelines were followed for post-transplantation filgrastim, antiemetics, antimicrobials, and blood product transfusions.
Trial Design
The primary endpoint of this phase 1 trial was to determine the maximum tolerated dose (MTD) of azacitidine combined with vorinostat/Gem/Bu/Mel based on dose-limiting toxicity (DLT), which was defined as any grade 4 nonhematologic and noninfectious toxicity or any grade 3 mucositis or skin toxicity lasting >3 days at peak severity. Secondary endpoints included the event-free survival (EFS) and overall survival (OS) rates, the overall response rate (ORR), and the CR rate in the different subgroups along with a description of the toxicity profile. For dose finding, we used the continual reassessment method with a target DLT probability per cohort of 25%.23 Azacitidine doses were chosen adaptively for successive cohorts with a minimum size of 2 patients. If the lowest dose level was identified as excessively toxic, then the trial would move to stage 2 using a lower dose of gemcitabine of 2475 mg/m2 daily. Toxicity scoring followed the National Cancer Institute Common Toxicity Criteria, version 3.0.24 The time to neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count ≥0.5 × 109/L, and the time to platelet engraftment was defined as the first of 7 consecutive days with a platelet count ≥20 × 109/L without platelet transfusion.
Correlative Studies of DNA Damage Response, Apoptosis, Histone Acetylation, and DNA Methylation
Mononuclear cells were purified from patient-derived cell samples using a lymphocyte separation medium (Mediatech, Manassas, Va), pelleted, and stored at −80°C until further use. The cells were lysed using lysis buffer (Cell Signaling Technology, Danvers, Mass), and proteins were resolved by polyacrylamide gel electrophoresis and assessed by Western blot analysis, as previously described.16 The phosphorylation status of histone 2AX (γ-H2AX) and the level of poly(ADP-ribose) polymerase 1 (PARP1) were used as indicators of DNA damage response and activation of apoptosis, respectively. Changes in acetylation of histone 3 at lysine 9 (Ac-H3K9) and in the level of DNMT3B also were determined. X-ray films were scanned with the EPSON Perfection V750 PRO (Epson America, Long Beach, Calif) and analyzed with UN-SCAN-IT software (Silk Scientific, Orem, Utah); all prechemotherapy samples were used as controls (with the value set at 1.0).
Statistical Methods
The ORR and the CR rate were calculated for patients who had measurable disease at HDC following the usual criteria.25 EFS was defined as the time from transplantation to either relapse, second tumors, or death, whichever occurred first, or last contact. OS was defined as the time from transplantation to death or last contact. Kaplan-Meier survival curves were used to estimate unadjusted time-to-event distributions.26 The log-rank test was used to compare EFS and OS between subgroups.27 Categorical variables were compared using a generalized Fisher exact test.28 All P values are 2-sided, an all calculations used the statistical software packages R (version 2.12.1; R Foundation for Statistical Computing, Vienna, Austria) and OpenBUGS (version 3.1.2, revision 668; Medical Research Council Biostatistics Unit, Cambridge, UK).
RESULTS
Patient Enrollment
Sixty patients were enrolled between November 2013 and May 2015 (Table 3). The median age was 41 years (range, 16–65 years). No patient had undergone prior transplantation. The diagnoses were DLBCL (N = 26), HL (N = 21), T-cell NHL (T-NHL) (N = 8), follicular lymphoma (N = 3), and mantel cell lymphoma (N = 2). Patients had received a median of 3 prior regimens (range, 2–7 prior regimens) and had extensive tumor involvement (median, 3 involved organs). Four patients had double-hit tumors with rearrangements of v-myc avian myelocytomatosis viral oncogene homolog (MYC) and B-cell lymphoma 2 (BCL2) identified by fluorescence in situ hybridization; 2 had triple rearrangements of MYC, BCL2, and B-cell lymphoma 6 protein (BCL6) identified by fluorescence in situ hybridization; and 4 had double protein expression of MYC and BCL2 identified by immunohistochemistry. Five patients had primary refractory tumors, 14 were in first relapse (9, 6, and 6 patients had a secondary IPI scores of 0–1, 2–3 and >3, respectively), and 7 had >1 prior relapse. In the HL subgroup, 12 patients (57%) had primary refractory tumors; extranodal disease and bulky tumor at relapse/disease progression were present in 11 and 14 patients, respectively; and 4 patients had >1 prior relapse. At the time of HDC, 32% of all patients had PET-positive tumors, and 7% had progressive disease.
TABLE 3.
Patient Characteristics, N = 60
| Characteristic | No. of Patients |
|---|---|
| Age: Median (range). y | 41 (16–65) |
| Sex | |
| Men | 36 |
| Women | 24 |
| Primary refractory tumora | 28% |
| Poor-risk or refractory relapsea | 62% |
| Primary refractory tumora | 28% |
| Poor-risk or refractory relapsea | 62% |
| Median no. of prior chemotherapy lines (range) | 3 (2–7) |
| Prior radiotherapy | 17% |
| Disease status at HDC | |
| CR | 68% |
| PR | 25% |
| Unresponsive | 7% |
| PET-positive at HDC | 32% |
| Diagnoses | |
| DLBCL | 26 |
| Double hit (MYC/BCL2) | 4 |
| Triple hit (MYC/BCL2/BCL6) | 2 |
| Double expression of MYC/BCL2 | 4 |
| Cell of origin | |
| Germinal center | 10 |
| Activated B cell | 11 |
| Primary mediastinal | 3 |
| Not determined | 2 |
| Primary refractory (induction PR/induction failure) | 5 |
| Relapsed | 21 |
| Secondary IPI score | |
| 0–1 | 9 |
| 2 | 6 |
| >3 | 6 |
| First relapse | 14 |
| Length of CR1 | |
| <12 mo | 8 |
| ≥12 mo | 6 |
| Second relapse or later | 7 |
| Primary CNS lymphoma | 1 |
| Transformed lymphoma | 2 |
| Hodgkin lymphoma | 21 |
| Primary refractory | 12 |
| Poor-risk relapse | 9 |
| First relapse | 5 |
| Second relapse or later | 4 |
| Extranodal disease at relapse/PD | 11 |
| Bulky tumor at relapse/PD | 14 |
| B-symptoms at relapse/PD | 1 |
| T-NHL | 8 |
| Histology | |
| PTCL NOS | 3 |
| ALCL | 2 |
| NK-T | 2 |
| AITL | 1 |
| Primary refractory | 0 |
| Primary relapsed | 8 |
| Follicular lymphoma | 3 |
| Mantle cell lymphoma | 2 |
Abbreviations: ALCL, anaplastic large-cell lymphoma; AITL angioimmunoblastic T-cell lymphoma; BCL2, B-cell lymphoma 2; BCL6, B-cell lymphoma 6 protein; CNS, central nervous system; CR, complete response; CR1, first complete response; DLBCL, diffuse large B-cell lymphoma; HDC, high-dose chemotherapy; IPI, International Prognostic Index; NK-T, natural killer-T cell; MYC, v-myc avian myelocytomatosis viral oncogene homolog; NOS, not otherwise specified; PD, progressive disease; PET, positron emission tomography; PR, partial response; PTCL, peripheral T-cell lymphoma; T-NHL, T-cell non-Hodgkin lymphoma.
For definitions of “primary refractory tumor,” “poor-risk relapse,” and “refractory relapse,” see the text (Materials and Methods, Patient Population).
Hematologic Recovery
The stem cell source was peripheral blood. Neutrophils and platelets were engrafted at a median on day +9 (range, days +7 to +11) and day +12 (range, days +8 to +64), respectively.
Regimen-Related Toxicities
Azacitidine was administered daily at 15 mg/m2 (level 1), 25 mg/m2 (level 2), and 35 mg/m2 (level 3). Two patients died from infectious complications (sepsis possibly caused by norovirus on level 3, respiratory syncythial virus pneumonia on level 1). There were no grade 4 regimen-related toxicities. The frequencies of DLTs at levels 1, 2, and 3 were 16%, 28%, and 48%, respectively. Because the target DLT frequency was 25%, level 1 was identified as the MTD. The MTD level was then expanded to a total of 37 patients to better characterize its toxicity profile at that dose:
Mucositis
Grade 2 and 3 mucositis was observed in 43% and 32% of patients, respectively. The rates of grade 3 mucositis (that did or did not meet DLT criteria) in the different dose levels were 32% (level 1), 44% (level 2), and 80% (level 3). Mucositis (all grades) started at a median on day +3 (range, days 0 to +7) and lasted at maximal severity for a median of 4 days (range, days +2 to +7).
Dermatitis
Grade 1 and 2 erythematous rash was observed in 30% and 14% of patients, respectively. All cases resolved either spontaneously or with topical sunburn remedies or topical steroids.
Hepatic side effects
Early self-limited transaminase elevation was frequent (18% grade 2, 25% grade 3), starting around day −1 and resolving within 1 week. The median maximum level was 134 IU/L (range, 57–914 IU/L). Transient hyperbilirubinemia at a median 2.8 mg/dL (range, 1.2–5.2 mg/dL) was observed in 26 patients in the first 10 days post-transplantation (16% grade 2, 18% grade 3). There were no cases of venoocclusive disease.
Pulmonary effects
The corrected single-breath carbon monoxide diffusing capacity value decreased from transplantation levels (median, 78.5% of predicted; range, 52%–152%) to 1-month to 3-month post-transplantation levels (median, 71%; range, 31%–150%; P = .002). There were 3 symptomatic cases of steroid-responsive grade 2 pneumonitis (5%) in the entire study.
Other toxicities
Diarrhea was mild, with only 7 and 5 episodes of grade 2 and grade 3 diarrhea, respectively. Two patients experienced grade 2 renal toxicity. No neurologic or cardiac toxicities were observed.
There was no correlation of preadmission C-reactive protein, B-type natriuretic peptide, ferritin, haptoglobin, or troponin values with toxicity (data not shown). Likewise, there was no significant effect of age on toxicity (data not shown).
Infections
All 60 patients developed grade 3 neutropenic fever. Two patients died of sepsis in the setting of norovirus intestinal infection and isolation of norovirus in blood (level 3) and respiratory syncythial virus pneumonia (level 1), respectively. Other documented infections included 2 episodes of Clostridium difficile diarrhea, and 1 episode each of cytomegalovirus pneumonia and Escherichia coli bacteremia.
Busulfan Pharmacokinetic Studies
Busulfan pharmacokinetics were calculated in all patients. The median (% coefficient of variation) clearance values after the test dose and the first therapeutic dose were 85 (14%) and 88 (12%) mL per minute per m2, respectively. Only 2 patients had a busulfan clearance that differed >20% when the first and test doses were compared. For the remaining 58 patients, the clearance variation was <20% between the test dose and the first therapeutic dose. The median (% coefficient of variation) population volume of distribution and plasma half-life from the first therapeutic dose were 24 L/m2 (10.7%) and 3.14 hours (12.9%), respectively. These population pharmacokinetics do not differ from those previously estimated with Bu/Mel,22 Gem/Bu/Mel,11 or vorinostat/Gem/Bu/Mel17 (data not shown).
Tumor Responses
The ORR and CR rate among patients who had DLBCL with measurable disease were 78% and 55%, respectively. Seven of 8 patients with measurable HL had a CR. Two of 2 patients with measurable T-NHL experienced a CR. These CRs were durable for the most part: 12 of 14 patients (5 of 6 with DLBCL, 5 of 6 with HL, and 2 of 2 with T-NHL) remained in unmaintained CR at 10 to 27 months after ASCT.
There were 3 patients who had less than a PR post-transplantation (all 3 had progressive disease). Two of these patients, who had DLBCL, died from progressive disease shortly afterward. The third patient, who had HL, was enrolled in a trial of nivolumab to which she responded and remained on therapy 20 months after transplantation.
Post-HDC Treatment
Six patients (4 with HL, 2 with DLBCL) who had bulky (>5 cm), PET-positive lesions pretransplantation received involved-site radiotherapy with good tolerance at doses from 30.6 to 42 grays (Gy) starting 1 to 2 months after transplantation. None of the patients with HL received maintenance brentuximab.
Patient Outcomes
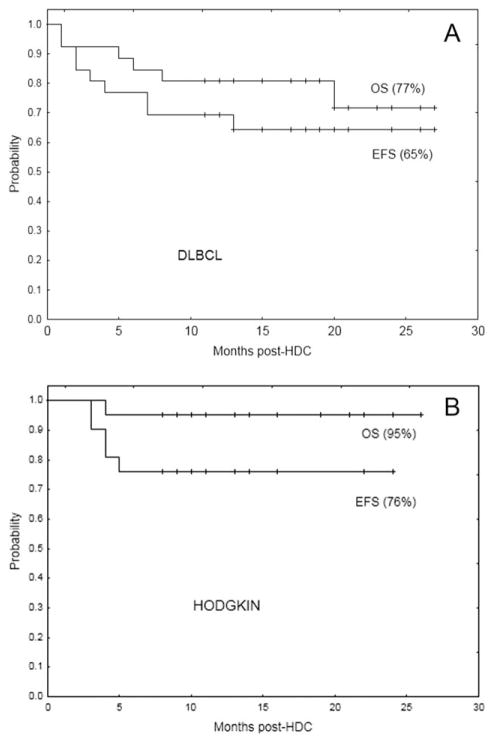
The median follow-up is 15 months (range, 8–27 months). Only 1 in 13 relapses occurred beyond 1 year post-HDC. The EFS and OS rates for the DLBCL group are 65% and 77%, respectively (Fig. 1A). The proportions of patients who remain alive in CR within the main different DLBCL subgroups are 2 of 3 with primary mediastinal tumors, 5 of 10 with double-hit tumors, 0 of 2 with transformed tumors, and 1 of 1 with a primary central nervous system tumor. According to the cell-of-origin type (determined by immunohistochemistry using the Hans algorithm29), 6 of 10 patients in the germinal center category and 6 of 11 patients in the activated B-cell category remain in CR.
Figure 1.
Event-free survival (EFS) and overall survival (OS) curves are shown for (A) the diffuse large B-cell lymphoma (DLBCL) subgroup (N = 26) and (B) the Hodgkin lymphoma (HL) subgroup (N = 21).
The EFS and OS rates among HL patients are 76% and 95%, respectively (Fig. 1B). Seven of the 8 patients with T-NHL are alive in CR at 10 to 17 months post-HDC. The 3 patients with follicular lymphoma and the 2 patients with mantel cell lymphoma are alive in CR at 10 to 23 months post-HDC.
It is noteworthy that patients who had PET-positive tumors at HDC demonstrated better than anticipated EFS in both the DLBCL group (50% vs 75% in the PET-negative subgroup; P = .16) and the HL group (71% vs 79% in the PET-negative subgroup; P = .6).
DNA Damage Response, Apoptosis, Histone Acetylation, and DNA Methylation Studies
Western blot analysis was used to determine the levels of γ-H2AX, PARP1, Ac-H3K9, and DNMT3B in peripheral blood mononuclear cells from 8 patients who received treatment at the MTD (Fig. 2). The level of γ-H2AX increased from baseline to day −1 by a median of 2.2-fold, indicating activation of the DNA damage response and consistent with our previous in vitro data. Likewise, PARP1 levels decreased by a median 41%, indicating apoptosis. Ac-H3K9 levels increased by 31%, and DNMT3B levels decreased in 6 of the 8 patients by a median of 68%. Overall, these results suggest the drug-induced activation of DNA damage response and apoptosis, which are correlated with histone acetylation and changes in the levels of proteins involved in DNA methylation.
Figure 2.
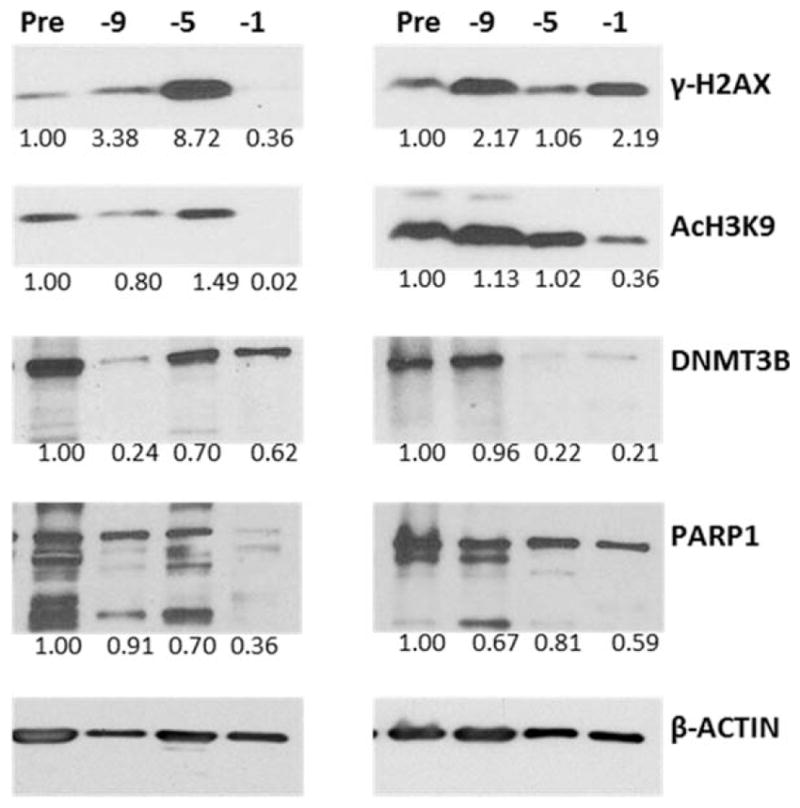
Western blot analysis of representative cases show changes in protein levels of γ-histone 2A, family member X (γ-H2AX); acetylation of histone 3 at lysine 9 (Ac-H3K9); DNA methyl transferase 3B (DNMT3B); and poly(ADP-ribose) polymerase 1 (PARP1) in peripheral blood mononuclear cells from patients who were treated at the maximum tolerated dose. The scanned band intensities were normalized to the β-actin signals and were analyzed relative to the pre-high–dose chemotherapy (HDC) sample (set at 1.0) on days −9, −5, and −1.
DISCUSSION
The current study demonstrates that azacitidine can be safely added to vorinostat/Gem/Bu/Mel. The schedule includes daily doses of azacitidine and vorinostat preceding full doses of Gem/Bu/Mel.
Confirming our preclinical studies, we observed high efficacy of azacitidine/vorinostat/Gem/Bu/Mel among patients with heavily pretreated and refractory lymphoma, mostly DLBCL and HL. At median follow-up of 15 months, the EFS rates in these 2 groups are 65% and 76%, respectively. Although longer follow-up is needed, these outcomes in such challenging populations are encouraging, because most relapses in these patients typically occur early after transplantation.4–10 Given the limitations of this study, which are intrinsic to early clinical trials, these results should be considered preliminary. Determining whether azacitidine/vorinostat/Gem/Bu/Mel is superior to traditional regimens like BEAM for any type of lymphoma will require a randomized study.
This study builds on our prior work on epigenetic modulation of HDC using vorinostat.17 We previously observed that vorinostat augmented the in vitro and clinical cytotoxicity of Gem/Bu/Mel in resistant B-cell and T-cell lymphomas.16,17 This effect could be mediated at least in part through activation of the DNA damage response signaling pathway, as demonstrated by increased levels of γ-H2AX. However, the ability of vorinostat to sensitize cells to DNA-damaging agents through chromatin remodeling still may not be maximized. Our prior experiments suggested that inhibition of DNA methyltransferases could further enhance the cytotoxicity of vorinostat-Gem/Bu/Mel. The current observations in our patient samples are also indicative of increased genomic injury that, among other responses, may lead to apoptosis.
Azacitidine is a pyrimidine nucleoside analog of cytidine. High doses are cytotoxic, whereas hypomethylation occurs at lower doses.30 The recommended hypomethylating dose as a single agent is 75 mg/m2 daily for 7 days, which is associated with significant myelosuppression but minimal nonhematologic toxicity.31 Previous reports of clinical combinations of azacitidine with standard-dose chemotherapy indicate their feasibility, allowing the administration of chemotherapy at full doses.32 In our current study, the MTD of azacitidine was identified at 15 mg/m2 daily for 10 days, which resulted in a major down-regulation of DNMT3B in most patients who received treatment at that dose. The side effects of azacitidine/vorinostat/Gem/Bu/Mel were manageable, including mucositis, dermatitis, and clinically silent elevation of liver function tests. A few patients (5%) developed steroid-responsive grade 2 pneumonitis. Although the incidence of mucositis was higher than usually expected with BEAM, the toxicity profile of azacitidine/vorinostat/Gem/Bu/Mel was similar to that we previously described with Gem/Bu/Mel11 and vorinostat/Gem/Bu/Mel.17
It would be useful to identify predictive markers of toxicity. Previous reports have correlated high pretransplantation values of C-reactive protein, ferritin, or B-type natriuretic peptide with severe transplantation-related toxicity.33–35 Likewise, haptoglobin levels have been inversely associated with gemcitabine hematologic toxicity.36 In contrast, we could not establish any correlation between those markers or any patient characteristic and toxicity. We recently demonstrated that polymorphic genetic variation of relevant enzymes involved in the metabolism of gemcitabine and DNA damage repair may predict toxicity after Gem/Bu/Mel.37 Finally, busulfan pharmacokinetics were similar to those we previously estimated with Gem/Bu/Mel,11 indicating no pharmacokinetic interaction between busulfan, vorinostat, and azacytidine.
In conclusion, azacitidine can be safely combined with vorinostat/Gem/Bu/Mel plus ASCT. This regimen induced high CR rates, promising early outcomes and encouraging correlative in vitro data from patients with refractory or poor prognosis relapsed HL and NHL. Further investigation of this combination is warranted.
TABLE 2.
Dose Escalation
| Dose Level | Azacitidine, mg/m2/da | Vorinostat, mg/d | Gemcitabine, mg/m2/db | Busulfan, μm-min/d | Melphalan, mg/m2/d | % DLT |
|---|---|---|---|---|---|---|
| 1 | 15 | 1000 | 2775 | 4000 | 60 | 16 |
| 2 | 25 | 1000 | 2775 | 4000 | 60 | 28 |
| 3 | 35 | 1000 | 2775 | 4000 | 60 | 40 |
Abbreviation: MTD, maximum tolerated dose.
The MTD of azacytidine was encountered at dose level 1.
A continuous infusion of gemcitabine was administered at 10 mg/m2 per minute.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
AUTHOR CONTRIBUTIONS
Yago Nieto: Designed study, enrolled and treated patients, analyzed data, and wrote manuscript. Benigno C. Valdez: Designed study, analyzed data, and wrote manuscript. Peter F. Thall: Designed study, analyzed data, and wrote manuscript. Roy B. Jones: Enrolled and treated patients, analyzed data, and wrote manuscript. Wei Wei: Designed study, analyzed data, and wrote manuscript. Alan Myers: Analyzed data and wrote manuscript. Chitra Hosing: Enrolled and treated patients. Sairah Ahmed: Enrolled and treated patients. Uday Popat: Enrolled and treated patients. Elizabeth J. Shpall: Enrolled and treated patients. Muzaffar Qazilbash: Enrolled and treated patients. Alison Gulbis: Enrolled and treated patients. Paolo Anderlini: Enrolled and treated patients. Nina Shah: Enrolled and treated patients. Amin Alousi: Enrolled and treated patients. Yasuhiro Oki: Enrolled and treated patients. Michelle Fanale: Enrolled and treated patients. Bouthaina Dabaja; Enrolled and treated patients. Chelsea Pinnix: Enrolled and treated patients. Richard Champlin: Enrolled and treated patients. Borje S. Andersson: Designed study, enrolled and treated patients, analyzed data, and wrote manuscript.
CONFLICT OF INTEREST DISCLOSURES
Yago Nieto reports research support from Otsuka Pharmaceuticals, Novartis Pharmaceuticals and Celgene Pharmaceuticals outside the submitted work. Chitra Hosing reports grants for clinical trials from Celgene Pharmaceuticals and personal fees from Sanofi, Aviara Pharmaceuticals, Seattle Genetics, Cardinal Health outside the submitted work. Qaiser Bashir reports research funding from Celgene Pharmaceuticals and Takeda Pharmaceuticals and personal fees from Takeda Pharmaceuticals and Spectrum Pharmaceuticals outside the submitted work. Richard Champlin reports research support from Sanofi and Celgene Pharmaceuticals outside the submitted work. Borje S. Andersson reports personal fees from Otsuka America Pharmaceutical, Inc, outside the submitted work. The remaining authors made no disclosures.
References
- 1.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin disease: results of a BNLI randomized trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous hemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin disease: a randomized trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 3.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 4.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blay J, Gomez F, Sebban C, et al. The International Prognostic Index correlates to survival in patients with aggressive lymphoma in relapse: analysis of the PARMA trial. Parma Group. Blood. 1998;92:3562–3568. [PubMed] [Google Scholar]
- 6.Guglielmi C, Gomez F, Philip T, et al. Time to relapse has prognostic value in patients with aggressive lymphoma enrolled onto the Parma trial. J Clin Oncol. 1998;16:3264–3269. doi: 10.1200/JCO.1998.16.10.3264. [DOI] [PubMed] [Google Scholar]
- 7.Caballero MD, Perez-Simon JA, Iriondo A, et al. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003;14:140–151. doi: 10.1093/annonc/mdg008. [DOI] [PubMed] [Google Scholar]
- 8.Vose JM, Zhang MJ, Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodgkin lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2011;19:406–413. doi: 10.1200/JCO.2001.19.2.406. [DOI] [PubMed] [Google Scholar]
- 9.Lazarus HM, Loberiza FR, Jr, Zhang MJ, et al. Autotransplants for Hodgkin disease in first relapse or second remission: a report from the Autologous Blood and Marrow Transplant Registry (ABMTR) Bone Marrow Transplant. 2001;27:387–396. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 10.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin lymphoma autografted after a first relapse. Ann Oncol. 2005;16:625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 11.Nieto Y, Thall P, Valdez B, et al. High-dose infusional gemcitabine combined with busulfan and melphalan with autologous stem-cell transplantation in patients with refractory lymphoid malignancies. Biol Blood Marrow Transplant. 2012;18:1677–1686. doi: 10.1016/j.bbmt.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunewald R, Kantarjian H, Du M, Faucher K, Tarassoff P, Plunkett W. Gemcitabine in leukemia: a phase 1 clinical, plasma, and cellular pharmacology study. J Clin Oncol. 1992;10:406–413. doi: 10.1200/JCO.1992.10.3.406. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi V, Plunkett W, Du M, Ayres M, Estey EH. Prolonged infusion of gemcitabine: clinical and pharmacodynamic studies during a phase 1 trial in relapsed acute myelogenous leukemia. J Clin Oncol. 2002;20:665–673. doi: 10.1200/JCO.2002.20.3.665. [DOI] [PubMed] [Google Scholar]
- 14.Nieto Y, Popat U, Anderlini P, et al. Autologous stem cell transplantation for refractory or poor-risk relapsed Hodgkin lymphoma: effect of the specific high-dose chemotherapy regimen on outcome. Biol Blood Marrow Transplant. 2013;19:410–417. doi: 10.1016/j.bbmt.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 16.Valdez BC, Nieto Y, Murray D, et al. Epigenetic modifiers the synergistic cytotoxicity of combined nucleoside analog-DNA alkylating agents in lymphoma cell lines. Exp Hematol. 2012;40:800–810. doi: 10.1016/j.exphem.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieto Y, Valdez BC, Thall PF, et al. Vorinostat combined with high-dose gemcitabine, busulfan and melphalan with autologous stem-cell transplantation in patients with refractory lymphomas. Biol Blood Marrow Transplant. 2015;21:1914–1920. doi: 10.1016/j.bbmt.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalac M, Scotto L, Marchi E, et al. HDAC inhibitors and decitabine are highly synergistic and associated with unique gene-expression and epigenetic profiles in models of DLBCL. Blood. 2011;118:5506–5516. doi: 10.1182/blood-2011-02-336891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meignan M, Gallamini A, Itti E, Barrington S, Haioun C, Polliack A. Report on the Third International Workshop on Interim Positron Emission Tomography in Lymphoma held in Menton, France, 26–27 September 2011 and Menton 2011 consensus. Leuk Lymphoma. 2012;53:1876–1881. doi: 10.3109/10428194.2012.677535. [DOI] [PubMed] [Google Scholar]
- 21.De Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 22.Kebriaei P, Madden T, Kazerooni R, et al. Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies. Biol Blood Marrow Transplant. 2011;17:412–420. doi: 10.1016/j.bbmt.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trial in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- 24.Cancer Therapy Evaluation Program. [Accessed May 13, 2016];Common Terminology Criteria for Adverse Events v3.0 (CTCAE) Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 25.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Mantel N. Evaluation of survival data and 2 new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;60:163–176. [PubMed] [Google Scholar]
- 28.Fisher R. On the interpretation of ×2 from contingency tables, and the calculation of P. J R Stat Soc. 1922;85:87–94. [Google Scholar]
- 29.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 30.Quintas-Cardama A, Santos FPS, Garcia-Manero G. Therapy with azanucleosides for myelodysplastic syndromes. Nat Rev Clin Oncol. 2010;7:433–444. doi: 10.1038/nrclinonc.2010.87. [DOI] [PubMed] [Google Scholar]
- 31.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomized, open-label, phase 3 study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krug U, Koschmieder A, Schwammbach D, et al. Feasibility of azacitidine added to standard chemotherapy in older patients with acute myeloid leukemia—a randomized SAL pilot study [serial online] PLoS One. 2012;7:e52695. doi: 10.1371/journal.pone.0052695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1209–1216. doi: 10.1016/j.bbmt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim ZY, Fiaccadori V, Gandhi S, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukemia receiving reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leuk Res. 2010;34:723–727. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Kataoka K, Nannya Y, Iwata H, et al. Plasma brain natriuretic peptide is associated with hepatic veno-occlusive disease and early mortality after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:1631–1637. doi: 10.1038/bmt.2010.26. [DOI] [PubMed] [Google Scholar]
- 36.Matsubara J, Ono M, Negishi A, et al. Identification of a predictive biomarker for hematologic toxicities of gemcitabine. J Clin Oncol. 2009;27:2261–2268. doi: 10.1200/JCO.2008.19.9745. [DOI] [PubMed] [Google Scholar]
- 37.Shinozuka K, Tang H, Jones RB, Li D, Nieto Y. Impact of polymorphic variations of gemcitabine metabolism, DNA damage repair, and drug resistance genes on the effect of high-dose chemotherapy for relapsed or refractory lymphoid malignancies. Biol Blood Marrow Transplant. 2016;22:843–849. doi: 10.1016/j.bbmt.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]