Abstract
Rationale
Neurofibromin 2 (NF2) is an established tumor suppressor that promotes apoptosis and inhibits growth in a variety of cell types, yet its function in cardiomyocytes remains largely unknown.
Objective
We sought to determine the role of NF2 in cardiomyocyte apoptosis and ischemia/reperfusion (I/R) injury in the heart.
Methods and Results
We investigated the function of NF2 in isolated cardiomyocytes and mouse myocardium at baseline and in response to oxidative stress. NF2 was activated in cardiomyocytes subjected to H2O2 and in murine hearts subjected to I/R. Increased NF2 expression promoted the activation of Mst1 and the inhibition of Yap, whereas knockdown of NF2 attenuated these responses following oxidative stress. NF2 increased apoptosis of cardiomyocytes that appeared dependent on Mst1 activity. Mice deficient for NF2 in cardiomyocytes, NF2 CKO, were protected against global I/R ex vivo and showed improved cardiac functional recovery. Moreover, NF2 CKO mice were protected against I/R injury in vivo and showed upregulation of Yap target gene expression. Mechanistically, we observed nuclear association between NF2 and its activator MYPT-1 in cardiomyocytes, and a subpopulation of stress-induced nuclear Mst1 was diminished in NF2 CKO hearts. Finally, mice deficient for both NF2 and Yap failed to show protection against I/R indicating that Yap is an important target of NF2 in the adult heart.
Conclusions
NF2 is activated by oxidative stress in cardiomyocytes and mouse myocardium and facilitates apoptosis. NF2 promotes I/R injury through activation of Mst1 and inhibition of Yap, thereby regulating Hippo signaling in the adult heart.
Keywords: Ischemia reperfusion injury, apoptosis, signal transduction
Introduction
Ischemic heart disease is a major component of cardiovascular disease and a leading cause of death worldwide 1, 2. Although much progress has been made in understanding the causes of ischemic heart disease, the molecular underpinnings that occur during ischemia/reperfusion (I/R) injury have not yet been fully elucidated, and the result is current treatments that are only partially effective.
Mammalian sterile 20-like kinase 1 (Mst1) is a ubiquitously expressed and highly conserved serine/threonine kinase 3 that is activated in the heart during stress conditions including I/R, myocardial infarction (MI) and pressure overload 4-6. Previous work from our group has shown that suppression of Mst1 inhibits I/R injury 4, 7 and prevents cardiac remodeling/dysfunction after chronic MI, 5 suggesting that Mst1 is a promising target of cardiac therapy for ischemic heart disease. Mst1 is the mammalian homolog of Drosophila hippo and the centerpiece of a signaling cascade that culminates in the phosphorylation and inactivation of the transcriptional co-factor yorkie (mammalian Yap). Our recent work demonstrated Yap to be a critical transcriptional co-factor that mediates cardioprotection and homeostasis of the adult heart 8, 9.
NF2 (also known as neurofibromin 2/schwannomin) is a widely expressed scaffold-like protein that is able to transduce intra- and extracellular signals to modulate various cellular processes 10-12. NF2 was originally described as a tumor suppressor protein and is linked to several human cancers 13, 14. However, its function in the heart remains largely unexplored. NF2 lacks catalytic function, and conformational changes determine its ability to interact with, and subsequently transduce signals through, effector proteins. NF2 structure is regulated by phosphorylation at Ser518, 15 a site that can be phosphorylated by PAK2 16, 17 and PKA, 18 and dephosphorylated by the myosin light chain phosphatase, MYPT-1 19. When Ser518 is phosphorylated, NF2 assumes an “open” conformation and can no longer associate with binding partners, effectively inhibiting its function 15, 20-22. Conversely, dephosphorylation of NF2 favors a “closed” conformation that promotes protein interaction and signal transduction. Studies in Drosophila have provided evidence that NF2 can regulate the activity of hippo/yorkie, thereby modulating cell proliferation and survival 23-25. Yet, to date, evidence linking NF2 and Mst1/Yap in mammalian systems is limited 26-28.
Herein we demonstrate that NF2 is activated by oxidative stress through dephosphorylation by the protein phosphatase targeting subunit MYPT-1. Active NF2 promotes cardiomyocyte apoptosis through activation of Mst1 and engagement of the Hippo signaling pathway. Interestingly, NF2 is present in both the cytosol and nucleus of cardiomyocytes and promotes phosphorylation and inactivation of Yap, thereby attenuating Yap target gene expression. Mice deficient for NF2 in cardiomyocytes (NF2flox/flox;CreαMHC ; NF2 CKO) show diminished Mst1 activation, increased Yap transcriptional activity, and are protected against I/R injury. These results provide evidence that NF2 modulates Hippo signaling in the mammalian heart to promote acute myocardial injury.
Methods
An expanded Methods section is available in the online Data Supplement.
Animal models
NF2 floxed mice29 were crossed with α-MHC-Cre transgenic mice30 to generate cardiac-specific knockout (NF2 CKO) mice. Yap floxed mice26 were bred to NF2 CKO mice to generate NF2flox/flox;Yapflox/+;CreαMHC mice. Mice were housed in a temperature-controlled environment with 12-hour light/dark cycles where they received food and water ad libitum. All protocols concerning the use of animals were approved by the Institutional Animal Care and Use Committee at Rutgers, The State University of New Jersey.
Statistics
All data are reported as mean ± SEM. Evaluation between three or more groups was done using one-way ANOVA. The statistical significance of the differences between groups was calculated using post hoc comparisons. Student's t test was used to evaluate the difference in means between two groups. Statistical analyses were performed using Graph Pad Prism 6.0. A p value less than 0.05 was considered significant.
Results
Regulation of NF2 by oxidative stress
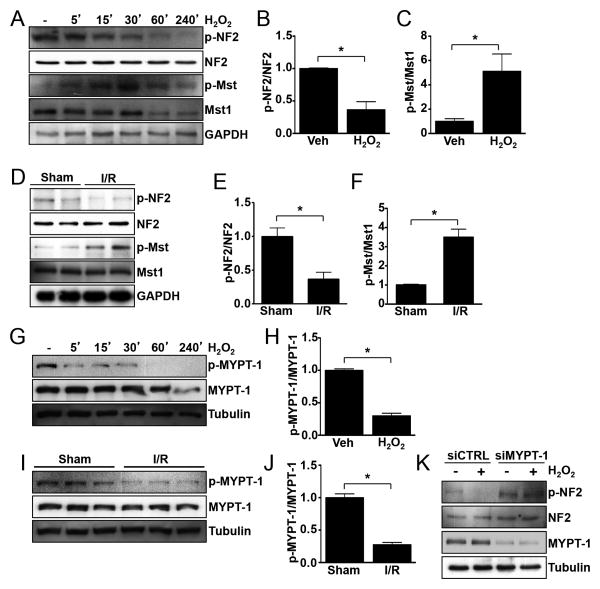
Our previous work demonstrated that Mst1 is activated in response to oxidative stress both in cultured cardiomyocytes and mouse myocardium in vivo 7, 31. Therefore, we first determined the activation status of NF2 using Ser518 phosphorylation as an indicator of the inactive conformation of NF2. Treatment of neonatal rat ventricular myocytes (NRVMs) with H2O2, to mimic oxidative stress during reperfusion, elicited activation of both NF2 and Mst1 (Figure 1A-C). To test the activation of NF2 in vivo, we subjected wild-type (WT) C57BL/6 mice to I/R and performed western blots to assay phosphorylation status. Similarly, we found that both NF2 and Mst1 were activated in the myocardium by I/R (Figure 1D-F). We also evaluated samples of failing human hearts and observed increased NF2 and Mst1 activation compared to healthy controls (Online Figure I). Since NF2 phosphorylation is decreased in response to oxidative stress, it is possible that increased phosphatase activity is responsible. To test this, we pretreated NRVMs with the phosphatase inhibitors okadaic acid or calyculin A, followed by stimulation with H2O2. The dephosphorylation of NF2 caused by H2O2 was partially attenuated in okadaic acid-treated cells at high concentration, but was fully prevented in calyculin A-treated cells indicating likely involvement of PP1 phosphatase (Online Figure II). Previous work identified PP1δ-MYPT-1 as an activator of NF2 through dephosphorylation of Ser518 in mammalian cells 19, 32 and in Drosophila 33. A lack of phosphorylation of MYPT-1 at Ser696 is indicative of its activation. We observed decreased MYPT-1 phosphorylation in response to oxidative stress both in NRVMs and mouse myocardium (Figure 1G-J). To directly test the involvement of MYPT-1 in NF2 regulation, we depleted NRVMs of endogenous MYPT-1 using siRNA. MYPT-1 downregulation was sufficient to attenuate NF2 activation by oxidative stress (Figure 1K). Taken together, these data indicate that MYPT-1 is activated by oxidative stress and mediates the dephosphorylation and activation of NF2 in cardiomyocytes.
Figure 1. Oxidative stress-induced activation of NF2 is mediated by MYPT-1.
(A) Neonatal rat ventricular myocytes (NRVMs) were treated with vehicle or H2O2 (100 mM) for the times indicated. Western blot analysis was performed to detect phosphorylated and total NF2 (Ser518) and Mst1 (Thr183). (B and C) Quantification of responses at 30′ time point, n = 3. *, P<0.05. (D) Wild-type C57BL/6 male mice were subjected to ischemia and reperfusion (30′/30′) or sham operation. Left ventricular homogenates were prepared and western blot was performed to detect phosphorylated and total NF2 and Mst1. (E and F) Quantitative analysis of results shown in panel D, n = 4 mice per group. *, P<0.05. (G) NRVMs were treated as described in panel A and westerns were performed to detect phosphorylated and total MYPT-1 (Ser696). (H) Quantification of response at 30′ time point, n = 3. *, P<0.05. (I) Wild-type C57BL/6 male mice were subject to sham or I/R as indicated in panel D and westerns were performed to detect phosphorylated and total MYPT-1. (J) Quantification of results in panel I, n = 3 mice per group. *, P<0.05. (K) NRVMs were transfected with siRNA targeted to MYPT-1 or scrambled control siRNA. 72 hours later, cells were stimulated with H2O2 (100 mM) or vehicle for 1 hour followed by western blot analysis. Representative images of 3 independent experiments are shown.
NF2 promotes Mst1 activation and cardiomyocyte apoptosis
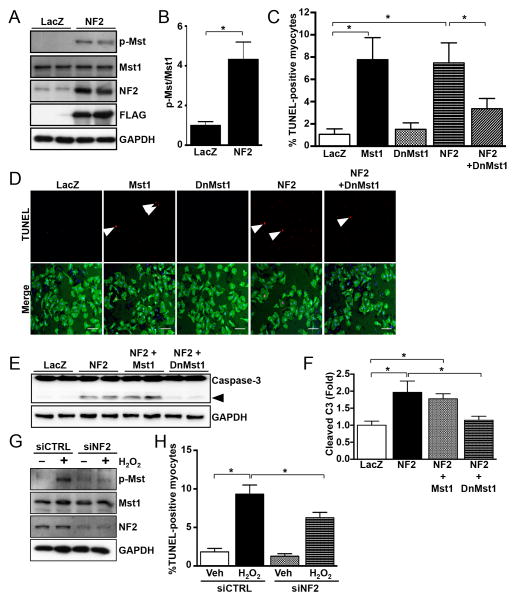
NF2 can activate the Hippo pathway in the liver26 and brain27. We sought to determine whether NF2 regulates Hippo signaling in the adult heart. Overexpression of NF2 in NRVMs stimulated Mst1 activation (Figure 2A and B). Mst1 is known to promote cell death; therefore, we evaluated cardiomyocyte apoptosis in response to NF2. We found that increased NF2 expression caused a significant increase in TUNEL-positive cardiomyocytes, and that this response was significantly attenuated by inhibition of Mst1 (Figure 2C and D). Similarly, NF2 elicited caspase-3 activation, which was significantly reduced by inhibition of Mst1 (Figure 2E and F). On the other hand, knockdown of endogenous NF2 using siRNA attenuated both the activation of Mst1 and cardiomyocyte apoptosis driven by H2O2 (Figure 2G and H). Taken together, these results indicate that NF2 can engage Hippo signaling at the level of Mst1 to promote cardiomyocyte apoptosis.
Figure 2. NF2 promotes activation of Mst1 and cardiomyocyte apoptosis.
(A) NRVMs were transduced with adenovirus engineered to express FLAG-NF2 or LacZ as a control. After 24 hours, cells were harvested and westerns were performed. (B) Quantification of results obtained from panel A, n = 3. *, P<0.05. (C) NRVMs were transduced with LacZ, Mst1, kinase-inactive (K59) dominant-negative DN-Mst1, or NF2 adenovirus and TUNEL was performed after 48 hours to determine DNA fragmentation as an indicator of apoptosis. (D) Representative images of TUNEL-positive nuclei (arrows), scale bar = 100 mm. (E) NRVMs were treated as described in panel C and westerns were performed to detect caspase-3 (arrow, cleaved 17kd fragment). (F) Quantification of cleaved caspase-3, n = 3. *, P<0.05. (G) NRVMs were transfected with siRNA targeted to Nf2 or scrambled control siRNA for 72 hours. Westerns were performed to detect Mst1 phosphorylation and extent of NF2 knockdown. (H) NRVMs were treated with siRNA against Nf2 or scrambled control and then stimulated with H2O2 (100 mM) or vehicle for 6-8 hours. TUNEL was used to measure apoptotic cardiomyocytes. *, P<0.05. n = 3 experiments. Representative images of 3 independent experiments are shown.
Subcellular localization of NF2 in cardiomyocytes
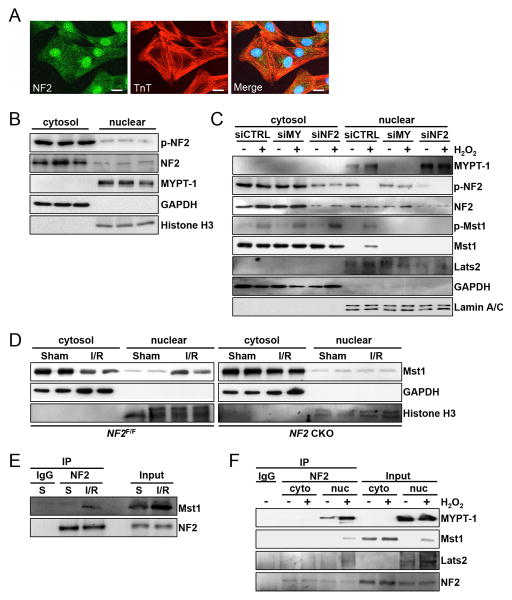
Previous work has demonstrated subcellular localization of NF2 at tight junctions 34, adherens junctions 35, desmosomes 28, the plasma membrane 36 and in the nucleus 37. We sought to investigate the localization of NF2 in NRVMs. Confocal imaging revealed distribution of NF2 in the cytosol, at the cell membrane, and a strong nuclear signal in NRVMs (Figure 3A, Online Figure IIIA). Detection of p-NF2 revealed a diffuse cytosolic and nuclear distribution with a relatively more pronounced presence at the plasma membrane (Online Figure IIIB). We also stained mouse heart sections and observed both nuclear and cytosolic localization of NF2 (Online Figure IVA). As a control, we evaluated HEK293 cells and found predominant plasma membrane distribution of NF2 and no appreciable nuclear signal (Online Figure IIIC). To investigate this biochemically, we separated mouse ventricular lysates into cytosolic and nuclear-enriched fractions. We detected endogenous NF2 and p-NF2 in both fractions (Figure 3B). Similarly, NF2 was observed in cytosolic and nuclear-enriched fractions generated from NRVMs and isolated adult mouse cardiomyocytes (AMCMs)(Figure 3C and Online Figure IVB). Although p-NF2 was detected in cytosolic and nuclear-enriched fractions of NRVMs, we did not observe a change in p-NF2 levels in the cytosol following H2O2 treatment, while nuclear p-NF2 levels decreased (Figure 3C). We also probed for MYPT-1 and detected it in the nuclear-enriched, but not in the cytosolic-enriched fractions, of heart homogenates and NRVMs (Figure 3B and C), consistent with a recent report 38. Subcellular fractionation of NRVMs into cytosolic and plasma membrane-enriched fractions revealed faintly detectable levels of NF2 at the plasma membrane, while p-NF2 was more evident (Online Figure IVC).
Figure 3. NF2 associates with Mst1 following oxidative stress.
(A) Confocal microscopy images of NRVMs. Endogenous NF2 (green) and troponin-T (red) were detected by immunofluorescence. Nuclei were stained with DAPI. Scale bar, 10 mm. (B) Cytosolic and nuclear-enriched fractions were prepared from ventricular homogenates of C57BL/6 wild-type mice and subjected to western blot analysis. (C) NRVMs were transduced with siRNA targeted to MYPT-1 (siMY), NF2 or scrambled control (siCTRL). 72 hours later, cells were treated with H2O2 (100 mM) or vehicle for 1 hour. Cytosolic and nuclear-enriched fractions were prepared and subjected to western analysis. (D) Control NF2 floxed (NF2F/F) and NF2 CKO mice were subjected to I/R (30′/30′) or sham operation and ventricular homogenates were separated into cytosolic and nuclear-enriched fractions. GAPDH and Histone H3 were used as markers of cytosolic and nuclear fractions respectively. (E) C57BL/6 wild-type mice were subjected to sham operation (S) or I/R (30′/30”). Homogenates were used for co-IP and subsequent western blotting. (F) NRVMs were treated with H2O2 (100 μM) or vehicle for 1 hour. Cytosolic and nuclear-enriched fractions were prepared and subjected to immunoprecipitation using anti-NF2 antibody or control IgG. Immunocomplexes and inputs were analyzed by western blot. Representative images of 3-4 independent experiments are shown.
NF2-dependent Mst1 localization
Because NF2 modified Mst1 activity, we investigated the subcellular localization of the Hippo kinases Lats2 and Mst1. Analysis of Lats2 distribution in cardiomyocytes demonstrated a largely nuclear presence (Figure 3C and Online Figure IVA and B). Mst1 showed a predominant cytosolic presence in NRVMs, AMCMs and heart sections; however, following oxidative stress we observed a nuclear subpopulation of Mst1 (Figure 3C and Online Figure IVB). Knockdown of either MYPT-1 or NF2 attenuated this response in NRVMs (Figure 3C). Furthermore, we observed Mst1 levels increase in nuclear-enriched fractions prepared from WT mouse myocardium subjected to I/R (Figure 3D). Nuclear localization of Mst1 was attenuated in hearts deficient for NF2, suggesting a causal role for NF2 in this process in vivo.
Association of NF2 with Hippo pathway components
Based on previous work demonstrating complexes comprised of NF2 and Hippo in Drosophila 39 and NF2 and Lats in mammalian cells 36, we examined whether NF2, Mst1 and Lats2 associate in cardiomyocytes. Co-IP studies showed an interaction between NF2 and Mst1 in hearts subjected to I/R (Figure 3E), and NRVMs subjected to oxidative stress (Online Figure VA). We performed additional co-IP experiments, this time stimulating NRVMs with H2O2 and using cytosolic and nuclear-enriched fractions to determine NF2 interactions. As demonstrated in Figure 3F, association between NF2 and MYPT-1, Mst1 and Lats2 was observed in nuclear-enriched fractions during oxidative stress. Phosphorylation status is important for NF2 conformation change and protein interactions. Therefore, we tested the ability of a phospho-resistant NF2 S518A mutant to associate with Mst1 and Lats2. Co-IP studies showed that NF2 S518A associated with Mst1 and Lats2, and to a greater extent than wild-type NF2, suggesting the importance of phosphorylation of Ser518 in modulating these protein associations (Online Figure VB).
NF2 deletion attenuates cardiac injury ex vivo
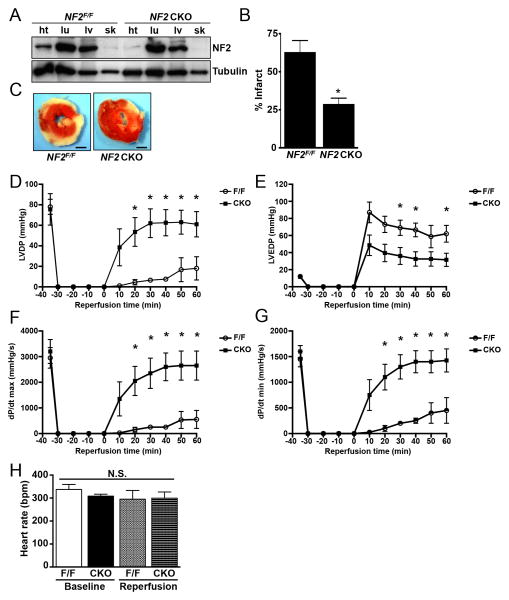
To test the physiological role of NF2, we crossed NF2 floxed mice29 with α-MHC-Cre transgenic mice30 to generate cardiac-specific knockout (NF2flox/flox;CreαMHC (NF2 CKO)) mice. These mice had depleted levels of NF2 protein in the myocardium but not in other tissues tested (Figure 4A). Hearts were isolated from NF2 CKO and control NF2flox/flox mice and subjected to global ischemia and reperfusion ex vivo using the Langendorff preparation. We observed that NF2 CKO hearts had significantly smaller infarct regions compared to control NF2flox/flox hearts (Figure 4B and C). Cardiac function was assessed at baseline prior to ischemia and serially every 10 minutes through 1 hour of reperfusion. While no differences were observed at baseline, we found that NF2 CKO hearts had significantly improved cardiac function during reperfusion (Figure 4D-G), while no significant difference in heart rate was observed (Figure 4H). Taken together, these data suggest that disruption of NF2 in cardiomyocytes confers protection against global I/R and preserves heart function.
Figure 4. NF2 CKO mouse hearts are protected against global I/R injury and have increased function during reperfusion.
(A) Ventricular (ht), lung (lu), liver (lv) and skeletal muscle (sk) homogenates were prepared from control NF2 floxed (NF2F/F) and NF2 CKO mice and subjected to western analysis. (B and C) Hearts from control and NF2 CKO mice were isolated and subjected to global I/R (30′/60′) using the Langendorff method. Infarct size was determined by TTC staining. Scale bar, 1 mm. (D-G) Parameters of ventricular function were determined during the reperfusion phase, including left ventricular developed pressure (LVDP), left ventricular end diastolic pressure (LVEDP), dP/dT max, and dP/dT min. Time 0 min represents the start of reperfusion. (H) Heart rate was determined at baseline and after 60 min of reperfusion. *, P<0.05 versus control NF2 floxed (NF2F/F). N.S. = not significant. N = 8 mice per group.
Deletion of NF2 affords cardioprotection against I/R injury
To determine whether disruption of NF2 also conferred protection of the myocardium in vivo, we subjected NF2 CKO and control NF2flox/flox mice to I/R and determined infarct size 24 hours later. We found no difference in the myocardial area at risk (AAR) following I/R; however, infarct size was significantly reduced in NF2 CKO mice indicating that NF2 deletion is cardioprotective (Figure 5A-C). We also subjected mice to I/R and examined heart function by echocardiography 2 weeks post-infarction. We observed that left ventricular ejection fraction (LVEF%) was significantly greater in NF2 CKO mice compared to controls (Figure 5D). Since NF2 regulates Hippo signaling in the mouse liver26 and brain27, we examined the activation of the conserved pathway components Mst1 and Yap. The phosphorylation of both Mst1 and Yap were increased in NF2flox/flox hearts in response to I/R, indicating Hippo pathway activation. However, this response was significantly attenuated in NF2 CKO hearts (Figure 5E-G). The activation of known cardioprotective targets AKT and ERK1/2 showed negligible differences between NF2flox/flox and NF2 CKO hearts (Figure 5E, H and I). Consistent with these findings, we also observed Yap downregulation in NRVMs overexpressing NF2, and increased Yap levels in NRVMs treated with siRNA targeting NF2 (Online Figure VI).
Figure 5. Myocardial Yap activity is increased and I/R injury is attenuated in NF2 CKO mice.
(A-C) Control NF2 floxed (NF2F/F) and NF2 CKO mice were subjected to I/R (30′/24hr) and area at risk (AAR) and infarct size were determined by Alcian blue and TTC staining respectively. Scale bar, 1 mm. *, P<0.05. N.S. = not significant. N = 5 mice per group. (D) Left ventricular ejection fraction (LVEF%) was determined by echocardiography prior to intervention and 2 weeks after I/R. *, P<0.05. N = 4-5 mice per group. (E) Ventricular homogenates from control NF2 floxed (NF2F/F) and NF2 CKO mice were prepared following sham operation and I/R (30′/30′) and western blot analysis performed. (F-I) Quantitative results from westerns in panel E. *, P<0.05. N.S. = not significant. (J) Real time quantitative PCR was performed using cDNA prepared from control NF2 floxed (NF2F/F) and NF2 CKO mice subjected to either sham or I/R (30′/120′). *, P<0.05 versus NF2F/F sham. #, P<0.05 versus NF2F/F I/R. N = 3-4 mice per group. Representative images for each experiment are shown.
Yap activation is upregulated in NF2 CKO myocardium
Yap is a transcriptional co-factor and pro-survival signaling molecule in cardiomyocytes9; therefore, we hypothesized that altered gene expression due to Yap modulation might explain the protective effect observed in NF2 CKO mice. We first tested whether established Yap target genes were altered following Yap modification in NRVMs. Overexpression of Yap in NRVMs caused a significant increase in mRNA levels of ctgf, cyr61, fgf2 and birc5 compared to LacZ control (Online Figure VIIA). Conversely, shRNA-mediated knockdown of endogenous Yap significantly reduced Yap target gene expression (Online Figure VIIB). To test whether NF2 affects Yap transcriptional activity in NRVMs, we used a TEAD luciferase reporter system and either overexpressed or silenced NF2, which resulted in a significant decrease and increase in reporter gene expression, respectively (Online Figure VIIC and D). We also examined expression of these Yap targets by qRT-PCR and observed a significant upregulation of the aforementioned genes in NF2 CKO hearts compared to controls (Figure 5J). Taken together these results suggest that Yap activates a genetic program in the heart similar to that observed in the liver26 and brain27, and indicate that Yap activity is upregulated in hearts deficient for NF2.
Genetic inhibition of Yap abolishes the protection observed in NF2 CKO mice
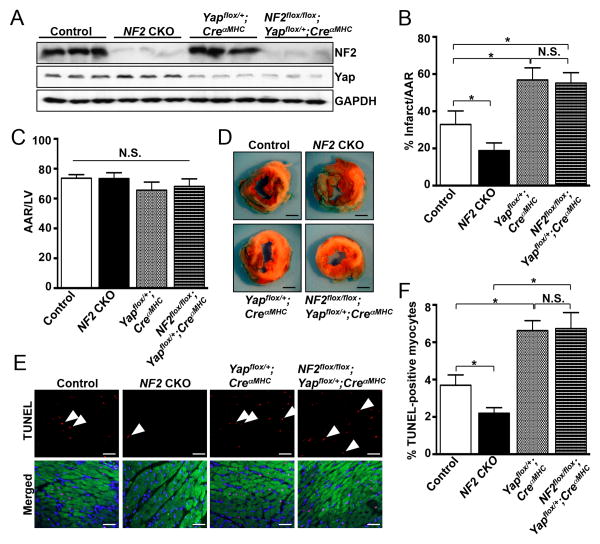
Finally, we tested whether Yap activity is required for the protective effect of NF2 depletion in vivo. NF2 CKO, Yapflox/+;CreαMHC , NF2-Yap double deficient (NF2flox/flox;Yapflox/+;CreαMHC ) and control (NF2flox/flox;Yapflox/+ ) mice were generated. The expected reduction in NF2 and Yap protein was confirmed by western blot (Figure 6A) and these mice were subjected to I/R. Consistent with our findings above, the NF2 CKO mice had significantly smaller infarcts and reduced TUNEL+ staining compared to control mice (Figure 6B, D and E). There were no differences in AAR/LV between the groups (Figure 6C). Interestingly, we observed a significant increase in infarct size in the Yapflox/+;CreαMHC and NF2flox/flox;Yapflox/+;CreαMHC myocardium versus controls (Figure 6B and D). Furthermore, we observed a significant increase in TUNEL+ cardiomyocyte nuclei in Yapflox/+;CreαMHC hearts compared to controls as well as in NF2flox/flox;Yapflox/+;CreαMHC hearts compared to NF2 CKO hearts following I/R (Figure 6E and F). Taken together, these results indicate a loss of cardioprotection in the NF2flox/flox;Yapflox/+;CreαMHC mice that is likely due to normalization of Yap activity.
Figure 6. Hemizygous deletion of myocardial Yap abrogates the cardioprotection observed in NF2 CKO hearts following I/R.
Control (NF2flox/flox;Yapflox/+) NF2 CKO (NF2flox/flox;CreaMHC), Yapflox/+;CreaMHC, and NF2-Yap double deficient mice (NF2flox/flox;Yapflox/+;CreaMHC) were generated. (A) Ventricular homogenates from all groups were subjected to western analysis to determine levels of NF2 and Yap protein. (B-D) All groups were subjected to I/R (30′/24hr) and infarct size and area at risk (AAR) determined by TTC and Alcian blue staining respectively. Scale bar, 1 mm. (E) Representative images from TUNEL staining of heart sections following I/R (30′/24hr) are shown. Sections were counterstained with DAPI (blue) and troponin-T (green) to identify nuclei and cardiomyocytes, respectively (Merged). Scale bar, 50 μm. (F) Average data as determined by TUNEL. *, P<0.05. N.S. = not significant. N = 5-8 mice per group.
Discussion
NF2 is a recognized tumor suppressor that has been shown to promote Hippo signaling in Drosophila 23-25. Subsequent work has demonstrated that NF2 can modulate mammalian Hippo signaling in the murine liver26 and brain27. In a recent report from Marian and colleagues, NF2 was shown to be activated in a mouse model of arrhythmogenic cardiomyopathy (AC) and in heart samples from AC patients28. Murine models of AC displayed increased activation of Hippo signaling and reduced Yap activation, which was shown to contribute to the enhanced adipogenesis observed. However, prior to our study, a loss-of-function mouse had not been used to test whether NF2 regulates Hippo signaling and contributes to myocardial injury caused by I/R. We demonstrate here that NF2 is activated in cardiomyocytes and mouse myocardium in response to oxidative stress and I/R, and contributes to cardiac injury through engagement of the Hippo pathway and subsequent inactivation of the transcriptional co-factor Yap.
Regulation of NF2 through multiple posttranslational modifications has been demonstrated previously. NF2 is negatively regulated via Ser518 phosphorylation by PKA18 and PAK216, 17, which promotes its inactive conformation, and thereby attenuates downstream signaling15, 22. Alternatively, dephosphorylation of Ser518 mediated by MYPT-1 activates NF219. NF2 function can also be modulated through Akt-mediated phosphorylation at Thr230 and Ser31540. These Akt-dependent modifications both inhibited the association of NF2 with effectors while also eliciting increased ubiquitin-mediated NF2 degradation. Our study demonstrates that MYPT-1 associates with NF2 and promotes its dephosphorylation, indicating that MYPT-1 is an important promoter of NF2 activity in cardiomyocytes. MYPT-1 was also activated (as determined by regulatory phosphorylation at Thr696) following I/R; however, establishing evidence that MYPT-1 is required for NF2 activation in vivo is a limitation of this study and warrants further investigation. More recent work has demonstrated that NF2 is also a substrate of sumoylation. It was reported that SUMO-1 modification at Lys76 led to decreased active conformation status, altered subcellular localization, and impaired tumor suppressor function suggesting that sumoylation is important for proper NF2 signaling41. Whether or not NF2 is sumoylated in cardiomyocytes, and if this modification modulates NF2 activity in the heart, remains to be determined.
The subcellular localization of NF2 has been investigated in multiple cell types with varied observations34-37, 42, 43. Recent findings from the Pan laboratory demonstrated that plasma membrane association of NF2 and direct binding with Lats1/2 is critical for engagement of Hippo signaling and subsequent inactivation of Yap36. In other work, Giancotti and colleagues have demonstrated that NF2 can shuttle between the cytosol and nucleus, an observation that may be mediated by a nuclear localization motif present in the N-terminal portion of NF2 44. It is likely that observed differences in subcellular localization of NF2 are due to cell type specificity, and that NF2 has important cellular functions in multiple locations. In NRVMs, AMCMs and mouse heart sections we observed NF2 in the cytosol and nucleus, and relatively lower levels at the plasma membrane, using both biochemical fraction enrichment and immunofluorescence-based approaches. In contrast, p-NF2 was detected in cytosolic and nuclear fractions, with relative enrichment at the plasma membrane. Importantly, nuclear p-NF2 levels decreased after oxidative stress indicating this is an important site of NF2 activation. We also observed a subpopulation of Mst1 in nuclear-enriched fractions of stressed cardiomyocytes and hearts, and Lats2 appeared almost exclusively nuclear, similar to our prior work8. The AC study mentioned above also examined the distribution of Hippo components by immunostaining heart sections28. In mouse myocardium, p-NF2 was reported to localize to cardiomyocyte desmosomes. This is not inconsistent with our current findings, which demonstrate p-NF2 at the plasma membrane. It should be noted that this prior study did not observe localization of p-Mst1 in the nucleus. It is possible that this apparent discrepancy concerning Mst1 is due to the type of stress imposed, or the potential for heterogeneity between cardiomyocytes in the heart. Indeed, our NF2 staining results suggest that nuclear NF2 may be more pronounced in a select subset of cardiomyocytes. We believe it is likely that NF2 serves important physiological functions in multiple subcellular regions. Based on our localization and immunoprecipitation results, we propose that the nucleus is one of perhaps several important focal points of NF2-Hippo signaling in the cardiomyocyte.
Our prior work demonstrated a deleterious role for Mst1 during I/R4, 7. These studies interrogated both upstream regulation and downstream effectors of Mst1 in this setting. We identified a K-Ras/RASSF1A/Mst1 complex present at mitochondria that mediated activation of Mst1 at this subcellular locale. We also found that active Mst1 phosphorylates Bcl-xL, thereby attenuating Bcl-xL-Bax interaction and increasing Bax activation and cardiomyocyte apoptosis7. Importantly, we demonstrated that this non-canonical Hippo signaling (i.e. not through Lats2/Yap) contributes to I/R injury45. Our current findings extend this work by demonstrating that NF2 is a regulator of canonical Hippo signaling in the myocardium and implicating the nucleus as a target organelle in which this signaling occurs. Based on our findings we hypothesize that NF2 and K-Ras/RASSF1A function to activate Mst1 in separate subcellular locations (mitochondria vs. nucleus) leading to engagement of different downstream effectors (Bcl-xL vs. Lats2/Yap). Mst1 has also been shown to inhibit cardiomyocyte autophagy and contribute to ischemic injury31. Although we found that NF2 promotes Mst1 activation, we did not observe a significant effect of NF2 on cardiomyocyte autophagy (Online Figure VIII), although further study is needed to examine this in greater detail. Taken together, we propose that inhibition of Mst1 likely confers cardioprotection by preventing multiple signaling mechanisms and would be advantageous versus targeting a single pathway in isolation.
Mst1 contains a regulatory phosphorylation site, Thr183, in its N-terminal catalytic domain46. Autophosphorylation of Thr183 has been shown to be important for Mst1 activation and homodimerization, the latter mediated by its C-terminal SARAH domain47. The SARAH domain also allows for heterodimerization between Mst1 and RASSF1A, NORE1 and Salvador (Sav1), known activators of the kinase. Structural studies of Mst1 demonstrated the importance of h1 and h2 helices for homodimerization of Mst1 monomers48; however, the precise molecular mechanism of Mst1 activation remains unclear and it is possible that additional kinases may play an important role49, 50. We observed association between NF2 and MYPT-1, Mst1 and Lats2 and increased phosphorylation of Mst1 following oxidative stress. Our results lead us to speculate the formation of a nuclear complex that could include additional components (e.g. Sav1, MOB1A/B, MAP4K/Happyhour)51, 52. We hypothesize that recruitment of Mst1 to this complex may be facilitated by Lats2, as a direct interaction between NF2 and Lats2 has been demonstrated previously36.
Based on the literature regarding the function of Yap in the mammalian heart, it is not entirely surprising that ablation of a negative regulator of Yap activity, in this case NF2, would prove cardioprotective against I/R injury. Our previous work demonstrated that inhibition of Lats2 in the myocardium afforded protection against I/R injury through increased Yap-FoxO1 transcriptional activation8. Through loss-of-function studies, Yap has been shown to be critical for adult heart homeostasis and protection against cardiomyocyte apoptosis, as well as a promoter of cardiomyocyte growth and proliferation9, 53, 54. Conversely, strategies to increase Yap expression and activity stimulate proliferation of cardiomyocytes and cardioprotection against MI and resection of the mouse heart53, 55. Our current study demonstrates that the cardioprotection observed in NF2 CKO mice is abrogated by Yap heterozygosity in cardiomyocytes. We believe this is strong evidence that Yap functions downstream of NF2; however, we cannot exclude the possibility that Yap acts in a parallel pathway that supersedes the NF2 effect in this setting.
Our results indicate that NF2 is activated by oxidative stress and has a presence in the nuclei of cardiomyocytes where it promotes activation of Mst1 and inhibitory phosphorylation of Yap (Figure 7). This work further supports the notion that increased Yap activity during acute stress/injury serves to protect the myocardium and highlights Yap as an attractive target for potential future therapies against MI.
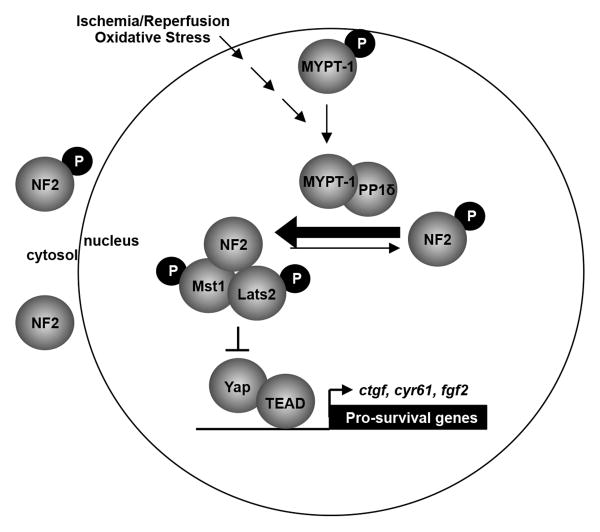
Figure 7. Schema illustrating a working hypothesis of NF2-related signaling in the cardiomyocyte during oxidative stress.
We propose that oxidative stress stimulates dephosphorylation of NF2 via MYPT-1-PP1δ thereby promoting an active conformation of NF2. Active NF2 associates with Mst1 and Lats2 in the cardiomyocyte nucleus, promotes Mst1 activation, and negatively regulates Yap target gene expression.
Supplementary Material
Novelty and Significance.
What Is Known?
Myocardial infarction (MI) results in cardiomyocyte loss and insufficient self-renewal, which contribute to injury and compromised heart function.
Increasing evidence suggests that Hippo-Yes-associated protein (Yap) signaling can modulate cardiomyocyte death and proliferation in the postnatal heart.
Neurofibromin 2 (NF2) has been linked to the Hippo-Yap pathway in mouse liver and brain, and is associated with arrhythmogenic cardiomyopathy (AC) in human and murine hearts.
What New Information Does This Article Contribute?
NF2 interaction with mammalian sterile 20-like kinase 1 (Mst1) and large tumor suppressor kinase 2 (Lats2) in the cardiomyocyte nucleus is associated with Mst1 activation following oxidative stress.
Phosphorylated (inactive) NF2 is decreased in failing human hearts and after ischemia/reperfusion in mouse hearts.
Cardiomyocyte-specific deletion of NF2 confers protection against ischemia/reperfusion injury in the mouse heart.
Yap transcriptional activity is upregulated in NF2 cardiomyocyte-specific knockout hearts, and evidence implicates heightened Yap function as a mediator of the observed cardioprotection.
Cardiomyocyte loss contributes to injury after MI. The Hippo-Yap pathway has emerged as an important modulator of cardiomyocyte survival and proliferation, yet mechanisms that underlie signaling initiation remain incompletely understood. These findings indicate that NF2 facilitates activation of the core Hippo pathway kinase Mst1, and negatively regulates the transcription co-factor Yap, to influence cardiomyocyte apoptosis and myocardial injury following ischemia/reperfusion.
Acknowledgments
The authors thank Dr. Giovannini (University of California, Los Angeles) for generously providing the NF2 floxed mice, L. Fritzky for confocal microscopy expertise, C. Brady for critical reading of the manuscript and M. Tong for assistance with adult mouse cardiomyocyte isolation.
Sources of Funding: This work was supported by grants from the NIH (HL127339 and HL122669 to D.P.D.; HL112330, HL102738, HL067724, HL091469, and AG023039 to J.S.), the Fondation Leducq Transatlantic Network of Excellence (J.S.), and an American Heart Association Scientist Development Grant (11SDG7240067 to D.P.D.).
Nonstandard Abbreviations and Acronyms
- αMHC
alpha myosin heavy chain
- AAR
area at risk
- AC
arrhythmogenic cardiomyopathy
- AMCMs
adult mouse cardiomyocytes
- CKO
cardiomyocyte-specific knockout
- IP
immunoprecipitation
- I/R
ischemia/reperfusion
- Lats2
large tumor suppressor kinase 2
- LV
left ventricle
- MI
myocardial infarction
- Mst1
mammalian sterile 20-like kinase 1
- MYPT-1
myosin phosphatase target subunit 1
- NF2
neurofibromin 2
- NRVMs
neonatal rat ventricular myocytes
- PP1δ
serine/threonine-protein phosphatase 1 delta
- RASSF1A
Ras association domain family protein 1A
- Sav1
Salvador
- TTC
triphenyltetrazolium chloride
- TUNEL
terminal deoxynucleotidyl transferase
- dUTP
nick end labeling
- WT
wild-type
- Yap
Yes-associated protein
Footnotes
Disclosures: None.
In May 2016, the average time from submission to first decision for all original research papers submitted to Circulation Research was 14.93 days.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto S, Yang G, Zablocki D, Liu J, Hong C, Kim SJ, Soler S, Odashima M, Thaisz J, Yehia G, Molina CA, Yatani A, Vatner DE, Vatner SF, Sadoshima J. Activation of mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111:1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odashima M, Usui S, Takagi H, Hong C, Liu J, Yokota M, Sadoshima J. Inhibition of endogenous mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ Res. 2007;100:1344–1352. doi: 10.1161/01.RES.0000265846.23485.7a. [DOI] [PubMed] [Google Scholar]
- 6.Del Re DP, Matsuda T, Zhai P, Gao S, Clark GJ, Van Der Weyden L, Sadoshima J. Proapoptotic rassf1a/mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J Clin Invest. 2010;120:3555–3567. doi: 10.1172/JCI43569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Re DP, Matsuda T, Zhai P, Maejima Y, Jain MR, Liu T, Li H, Hsu CP, Sadoshima J. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of bcl-xl. Mol Cell. 2014;54:639–650. doi: 10.1016/j.molcel.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, Lim DS, Pan D, Sadoshima J. A functional interaction between hippo-yap signalling and foxo1 mediates the oxidative stress response. Nature communications. 2014;5:3315. doi: 10.1038/ncomms4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, Sadoshima J. Yes-associated protein isoform 1 (yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013;288:3977–3988. doi: 10.1074/jbc.M112.436311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis--the nf2 tumor suppressor, merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 11.Okada T, You L, Giancotti FG. Shedding light on merlin's wizardry. Trends in cell biology. 2007;17:222–229. doi: 10.1016/j.tcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Shaw RJ, McClatchey AI, Jacks T. Regulation of the neurofibromatosis type 2 tumor suppressor protein, merlin, by adhesion and growth arrest stimuli. J Biol Chem. 1998;273:7757–7764. doi: 10.1074/jbc.273.13.7757. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi AB, Hara T, Ramesh V, Gao J, Klein-Szanto AJ, Morin F, Menon AG, Trofatter JA, Gusella JF, Seizinger BR, et al. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet. 1994;6:185–192. doi: 10.1038/ng0294-185. [DOI] [PubMed] [Google Scholar]
- 14.McClatchey AI, Saotome I, Mercer K, Crowley D, Gusella JF, Bronson RT, Jacks T. Mice heterozygous for a mutation at the nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 1998;12:1121–1133. doi: 10.1101/gad.12.8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for nf2 growth suppression. Oncogene. 2004;23:8447–8454. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- 16.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10394–10399. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 17.Xiao GH, Beeser A, Chernoff J, Testa JR. P21-activated kinase links rac/cdc42 signaling to merlin. J Biol Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 18.Alfthan K, Heiska L, Gronholm M, Renkema GH, Carpen O. Cyclic amp-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin-ezrin heterodimerization. J Biol Chem. 2004;279:18559–18566. doi: 10.1074/jbc.M313916200. [DOI] [PubMed] [Google Scholar]
- 19.Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by cpi-17 through inhibition of a merlin phosphatase. Nature. 2006;442:576–579. doi: 10.1038/nature04856. [DOI] [PubMed] [Google Scholar]
- 20.LaJeunesse DR, McCartney BM, Fehon RG. Structural analysis of drosophila merlin reveals functional domains important for growth control and subcellular localization. J Cell Biol. 1998;141:1589–1599. doi: 10.1083/jcb.141.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surace EI, Haipek CA, Gutmann DH. Effect of merlin phosphorylation on neurofibromatosis 2 (nf2) gene function. Oncogene. 2004;23:580–587. doi: 10.1038/sj.onc.1207142. [DOI] [PubMed] [Google Scholar]
- 22.Sherman L, Xu HM, Geist RT, Saporito-Irwin S, Howells N, Ponta H, Herrlich P, Gutmann DH. Interdomain binding mediates tumor growth suppression by the nf2 gene product. Oncogene. 1997;15:2505–2509. doi: 10.1038/sj.onc.1201418. [DOI] [PubMed] [Google Scholar]
- 23.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes nf2/merlin and expanded act through hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 24.Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, Tao C, Zhang X, Halder G. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of yap oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The merlin/nf2 tumor suppressor functions through the yap oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavado A, He Y, Pare J, Neale G, Olson EN, Giovannini M, Cao X. Tumor suppressor nf2 limits expansion of the neural progenitor pool by inhibiting yap/taz transcriptional coactivators. Development. 2013;140:3323–3334. doi: 10.1242/dev.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res. 2014;114:454–468. doi: 10.1161/CIRCRESAHA.114.302810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G. Conditional biallelic nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–1630. [PMC free article] [PubMed] [Google Scholar]
- 30.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maejima Y, Kyoi S, Zhai P, Liu T, Li H, Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, Lim DS, Isobe M, Sadoshima J. Mst1 inhibits autophagy by promoting the interaction between beclin1 and bcl-2. Nat Med. 2013;19:1478–1488. doi: 10.1038/nm.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the hippo tumour suppressor pathway by integrin-linked kinase. Nature communications. 2013;4:2976. doi: 10.1038/ncomms3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Primrose DA, Leung AC, Fitzsimmons RB, McDermand MC, Missellbrook A, Haskins J, Smylie AS, Hughes SC. The pp1 phosphatase flapwing regulates the activity of merlin and moesin in drosophila. Dev Biol. 2012;361:412–426. doi: 10.1016/j.ydbio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, Persson NL, Shimono A, Speicher DW, Marmorstein R, Holmgren L, Kissil JL. A tight junction-associated merlin-angiomotin complex mediates merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19:527–540. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The nf2 tumor suppressor, merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of hippo signaling at the plasma membrane mediated by the tumor suppressor merlin/nf2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, Ishii R, Giovannini M, Hanemann CO, Long SB, Erdjument-Bromage H, Zhou P, Tempst P, Giancotti FG. Merlin/nf2 suppresses tumorigenesis by inhibiting the e3 ubiquitin ligase crl4(dcaf1) in the nucleus. Cell. 2010;140:477–490. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan T, Shelton M, Lambert JP, Malecova B, Boisvenue S, Ruel M, Figeys D, Puri PL, Skerjanc IS. Myosin phosphatase modulates the cardiac cell fate by regulating the subcellular localization of nkx2.5 in a wnt/rho-associated protein kinase-dependent pathway. Circ Res. 2013;112:257–266. doi: 10.1161/CIRCRESAHA.112.275818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates hippo signaling in conjunction with merlin and expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang X, Jang SW, Wang X, Liu Z, Bahr SM, Sun SY, Brat D, Gutmann DH, Ye K. Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol. 2007;9:1199–1207. doi: 10.1038/ncb1641. [DOI] [PubMed] [Google Scholar]
- 41.Qi Q, Liu X, Brat DJ, Ye K. Merlin sumoylation is required for its tumor suppressor activity. Oncogene. 2014;33:4893–4903. doi: 10.1038/onc.2013.438. [DOI] [PubMed] [Google Scholar]
- 42.Shaw RJ, McClatchey AI, Jacks T. Localization and functional domains of the neurofibromatosis type ii tumor suppressor, merlin. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1998;9:287–296. [PubMed] [Google Scholar]
- 43.McCartney BM, Fehon RG. Distinct cellular and subcellular patterns of expression imply distinct functions for the drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J Cell Biol. 1996;133:843–852. doi: 10.1083/jcb.133.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D, Snuderl M, Ladanyi M, Hanemann CO, Zhou P, Karajannis MA, Giancotti FG. Merlin/nf2 loss-driven tumorigenesis linked to crl4(dcaf1)-mediated inhibition of the hippo pathway kinases lats1 and 2 in the nucleus. Cancer Cell. 2014;26:48–60. doi: 10.1016/j.ccr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura M, Zhai P, Del Re DP, Maejima Y, Sadoshima J. Mst1-mediated phosphorylation of bcl-xl is required for myocardial reperfusion injury. JCI Insight. 2016;1 doi: 10.1172/jci.insight.86217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KK, Yonehara S. Phosphorylation and dimerization regulate nucleocytoplasmic shuttling of mammalian ste20-like kinase (mst) J Biol Chem. 2002;277:12351–12358. doi: 10.1074/jbc.M108138200. [DOI] [PubMed] [Google Scholar]
- 47.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the mst1 kinase by autophosphorylation, by the growth inhibitory proteins, rassf1 and nore1, and by ras. Biochem J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang E, Ryu KS, Paakkonen K, Guntert P, Cheong HK, Lim DS, Lee JO, Jeon YH, Cheong C. Structural insight into dimeric interaction of the sarah domains from mst1 and rassf family proteins in the apoptosis pathway. Proc Natl Acad Sci U S A. 2007;104:9236–9241. doi: 10.1073/pnas.0610716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates hippo/mst kinases to regulate the hippo-salvador-warts tumor suppressor pathway. Dev Cell. 2011;21:888–895. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase tao-1 controls tissue growth by regulating the salvador-warts-hippo pathway. Dev Cell. 2011;21:896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of happyhour/map4k as alternative hpo/mst-like kinases in the hippo kinase cascade. Dev Cell. 2015;34:642–655. doi: 10.1016/j.devcel.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, Flores F, Yu FX, Halder G, Guan KL. Map4k family kinases act in parallel to mst1/2 to activate lats1/2 in the hippo pathway. Nature communications. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT. Yap1, the nuclear target of hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012;109:2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT. Cardiac-specific yap activation improves cardiac function and survival in an experimental murine myocardial infarction model. Circ Res. 2014;115:354–363. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.