Abstract
Cellular crosstalk is a process through which a message is transmitted within an individual cell (intracellular crosstalk) or between different cells (intercellular crosstalk). Intercellular crosstalk within the liver microenvironment is critical for the maintenance of normal hepatic functions and for cells survival. Hepatic cells are closely connected to each other, work in synergy, and produce molecules that modulate their differentiation and activity. This review summarises the current knowledge regarding paracrine communication networks in parenchymal and non-parenchymal cells in liver fibrosis due to chronic injury, and regeneration after partial hepatectomy.
Keywords: Liver sinusoidal endothelial cells, LSEC, hepatic stellate cells, HSC, Kupffer cells, Hepatocytes, cirrhosis, portal hypertension, ischemia/reperfusion, transplantation, regeneration
1- Introduction: Liver sinusoidal cells and cellular crosstalk
The hepatic sinusoid represents a well-organized vascular matrix that provides the structural and biochemical environment in which non-parenchymal liver cells live and communicate. The space of Disse separates sinusoidal cells from parenchymal cells and contains extracellular matrix (ECM) components. Thus, the liver microenvironment can be described as a multidirectional interaction complex (cell-matrix-cell) organized to manage the delivery of molecular signals, where every piece has a crucial role. It is due to this particular structure that hepatic cells, composed mainly of hepatocytes, liver sinusoidal endothelial cells (LSEC), hepatic stellate cells (HSC), and Kupffer cells (KC), precisely “talk” to each other.
The word crosstalk was firstly used in electronics and technology communication to explain any phenomena by which a signal transmitted on one circuit creates an undesired effect in another circuit. In biomedical science we do not view this phenomenon as undesired but, consider it a mechanism cells have to transmit “instructions” both in physiological and pathophysiological situations. In the latter scenario instructions are considered as transmitted errors, and are therefore an event or multi-events that could be considered as biological targets.
LSEC, the hepatic cell population that interfaces blood components and forms the barrier of sinusoids, are pivotal regulators of the liver microcirculation and have a key role in sinusoidal crosstalk. Their peculiarities (small cells with non-diaphragmed fenestrae and without a basement membrane) make them a vital link in the complex network of hepatic cellular interactions both in health and disease. Fenestrae, arranged in sieve plates, contribute to LSEC permeability and functions [1], facilitating oxygenation of hepatocytes and enhancing hepatocytes exposure to macromolecules from the portal circulation. They are capable of contracting or dilating, can change in size, porosity (number of fenestrae per μm2), and frequency, acting like a filter for the transit of substances. Modifications in fenestrae properties, a process known as pseudocapillarization, are associated with aging and hypoxia [2, 3], while capillarization (complete loss of fenestrae) [4] seems to precede the development of most chronic liver diseases [5, 6]. Note that the crude term “capillarization” in this specific context means that the unique and highly specialized phenotype of LSEC is lost and cells become ordinary non-specialized endothelial cells, or endothelial cells of an ordinary capillary.
LSEC are the main source of the endothelium-derived nitric oxide (NO), an important modulator of vascular tone, where it is produced by the endothelial nitric oxide synthase (eNOS). Moreover, LSEC express singular sets of adhesion molecules that correlate with the micro-environmental characteristics of the sinusoidal wall [7]. These adhesion molecules include ICAM-1 (Intercellular Adhesion Molecule 1), VCAM-1 (vascular cell adhesion molecule 1), and selectins, are regulated by inflammatory cytokines, and influence cell-to-cell interactions [8]. Interestingly, LSEC phenotype is maintained, at least in part, by paracrine secretion of vascular endothelial growth factor (VEGF) by hepatocytes and HSC [9].
HSC, localized in the space of Disse, are the main collagen-synthesizers of the liver [10], and contribute to its architecture and functions by interaction with neighbouring cells [11]. They preserve retinoid storage and homeostasis, ECM metabolism, and sinusoidal lumen diameter [12]. A single stellate cell can wrap up to 4 sinusoids and regulate sinusoidal blood flow by contraction. Injuries to the hepatic microvasculature activate the trans-differentiation of HSC conferring them a proliferative and fibrogenic myofibroblast-like phenotype. HSC activities mainly depend on their interactions with ECM components, endothelial cells and hepatocytes [11, 13].
KC are hepatic macrophages that have an essential role in the liver immune system [14] and inflammation [15]. They are attached to the sinusoidal endothelial layer, where they uniquely capture signals from the blood and contribute to hepatic blood flow regulation [16]. They are mainly the source rather than the target of soluble mediators [14], eliciting a physiological response to all other liver cells.
Hepatocytes represent the most abundant cell type within the liver and, although they are the major functional hepatic cells, their interactions with sinusoidal cells are totally necessary for their multiple activities. While consequences of endothelial capillarization and HSC phenotype deregulation on liver function are well characterised, the exact process of hepatocyte dysfunction in chronic liver disease is not completely known. Indeed, the liver can function normally with less than half of its hepatocytes, although they have the unique ability for continuous turnover and replenishment [17].
As a consequence of parenchymal and non-parenchymal interactions cells grow and proliferate, migrate and differentiate, and preserve their normal cellular phenotype. In this interactive network, healthy or injured cells become the positive or negative regulators of the closest neighbouring cells (via juxtacrine signalling and receptor-ligand complexes or through indirect contact), or of the hypothetically most distant cells (via a variety of soluble paracrine and endocrine factors, such as cytokines, growth factors, second messengers and hormones) (Table 1). In this regard, ECM is considered a depository for growth factors, cytokines and other proteins that can be released when required to be used by proximal cells, contributing to cellular programming [18, 19]. Understanding the intercellular crosstalk within the sinusoids is critical for a better knowledge of the progression, aggravation, and resolution of liver disease, and modifications in the coordinated interactions may lead to disease development/improvement.
Table 1.
Paracrine crosstalk agents.
| cytokines and growth factors | LGF, VEGF, FGF, IGF, HB-EGF, PDGF, HGF, TGF-β, CTGF, SDF-1α, MCP-1, midkine and pleiotrophin |
| second messengers | ET-1, Angiotensin II, NO, angiopoientin, apelin, leptin, adenosine, cGMP |
| hormones | aldosterone, prostaglandins, adiponectin, intermedin |
| cellular particles | microparticles, exosomes |
LGF: liver growth factor; VEGF: vascular endothelial growth factor; FGF: fibroblast growth factor; IGF: insulin growth factor; HB-EGF: heparin-binding EGF-like growth factor; PDGF: platelet derived growth factor; HGF: hepatocyte growth factor; TGF-β: transforming growth factor beta; CTGF: connective tissue growth factor; SDF-1α: Stromal cell-derived factor 1; MCP-1: Monocyte Chemoattractant Protein-1; ET-1: endothelin-1; NO: nitric oxide; cGMP: cyclic guanosine monophosphate
2- Sinusoidal crosstalk in fibrosis, cirrhosis and portal hypertension
Fibrosis is characterised by intra-hepatic accumulation of ECM, mainly in the perisinusoidal space of Disse and portal tracts [20]. The formation of abnormal nodules with consequent distortion of the liver architecture, inflammation, vascular occlusion, and intra-hepatic angiogenesis aggravate the fibrotic state leading to the development of cirrhosis [21]. Alterations in the normal crosstalk cause progressive microvascular dysfunction in the cirrhotic liver, increase in hepatic vascular resistance and development of the main complication of cirrhosis, portal hypertension [22]. All sinusoidal cells take part in this process: they communicate and acquire a vasoconstrictor phenotype that is further exacerbated in response to biomechanical, pathogenic and inflammatory stimuli [23, 24]. However, our understanding of the mechanisms underlying the changes in phenotype of sinusoidal cells and the divergent cellular communication during cirrhosis progression is far from complete. Unfortunately, the only treatment for end-stage cirrhosis is transplantation [25], and the need to develop anti-fibrotic compounds is a must.
2.1. Liver fibrosis is initiated by crosstalks from LSEC & Hepatocytes
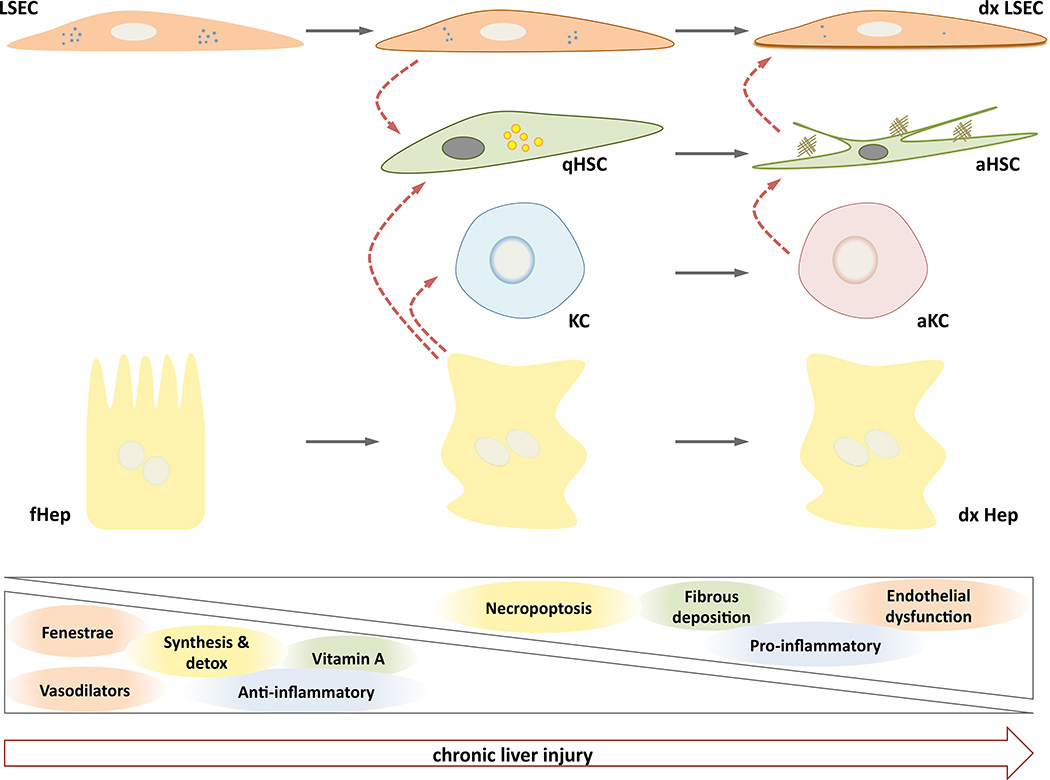
The fibrogenic reaction is initiated by two major intercellular crosstalks that are ultimately connected. In the presence of hepatic injury, LSEC become rapidly de-regulated and start dedifferentiation towards a capillarized phenotype, accompanied by the production and release of soluble factors that rapidly travel to neighbouring cells affecting their phenotype [26]. As an example, it has been demonstrated that LSEC-derived fibronectin affects HSC phenotype, promoting their activation [27]. In parallel, exogenous hepatic injury significantly modifies hepatocytes transcriptional programs promoting their proliferation and death. Hepatocyte apoptosis results in the formation of apoptotic bodies that once captured by the non-parenchymal cells (HSC and KC) contribute to their activation [17, 28, 29]. In turn, HSC begin to proliferate, contract and deposit elevated amount of collagen fibers and extracellular matrix molecules in the hepatic parenchyma, contributing to the stiffening of the organ and perturbing all cellular functions. Interestingly, collagen accumulation in the space of Disse may contribute to the loss of endothelial fenestrations, aggravating hepatic fibrosis [30]. Thus, a self-perpetuating cycle between collagen-producing activated HSC and capillarized LSEC stimulate each other, further contributing to liver fibrosis (Figure 1).
Figure 1. Sinusoidal crosstalk during chronic liver injury.
Initial de-regulations in functional Hepatocytes (fHep) and Liver Sinusoidal Endothelial Cells (LSEC) due to liver damage lead to complex paracrine interactions (red dotted arrows) with quiescent Hepatic Stellate Cells (qHSC) and Kupffer Cells (KC), ultimately creating a dysfunctional sinusoidal microenvironment composed by dysfunctional LSEC (dxLSEC), activated HSC (aHSC), activated KC (aKC) and dysfunctional and necroptotic hepatocytes (dxHep). Phenotypic characteristics of each cell type are progressively lost during the progression of the liver disease, and new pathologic properties appear.
Hepatocytes and LSEC also communicate with each other during fibrogenesis via the VEGF-A/VEGFR2 pathway [31]. It has been recently observed that CD147, a transmembrane glycoprotein linked to liver fibrosis [32], promotes the expression and secretion of VEGF-A via the PI3K/Akt signalling pathway in hepatocytes, and up-regulates VEGFR2 expression in LSEC, augmenting their proliferation and migration. Interestingly, anti-CD147 antibodies inhibited the VEGF-A/VEGFR-2-mediated angiogenesis with a consequent attenuation of liver fibrosis progression. The synchronistic expression of CD147 in both hepatocytes and LSEC suggest a regulatory role for CD147 in hepatocyte–LSEC interactions [33]. Therapeutic trials blocking CD147-induced angiogenesis may be promising for the treatment of liver fibrosis.
Considering the cellular communications initiating the fibrogenesis reaction, protection of LSEC and/or hepatocytes may be an effective strategy to avoid fibrosis progression. Potentially, drugs specifically designed to ameliorate LSEC phenotype may also prevent the progression of fibrosis, and its derived complications. Although this idea has been demonstrated in experimental models of cirrhosis regression [34, 35], it has not been validated in situations of liver fibrosis progression. Regarding hepatocytes, apoptosis occurs in physiological conditions with no release of pro-inflammatory cytokines resulting in a minimal immune response. It would be reasonable to extrapolate that under pathophysiological situations, the mechanisms that would otherwise protect neighbouring cells fail. Inhibiting hepatocyte apoptosis following injury does not convey benefit [36], and an alternative strategy could be aimed at either blocking the capturing of the apoptotic bodies by non-parenchymal cells, or building a ‘screen’ between hepatocytes and HSC/KC. To test these hypotheses however, we first need to understand when cell dysfunction initially occurs, and to which cell.
2.2 Communications between hepatic cells and ECM
As stated, LSEC have a major role in hepatic fibrogenesis: they are contributors of extracellular matrix (ECM) deposition [37] and regulators of ECM metabolism [38], however, LSEC phenotype is strongly modulated by ECM itself. Ford and colleagues demonstrated how the phenotype of LSEC changes in response to matrix stiffness. Using collagen hydrogels with two different values for elastic modulus they reproduced healthy (6 kPa) and fibrotic (36 kPa) tissue matrix. LSEC seeded on 6 kPa substrates exhibited well-defined fenestrae arranged in sieve plate structures, and underwent pseudocapillarization after 96h. However, as a result of increasing elastic modulus, LSEC seeded on 36 kPa substrates completely lose fenestrae and express CD31 at the surface just after 24 h [39], thus confirming the importance of HSC-derived ECM on LSEC phenotype [18].
Liver ECM also markedly influences the behaviour of other non-parenchymal cells, especially portal myofibroblasts (PMF) and HSC. When cultured on stiff rather than soft collagen lattices PMF increase their expression of fibrillin-1 [40], and HSC become highly activated loosing their lipid droplets and expressing high levels of α-SMA [41], demonstrating that the stiffness of the matrix has significant functional relevance for the contractile non-parenchymal cells. Interestingly, a recent paper further depicts the underlying mechanisms partly responsible for such activation revealing a negative regulation between RhoA and the cytosolic tyrosine kinase c-SCR [42].
In addition to ECM stiffness, its chemical components can also actively modulate cells status. Hyaluronic acid (HA) is one of the main components of the ECM and influences cell proliferation and migration, and it has been proposed as a marker of liver fibrosis since high serum levels correlate with fibrotic stages [43]. Within the liver, HA is mostly synthesized by HSC and degraded by LSEC [44]. This efficient homeostasis is guaranteed by the activity of KC, since their depletion does not allow HA uptake by LSEC [45], suggesting that maintaining KC in a competent state could control ECM metabolism and contribute to fibrosis resolution. Conversely, KC inactivation using gadolinium chloride prevents the development of cirrhosis in experimental models of fibrosis [46]. KC also play a key role modulating the phenotype of neighbouring non-parenchymal cells. This relationship has been characterised by Nieto and colleagues: in a seminal paper they described how KC negatively affect neighbouring HSC, promoting their activation defined as high proliferation, high expressions of α-SMA and collagen-I, and reduced capability to degrade extracellular collagen [47]. Furthermore, the paper described an H2O2-IL-6-dependent mechanism responsible for such intercellular communication. Subsequent studies validated the KC-HSC crosstalk when M2 phenotype-like KC were co-cultured with HSC, thus confirming such communications in an injured microenvironment [48].
In the clinic liver stiffness can be measured with transient elastography and provide a measure of fibrosis [49], thus a hypothetical degree of cell-matrix-cell crosstalk potentially could be estimated at the bedside due to its quantifiable association with liver stiffness.
2.3 LSEC & HSC communications in fibrosis and cirrhosis
The interaction between LSEC and HSC in fibrosis/cirrhosis has been extensively investigated. In 2004 DeLeve and colleagues showed that the phenotype of rat LSEC was maintained by autocrine and paracrine regulation exerted by either healthy hepatocytes or stellate cells, and that there was no added effect of co-culturing both hepatocytes and stellate cells together with LSEC [9]. Their experiments suggested the requirement of an autocrine production of NO by LSEC, since the addition of the inhibitor of eNOS L-NAME (L-NG-Nitroarginine Methyl Ester) was associated with a low number of surface CD31 positive cells, a marker of LSEC de-differentiation. Subsequently, they demonstrated that the maintenance of differentiated LSEC phenotype (with regular fenestrae) was due to both a VEGF-stimulated-NO-independent pathway and a VEGF-stimulated-NO-dependent pathway, and that restoration of LSEC differentiation in vivo promoted HSC quiescence, prevention of fibrosis progression, and regression of mild fibrosis [50, 51]. Studies from our group further investigated the HSC/LSEC paracrine interactions, especially in pathological conditions, and demonstrated that co-culture of differentiated LSEC with HSC in which eNOS was inhibited by L-NAME blocked the ability of LSEC to maintain HSC quiescence [52]. We proposed that the transcription factor Kruppel-Like Factor 2 (KLF2) is crucial in safeguarding the hepatic sinusoid via a bidirectional protective cell-to-cell communication in which growth factors and second messengers play a key role. Using in vitro and in vivo experimental models of liver cirrhosis, we demonstrated that KLF2-mediated improvement in HSC phenotype paracrinally ameliorates the dysfunctional phenotype of LSEC and vice versa, leading to a significant regression of liver cirrhosis and a marked reduction in portal pressure [52, 53].
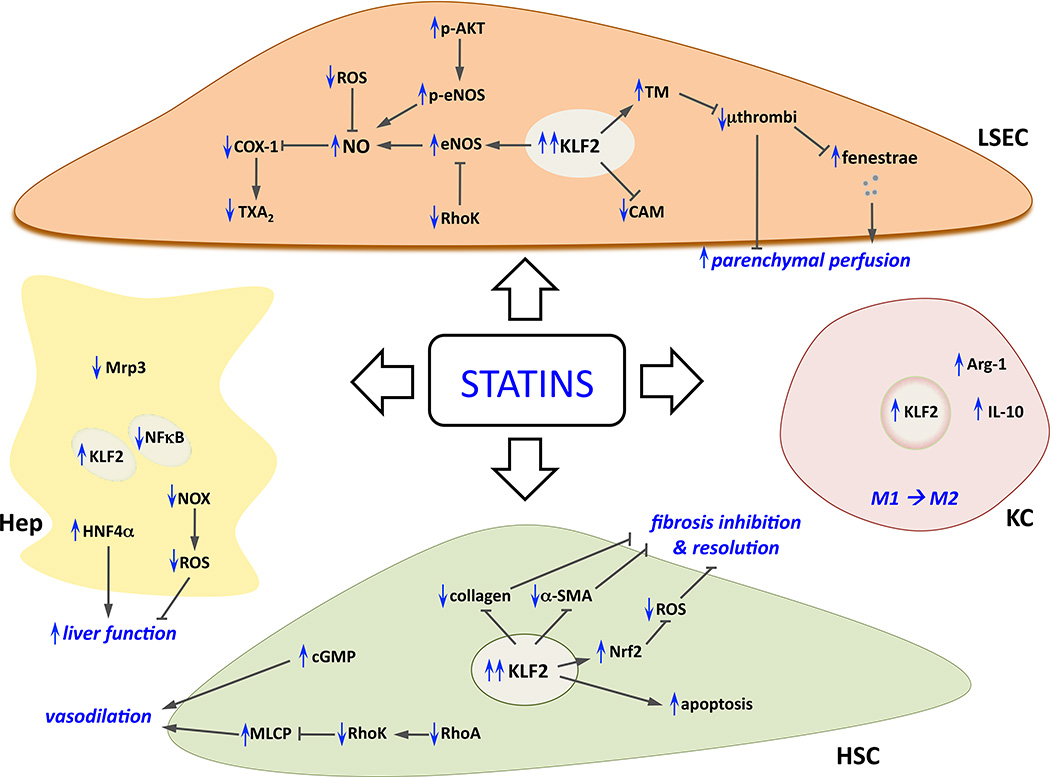
In these recent papers, following the research initiated 20 years ago by Jaime Bosch [54] and conducted subsequently by our group and others [55–61], some of the mechanisms through which statins have beneficial effects on dysfunctional sinusoidal cells have been described. We observed that simvastatin is the optimal statin [52] to increase NO bioavailability selectively in the liver [55] and that its anti-oxidant, anti-inflammatory, vasodilatory and anti-fibrotic effects were due to KLF2 activity [53, 62]. Interestingly, results from a recently published placebo-controlled randomized clinical trial demonstrate that simvastatin reduces mortality in patients with cirrhosis who have survived an episode of variceal haemorrhage [63]. Other NO donors have undesired systemic effects that prevent their use at the bedside [64–67], but simvastatin, an already established medication, appears to be a good anti-fibrotic candidate due to simultaneous effects on different hepatic cells (Figure 2). This potentially occurs through the ability of simvastatin to activate the appropriate molecules, such as KLF2, needed for cell communication [68, 69].
Figure 2. Effects of statins on sinusoidal cells.
Summary of cellular and molecular mechanisms underlying the beneficial effects of statins in liver cells (in blue, modifications due to statins administration). Please note that to simplify the figure paracrine interactions between statin-improved cells are intentionally omitted, complete explanation can be found along the text. α-SMA; smooth muscle actin alpha; Arg-1, arginase-1; CAM, cell adhesion molecules; cGMP, cyclic guanosine monophosphate; COX-1, cyclooxygenase-1; eNOS, endothelial nitric oxide synthase; HNF4α, hepatocyte nuclear factor 4 alpha; KLF2, Kruppel-like factor 2; MLCP, myosin light chain phosphatase; Mrp3, ATP-binding cassette sub-family C member 3; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; NOX, NADPH oxidase; Nrf2, nuclear factor (erythroid-derived 2)-like 2; ROS, radical oxygen species; TM, thrombomodulin; TXA2, thromboxane A2.
Additionally, recent data support the concept that sinusoidal communication occurs not only via the release of soluble molecules but also through the liberation of cellular microvesicles. Two papers describe the importance of this type of paracrine interactions in cirrhosis and portal hypertension [70, 71]. In the first study, using in vitro co-culture models of PMF and endothelial cells, authors describe the release of microparticles rich in VEGF by PMF, which in turn promote an increase in the pro-angiogenic activity of endothelial cells. The second study further supports the exosomal sinusoidal paracrine communication, demonstrating that secretion of exosome-packaged Sphingosine kinase-1 by endothelial cells paracrinally act on HSC, promoting their activation (in terms of elevated migration). These papers add key information to our understanding of sinusoidal communications, opening new avenues of research.
In summary, findings confirm that LSEC act as “protective gatekeepers” of the microvascular milieu, and keeping LSEC in a healthy condition is an acute strategy for the development of new hepatoprotective drugs. Additionally, therapies aimed at controlling KC or HSC activation may also prevent the development of hepatic cirrhosis, probably in part due to the re-activation of proper hepatic crosstalk. To date, various drugs with anti-inflammatory or anti-oxidant properties have shown promising results on fibrosis at the benchside, but limited outcomes in clinical trials [72–76], likely because of unknown effects on cellular communication. Future studies are needed to discover new mechanisms of cellular crosstalk to determine at which stage of cirrhosis progression these interactions occur. The mechanism of microvesicle transport could potentially be productive in this regard.
3- Sinusoidal crosstalk in liver regeneration and transplantation
Parenchymal and non-parenchymal cells tightly regulate their proliferation. Sinusoidal cells are a key source of cytokines and growth factors needed by the hepatocytes, and sinusoidal cells growth is modulated by hepatocytes and other non-parenchymal cells [77, 78]. The maintenance of these intercellular relationships is essential to promote the replacement of missing hepatocytes during liver regeneration, a process that occurs more frequently after ischaemic or toxic injury. When the liver is damaged beyond its ability to regenerate, liver transplantation is the treatment of choice. Living-donor transplantation, bioengineered liver support systems or patient-specific hepatic cell transplantation are current alternatives to whole liver transplantation. Understanding the effects of sinusoidal cells on hepatocytes phenotype will help clarify sequalae associated with liver injury due to the ischemia/reperfusion occurring during hepatic resection and liver transplantation.
3.1. Liver regeneration – classic & new views
Most of the data regarding liver regeneration derives from experimental partial hepatectomy, which is the accepted animal model to mimic human major hepatic resection. However, the positive results obtained in the laboratory are difficult to successfully translate to clinical therapies probably because the regenerative program in humans differs greatly following damage by drug overdose, viral infection, or excessive alcohol consumption [79].
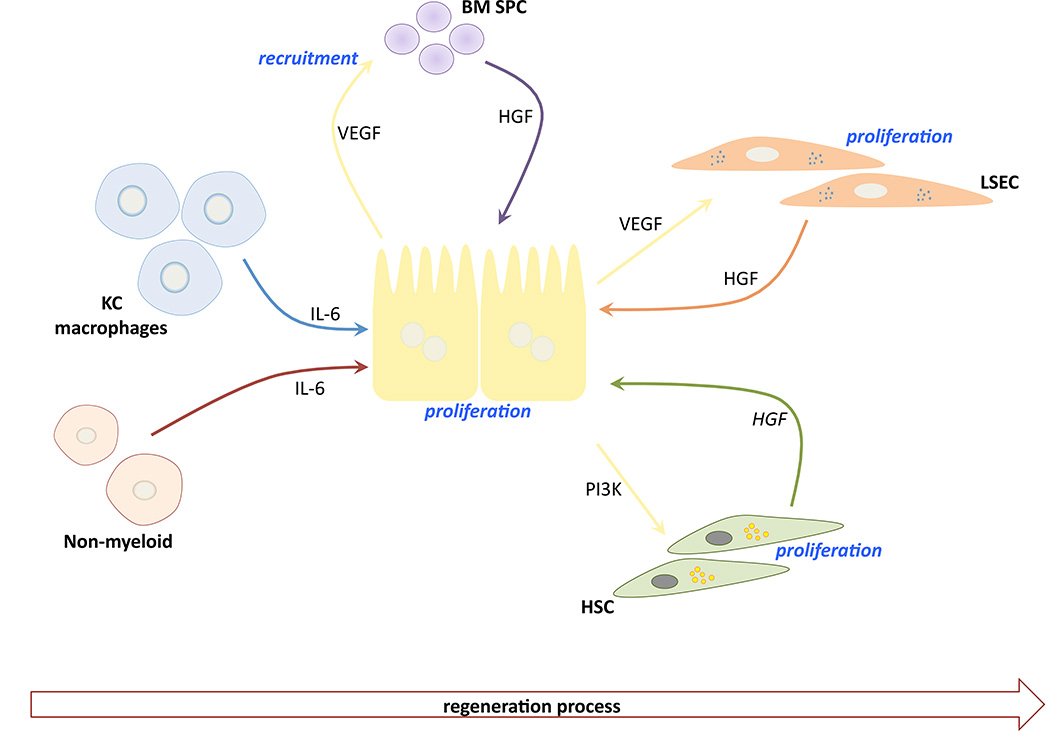
The initiation of liver regeneration after conventional two-thirds partial hepatectomy appears macrophage driven (Figure 3). Peak macrophage proliferative response is at 48–72h [80], at which point they begin to communicate with hepatocytes mostly through the production of IL-6 [81, 82]. LSEC and HSC divide approximately 96h after partial hepatectomy, primarily due to hepatocyte-derived VEGF/angiopoietin stimulation, and proteins relying on phosphoinositide 3-kinase for their mitogenic effect, respectively [83–85]. There is an evident cooperation among non-parenchymal and parenchymal cells during regeneration [86], however, the idea that bone marrow-derived endothelial sinusoidal progenitor cells (BM SPC), rich in hepatocyte growth factor (HGF), are also recruited to the site of the injury is now emerging [87, 88]. Recently, Katagiri and colleagues observed that a small representation (1–2%) of bone marrow mesenchymal stem cells, named Muse, differentiate into liver-lineage cells and repair tissue. After their infusion in a model of partial hepatectomy, they integrated into regenerating areas and expressed liver progenitor markers during the early phase of regeneration to then differentiate into hepatocytes, KC and LSEC [89]. Muse cells can be obtained easily from patients and donors, and could be used for clinical applications. Their differentiation into a specific hepatic cell may depend on cell-matrix-cell interactions; reinforcing the concept that liver matrix is much more than a simple scaffold.
Figure 3. Sinusoidal crosstalk during liver regeneration.
Upon hepatectomy, initial signals (mainly IL-6) from Kupffer cells (KC), macrophages and other non-myeloid cells lead to the first burst of hepatocytes proliferation, which in turn will produce vascular endothelial growth factor (VEGF) to recruit bone marrow-derived sinusoidal progenitor cells (BM SPC) as a key source of hepatocyte growth factor (HGF). Latterly, hepatic stellate cells (HSC) and liver sinusoidal endothelial cells (LSEC) contribute to the regeneration process.
3.2 Role of LSEC in liver repopulation
Improved understanding of the cellular crosstalk occurring during liver regeneration, both in space and time, may provide new strategies to promote liver repopulation. In the experimental field of hepatocyte transplantation, Gupta and colleagues observed that cell engraftment in the liver involves the disruption of endothelial structures, entry of transplanted cells into the liver plates, reconstitution of plasma membrane structures with restoration of cell polarity, and integration of cells in the liver parenchyma [90]. Interestingly, transplanted and host hepatocytes produce VEGF before the disruption of the endothelial layer, reaching a peak around 8h post hepatocyte injection, but totally disappearing after 24h. These observations correlate with more recent work in which loss of LSEC viability occurs after 24h of liver injury, but before hepatocyte necrosis, suggesting that a VEGF-regulated engraftment of BM SPC, an alternative source of HGF, may latterly stimulate hepatocyte proliferation and liver regeneration [91]. Certainly, VEGF knockdown significantly decreases partial hepatectomy-induced BM SPC proliferation and engraftment to the liver [87]. These studies suggest that loss of LSEC functionality seems to be the input signalling in the bone marrow-liver crosstalk, which will promote the release of BM SPC, regeneration of the liver, and at a very final step reconstitution of LSEC structures will occur.
The role of VEGF in the healthy and diseased liver is ambiguous, however, its relevance in liver regeneration was hypothesized two decades ago [92], and refined more recently when it was demonstrated that VEGF promotes proliferation of hepatocytes through the reconstruction of the liver sinusoids by proliferation of sinusoidal endothelial cells [83]. A recent paper by Hu and colleagues highlighted the underlying mechanisms involved in the space-temporal regulation of cellular crosstalk during liver regeneration [93]. They demonstrated how LSEC-angiopoetin 2 expression is dynamically regulated during the regeneration process to promote hepatocytes proliferation during the early phase, and to stimulate angiogenesis during the late phase. In this new vision of the regeneration puzzle, the exact role of the previously described BM SPC remains to be defined. Nevertheless, VEGF has to be considered as the signalling molecule par excellence involved in the hepatic intercellular crosstalk occurring during liver regeneration.
3.3 Other non-parenchymal cells in liver regeneration
Gupta et al investigated whether transplanted hepatocytes could additionally interact with HSC, aware that cell-to-cell interactions could modulate their engraftment in the liver [94]. This study demonstrated that HSC (as well as KC) become activated after only 24h following hepatocyte transplantation, reaching a peak after 3 days. Interestingly, activated cells were in the immediate proximity of transplanted hepatocytes, suggesting they could interact with each other. Additionally, but perhaps counterintuitively, HSC and KC contributed to correct cell engraftment. Future works aimed to elucidate the exact role of every other non-parenchymal cell, in space and time, during the communication process that occurs in liver regeneration and transplantation will be of significant interest.
5- Conclusions and future research
Summarised is the current knowledge about the sinusoidal crosstalk occurring in two pathological situations with similar cellular and molecular mechanisms, but with a significant difference: liver regeneration after hepatectomy progresses without signs of fibrosis, but liver repair in response to chronic liver injury is associated with fibrosis. Although research and knowledge has increased in recent years, our understating of these processes is still far from complete. Further investigation is required, particularly in cirrhosis, where we still do not know the exact spatial-temporal sinusoidal communications during the progression and [importantly] regression of the disease, especially considering the probable aetiology dependent differences. Improved knowledge of these essential processes would help develop efficient strategies to promote fibrosis resolution. The most recent studies in hepatic regeneration biology proposed new, but possibly incompatible, explanations of crosstalk and proliferation of hepatic cells in response to hepatectomy [93, 95], and therefore further validation is required.
In addition to disease-specific limitations, we should consider two important points that need to be addressed. Firstly, the possible experimental discrepancies between research groups (i.e. experimental models of hepatectomy or fibrosis induction), which may lead to divergent conclusions, and therefore inadvertently protract the development of proficient therapeutic strategies. Secondly, most data is obtained using in vitro methods of cell culturing, which may differ greatly to actual pathological mechanisms. For instance, sinusoidal cells rapidly dedifferentiate after isolation, require specific matrices, and the endothelial layer needs biomechanical stimulation. New methods are needed to better explore the mechanisms underlying physiological and pathophysiological intercellular crosstalk. In this sense, the newest liver on a chip platforms [96, 97] may have the potential to reproduce the exact liver-like micronetwork, taking into account the importance of hemodynamic and mechanical forces, and the liver architecture.
In conclusion, acquiring more data inherent to intercellular crosstalk will certainly improve knowledge of the progression of liver disease, allowing the creation of in vitro models for the development of therapeutic strategies.
Key Point Box.
-
-
Liver cells, mainly LSEC, KC, HSC and Hepatocytes keenly interact each other, thus conforming a highly efficient signalling network that maintains sinusoidal homeostasis.
-
-
Sinusoidal paracrine interactions are rapidly de-regulated upon liver injury, actively contributing to fibrogenesis and hepatic microvascular dysfunction. Statins represent the most promising therapy to improve the sinusoidal milieu in chronically injured livers.
-
-
In response to hepatectomy, liver sinusoidal and bone marrow-derived stem cells orchestrate a precise response to promote hepatocyte regeneration. VEGF plays a key role in such process.
Acknowledgments
Financial support: This work was supported by grants FIS PI14/00029 & ExploraBIO2014-61377 from the Instituto de Salud Carlos III, Funds FEDER “una manera de hacer Europa”, and Ministerio de Economía y Competitividad, Spain (Gracia-Sancho), R01 AA021171 & R01 DK59615 from the National Institutes of Health, USA (Shah), and the Sheila Sherlock Postdoctoral Fellowship from the European Association for the Study of the Liver (Marrone).
Authors acknowledge Tom Shepherd (UCL) for the throughout revision of the manuscript.
Abbreviations
- ECM
extracellular matrix
- LSEC
liver sinusoidal endothelial cells
- HSC
hepatic stellate cells
- KC
Kupffer cells
- eNOS
endothelial nitric oxide synthase
- VEGF
vascular endothelial growth factor
- α-SMA
alpha-smooth muscle actin
- KLF2
kruppel-like factor 2
- PMF
portal myofibroblast
- BM SPC
bone marrow-derived endothelial sinusoidal progenitor cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: None to declare.
Authors’ contributions: G.M. literature search, writing and revision of the manuscript, and figure illustrations. V.H.S. conception of the paper and critically revision of the manuscript. J.G.-S. conception of the paper, and writing and revision of the manuscript.
Reference List
- 1.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cogger VC, Warren A, Fraser R, Ngu M, McLean AJ, le Couteur DG. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp Gerontol. 2003;38:1101–1107. doi: 10.1016/j.exger.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 3.McLean AJ, Cogger VC, Chong GC, Warren A, Markus AM, Dahlstrom JE, et al. Age-related pseudocapillarization of the human liver. J Pathol. 2003;200:112–117. doi: 10.1002/path.1328. [DOI] [PubMed] [Google Scholar]
- 4.Schaffner F, Poper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239–242. 239–242. [PubMed] [Google Scholar]
- 5.Urashima S, Tsutsumi m, Nakase K, Wang JS, Takada A. Studies on capillarization of the hepatic sinusoids in alcoholic liver disease. Alcohol Alcohol Suppl. 1993;1B:77–84. doi: 10.1093/alcalc/28.supplement_1b.77. 77–84. [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Broome U, Uzunel M, Nava S, Ge X, Kumagai-Braesch M, et al. Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol. 2003;163:1275–1289. doi: 10.1016/S0002-9440(10)63487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geraud C, Evdokimov K, Straub BK, Peitsch WK, Demory A, Dorflinger Y, et al. Unique cell type-specific junctional complexes in vascular endothelium of human and rat liver sinusoids. PLoS One. 2012;7:e34206. doi: 10.1371/journal.pone.0034206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essani NA, McGuire GM, Manning AM, Jaeschke H. Differential induction of mRNA for ICAM-1 and selectins in hepatocytes, Kupffer cells and endothelial cells during endotoxemia. Biochem Biophys Res Commun. 1995;211:74–82. doi: 10.1006/bbrc.1995.1780. [DOI] [PubMed] [Google Scholar]
- 9.DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G757–G763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 10.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- 12.Pinzani M, Gentilini P. Biology of hepatic stellate cells and their possible relevance in the pathogenesis of portal hypertension in cirrhosis. Semin Liver Dis. 1999;19:397–410. doi: 10.1055/s-2007-1007128. [DOI] [PubMed] [Google Scholar]
- 13.Wake K. Structure of the sinusoidal wall in the liver. In: Wisse E, Knook DL, Wake K, editors. Cells of the Hepatic Sinusoid. Leiden: The Kupffer Cell Foundation; 1995. pp. 241–246. [Google Scholar]
- 14.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 15.Liaskou E, Wilson DV, Oo YH. Innate immune cells in liver inflammation. Mediators Inflamm. 2012;2012:949157. doi: 10.1155/2012/949157. Epub;%2012 Aug 9.:949157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCuskey RS. Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver. 2000;20:3–7. doi: 10.1034/j.1600-0676.2000.020001003.x. [DOI] [PubMed] [Google Scholar]
- 17.Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 19.March S, Hui EE, Underhill GH, Khetani S, Bhatia SN. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology. 2009;50:920–928. doi: 10.1002/hep.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 21.Pinzani M, Rosselli M, Zuckermann M. Liver cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25:281–290. doi: 10.1016/j.bpg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Pagan JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458–461. doi: 10.1016/j.jhep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, Garcia-Caldero H, Bosch J, Garcia-Pagan JC. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 2007;47:220–227. doi: 10.1016/j.jhep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Steib CJ, Gerbes AL, Bystron M, op den Winkel M, Hartl J, Roggel F, et al. Kupffer cell activation in normal and fibrotic livers increases portal pressure via thromboxane A(2) J Hepatol. 2007;47:228–238. doi: 10.1016/j.jhep.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Said A, Lucey MR. Liver transplantation: an update 2008. Curr Opin Gastroenterol. 2008;24:339–345. doi: 10.1097/MOG.0b013e3282f8e27e. [DOI] [PubMed] [Google Scholar]
- 26.DeLeve LD. The Hepatic Sinusoidal Endothelial Cell: Morphology, Function, and Pathobiology. In: Arias IM, editor. The Liver: Biology and Pathobiology. 5th. Hokoben: Wiley & Sons; 2009. pp. 371–388. [Google Scholar]
- 27.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 29.Jiang JX, Mikami K, Venugopal S, Li Y, Torok NJ. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol. 2009;51:139–148. doi: 10.1016/j.jhep.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire RF, Bissell DM, Boyles J, Roll FJ. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology. 1992;15:989–997. doi: 10.1002/hep.1840150603. [DOI] [PubMed] [Google Scholar]
- 31.Yamane A, Seetharam L, Yamaguchi S, Gotoh N, Takahashi T, Neufeld G, et al. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1) Oncogene. 1994;9:2683–2690. [PubMed] [Google Scholar]
- 32.Zhang DW, Zhao YX, Wei D, Li YL, Zhang Y, Wu J, et al. HAb18G/CD147 promotes activation of hepatic stellate cells and is a target for antibody therapy of liver fibrosis. J Hepatol. 2012;57:1283–1291. doi: 10.1016/j.jhep.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 33.Yan Z, Qu K, Zhang J, Huang Q, Qu P, Xu X, et al. CD147 promotes liver fibrosis progression via VEGF-A/VEGFR2 signalling-mediated cross-talk between hepatocytes and sinusoidal endothelial cells. Clin Sci (Lond) 2015;129:699–710. doi: 10.1042/CS20140823. [DOI] [PubMed] [Google Scholar]
- 34.Di Pascoli M, Divi M, Rodriguez-Vilarrupla A, Rosado E, Gracia-Sancho J, Vilaseca M, et al. Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2013;58:904–910. doi: 10.1016/j.jhep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Guillaume M, Rodriguez-Vilarrupla A, Gracia-Sancho J, Rosado E, Mancini A, Bosch J, et al. Recombinant human manganese superoxide dismutase reduces liver fibrosis and portal pressure in CCl4-cirrhotic rats. J Hepatol. 2013;58:240–246. doi: 10.1016/j.jhep.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Bannert K, Kuhla A, Abshagen K, Vollmar B. Anti-apoptotic therapeutic approaches in liver diseases: do they really make sense? Apoptosis. 2014;19:1243–1253. doi: 10.1007/s10495-014-1004-1. [DOI] [PubMed] [Google Scholar]
- 37.Wells RG. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis. 2008;12:759–768. viii. doi: 10.1016/j.cld.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers PR, Tanner MA. Vascular endothelial cell regulation of extracellular matrix collagen: role of nitric oxide. Arterioscler Thromb Vasc Biol. 1998;18:717–722. doi: 10.1161/01.atv.18.5.717. [DOI] [PubMed] [Google Scholar]
- 39.Ford AJ, Jain G, Rajagopalan P. Designing a fibrotic microenvironment to investigate changes in human liver sinusoidal endothelial cell function. Acta Biomater. 2015;24:220–227. doi: 10.1016/j.actbio.2015.06.028. Epub;%2015 Jun 24.:220–227. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 41.Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–G118. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gortzen J, Schierwagen R, Bierwolf J, Klein S, Uschner FE, van d V, et al. Interplay of Matrix Stiffness and c-SRC in Hepatic Fibrosis. Front Physiol. 2015;6:359. doi: 10.3389/fphys.2015.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halfon P, Bourliere M, Penaranda G, Deydier R, Renou C, Botta-Fridlund D, et al. Accuracy of hyaluronic acid level for predicting liver fibrosis stages in patients with hepatitis C virus. Comp Hepatol. 2005;4:6. doi: 10.1186/1476-5926-4-6. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tangkijvanich P, Kongtawelert P, Pothacharoen P, Mahachai V, Suwangool P, Poovorawan Y. Serum hyaluronan: a marker of liver fibrosis in patients with chronic liver disease. Asian Pac J Allergy Immunol. 2003;21:115–120. [PubMed] [Google Scholar]
- 45.Deaciuc IV, Bagby GJ, Lang CH, Spitzer JJ. Hyaluronic acid uptake by the isolated, perfused rat liver: an index of hepatic sinusoidal endothelial cell function. Hepatology. 1993;17:266–272. [PubMed] [Google Scholar]
- 46.Muriel P, Escobar Y. Kupffer cells are responsible for liver cirrhosis induced by carbon tetrachloride. J Appl Toxicol. 2003;23:103–108. doi: 10.1002/jat.892. [DOI] [PubMed] [Google Scholar]
- 47.Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology. 2006;44:1487–1501. doi: 10.1002/hep.21427. [DOI] [PubMed] [Google Scholar]
- 48.Cubero FJ, Nieto N. Ethanol and arachidonic acid synergize to activate Kupffer cells and modulate the fibrogenic response via tumor necrosis factor alpha, reduced glutathione, and transforming growth factor beta-dependent mechanisms. Hepatology. 2008;48:2027–2039. doi: 10.1002/hep.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696–703. doi: 10.1016/j.jhep.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 50.DeLeve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920–930. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142:918–927. doi: 10.1053/j.gastro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marrone G, Russo L, Rosado E, Hide D, Garcia-Cardena G, Garcia-Pagan JC, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J Hepatol. 2013;58:98–103. doi: 10.1016/j.jhep.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 53.Marrone G, Maeso-Díaz R, Garcia-Cardena G, Garcia-Pagan JC, Bosch J, Gracia-Sancho J. KLF2 exerts anti-fibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut. 2015;64:1434–1443. doi: 10.1136/gutjnl-2014-308338. [DOI] [PubMed] [Google Scholar]
- 54.Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernandez M, Garca-Pagan JC, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–755. doi: 10.1053/j.gastro.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Abraldes JG, Rodriguez-Vilarrupla A, Graupera M, Zafra C, Garcia-Caldero H, Garcia-Pagan JC, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl(4) cirrhotic rats. J Hepatol. 2007;46:1040–1046. doi: 10.1016/j.jhep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–253. doi: 10.1002/hep.21673. [DOI] [PubMed] [Google Scholar]
- 57.Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702–712. doi: 10.1016/j.jhep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 58.Gracia-Sancho J, Russo L, Garcia-Caldero H, Garcia-Pagan JC, Garcia-Cardena G, Bosch J. Endothelial expression of transcription factor Kruppel-like factor 2 and its vasoprotective target genes in the normal and cirrhotic rat liver. Gut. 2011;60:517–524. doi: 10.1136/gut.2010.220913. [DOI] [PubMed] [Google Scholar]
- 59.Klein S, Klosel J, Schierwagen R, Korner C, Granzow M, Huss S, et al. Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats. Lab Invest. 2012;92:1440–1450. doi: 10.1038/labinvest.2012.106. [DOI] [PubMed] [Google Scholar]
- 60.Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci. 2014;59:1958–1965. doi: 10.1007/s10620-014-3179-2. [DOI] [PubMed] [Google Scholar]
- 61.Souk K, Al-Badri M, Azar ST. The Safety and Benefit of Statins in Liver Cirrhosis: a Review. Exp Clin Endocrinol Diabetes. 2015;123:577–580. doi: 10.1055/s-0035-1564093. [DOI] [PubMed] [Google Scholar]
- 62.Bosch J, Forns X. Therapy. Statins and liver disease: from concern to 'wonder' drugs? Nat Rev Gastroenterol Hepatol. 2015;12:320–321. doi: 10.1038/nrgastro.2015.78. [DOI] [PubMed] [Google Scholar]
- 63.Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Bellis L, Berzigotti A, Abraldes JG, Moitinho E, Garcia-Pagan JC, Bosch J, et al. Low doses of isosorbide mononitrate attenuate the postprandial increase in portal pressure in patients with cirrhosis. Hepatology. 2003;37:378–384. doi: 10.1053/jhep.2003.50053. [DOI] [PubMed] [Google Scholar]
- 65.Fiorucci S, Antonelli E, Brancaleone V, Sanpaolo L, Orlandi S, Distrutti E, et al. NCX-1000, a nitric oxide-releasing derivative of ursodeoxycholic acid, ameliorates portal hypertension and lowers norepinephrine-induced intrahepatic resistance in the isolated and perfused rat liver. J Hepatol. 2003;39:932–939. doi: 10.1016/s0168-8278(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 66.Bosch J. Decreasing hepatic vascular tone by liver-specific NO donors: wishful thinking or a promising reality? J Hepatol. 2003;39:1072–1075. doi: 10.1016/j.jhep.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Berzigotti A, Bellot P, De GA, Garcia-Pagan JC, Gagnon C, Spenard J, et al. NCX-1000, a Nitric Oxide-Releasing Derivative of UDCA, Does Not Decrease Portal Pressure in Patients With Cirrhosis: Results of a Randomized, Double-Blind, Dose-Escalating Study. Am J Gastroenterol. 2009 doi: 10.1038/ajg.2009.661. [DOI] [PubMed] [Google Scholar]
- 68.Ray K. Liver: Sussing out statins in cirrhosis--KLF2 is the key. Nat Rev Gastroenterol Hepatol. 2015;12:64. doi: 10.1038/nrgastro.2014.233. [DOI] [PubMed] [Google Scholar]
- 69.Hide D, Ortega-Ribera M, Garcia-Pagan JC, Peralta C, Bosch J, Gracia-Sancho J. Effects of warm ischemia and reperfusion on the liver microcirculatory phenotype of rats: underlying mechanisms and pharmacological therapy. Sci Rep. 2016;6:22107. doi: 10.1038/srep22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lemoinne S, Cadoret A, Rautou PE, El MH, Ratziu V, Corpechot C, et al. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology. 2015;61:1041–1055. doi: 10.1002/hep.27318. [DOI] [PubMed] [Google Scholar]
- 71.Wang R, Ding Q, Yaqoob U, de Assuncao TM, Verma VK, Hirsova P, et al. Exosome Adherence and Internalization by Hepatic Stellate Cells Triggers Sphingosine 1-Phosphate-dependent Migration. J Biol Chem. 2015;290:30684–30696. doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelson DR, Tu Z, Soldevila-Pico C, Abdelmalek M, Zhu H, Xu YL, et al. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38:859–868. doi: 10.1053/jhep.2003.50427. [DOI] [PubMed] [Google Scholar]
- 73.Lebrec D, Thabut D, Oberti F, Perarnau JM, Condat B, Barraud H, et al. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138:1755–1762. doi: 10.1053/j.gastro.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 74.Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277–286. [PubMed] [Google Scholar]
- 75.Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome* study. Hepatology. 2014;60:1211–1221. doi: 10.1002/hep.27289. [DOI] [PubMed] [Google Scholar]
- 76.Reverter E, Mesonero F, Seijo S, Martinez J, Abraldes JG, Penas B, et al. Effects of Sapropterin on Portal and Systemic Hemodynamics in Patients With Cirrhosis and Portal Hypertension: A Bicentric Double-Blind Placebo-Controlled Study. Am J Gastroenterol. 2015;110:985–992. doi: 10.1038/ajg.2015.185. [DOI] [PubMed] [Google Scholar]
- 77.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao SA, Glorioso JM, Nyberg SL. Liver regeneration. Transl Res. 2014;163:352–362. doi: 10.1016/j.trsl.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Widmann JJ, Fahimi HD. Proliferation of mononuclear phagocytes (Kupffer cells) and endothelial cells in regenerating rat liver. A light and electron microscopic cytochemical study. Am J Pathol. 1975;80:349–366. [PMC free article] [PubMed] [Google Scholar]
- 81.Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy 6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1570–G1577. doi: 10.1152/ajpgi.00399.2006. [DOI] [PubMed] [Google Scholar]
- 82.Xu CS, Jiang Y, Zhang LX, Chang CF, Wang GP, Shi RJ, et al. The role of Kupffer cells in rat liver regeneration revealed by cell-specific microarray analysis 8. J Cell Biochem. 2012;113:229–237. doi: 10.1002/jcb.23348. [DOI] [PubMed] [Google Scholar]
- 83.Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem. 2001;49:121–130. doi: 10.1177/002215540104900112. [DOI] [PubMed] [Google Scholar]
- 84.Sato T, El-Assal ON, Ono T, Yamanoi A, Dhar DK, Nagasue N. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J Hepatol. 2001;34:690–698. doi: 10.1016/s0168-8278(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 85.Mabuchi A, Mullaney I, Sheard PW, Hessian PA, Mallard BL, Tawadrous MN, et al. Role of hepatic stellate cell/hepatocyte interaction and activation of hepatic stellate cells in the early phase of liver regeneration in the rat. J Hepatol. 2004;40:910–916. doi: 10.1016/j.jhep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Roskams T. Relationships among stellate cell activation, progenitor cells, and hepatic regeneration. Clin Liver Dis. 2008;12:853–860. ix. doi: 10.1016/j.cld.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 87.Wang L, Wang X, Wang L, Chiu JD, Van d V, Gaarde WA, et al. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology. 2012;143:1555–1563. doi: 10.1053/j.gastro.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123:1861–1866. doi: 10.1172/JCI66025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katagiri H, Kushida Y, Nojima M, Kuroda Y, Wakao S, Ishida K, et al. A Distinct Subpopulation of Bone Marrow Mesenchymal Stem Cells, Muse Cells, Directly Commit to the Replacement of Liver Components. Am J Transplant. 2015;10 doi: 10.1111/ajt.13537. [DOI] [PubMed] [Google Scholar]
- 90.Gupta S, Rajvanshi P, Sokhi R, Slehria S, Yam A, Kerr A, et al. Entry and integration of transplanted hepatocytes in rat liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology. 1999;29:509–519. doi: 10.1002/hep.510290213. [DOI] [PubMed] [Google Scholar]
- 91.Wang L, Wang X, Xie G, Wang L, Hill CK, DeLeve LD. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest. 2012;122:1567–1573. doi: 10.1172/JCI58789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mochida S, Ishikawa K, Inao M, Shibuya M, Fujiwara K. Increased expressions of vascular endothelial growth factor and its receptors, flt-1 and KDR/flk-1, in regenerating rat liver. Biochem Biophys Res Commun. 1996;226:176–179. doi: 10.1006/bbrc.1996.1329. [DOI] [PubMed] [Google Scholar]
- 93.Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 94.Benten D, Kumaran V, Joseph B, Schattenberg J, Popov Y, Schuppan D, et al. Hepatocyte transplantation activates hepatic stellate cells with beneficial modulation of cell engraftment in the rat. Hepatology. 2005;42:1072–1081. doi: 10.1002/hep.20889. [DOI] [PubMed] [Google Scholar]
- 95.Fernandez-Mena C, Almagro J, Puerro M, Quintana A, Hidalgo J, Bañares R, et al. Effects of myeloid cell-selective deficiency of IL-6 for liver regeneration after partial hepatectomy (PH) in mice. Hepatology. 2016;62:848A. [Google Scholar]
- 96.Illa X, Vila S, Yeste J, Peralta C, Gracia-Sancho J, Villa R. A novel modular bioreactor to in vitro study the hepatic sinusoid. PLoS One. 2014 doi: 10.1371/journal.pone.0111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prodanov L, Jindal R, Bale SS, Hegde M, McCarty WJ, Golberg I, et al. Long-term maintenance of a microfluidic 3D human liver sinusoid. Biotechnol Bioeng. 2016;113:241–246. doi: 10.1002/bit.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]